Abstract

Co-sensitization is an important strategy toward efficiency enhancement of solar cells by enabling better light harvesting across the solar spectrum. Betanin is a natural dye which absorbs light in the major portion of the incident solar spectrum (green region) and is the most efficient natural pigment used in dye-sensitized solar cells. This study investigates the performance enhancement of a betanin solar cell by co-sensitizing it with two natural pigments which show complementary light absorption, i.e., indigo and lawsone, absorbing in the red and blue regions of the solar spectrum, respectively. The calculated highest occupied molecular orbital and lowest unoccupied molecular orbital energies of the pigment molecules, derived from density functional theory (DFT) simulations, confirmed their optimal alignment with respect to the conduction band energy of the TiO2 semiconductor and reduction potential energy level of the I–/I3– electrolyte, a necessary requirement for optimal device performance. Lawsone solar cells displayed better performance, showing average efficiencies of 0.311 ± 0.034%, compared to indigo solar cells showing efficiencies of 0.060 ± 0.004%. Betanin was co-sensitized with indigo and lawsone, and the performances of the co-sensitized solar cells were compared. The betanin/lawsone co-sensitized solar cell showed a higher average efficiency of 0.793 ± 0.021% compared to 0.655 ± 0.019% obtained for the betanin/indigo co-sensitized solar cell. An 11.7% enhancement in efficiency (with respect to betanin) was observed for the betanin/indigo solar cell, whereas a higher enhancement of 25.5% was observed for the betanin/lawsone solar cell. Electrochemical impedance spectroscopy studies confirmed that the higher efficiency can be attributed to the higher electron lifetime of 313.8 ms in the betanin/lawsone co-sensitized solar cell compared to 291.4 ms in the betanin/indigo solar cell. This is due to the energy levels being more optimally aligned in lawsone compared to that of indigo, as observed in the DFT studies, and the lack of dipole moment in indigo, resulting in more efficient charge separation and charge transfer in lawsone.
Introduction
Pioneering dye-sensitized solar cells (DSSCs) were realized by O’Regan and Grätzel in 1991, which have now garnered significant attention owing to their facile fabrication, making them an attractive alternative to first and second generation solar cells. A DSSC typically consists of a photoanode and a counter electrode, sandwiching a redox couple electrolyte in between. The photoanodes are primarily transparent conductive oxide [such as indium-doped tin oxide or fluorine-doped tin oxide (FTO)]-coated glass substrates upon which a wide band gap semiconductor such as titanium dioxide (TiO2) is coated. The mesoporous titanium dioxide layer plays the role of a scaffold, enabling the adsorption of pigment molecules and charge transfer.1,2 Platinum-coated FTO substrates are widely used as catalytic counter electrodes. In between the photoanode and the counter electrode is filled a suitable redox electrolyte that helps in the reduction of the oxidized dye molecules and in shuttling electrons from the counter electrode.
The photoanode plays the major role of light absorption, generation of excitons, and charge transfer, and hence the photosensitizer is a particularly important component of the DSSC.3,4 Ruthenium- and osmium-based polypyridyl complex dyes have so far resulted in the best efficiencies of ∼11% and stability.1,5 These photosensitizers possess the general structure ML2(X)2, where M, L, and X represent Ru or Os, 2,2′bipyridyl-4,4-dicarboxylic acid, and halide/cyanide/thiocyanate/acetylacetonate/thiocarbamate/water groups, respectively. The N3 dye, i.e., cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato ruthenium(II), is the pioneering dye used in DSSCs since 1993, whose performance remained unmatched for several years.6 Ruthenium complex-based dyes, though high-performing, are extremely expensive because of the rarity of the ruthenium metal and the complex preparatory processes. Moreover, they are highly toxic, and therefore, in the long run, their disposal would pose a serious problem.
In contrast, natural dyes are nontoxic, environment-friendly, and easily extracted from various plant parts such as leaves, fruits, flowers, and roots. Therefore, they are an excellent alternative for application as photosensitizers in DSSCs.7,8 Numerous natural dyes such as chlorophylls,9,10 carotenoids,11 flavonoids such as anthocyanins12,13 and betalains,7,14−16 and tannins17−19 have been studied as photosensitizers in DSSCs. However, poor efficiencies and limited life span are the primary issues faced by solar cells prepared using natural pigments. Till date, mean photoelectric conversion efficiencies of ∼0.4% have been demonstrated by these natural DSSCs.7,9−15,18,20 The underwhelming performance of natural dye solar cells is primarily because of the narrow absorption bands and low absorption coefficients of the dyes compared to the conventionally used synthetic dyes.21
Previous studies indicate that betanin is a comparatively more efficient plant pigment and has demonstrated the best efficiencies (1.5–2%) among natural dye-based solar cells.15,22 Betanin is an interesting plant pigment absorbing in the 476–600 nm range, thereby harnessing solar radiation in the green portion of the visible spectrum, where most of the incident solar radiation falls. The broad-band absorption of betanin in covering the green region of the visible spectrum, combined with the presence of strong anchoring groups, could be the reason for its relatively higher solar cell efficiencies compared to other natural pigments.
Augmenting the efficiencies achieved by betanin solar cells would render these “green” solar cells an appealing technology, suitable for application in disposable portable electronics.
Efficiency enhancement by widening the absorption band23,24 using multiple materials is a strategy that has been explored long since the inception of multijunction solar cells.25 Combining betanin along with pigments absorbing in the complementary regions of the solar spectrum is a promising strategy for enhancing the efficiency of betanin solar cells. Our previous study involving the co-sensitization of betanin with chlorophyll26 indicated that betanin solar cells showed a relatively better performance, giving average efficiencies of 0.538 ± 0.021%; however, chlorophyll was observed to be an inefficient photosensitizer and did not contribute much to the overall efficiency. In this study, alternate natural pigments, which absorb light in regions complementary to betanin, have been explored (following methods similar to those described in our previous publication26) to study efficiency enhancement via co-sensitization. In this study, we compare the performance enhancement of betanin (λmax = 535 nm,27 the green region) through co-sensitization with (i) indigo (λmax = 625 nm,28 the red region) and (ii) lawsone (λmax = 410 nm,17 the blue region). Indigo and lawsone are natural pigments and were extracted from the plant sources—Indigofera tinctoria and Lawsonia inermis, and used in this study. The aim of this study is to explore plant pigments that complement the absorption spectrum of betanin for performance enhancement in DSSCs. Lawsone would complement betanin by extending the light harvesting to the blue region, and indigo would complement betanin by extending the light-harvesting to the red region. Several factors involved in the fabrication of natural dye solar cells have been optimized in this work.
So as to make certain that the performance of solar cells was optimal, the band gap and the appropriate alignment of the pigments were confirmed using DFT (density functional theory) simulations29 using Gaussian0930 software. Although DFT studies of the betanin molecule have previously been explored,26 the DFT studies of indigo and lawsone are limited in the literature. The computational studies helped in predicting the positions of the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) levels and thereby the band gap of the molecules. Moreover, it helped in understanding the intramolecular electron density shift, caused by photoexcitation in the donor–linker–acceptor groups. The dipole moments and charge distribution of the pigments were also determined from the simulations, which helped in predicting how well the pigment would interact and bind with TiO2. The DFT simulations of the pigment molecules helped gain better insights into their electronic features and predict their feasibility of application in DSSCs. The feasibility of application of these dyes predicted by the DFT simulations was confirmed to be accurate by the performance of the solar cells, proving this method to be an invaluable tool in selecting the right dyes from the vast array of pigments available in nature.
In this paper, we report the comparison of performance enhancement by the co-sensitization of betanin with indigo and with lawsone, the natural pigments derived from plant sources—I. tinctoria and L. inermis. Individual and co-sensitized configurations were fabricated and their performances were assessed. As expected, the co-sensitized solar cell configurations demonstrated better efficiencies than the individually sensitized configurations because of the broader absorption of incident light resulting in a higher current density. On comparing the co-sensitized configurations, the betanin/lawsone co-sensitized solar cell had a higher average efficiency of 0.793 ± 0.021%, whereas an average efficiency of 0.655 ± 0.019% was demonstrated by the betanin/indigo co-sensitized solar cell. Electrochemical impedance spectroscopy (EIS) of the solar cells was carried out to understand the internal resistances and electron lifetimes of the solar cells31 and correlate them with their performance characteristics. The dye degradation of indigo and lawsone because of the photocatalytic activity (PCA) of TiO2 and its effect on the solar cell lifetimes has also been investigated.
Results and Discussion
Structures of the Two Natural Dye Co-Sensitized Solar Cells
The diagrammatic representations of both the natural dye co-sensitized solar cell configurations, betanin/indigo and betanin/lawsone solar cells, are shown in Figure 1. A TiO2 mesoporous film is coated onto the FTO conductive glass substrate. The two configurations of solar cells consist of the TiO2 film co-sensitized using the betanin pigment with complementary dyes: (a) with indigo in one configuration and (b) with lawsone in the other configuration. The FTO glass substrate coated with the TiO2 film and sensitized by the dye functions as the photoanode. Pt-coated FTO electrodes have been employed as the counter electrodes. Between both the electrodes is the iodide/triiodide redox mediator.
Figure 1.
Illustration of the device showing the complementary absorption by the co-sensitized pigments (a) betanin and indigo and (b) betanin and lawsone (image source: pictures of the pigment sources shown as insets are from the public domain).
Betanin consists of the betaine moiety, i.e., an indole-2-carboxylic acid, N-linked through an acetyl group to the betalamic acid group, i.e., a pyridine dicarboxylic acid group. The electron transitions occurring in the conjugated systems cause light absorption by betanin in the green region of the visible-light spectrum. The molecule possesses many carboxylic acid groups, which enable strong anchoring onto TiO2 with good dipole interactions. The mechanism of binding of betanin with TiO2 is through bidentate chelation32 via the carboxyl groups. Indican is a colorless, naturally occurring water-soluble derivative of tryptophan (amino acid), found in I. tinctoria plants. It is the precursor of the dye, indigo. On hydrolysis of indican, β-d-glucose and indoxyl are released. Oxidation of indoxyl, on exposure to air, converts it to indigo. Indican was extracted from I. tinctoria leaves by fermentation, which converts the glycoside indican (0.2–0.8% of this compound is present in I. tinctoria leaves) to the compound indigotin, responsible for the blue color of the indigo dye. Lawsone, also known as hennotannic acid, is chemically 2-hydroxy-1, 4-naphthoquinone. The lawsone molecule consists of two oxygen atoms, which are attached to the naphthalene carbons at positions 1 and 4 forming 1,4-naphthoquinone, and a hydroxyl (−OH) group attached at position 2. Lawsone was extracted from L. inermis (henna) leaves.
The extracted dyes were purified and used as such. No chemical modifications were performed on the dyes. For the best solar cell performances, the combination of pigments used in the solar cells should absorb at wavelengths that coincide well with the incident solar spectrum in the 300–1000 nm range.33 This was confirmed using UV–vis absorption spectroscopic studies, elucidated in the following section.
Absorption Studies of the Dyes
UV–vis absorption spectroscopy of the pigments was performed at wavelengths between 300 and 800 nm. Indigo and lawsone were extracted from I. tinctoria and L. inermis leaves using deionized water and acetone, respectively. The individual absorption spectra of betanin, indigo, and lawsone are shown in Figure 2a. The peak absorbance for indigo and lawsone was obtained at 625 and 410 nm, respectively (Figure 2a), and matched with the values found in the literature.17,28 In the absorption spectrum of lawsone, the first absorption maximum at 338 nm is observed because of the HOMO → LUMO transitions which take place in the C=C (π → π*) and C=O (n → π*) regions of the quinoidal ring of lawsone, and the absorption peak seen in the visible region at 410 nm arises from the n → π* transitions localized mainly around the oxygen atom of the quinoidal ring. In the absorption spectrum of indigo, the absorption maximum at 625 nm is observed because of the n → π* transitions which occur between the electron donor NH and the electron acceptor C=O groups. As determined in our earlier studies, betanin has its absorption maximum at 535 nm.26
Figure 2.
Absorption spectra of (a) liquid extracts of betanin, indigo, and lawsone, (b) photoanode co-sensitized with a mixture of betanin and indigo, and (c) photoanode co-sensitized with a mixture of betanin and lawsone. The chemical structures of betanin, indigo, and lawsone are shown as insets.
To effectively complement the absorption spectrum of betanin, indigo and lawsone were mixed in a 1:1 ratio with betanin, and the absorption spectra of the betanin/indigo and betanin/lawsone mixtures were characterized. As expected, broad absorption bands were observed in both cases (seen in Figure 2b,c), and the combined absorption spectrum is a broad band covering a significant portion of the incident solar radiation (in 300–1000 nm range).33 When incident light falls on the pigments, they generate photocarriers. For an effective transfer of the photoexcited electrons, it is essential to confirm the optimal alignment of the LUMO and HOMO energy levels of the dye molecules with the conduction band of TiO2 and the redox potential of the electrolyte, respectively.
DFT Simulations of the Dyes
To ensure effective charge transfer, it is necessary to have sufficient overlapping of the LUMO of the pigment molecules with the conduction band of titanium dioxide.32 DFT simulations were performed using Gaussian09 software to verify the alignment of the HOMO and LUMO energy levels of the pigment molecules and to determine their band gaps.
The simulations were done using the Becke-3-Lee–Yang–Parr (B3LYP) hybrid functional and the 6-31G(d,p) basis set, as these offer good accuracy for a wide range of organic molecules. By performing energy optimization, the pigment molecules were optimized into energy-favorable conformations. The convergence criterion for energy optimization was set as 10–6 Hartree. The probability distribution of electron density at the molecular orbitals, the energy levels of molecular orbitals (with respect to vacuum), the charge distribution, and the dipole moment of the pigment molecules were obtained from the DFT simulations. The HOMO and LUMO energy levels were determined and used to calculate the band gap of indigo and lawsone.
The band gap of indigo was deduced to be 1.8 eV from the HOMO and LUMO levels obtained from the simulations, closely corresponding to 1.9 eV, which was experimentally determined from the absorption maximum of indigo at 625 nm (also reported in the literature34). The analysis of the molecular orbitals showed that the probability distribution of electron density at the HOMO and LUMO levels is predominantly around the NH and C=O groups in the molecule. The two nonbonding electrons on the N atom participating in the delocalization of the π-electrons of the conjugated systems correspond to the HOMO energy level (Figure 3a) and the antibonding π* orbitals form the LUMO level of indigo (Figure 3b). The photoexcitation of the nonbonding electrons from the electron donor NH group to the antibonding π* orbital in the electron acceptor C=O group form the n → π* transitions. The C=O group helps the pigment anchor with TiO2. The electron density map shown in Figure 3c depicts the distribution of charge on the indigo molecule (green and red colors represent electropositivity and electronegativity, respectively). As the molecule is symmetric, the net dipole moment is negligible and is equal to 0.0053 D.
Figure 3.
Electron density corresponding to the (a) HOMO energy level, (b) LUMO energy level, and (c) charge distributions of indigo. Electron density corresponding to the (d) HOMO energy level, (e) LUMO energy level, and (f) charge distributions of lawsone. (g) Alignment of the energy levels (with respect to vacuum) of the materials with respect to each other.
The band gap of lawsone was deduced to be 2.7 eV from the HOMO and LUMO energy levels, which is close to 2.9 eV, experimentally determined from the absorption peak of lawsone at 410 nm.17 The probability distribution of the electron density corresponding to the HOMO and LUMO levels is located around the benzoid and the quinoid moieties, respectively (Figure 3d,e). The first absorption peak at 338 nm mainly involves HOMO → LUMO transitions within the C=C (π → π*) and C=O (n → π*) regions of the quinoidal ring of lawsone. The absorption peak seen in the visible region at 410 nm arises from the n → π* transitions localized mainly around the oxygen atom of the quinoidal ring. Figure 3f shows the distribution of charge on the lawsone molecule. The carbonyl carbons of C=O groups can be observed to be highly electropositive, indicating the strong electron-withdrawing nature. The C=O group anchors the lawsone molecule onto TiO2. The lawsone molecule shows a net dipole moment equal to 5.78 D.
DFT studies of betanin molecule have already been performed in our previous work.26 The HOMO and LUMO levels and the dipole moment obtained from this study have been listed in Table 1 and are used to plot the relative energy levels in Figure 3g. The HOMO and LUMO energy levels of betanin, indigo, and lawsone determined from the DFT calculations match the energy levels that have been previously determined experimentally using the cyclic voltammetry technique by various research groups,35,36 thus validating these results.
Table 1. HOMO Energies (EHOMO), LUMO Energies (ELUMO), and the Dipole Moment of the Natural Pigments.
| dye molecule | EHOMO (eV) | ELUMO (eV) | dipole moment (μ) (Debye) |
|---|---|---|---|
| indigo | –5.7 | –3.9 | 0.0053 |
| lawsone | –5.3 | –2.6 | 5.7816 |
| betanin26 | –5.2 | –3.1 | 8.6895 |
The difference between the oxidation and reduction potentials with respect to the normal hydrogen electrode (NHE) reference is the band gap of the material. On the basis of this, the energy levels of various dyes used in this study can be determined with respect to vacuum37 and to predict their alignment with respect to one another. The HOMO–LUMO energy levels and the dipole moments of indigo and lawsone molecules obtained from the simulations are listed in Table 1.
For optimal working of the device, the LUMO energy level of the pigment molecules should be positioned above the conduction band of TiO2, and their HOMO energy levels should be positioned below the redox potential of the electrolyte. The band diagram shows an appropriate alignment of the energy levels of lawsone and indigo with respect to those of TiO2 and the electrolyte. However, in the case of indigo, the alignment is not very optimal as the HOMO level is far beneath the redox potential of the electrolyte and the LUMO is too close to the conduction band of TiO2. Moreover, indigo seems to have an almost negligible dipole moment, indicating that its interaction with TiO2 may be weak and the charge transfer may not be effective. This could hamper the solar cell performance.
Optimization of the Sensitization of the Dyes
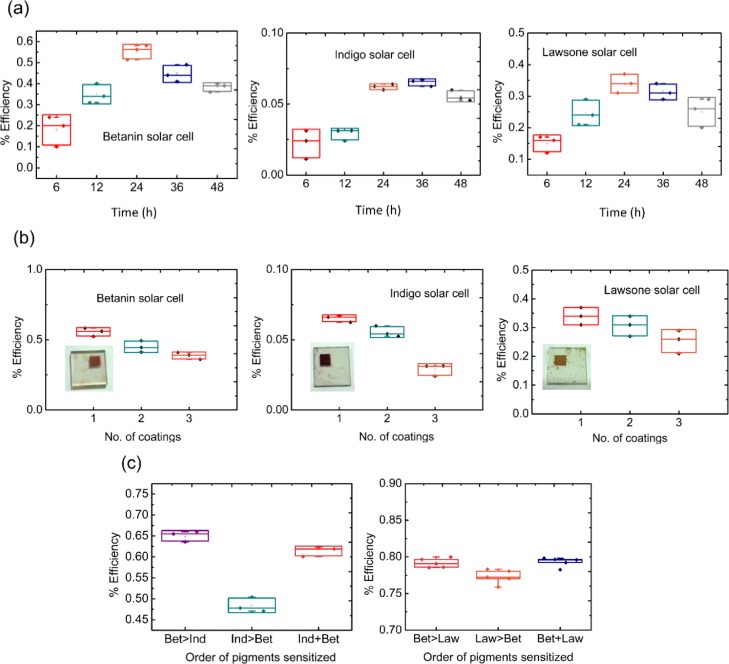
The time of sensitization and number of dye coatings for the solar cell configurations fabricated with betanin, indigo, and lawsone individually were optimized. After this, the sequence of sensitization was optimized in the co-sensitized solar cell configurations. The distribution of cell performance over multiple samples of each configuration is depicted using the box plots (Figure 4a–c).
Figure 4.
Optimization of the sensitization of betanin, indigo, and lawsone pigments: (a) time of sensitization, (b) number of coatings, and the (c) sequence of the sensitization of the pigments in the co-sensitized configuration with betanin [image source: pictures of the photoelectrodes shown as inset in (b) were photographed by the first author].
Betanin, indigo, and lawsone solar cells were fabricated and tested for a time of sensitization of 6, 12, 24, 36, and 48 h. The time 24 h was found to be an optimum time for sensitization in the case of betanin and lawsone solar cells, and 36 h was observed to be optimum in the case of indigo solar cells. The optimum time of sensitization for the best performance of a particular dye is dependent on the rate of dye anchoring up to an optimum concentration. Lawsone and betanin have higher dipole moments favoring the dipole–dipole interaction with TiO2; moreover, they possess more favorable functional groups (−COOH and −OH) compared to indigo (with =CO groups) which will enable a higher rate of anchoring. The dipole moments were calculated using the DFT studies and have been listed in Table 1. Studies by Rajab et al. show that the energy conversion efficiency increased with the dye adsorption time.38 Chang et al. demonstrated that with the increasing dye adsorption time, the chemisorbed dye layer increases; however, beyond an optimum concentration, the increase of the dye layer obstructs the charge transfer from the conduction band of the TiO2 surface to the FTO electrolyte.39 In the case of betanin and lawsone, the binding rates are faster because of the aforementioned reasons; thereby, the optimum concentration of the dye layer at which the dye performs best in the DSSC is achieved faster compared to that of indigo. Three samples of each configuration were tested, and the distribution of the solar cell efficiencies is shown in Figure 4a.
With respect to the number of coatings, in case of all the three solar cell configurations (betanin, indigo, and lawsone), more than one coating proved detrimental to the solar cell performance, possibly because of dye aggregation. The physical aggregation of the dye inhibits the chemical adsorption of the dye with TiO2, reducing the effective charge transfer. Three samples of each configuration were tested, and the distribution of the solar cell efficiencies is shown in Figure 4b.
To determine the optimum sequence of sensitization for the co-sensitized solar cell configurations, the following configurations were fabricated: (i) betanin followed by indigo/lawsone, (ii) indigo/lawsone followed by betanin, and (iii) a mixture of betanin and indigo/lawsone. Five samples of each configuration were tested, and the distribution of the solar cell efficiencies is shown in Figure 4c. It was observed that the premixed solution resulted in slightly higher efficiencies in the case of the betanin/lawsone configuration, whereas sensitizing betanin followed by indigo showed a better performance in the case of the betanin/indigo configuration, as observed from Figure 4c. The optimized solar cell configurations were used for further testing and characterization.
Triple-dye co-sensitized solar cells using all the three dyes were also tested in various orders of sensitization as well as using a mixture. However, the efficiencies of these triple-dye co-sensitized solar cells were lower than that of the betanin solar cell, and hence this was not pursued further. The plausible reasons for the detrimental effect on combining all three dyes could be: (i) too much competition among the dyes for the binding sites with TiO2; this can be inferred by the fact that the efficiency of the mixed solar cell was found to be lower than that of the betanin solar cell; (ii) the presence of additional trap levels where the photoexcited electron can recombine without injection into TiO2, which could cause the rate of back-reactions and recombination to be higher than the favorable kinetics. Hence, these three dyes are not ideal for triple sensitization, though the reason for this is presently unclear. However, there might exist other combinations of dyes that can be explored, which will result in a combined efficiency that is higher than their individual efficiencies.
Photoelectric Conversion Efficiency of the Solar Cells
After optimization, solar cells of five configurations were fabricated: indigo solar cell, lawsone solar cell, betanin solar cell, betanin/indigo co-sensitized solar cell, and betanin/lawsone co-sensitized solar cell. The performance characteristics of the solar cells were measured using a solar simulator under 1 sun illumination. The efficiencies of the solar cells were measured by defining a photoactive area of 0.25 cm2 using a black mask. Five samples were tested for each configuration. The following performance parameters were obtained from the J–V characteristic curves of the solar cells: open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), voltage (Vmax) and current density (Jmax) corresponding to the maximum power extraction, and the photoelectric conversion efficiency (η)and are listed in Table 2.
Table 2. Average Performance Characteristics of the Three Solar Cells Studied.
| performance
characteristics |
||||||
|---|---|---|---|---|---|---|
| Cell | Voc (V) | Jsc (mA cm–2) | Vmax (V) | Jmax (mA cm–2) | FF (%) | efficiency (%) |
| betanin (Bet) | 0.54 | 1.05 | 0.44 | 0.98 | 76.15 | 0.538 ± 0.021 |
| indigo (Ind) | 0.51 | 0.16 | 0.36 | 0.13 | 57.01 | 0.060 ± 0.004 |
| lawsone (Law) | 0.54 | 0.92 | 0.38 | 0.65 | 50.20 | 0.311 ± 0.034 |
| Bet-Ind | 0.54 | 1.52 | 0.43 | 1.20 | 63.88 | 0.655 ± 0.019 |
| Bet-Law | 0.54 | 1.72 | 0.44 | 1.46 | 68.50 | 0.793 ± 0.021 |
The J–V and P–V characteristics of the five samples fabricated in each configuration are shown in Figure 5a,b. A box plot showing the distribution of the efficiencies achieved for the various configurations is shown in Figure 5c. Lawsone solar cells showed better performance, giving average efficiencies of 0.311 ± 0.034% compared to indigo solar cells showing 0.060 ± 0.004%. The lawsone solar cells show higher current density compared to the indigo solar cells.
Figure 5.
Performance characteristics of the five solar cell configurations: betanin (Bet), indigo (Ind), lawsone (Law), betanin/indigo (Bet/Ind), betanin/lawsone (Bet/Law): (a) I–V characteristics, (b) P–V characteristics, and (c) box plot showing the distribution of efficiencies of five samples of each configuration, mean, and deviation.
Betanin was co-sensitized with indigo and lawsone, and the performances of the co-sensitized solar cells were assessed. As expected, the co-sensitized solar cell configurations displayed better performances than the individually sensitized solar cells attributed to their light harvesting over a broader wavelength range, causing an increased current density. Among the two co-sensitized configurations, the betanin/lawsone co-sensitized solar cell gave the best average efficiency of 0.793 ± 0.021% compared to 0.655 ± 0.019% obtained for the betanin/indigo co-sensitized solar cell. An efficiency enhancement (with respect to betanin) of 11.7% was observed for the betanin/indigo solar cell, whereas an enhancement of 25.5% was observed for the betanin/lawsone solar cell.
Study of Electron Lifetime in the Solar Cells
The better performance by lawsone solar cells compared to indigo solar cells and hence the better enhancement observed in betanin/lawsone solar cells can be understood by EIS. EIS is a tool which helps in the better understanding of the electron transfer and recombination processes occurring within DSSCs and can be used to determine the electron lifetimes and correlating the physical processes with the performance of the device.
The Nyquist plots of the solar cell configurations were measured at a frequency range of 100 kHz to 10 mHz, keeping constant a dc voltage bias of 0.5 V (∼Voc of the DSSC). Typical Nyquist plots of DSSCs consist of two semicircles, wherein the semicircle with the larger real part describes the electron recombination in the TiO2 film and that with the smaller real part describes the transfer of electrons at the Pt–FTO electrode/electrolyte interface and from the TiO2 layer to FTO. The physical parameters of DSSCs were extracted by fitting the EIS spectra into a suitable equivalent circuit model that mimics the charge transfer and recombination processes in the device.
The equivalent circuit model depicted in Figure 6a describes the typical internal resistances and capacitances present in a DSSC40 and was used for the fitting of the measured Nyquist plots.
Figure 6.
(a) Equivalent circuit model used for the fitting of Nyquist plots. (b) Nyquist plots generated from the equivalent circuit model fit with the measured data for the five solar cell configurations: betanin (Bet), indigo (Ind), lawsone (Law), betanin/indigo (Bet/Ind), and betanin/lawsone (Bet/Law).
The impedance Z(f) of the model is described by the below equation40
| 1 |
where Rs describes the series resistance in FTO and the loss because of transport in the electrolyte layer, RPt describes the resistance to the electron transfer at the Pt electrode/electrolyte interface, RTiO2 describes the recombination resistance at the TiO2/electrolyte interface, QPt describes the capacitance at the Pt electrode/electrolyte interface, and QTiO2 describes the chemical capacitance of the double layer formed at the TiO2/electrolyte interface.41Q represents the constant phase element (CPE) which is used for describing imperfect capacitive processes. Pure capacitance with the CPE index α equaling 1 occurs very rarely in DSSCs, where often α is lesser than 1. In such devices, the capacitive processes are depicted by CPE elements, so that data fitting is accurate.40
The fitting was done using the Z-Fit function of E-C Lab software by choosing the model shown in Figure 6a, and the χ2 value was minimized to establish the goodness-of-fit of the model. χ2 (Chi square) values having orders <10–4 and error < 1% are regarded as a good fit. The Nyquist plots of the five configurations are shown in Figure 6b. The extracted EIS parameters and the calculated electron lifetimes of the five solar cell configurations are listed in Table 3.
Table 3. Parameters Determined from the EIS Spectra of the DSSCs.
| parameters |
|||||||
|---|---|---|---|---|---|---|---|
| cell | Rs (Ω) | RTiO2 (Ω) | QTiO2 (F sα–1) | α | % error | χ2 | τ (ms) |
| Bet | 15.23 | 32.69 | 0.004 | 0.700 | <0.54 | 2.94 × 10–5 | 54.67 |
| Law | 15.40 | 28.48 | 0.004 | 0.625 | <0.61 | 3.82 × 10–5 | 34.61 |
| Ind | 15.17 | 27.33 | 0.003 | 0.709 | <0.58 | 3.36 × 10–5 | 29.44 |
| Bet/Ind | 15.57 | 53.53 | 0.008 | 0.698 | <0.51 | 4.08 × 10–5 | 291.40 |
| Bet/Law | 16.12 | 57.83 | 0.007 | 0.728 | <0.56 | 3.15 × 10–5 | 313.80 |
The photoanode is of primary interest in this study. EIS helps in relating the internal resistances and electron lifetimes with the performance of DSSCs. For optimum working, a DSSC should possess a high resistance to electron recombination processes. It is known that the electron lifetime (τ) in the photoanode is a function of charge-transfer resistance at the photoanode–electrolyte interface. Greater values of charge-transfer resistance and capacitance predict that the electron lifetime would be longer and that the occurrence of unwanted electron recombination processes at the electrode–electrolyte interface would have lesser odds.42 The efficiency of the solar cell is negatively affected by the recombination of electrons from the conduction band of TiO2 with the redox electrolyte. Therefore, longer electron lifetimes are favorable. Table 3 lists the EIS parameters and the electron lifetimes of the five solar cell configurations, calculated using eq 2.43
| 2 |
A higher electron lifetime is observed in lawsone solar cells (34.61 ms) compared to indigo solar cells (29.44 ms). This is possibly because of the better optimal alignment of energy levels in lawsone compared to indigo as observed in the DFT studies and the lack of dipole moment in indigo, resulting in more efficient charge separation and charge transfer in lawsone. The lower value of RTiO2 and QTiO2 in indigo solar cells (27.33 Ω, 0.003 F sα–1) compared to that in lawsone solar cells (28.48 Ω, 0.004 F sα–1) and in betanin solar cells (32.69 Ω, 0.004 F sα–1) shows that the probability of recombination at the TiO2/dye electrolyte interface is higher in indigo solar cells. Therefore, the electron lifetime in indigo solar cells is lower (at 29.44 ms) compared to that in lawsone solar cells and betanin solar cells. As a result, the better performance in the betanin/lawsone co-sensitized solar cell can be attributed to the greater electron lifetime (313.8 ms) in it, compared to that (291.4 ms) attained in the betanin/indigo solar cell. The photocurrent generation by the different photoanodes and the photo-induced charge transfer can be analyzed further by measuring the incident photon conversion efficiency and performing ultrafast transient absorption pump–probe spectroscopy.
Dye Degradation and Solar Cell Lifetime Studies
The following study examines the degradation of the dye-sensitized onto TiO2 as a result of its inherent photocatalytic nature and the subsequent effect this imposes on the lifetime of the solar cell. PCA of TiO2 occurs when high-energy UV radiation from the solar spectrum is absorbed by TiO2, exciting electrons from its valence band to the conduction band. The resulting photoexcited electrons are of high energy, and upon their interaction with moisture, they result in the generation of reactive OH radicals. These reactive species result in the PCA of TiO2 causing disintegration of organic matter which is in close proximity.44,45 Heat-based sealing techniques normally employed in conventional DSSCs cannot be used in natural dye solar cells, which means that oxygen and moisture can infiltrate the solar cells through minute gaps which may be present at the unsealed edges between the sandwiched electrodes, making the pigments vulnerable to photocatalytic degradation.
The degradation of the pigments lawsone and indigo as a result of the PCA of TiO2 was observed using UV–vis spectroscopic studies. TiO2-coated substrates were placed fully immersed in lawsone and indigo pigment solutions of 5 mg L–1 as the initial concentration. The samples were exposed to a light intensity of 1 sun (using a solar simulator) for 5 h.
The effect of PCA of TiO2 on the dyes was studied by measuring their absorption spectra at intervals of 1 h.
Lawsone and indigo are colored compounds absorbing visible light, having conjugated double bonds which means that low-energy electronic transitions occur in these molecules. As these molecules break down with photodegradation, the degree of delocalization decreases causing an increase in the required energy to result in electron transitions.46 Hence, this results in a blue shift of λmax of both indigo and lawsone. The intensity of absorbance also decreases with time, indicating the degradation of the dye molecules occurring with time as a result of PCA of TiO2. This can be seen in Figure 7a,b.
Figure 7.

PCA of TiO2 resulting in a decrease in the intensity of absorbance by (a) indigo and (b) lawsone; (c) photocatalytic degradation of the dyes following first-order kinetics; and the (d) percentage decrease in the concentration of the dyes with time.
Pigment degradation follows the first-order kinetics47 described using the following equation47
| 3 |
where t is the irradiation time, C0 represents the initial concentration, Ct represents the concentration of the dye at a time “t”, and k is the rate constant.
Figure 7c shows the photocatalytic degradation kinetics of indigo and lawsone. The adjusted R2 value of the linear fit of the plot was determined as 0.9686, and the “k” values of indigo and lawsone were found to be 0.0027 and 0.0017, respectively. The study showed that, over a period of 5 h, 90% of degradation is observed in indigo, whereas 70% of degradation is observed in the case of lawsone. The photocatalytic degradation of betanin has been studied in our previous publication,26 which showed betanin reaches 90% degradation mark in 4 h. It can be inferred that the rate of degradation of lawsone is slower than that of indigo (and also betanin). This could be because of the fact that lawsone is capable of strongly absorbing in the UV region at 335 nm (which results in high energy π → π* transitions)17 without breaking down its molecular structure and properties. Although the mechanism of the relative stability of lawsone to PCA of TiO2 is unclear as yet, the substance has been used as an effective sunscreen since ancient times because of this property.48Figure 7d shows the percentage decrease in the concentration of the pigments with respect to time, which was calculated as follows
| 4 |
where ρ is the decrease in the concentration of the pigments with respect to time, t is the irradiation time, C0 represents the initial concentration, and Ct represents the concentration of the dye at a time “t”.
The five solar cell configurations were fabricated and kept under constant illumination of 1 sun, under the solar simulator for 5 h. At 30 min intervals, the efficiencies of the solar cells were recorded. It was observed that the solar cell efficiencies reduce with time (as seen in Figure 8a,b). The reason for this efficiency decrease may be attributed to either the interaction with TiO2 or with the electrolyte. However, it appears that the effect of PCA of TiO2 is a major factor contributing to this negative effect, as observed in the study described in the previous section. The efficiency decrease observed in lawsone solar cells of 51% (in 5 h, 0.321% decreased to 0.159%) is noted to be slightly lower compared to the 53% efficiency decrease observed in the indigo solar cells (in 5 h, 0.0629% decreased to 0.0298%). On studying the lifetimes of the two co-sensitized solar cell configurations, it was observed that the betanin/lawsone co-sensitized solar cell demonstrates a slightly higher lifetime, with only a 40.8% efficiency decrease (in 5 h, 0.798% decreased to 0.473%), whereas a 46.3% efficiency decrease (in 5 h, 0.66% decreased to 0.355%) was observed in the betanin/indigo co-sensitized solar cell. The study shows that betanin/lawsone co-sensitized solar cells offer a comparatively higher combined efficiency and lifetime.
Figure 8.

Change in the efficiency of the five configurations of solar cells exposed to a constant illumination of 1 sun for 5 h: (a) absolute decrease in efficiency and (b) normalized decrease in efficiency.
The components apart from the dyes used at present in the NDSSCs are optimized for synthetic dyes and do not take into consideration the negative effect they may have on natural dyes. Optimization or replacement of these components (such as the photocatalytic TiO2 and the electrolyte) to render them more compatible with natural dyes to improve their stability is the way forward for further efficiency enhancement of the natural DSSCs. The stability and lifetimes of the NDSSCs require improvement. It may be possible to inhibit the photocatalytic degradation of natural photosensitizers by using suitable stabilizers and robust encapsulation techniques.
Conclusions
A comparison of co-sensitization of two combinations of natural photosensitizers has been reported in this work. Betanin, a natural dye absorbing in the major portion of the incident solar spectrum—the green region, is the most efficient natural pigment for DSSCs. To broaden the absorption band, betanin was co-sensitized with two pigments which absorb light in regions complementary to betanin/indigo and lawsone, the natural pigments derived from plant sources—I. tinctoria and L. inermis, with the absorption bands in the red and blue portions of the solar spectrum, respectively. The absorption studies of the extracted and purified pigments showed the characteristic absorption peaks of lawsone and indigo at 410 nm (blue) and 625 nm (red), respectively. The theoretically calculated HOMO and LUMO energies of the pigment molecules, obtained from DFT simulations, showed their optimal alignment with respect to the conduction band of the TiO2 semiconductor and the reduction potential energy of the I–/I3– electrolyte, required for the optimal working of the device. The fabrication of the devices was optimized, and the performance characteristics of the various solar cell configurations have been elucidated. Lawsone solar cells gave a better performance, giving average efficiencies of 0.311 ± 0.034% compared to indigo solar cells showing 0.060 ± 0.004%. This is also corroborated by the EIS studies, which showed higher electron lifetime in lawsone solar cells (34.61 ms) compared to that in indigo solar cells (29.44 ms). This may be attributed to the more optimal alignment of energy levels of lawsone molecules compared to that of the indigo molecules, as observed in the DFT studies. Moreover, the lack of dipole moment in indigo, compared to the strong dipole moment present in lawsone, resulted in more efficient charge separation and charge transfer in lawsone. The lawsone solar cells show higher current density compared to the indigo solar cells. Betanin was co-sensitized with indigo and lawsone, and the performances of the co-sensitized solar cells were assessed. The betanin/lawsone co-sensitized solar cell demonstrated a higher average efficiency of 0.793 ± 0.021% compared to 0.655 ± 0.019% obtained for the betanin/indigo co-sensitized solar cell. An 11.7% enhancement of efficiency (with respect to betanin) was observed for the betanin/indigo solar cell, whereas a higher enhancement of 25.5% was observed for the betanin/lawsone solar cell. It could be concluded from the EIS studies that the better performance of the betanin/lawsone solar cell is a result of the longer electron lifetime in it (313.8 ms) compared to that in the betanin/indigo solar cell (291.4 ms).
The future scope of this study will include fabrication of tandem devices and comparison of their performances with that of the existing configuration for solar cells using multiple natural dyes. The results from the various studies are encouraging enough to continue exploring avenues of increasing the efficiency and lifetime of natural pigment solar cells to be able to harness their potential for practical applications in the future.
Methods
Computational Studies of the Dye Molecules Using Gaussian09
Guassian09 software was used to perform energy optimization of the pigment molecules by employing the DFT hybrid functional B3LYP and the 6-31G(d,p) basis set.30 The studies helped in determining the probability distribution of electron densities corresponding to HOMO and LUMO of the dye molecules and their energy levels with respect to vacuum. On the basis of the difference between the HOMO and LUMO energy levels, the band gap of the pigment molecules was determined. The charge distribution and the dipole moments of indigo and lawsone molecules were determined from the simulations that helped predict how the dye molecules would interact with TiO2 and perform in the solar cell.
Preparation of the Solar Cell
The photoanodes were prepared using FTO substrates (dimensions = 15 mm × 15 mm × 1.1 mm, ρ < 10 Ω cm–1, and % T > 83%, purchased from Solaronix) and used for the experiments. For the photoanode preparation, successive ultrasonication of the FTO substrates was carried out for 15 min each in deionized water, detergent solution, and ethanol.49 The TiO2 paste was prepared following the optimized methods available in the literature.50 To fabricate the photoanode, the prepared TiO2 suspension was doctor-bladed onto the conducting face of the FTO plate and then calcined for 1 h at 450 °C to remove the organics present.49 All preparatory works were carried out at room temperature in ambient conditions.
Following this, the prepared photoanodes were subjected to TiCl4 treatment according to the optimized protocols available in the literature.15,50 The TiCl4 treatment is an essential step in the preparation of the photoanode that ensures higher performance. As a result of this step, TiO2 nanoparticles are formed caused by the hydrolyzation of the TiCl4 solution. When the photoanodes are annealed, these nanoparticles crystallize and create better contact interface within the mesoporous TiO2 film that facilitates better electron transfer across the film. This helps in reducing defect sites, thereby reducing the recombination rate of electrons, and hence enhancing their collection.51,52 In addition, the TiCl4 treatment helps in providing more sites for the binding of the photosensitizer with TiO2.15,50 The TiO2 coating was performed by optimized methods,50 and the thickness was verified to be at an average optimum thickness of 22 μm measured using the Z-stack option in the Carl Zeiss SteREO Discovery V20 optical microscope. A thickness range of 20–25 μm for the TiO2 coating has been previously determined as optimal for the best performance of DSSCs, through rigorous optimization, by Ito et al., 2007.50
The betanin, indigo, and lawsone pigments used in the experiments were extracted and purified following the optimized methods.53−55 The photoanodes were left to stand immersed in the extracted pigments for 24 h in the case of betanin and lawsone and for 36 h in the case of indigo. The sensitization process has been carefully optimized by varying the number of dye coatings, the time of sensitization, and the order of sensitization while quantifying the solar cell performance as described in the section Optimization of the Sensitization of Dyes. Following this step, they were gently rinsed using ethanol to remove moisture and debris and then dried. The color of each pigment adsorbed onto TiO2 could be observed visually. Pt-coated FTO substrates (Solaronix) were employed as the counter electrodes. The electrolyte used in the experiments was gel-type and prepared by dissolving LiI/I2 (1.0 M/0.1 M), the iodine redox mediator, in N-methyl-2-pyrrolidone mixed with 6% (w/w) polyvinylidene fluoride which works as a binder.56 The solar cell was fabricated by fixing the photoanode over the counter electrode and injecting the prepared gel electrolyte into the space between the electrodes set by a spacer.
Five different solar cell configurations were prepared: indigo solar cell, lawsone solar cell, betanin solar cell, betanin/indigo solar cell, and betanin/lawsone solar cell. A black mask was employed to define an active area of 0.25 cm2 for all the experiments, and the reverse face of the Pt-FTO electrode was covered with a copper sheet to arrest transmission losses.
Characterization and Measurements
The absorption characteristics of indigo, lawsone, betanin/indigo, and betanin/lawsone mixtures were measured in the wavelengths between 300 and 800 nm using a Shimadzu UV-2400 PC series spectrophotometer, set at a sampling interval of 1 nm and a slit width of 5 nm.
EIS of the solar cells were performed at ambient conditions using a Bio-Logic electrochemical workstation, between frequencies of 100 kHz and 10 mHz, keeping 0.5 V (∼Voc of the DSSC) as a constant DC voltage bias. The method of carrying out EIS studies of DSSCs and their interpretation has been systematically established by researchers in the past.40,41,57−59 The EIS spectra were analyzed using the Z-Fit function in the E-C Lab software, by selecting a suitable equivalent circuit model to extract the EIS parameters: electron transport resistances, electron recombination, and the chemical capacitance of the solar cells.
The performance characteristics of the solar cells were studied using a solar simulator (Newport Corporation, Oriel Class AAA). Keithley 2440 5A (Keithley, Inc.) source meter was used to obtain the current density–voltage characteristics of the solar cells. All the cells were tested at an illumination of 1 sun (AM 1.5G standard test conditions at 1000 W cm–2). Five samples of each configuration were fabricated and tested multiple times to ensure the repeatability of the solar cell performances.
Acknowledgments
The first author thanks CSIR for the award of Senior Research Fellowship (award no. 31/57(002)/2015-EMRI) to pursue research at CSIR-CEERI. The authors thank Dr. K. Ramesha, Principal Scientist, CSIR-CECRI for the kind support and facilities provided to carry out the work.
Glossary
Abbreviations
- HOMO
highest occupied molecular orbital
- LUMO
lowest unoccupied molecular orbital
- DFT
density functional theory
- EIS
electrochemical impedance spectroscopy
- DSSC
dye-sensitized solar cell
- FTO
fluorine-doped tin oxide
- UV–vis
ultraviolet–visible
- ITO
indium-doped tin oxide
- NHE
normal hydrogen electrode
- FF
fill factor
- DC
direct current
- CPE
constant phase element
- PCA
photocatalytic activity
- B3LYP
Becke-3-Lee–Yang–Parr
- AM
air mass
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01875.
Extraction and purification of betanin, indigo, and lawsone and their effect on photovoltaic performance in DSSCs (PDF)
Author Contributions
S.S. carried out the experiments pertaining to this research work as part of her doctoral thesis work and wrote the manuscript. B.P. supervised the research work and contributed to multiple revisions of the manuscript.
This work was supported by the CSIR Senior Research Fellowship award no. 31/57(002)/2015-EMRI provided by the Department of Science and Technology, Government of India.
The authors declare no competing financial interest.
Supplementary Material
References
- Hardin B. E.; Snaith H. J.; McGehee M. D. The Renaissance of Dye-Sensitized Solar Cells. Nat. Photonics 2012, 6, 162–169. 10.1038/nphoton.2012.22. [DOI] [Google Scholar]
- Grätzel M. The advent of mesoscopic injection solar cells. Prog. Photovolt. Res. Appl. 2006, 14, 429–442. 10.1002/pip.712. [DOI] [Google Scholar]
- Shah S. A. A.; Sayyad M. H.; Wahab F.; Khan K. A.; Munawar M. A.; Elbohy H.; Qiao Q. Synthesis, Modeling and Photovoltaic Properties of a Benzothiadiazole Based Molecule for Dye-Sensitized Solar Cells. J. Mater. Sci.: Mater. Electron. 2016, 27, 4501–4507. 10.1007/s10854-016-4324-9. [DOI] [Google Scholar]
- Xie Y.; Joshi P.; Ropp M.; Galipeau D.; Zhang L.; Fong H.; You Y.; Qiao Q. Structural Effects of Core-Modified Porphyrins in Dye-Sensitized Solar Cells. J. Porphyrins Phthalocyanines 2009, 13, 903–909. 10.1142/s1088424609001145. [DOI] [Google Scholar]
- Green M. A.; Hishikawa Y.; Dunlop E. D.; Levi D. H.; Hohl-Ebinger J.; Yoshita M.; Ho-Baillie A. W. Y. Solar Cell Efficiency Tables (Version 53). Prog. Photovolt. Res. Appl. 2019, 27, 3–12. 10.1002/pip.3102. [DOI] [Google Scholar]
- Nazeeruddin M. K.; Liska P.; Moser J.; Vlachopoulos N.; Grätzel M. Conversion of Light into Electricity with Trinuclear Ruthenium Complexes Adsorbed on Textured TiO2Films. Helv. Chim. Acta 1990, 73, 1788–1803. 10.1002/hlca.19900730624. [DOI] [Google Scholar]
- Richhariya G.; Kumar A.; Tekasakul P.; Gupta B. Natural Dyes for Dye Sensitized Solar Cell: A Review. Renewable Sustainable Energy Rev. 2017, 69, 705–718. 10.1016/j.rser.2016.11.198. [DOI] [Google Scholar]
- Calogero G.; Bartolotta A.; Di Marco G.; Di Carlo A.; Bonaccorso F. Vegetable-Based Dye-Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 3244–3294. 10.1039/c4cs00309h. [DOI] [PubMed] [Google Scholar]
- Al-Alwani M. A. M.; Ludin N. A.; Mohamad A. B.; Kadhum A. A. H.; Sopian K. Extraction, Preparation and Application of Pigments from Cordyline Fruticosa and Hylocereus Polyrhizus as Sensitizers for Dye-Sensitized Solar Cells. Spectrochim. Acta, Part A 2017, 179, 23–31. 10.1016/j.saa.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Nan H.; Shen H.-P.; Wang G.; Xie S.-D.; Yang G.-J.; Lin H. Studies on the Optical and Photoelectric Properties of Anthocyanin and Chlorophyll as Natural Co-Sensitizers in Dye Sensitized Solar Cell. Opt. Mater. 2017, 73, 172–178. 10.1016/j.optmat.2017.07.036. [DOI] [Google Scholar]
- Orona-Navar A.; Aguilar-Hernández I.; Cerdán-Pasarán A.; López-Luke T.; Rodríguez-Delgado M.; Cárdenas-Chávez D. L.; Cepeda-Pérez E.; Ornelas-Soto N. Astaxanthin from Haematococcus Pluvialis as a Natural Photosensitizer for Dye-Sensitized Solar Cell. Algal Res. 2017, 26, 15–24. 10.1016/j.algal.2017.06.027. [DOI] [Google Scholar]
- Chaiamornnugool P.; Tontapha S.; Phatchana R.; Ratchapolthavisin N.; Kanokmedhakul S.; Sang-aroon W.; Amornkitbamrung V. Performance and Stability of Low-Cost Dye-Sensitized Solar Cell Based Crude and Pre-Concentrated Anthocyanins: Combined Experimental and DFT/TDDFT Study. J. Mol. Struct. 2017, 1127, 145–155. 10.1016/j.molstruc.2016.07.086. [DOI] [Google Scholar]
- Talip L. F. A.; Ramli M. M.; Isa S. S. M.; Halin D. S. C.; Mazlan N. S.; Anhar N. A. M.; Danial N. A.; Muda M. R. Hybrid TiO2-Gigantochloa Albociliata Charcoal in Dye Sensitized Solar Cell. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 209, 012086. 10.1088/1757-899x/209/1/012086. [DOI] [Google Scholar]
- Ramamoorthy R.; Radha N.; Maheswari G.; Anandan S.; Manoharan S.; Victor Williams R. Betalain and Anthocyanin Dye-Sensitized Solar Cells. J. Appl. Electrochem. 2016, 46, 929–941. 10.1007/s10800-016-0974-9. [DOI] [Google Scholar]
- Sandquist C.; McHale J. L. Improved Efficiency of Betanin-Based Dye-Sensitized Solar Cells. J. Photochem. Photobiol., A 2011, 221, 90–97. 10.1016/j.jphotochem.2011.04.030. [DOI] [Google Scholar]
- Sreeja S.; Pesala B.. Efficiency Enhancement of Betanin Dye Sensitized Solar Cells Using Plasmon Enhanced Silver Nanoparticles. Springer Proceedings in Energy; Springer: Presented at the International Conference on Advances in Energy Research, IIT Bombay, 2017.
- Khadtare S. S.; Ware A. P.; Salunke-Gawali S.; Jadkar S. R.; Pingale S. S.; Pathan H. M. Dye Sensitized Solar Cell with Lawsone Dye Using ZnO Photoanode: Experimental and TD-DFT Study. RSC Adv. 2015, 5, 17647–17652. 10.1039/c4ra14620d. [DOI] [Google Scholar]
- Al-Bat’hi S. A. M.; Alaei I.; Sopyan I. Natural Photosensitizers for Dye Sensitized Solar Cells. Int. J. Renew. Energy Res. 2013, 3, 138–143. [Google Scholar]
- Espinosa R.; Zumeta I.; Santana J. L.; Martínez-Luzardo F.; González B.; Docteur S.; Vigil E. Nanocrystalline TiO2 Photosensitized with Natural Polymers with Enhanced Efficiency from 400 to 600 Nm. Sol. Energy Mater. Sol. Cells 2005, 85, 359–369. 10.1016/j.solmat.2004.05.006. [DOI] [Google Scholar]
- Narayan M. R. Review: Dye Sensitized Solar Cells Based on Natural Photosensitizers. Renewable Sustainable Energy Rev. 2012, 16, 208–215. 10.1016/j.rser.2011.07.148. [DOI] [Google Scholar]
- Kim S.-H.Functional Dyes, 1st ed.; Elsevier, 2006. [Google Scholar]
- Ghann W.; Kang H.; Sheikh T.; Yadav S.; Chavez-Gil T.; Nesbitt F.; Uddin J. Fabrication, Optimization and Characterization of Natural Dye Sensitized Solar Cell. Sci. Rep. 2017, 7, 41470. 10.1038/srep41470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M. III-V compound multi-junction solar cells: present and future. Sol. Energy Mater. Sol. Cells 2003, 75, 261–269. 10.1016/s0927-0248(02)00168-x. [DOI] [Google Scholar]
- Colonna D.; Capogna V.; Lembo A.; Brown T. M.; Reale A.; Carlo A. D. Efficient Cosensitization Strategy for Dye-Sensitized Solar Cells. Appl. Phys. Express 2012, 5, 022303. 10.1143/apex.5.022303. [DOI] [Google Scholar]
- Taranekar P.; Qiao Q.; Jiang H.; Ghiviriga I.; Schanze K. S.; Reynolds J. R. Hyperbranched Conjugated Polyelectrolyte Bilayers for Solar-Cell Applications. J. Am. Chem. Soc. 2007, 129, 8958–8959. 10.1021/ja073216a. [DOI] [PubMed] [Google Scholar]
- Sreeja S.; Pesala B. Co-Sensitization Aided Efficiency Enhancement in Betanin–Chlorophyll Solar Cell. Renew. Sustain. Energy 2018, 7, 25. 10.1007/s40243-018-0132-x. [DOI] [Google Scholar]
- Dumbravǎ A.; Enache I.; Oprea C. I.; Georgescu A.; Gîrţu M. A. Toward a More Efficient Utilisation of Betalains as Pigments for Dye-Sensitized Solar Cells. Dig. J. Nanomater. Biostructures 2012, 7, 339–351. [Google Scholar]
- Wambuguh D.; Chianelli R. R. Indigo Dye Waste Recovery from Blue Denim Textile Effluent: A by-Product Synergy Approach. New J. Chem 2008, 32, 2189–2194. 10.1039/b806213g. [DOI] [Google Scholar]
- Kumbar M. N.; Sannaikar M. S.; Shaikh S. K. J.; Kamble A. A.; Wari M. N.; Inamdar S. R.; Qiao Q.; Revanna B. N.; Madegowda M.; Dasappa J. P.; Kamble R. R. Synthesis, Photophysical and Computational Study of Novel Coumarin-Based Organic Dyes. Photochem. Photobiol. 2018, 94, 261–276. 10.1111/php.12852. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian J. V.. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford CT, 2009.
- Shah S. A. A.; Sayyad M. H.; Nasr N.; Toor R. A.; Sajjad S.; Elbohy H.; Qiao Q. Photovoltaic Performance and Impedance Spectroscopy of a Purely Organic Dye and Most Common Metallic Dye Based Dye-Sensitized Solar Cells. J. Mater. Sci.: Mater. Electron. 2017, 28, 6552–6559. 10.1007/s10854-017-6344-5. [DOI] [Google Scholar]
- Park K.-H.; Kim T.-Y.; Park J.-Y.; Jin E.-M.; Yim S.-H.; Choi D.-Y.; Lee J.-W. Adsorption characteristics of gardenia yellow as natural photosensitizer for dye-sensitized solar cells. Dyes Pigm. 2013, 96, 595–601. 10.1016/j.dyepig.2012.10.005. [DOI] [Google Scholar]
- ASTM Reference Solar Spectral Irradiance: Air Mass 1.5. Terrestrial Reference Spectra for Photovoltaic Performance Evaluation, 2003.
- Amat A.; Rosi F.; Miliani C.; Sgamellotti A.; Fantacci S. Theoretical and Experimental Investigation on the Spectroscopic Properties of Indigo Dye. J. Mol. Struct. 2011, 993, 43–51. 10.1016/j.molstruc.2010.11.046. [DOI] [Google Scholar]
- Leonat L.; Sbârcea G.; Branzoi I. V. Cyclic Voltammetry for Energy Levels Estimation of Organic Materials. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2013, 75, 111–118. [Google Scholar]
- Kavitha S.; K P.; M L. A New Method to Evaluate the Feasibility of a Dye in DSSC Application. Int. J. Energy Res. 2017, 41, 2173–2183. 10.1002/er.3778. [DOI] [Google Scholar]
- Amemiya S.Handbook of Electrochemistry, 1st ed.; Elsevier Science, USA, 2007. [Google Scholar]
- Rajab F. M. Effect of Solvent, Dye-Loading Time, and Dye Choice on the Performance of Dye-Sensitized Solar Cells. J. Nanomater. 2016, 2016, 370316. 10.1155/2016/3703167. [DOI] [Google Scholar]
- Chang W.-C.; Lee C.-H.; Yu W.-C.; Lin C.-M. Optimization of Dye Adsorption Time and Film Thickness for Efficient ZnO Dye-Sensitized Solar Cells with High at-Rest Stability. Nanoscale Res. Lett. 2012, 7, 688. 10.1186/1556-276x-7-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisquert J.; Fabregat-Santiago F. Impedance Spectroscopy: A General Introduction and Application to Dye-Sensitized Solar Cells. Dye. Sol. Cells 2010, 604. [Google Scholar]
- Fabregat-Santiago F.; Bisquert J.; Garcia-Belmonte G.; Boschloo G.; Hagfeldt A. Influence of Electrolyte in Transport and Recombination in Dye-Sensitized Solar Cells Studied by Impedance Spectroscopy. Sol. Energy Mater. Sol. Cells 2005, 87, 117–131. 10.1016/j.solmat.2004.07.017. [DOI] [Google Scholar]
- Sarker S.; Ahammad A. J. S.; Seo H. W.; Kim D. M. Electrochemical Impedance Spectra of Dye-Sensitized Solar Cells: Fundamentals and Spreadsheet Calculation. Int. J. Photoenergy. 2014, 2014, 851705. 10.1155/2014/851705. [DOI] [Google Scholar]
- Wang M.; Chen P.; Humphry-baker R.; Zakeeruddin S. M.; Grätzel M. The Influence of Charge Transport and Recombination on the Performance of Dye-Sensitized Solar Cells. ChemPhysChem 2009, 10, 290–299. 10.1002/cphc.200800708. [DOI] [PubMed] [Google Scholar]
- Raja V.; Shiamala L.; Alamelu K.; Jaffar Ali B. M. A Study on the Free Radical Generation and Photocatalytic Yield in Extended Surfaces of Visible Light Active TiO2compounds. Sol. Energy Mater. Sol. Cells 2016, 152, 125–132. 10.1016/j.solmat.2016.03.008. [DOI] [Google Scholar]
- Mukhlish M. Z. B.; Najnin F.; Rahman M. M.; Uddin M. J. Photocatalytic Degradation of Different Dyes Using TiO2 with High Surface Area: A Kinetic Study. J. Sci. Res. 2013, 5, 301–314. 10.3329/jsr.v5i2.11641. [DOI] [Google Scholar]
- Yan Z.; Gong W.; Chen Y.; Duan D.; Li J.; Wang W.; Wang J. Visible-Light Degradation of Dyes and Phenols over Mesoporous Titania Prepared by Using Anthocyanin from Red Radish as Template. Int. J. Photoenergy 2014, 2014, 968298. 10.1155/2014/968298. [DOI] [Google Scholar]
- Hamad H. A.; Sadik W. A.; Abd El-latif M. M.; Kashyout A. B.; Feteha M. Y.; Feteha M. Photocatalytic Parameters and Kinetic Study for Degradation of Dichlorophenol-Indophenol (DCPIP) Dye Using Highly Active Mesoporous TiO2 Nanoparticles. J. Environ. Sci. 2016, 43, 26–39. 10.1016/j.jes.2015.05.033. [DOI] [PubMed] [Google Scholar]
- Watkinson A. C.; Brain K. R.; Walters K. A.; Hadgraft J. Prediction of the Percutaneous Penetration of Ultra-Violet Filters Used in Sunscreen Formulations. Int. J. Cosmet. Sci. 1992, 14, 265–275. 10.1111/j.1467-2494.1992.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Naphade R. A.; Tathavadekar M.; Jog J. P.; Agarkar S.; Ogale S. Plasmonic Light Harvesting of Dye Sensitized Solar Cells by Au-Nanoparticle Loaded TiO2 Nanofiber. J. Mater. Chem. A 2014, 2, 975–984. 10.1039/c3ta13246c. [DOI] [Google Scholar]
- Ito S.; Chen P.; Comte P.; Nazeeruddin M. K.; Liska P.; Gra M. Fabrication of Screen-Printing Pastes From TiO2 Powders for Dye-Sensitised Solar Cells. Prog. Photovolt. Res. Appl. 2007, 15, 6013–6612. 10.1002/pip.768. [DOI] [Google Scholar]
- O’Regan B. C.; Durrant J. R.; Sommeling P. M.; Bakker N. J. Influence of the TiCl4 Treatment on Nanocrystalline TiO2 Films in Dye-Sensitized Solar Cells. 2 .Charge Density, Band Edge Shifts, and Quantification of Recombination Losses at Short Circuit. J. Phys. Chem. C 2007, 111, 14001–14010. 10.1021/jp073056p. [DOI] [Google Scholar]
- Lee S.-W.; Ahn K.-S.; Zhu K.; Neale N. R.; Frank A. J. Effects of TiCl4 Treatment of Nanoporous TiO2 Films on Morphology, Light Harvesting, and Charge-Carrier Dynamics in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 21285–21290. 10.1021/jp3079887. [DOI] [Google Scholar]
- Buscio V.; Crespi M.; Gutiérrez-Bouzán C. A Critical Comparison of Methods for the Analysis of Indigo in Dyeing Liquors and Effluents. Materials 2014, 7, 6184–6193. 10.3390/ma7096184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D.; Mondal B.; Mukherjee K. Visible Light Absorption and Photo-Sensitizing Properties of Spinach Leaves and Beetroot Extracted Natural Dyes. Spectrochim. Acta, Part A 2015, 148, 85–92. 10.1016/j.saa.2015.03.120. [DOI] [PubMed] [Google Scholar]
- Anju D.; Kavita S.; Jugnu G.; Munish G.; Asha S. Determination of Lawsone Content in Fresh and Dried Leaves of Lawsonia Inermis Linn and its Quantitative Analysis by HPTLC. J. Pharm. Sci. Innovation 2012, 1, 17–20. [Google Scholar]
- Man-Gu K.; Park N.-G.; Kim K.-M.; Chang S.-H.. Dye Sensitized Solar Cells Including Polymer Electrolyte Gel Containg Poly(Vinyldene Fluoride). U.S. Patent 006,756,537 B2, 2004.
- Fabregat-Santiago F.; Bisquert J.; Palomares E.; Otero L.; Kuang D.; Zakeeruddin S. M.; Grätzel M. Correlation between Photovoltaic Performance and Impedance Spectroscopy of Dye-Sensitized Solar Cells Based on Ionic Liquids. J. Phys. Chem. C 2007, 111, 6550–6560. 10.1021/jp066178a. [DOI] [Google Scholar]
- Kern R.; Sastrawan R.; Ferber J.; Stangl R.; Luther J. Modeling and Interpretation of Electrical Impedance Spectra of Dye Solar Cells Operated under Open-Circuit Conditions. Electrochim. Acta 2002, 47, 4213–4225. 10.1016/s0013-4686(02)00444-9. [DOI] [Google Scholar]
- Zheng D.; Ye M.; Wen X.; Zhang N.; Lin C. Electrochemical Methods for the Characterization and Interfacial Study of Dye-Sensitized Solar Cell. Sci. Bull. 2015, 60, 850–863. 10.1007/s11434-015-0769-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.