Short abstract
The blood–brain barrier (BBB) and blood–nerve barrier ensure protection of the nervous system but pose a challenge for the treatment of pain since it restricts passage of many therapeutic drugs. Although it is unknown which blood–neural barrier is more relevant, or whether permeabilities are the same for different barriers, we proposed that the inefficiency of thiazolidinedione-type agonists for peroxisome proliferator-activated receptor gamma (PPARɣ) is due to their difficulty in passage through the BBB. We developed a new highly BBB penetrable PPARɣ agonist for the treatment of neuropathic pain, assuming BBB permeability is a rule of thumb to estimate the overall permeability of relevant blood–neural barriers. As an index of brain penetration, the brain–plasma ratio (Kp) of ELB00824 is 5.13, suggesting very high brain bioavailability, which is 58-fold that of pioglitazone. The series of studies presented here indicate that ELB00824 may be the most potent PPARɣ agonist currently known for acute reduction of neuropathic pain in trigeminal nerve in rat and mouse models. Low-dose PPARɣ agonist, ELB00824 (10 mg/kg), effectively decreased neuropathic hypersensitivity in mice and rats at both acute and chronic time points, a dose 100-fold lower than the effective dose (1000 mg/kg, i.p.) of pioglitazone. Comparisons of ELB00824 alone or in combination with gabapentin or carbamazepine are provided. While PPARɣ agonists used to treat Type 2 diabetes produce several adverse side effects, sub-chronic oral toxicity study provided promising results that ELB00824 does not produce any significant short-term toxicity. The study animals of either sex remained alive and healthy with no significant alteration of body weight long term. Toxicity study results obtained were satisfactory, with no significant alterations in any serum biochemistry parameters.
Keywords: Non-opioid therapy, chronic pain, hypersensitivity, anxiety, infraorbital nerve, mice, rats
Introduction
Trigeminal neuropathic pain (TNP) is a chronic neuropathic pain (NP) disorder that affects the trigeminal nerve and is evoked by blunt force facial trauma, oral surgery, inflammation, or unknown causes. Typical pain medications do not work well for TNP patients. The treatment for TNP usually starts with anticonvulsants (e.g., gabapentin, carbamazepine). However, over time, some people may stop responding to medications, or they may experience unpleasant side effects. Therefore, the development of mechanism-based novel treatments to prevent or reverse this NP is an urgent need that requires an in-depth understanding of the underlying model of actions.
Peroxisome proliferator-activated receptor gamma (PPARɣ) is a potential therapeutic target for the treatment of NP. PPARɣ agonists were reported to modulate NP in several animal studies, including two TNP models.1,2 Positive effects for non-thiazolidinedione (TZD) type PPARɣ agonist ATx08-001 have been shown in patients with postherpetic NP.3 Animal study indicated that acute (within 60 min) TZD type PPARɣ agonist pioglitazone inhibits NP in part by reducing astrocyte activation,GFAP upregulation, and via both genomic and non-genomic PPARɣ mechanisms.4 Immune mediators released from activated astrocytes and microglia can powerfully enhance neuronal excitability and pain sensitivity, and anti-inflammatory drugs ibudilast and minocycline have shown signs of efficacy in relieving pain in a small cohort of patients.5
A selection of blood–neural barriers ensures the protection of the nervous system while restricting the drug delivery, which include the blood–brain barrier (BBB), blood–spinal cord barrier, blood–nerve barrier (BNB), and so on. Among them, BBB is well documented, and current clinically used TZD type PPARɣ agonists either do not pass the BBB (rosiglitazone) or do so poorly (pioglitazone).6 Previous studies showed that NP was attenuated only by prohibitively high-dose oral treatment of pioglitazone (600 mg/kg in mouse) or intrathecal administration of rosiglitazone,1,7 which hampers their practical use. We proposed that the inefficiency of TZD type PPARɣ agonists is due to their difficulties to pass certain BNB. Although it is unknown which blood–neural barrier is relevant, or whether the compound permeabilities are the same for different barriers, we developed a series of highly BBB penetrable compounds, assuming BBB permeability is a rule of thumb to estimate the overall permeability of relevant blood–neural barriers.
In this study, a new BBB penetrable non-TZD type PPARɣ agonist called ELB00824 was synthesized, and two rodent species were used to investigate the effects on the relief of acute trigeminal NP, including rat distal infraorbital nerve (dIoN) model and mouse trigeminal inflammatory compression (TIC) injury model, and the antinociception was then compared to those of existing drugs (pioglitazone, gabapentin, and carbamazepine). The PPARɣ-dependent molecular basis of its effects was also explored. The relief of the acute and chronic TNP found in the current study will pave the way for preclinical and clinical investigations and validation of ELB00824 in trigeminal as well as other types of NPs.
Materials and methods
Animals
The procedures in this study were approved by the Institutional Animal Care and Use Committees of University of New Mexico, Shanghai Elixiria Biotech Co. Ltd. and Joinn laboratories Co. Ltd. (China) and are in accordance with the Helsinki Declaration of 1975. All animals were housed in a well-ventilated rodent housing room (maintained at 20°C–22°C) with a reversed 10/14 h dark/light cycle so that testing could be performed in their active period. All animals were housed for one week before the experiments. All animals had access to food and water ad libitum throughout the duration of the experiment. Low soybean content normal chow diet was provided.
Chemical
All chemicals were purchased from Sigma-Aldrich (St Louis, MO) excepting ELB00824, including gabapentin (CAS: 60142–96-3), carbamazepine (CAS: 298–46-4), pioglitazone hydrochloride (CAS: 112529–15-4), GW9662 (CAS: 22978–25-2), rosiglitazone (CAS: 122320–73-4), Cremophor@EL (CAS: 61791–12-6), Miglyol 812 (CAS: 37332–31-3), carbopol (CAS: 9007–20-9), and so on.
In p.o. and i.p. treatment, unless specified, all chemicals were dissolved in vehicle, which is 30% DMSO, 15% Cremophor@EL, and 9% ethanol in phosphate-buffered saline (PBS) buffer (pH 7.4), and vortexed for 30 s before administration.
In topical treatment, the composition of a cream was prepared by mixing together all components of oil phase (miglyol 812, cetyl alcohol, glycerol monostearate, oleyl alcohol, stearyl alcohol, and carbopol) and compound ELB00824, and heating to 45°C until all components are dissolved. The water phase (sodium cetostearyl sulphate, benzyl alcohol, and citric acid) was prepared separately and its pH was adjusted to 5.5 with sodium hydroxide. The water phase was then added to the oil phase and stirred in the same clockwise until all components are dissolved. The 7% cream contains ELB00824 (7%), miglyol 812 (15%), cetyl alcohol (4%), glycerol monostearate (2%), oleyl alcohol (10%), stearyl alcohol (4%), carbopol (1%), sodium cetostearyl sulphate (1%), benzyl alcohol (1%), and citric acid (1%). All abovementioned units of concentration are w/w.
Synthesis and 1 H NMR Spectroscopic Elucidation of ELB00824
ELB00824, a novel PPARɣ agonist with an international patent pending (PCT/CN2019/072302), was synthesized by the following procedure. Under argon, tetramethylethylenediamine (5.00 g, 0.043 mol) was added dropwise to 200 mL of anhydrous tetrahydrofuran solution containing magnesium powder (1.04 g, 0.043 mol) and ferric chloride (300 mg, 0.00178 mol) and stirred at room temperature for 20 min. Then p-ethyl bromobenzene (6.6 g, 0.036 mol) and 4-bromo-1-butene (5.8 g, 0.043 mol) were slowly added dropwise at 0°C and stirred for 3 h. After completion of the reaction as indicated by thin-layer chromatography (TLC), the reaction was quenched and extracted. The crude product was concentrated and purified by column chromatography (organic phase: petroleum ether) to obtain 3 g (52%) of colorless liquid intermediate compound [1-(but-3-en-1-yl)-4-ethylbenzene]. Under argon, 6-bromo-1-indanone (112 mg, 0.53 mmol), 1-(but-3-en-1-yl)-4-ethylbenzene (140 mg, 0.80 mmol), palladium acetate (6 mg, 0.027 mmol), and tris (o-methylphenyl) phosphine (16 mg, 0.053 mmol) were weighed into a 25 mL three-port bottle. Triethylamine (1.5 mL) and N, N-dimethylformamide (1.5 mL) were added as the solvent. The reaction system was heated to 100°C and stirred for 6 h. After completion of the reaction as indicated by TLC, the reaction was quenched, extracted, and concentrated. The crude product was purified by column chromatography (mobile phase: ethyl acetate: petroleum ether = 1: 5) to obtain 70 mg (44%) of pale yellow solid [(E)-6–(4-(4-ethylphenyl)but-1-en-1-yl)-2,3-dihydro-1H-inden-1-one, named ELB00824]. Recrystallization is used to purify the ELB00824 to 95% purity. 1 H NMR (400 MHz,CDCl3) δ(ppm): 1.25(t3,H), 2.23(dd,2H), 2.60(m,4H), 2.71(m,2H), 3.12(m,2H), 6.38(m,2H), 7.11(m,4H), 7.65(m,3H).
Preliminary 14-Day Repeat Dose Toxicity Study in Rats
Subchronic toxicity studies were conducted for ELB00824 with 14-day dose range finding studies in rats. Thirty healthy Sprague-Dawley rats (190–200 g, 12 males and 12 females) were housed and monitored daily by animal care specialists at the Primate Utility, Joinn Laboratories Co. Ltd. Beijing, China. The care of experimental animals was in accordance with institutional guidelines. The rats were randomly grouped into four experimental and one control groups, containing six rats per group (three males and three females in separate cage). Animals of different sexes were placed in the separate cages. The animals in the experimental groups (Groups I, II, III, IV) were treated with ELB00824 orally at doses of 0, 30, 120, and 450 mg/kg/day in vehicle with the intervals of 24 h for 14 days. During the administration period, the rats were observed for any physical, behavioral alterations and signs of abnormalities daily, and body weights, food intake, and water consumption were recorded weekly. The rats were anesthetized with isoflurane one day after the final gavage. Blood samples were collected via the posterior vena cava. The hematology parameters were measured using an automatic Siemens Advia2120i Hematology Analyzer (Dublin, Ireland) and Sysmex CS1300 blood coagulation analyzer (Sysmex Corporation, Kobe, Hyogo, Japan) for the following parameters: WBC (white blood cell count), hemoglobin, erythrocyte, hematocrit, MCV (mean corpuscular volume), MCHC (mean corpuscular hemoglobin concentration), MCH (mean corpuscular hemoglobin), Platelet absolute count, MPV (mean platelet volume), PDW (platelet distribution width), neutrophils percent, lymphocyte percent, mononuclear percent, eosinophil percent, basophil percent, LUC percent (large unstained cell percent), absolute neutrophil count, absolute lymphocyte count, absolute mononuclear count, absolute basophil count, absolute eosinophil count, LUC count (absolute large unstained cell count), reticulocyte percent, absolute reticulocyte count, CHCM (cell hemoglobin concentration mean), erythrocyte percent, HDW (hemoglobin distribution width), WBCP (white blood cell perox count), platelet percent (%), LI (lobularity index), MPXI (myeloperoxidase index), PT (prothrombin time), APTT (activated partial thromboplastin time), and fibrinogen. Standard serum biochemistry parameters were analyzed with an automatic Chemistry Analyzer (Toshiba TBA-120FR, Toshiba Medical Systems, Inc., Tokyo) to evaluate the following serum biochemistry parameters: ALT (alanine transaminase), AST (aspartate transaminase), total protein, albumin, creatinine, ALP (alkaline phosphatase), calcium, glucose, total bilirubin, GGT (γ-glutamyltransferase), LDH (lactate dehydrogenase), CK (creatine kinase), urea, TC (total cholesterol), TG (triglyceride), phosphorus, A/G (albumin/globulin), globulin, sodium ion, potassium, and chloride ion.
Toxicokinetic (or Pharmacokinetic) study and BBB permeability in rats
Healthy Sprague-Dawley rats (200–250 g, male) were housed and monitored daily by animal care specialists at Shanghai Elixiria Biotech Co. Ltd., Shanghai, China. The care of experimental animals was in accordance with institutional guidelines. ELB00824, pioglitazone, and rosiglitazone concentrations were determined in rat brain tissue and plasma. Rats were treated with 10 mg/kg compounds by intraperitoneal injection. After 0.5, 1, 2, 4, 6, 10, 24 h, rats (n = 5) were anesthetized with carbon dioxide and sacrificed by decapitation. The brains were dissected, heated immediately at 90°C for 2 min, homogenized with four volumes of methanol (pH 9), and added with Internal Standard (IS). For plasma isolation after decapitation, whole blood was collected, centrifuged at 1500 g for 15 min, and added with IS and four volumes of methanol (pH 9). The IS for pioglitazone is rosiglitazone, and vice versa, while the IS for ELB00824 is its derivative, ELB01115b. The following steps are the same for brain and plasma samples. The protein was precipitated by centrifugation at 1500 g for 10 min, and 600 µl of the upper layer was dried under vacuum, and then resuspended in 200 µl of 75% methanol in PBS. In addition, 20 µl of sample were then load into Shimadzu Essentia LC-16P high-performance liquid chromatographic (HPLC) system coupled with SPD-16 ultraviolet (UV) detector and RF-20A XS fluorescence detector (Shimadzu, Japan). For calibration standards, blank brain homogenates or plasma (spiked with 0.1, 1, 10, and 30 µM compounds) were prepared in a similar manner and used to build a calibration curve for each sample type. HPLC was performed using a Welchrom C18 5 μm, 4.6 × 150 mm column (Welch Materials, Inc., Shanghai, China). Column temperature was maintained at 30°C. For ELB00824, the mobile phases consisted of 75% methanol and 0.4% acetic acid in Milli-Q water (solvent A), and 50% acetonitrile, 37.5% methanol, 12.5% tetrahydrofuran, 0.4% acetic acid in acetonitrile (solvent B), delivered at 1 mL/min. The elution conditions used were as follows: gradient of 5% to 15% solvent B (0.1–10 min) and isocratic at 15% solvent B (10–20 min). For pioglitazone and rosiglitazone, the mobile phases consisted of 0.1% trifluoroacetic acid in Milli-Q water (solvent A) and 0.1% trifluoroacetic acid in acetonitrile (solvent B) delivered at 1 mL/min. The elution conditions used were as follows: gradient of 5% to 30% solvent B (0.1–10 min), isocratic at 30% solvent B (10–17 min). To monitor analyte peak elution, the UV detector was set to a wavelength of 240 nm or 269 nm for ELB00824 or pioglitazone, respectively; and the fluorescence detector was set to an emission wavelength of 247 nm with excitation at 367 nm for rosiglitazone. Using LabSolutions Analysis Data System software (Shimadzu, Japan), integration of peak areas and data processing were carried out, which were used to construct calibration plots and then to determine actual concentrations in experimental samples. Concentration maximum (Cmax), time when the maximum concentration was reached (Tmax), and area under the curve from time 0 to 24 h (AUC0–24h) were determined by identifying the highest measured concentration in each subject’s chemical concentration–time profile. Kp is the total brain/plasma concentration ratio, calculated from the ratio of AUCbrain to AUCplasma, which was used to examine and predict of brain penetration of chemicals.
In vitro effect on PPARɣ receptor
The agonistic potencies (EC50) of tested compounds against human PPARɣ receptor were measured as transactivation activity in HEK 293 T cells. Compounds were dissolved in DMSO (<0.5% v/v). The lentiviral vector pHBLV-CMVIE-ZsGreen-Puro was purchased from Hanbio Biotech Co., Ltd (Shanghai, China) containing cytomegalovirus immediate early (CMVie) promoter/enhancer, and expressing ZsGreen and Puro dual reporter. The PPARɣ expression plasmid was constructed by cloning full-length coding sequences of the pertinent receptors into pHBLV-CMVIE-ZsGreen-Puro vector. To construct the luciferase (luc) reporter plasmid, the CMV promoter in plasmid pHBLV-CMVIE-ZsGreen-Puro was first replaced by a peroxisome proliferator responsive element (PPRE) containing fragment8 comprised the nucleotide sequence 5′-GTCGACAG GGGACCAGGACA AAGGTCACGTTCGGG AGT CGAC, repeated four times in tandem, with consensus PPRE underlined, to generate a new plasmid, at a multiple cloning site of which the luc gene was then inserted. HEK 293 T cells were grown in DMEM containing 10% FBS at 37°C and 5% CO2. Cells were distributed in 10-cm tissue culture plates at a density of 5.5 million cells per plate, and then transfected using LipoFiter™ reagent (60 µl/plate, Hanbio Biotech., Shanghai, China) with the luciferase reporter plasmid (6 ng/plate) and the PPARɣ expression plasmid (18 ng/plate). Cells were harvested by trypsinization 48 h post-transfection, and then distributed in 96-well plates at a density of 20,000 cells per well in 85 μl of the corresponding medium. After incubation for 6 h, 5 µl of compounds or control with different concentration were placed into each well, resulting in a final concentration of 0.5% DMSO and 0.1, 0.3, 1, 3, 10, 30 µM of compounds. Compound H, i.e., (N-(2-chlorophenyl)-1H-indole-3-carboxamide), identified in previous studies9 was used as a positive control. Cells were exposed to the compound overnight. Twenty-one hours later, promoter activation was detected by the addition of SteadyLite (PerkinElmer, Waltham, MA) to medium overlaying cells. Plates were read on a GloMax® 96 Microplate Luminometer (Promega, Madison, WI). Conditions were optimized to minimize well-to-well and plate-to-plate variability in luciferase signal, so that the CV (coefficient of variation) for any group is below 5%. Each condition was tested in triplicate wells. Activation of PPARɣ activity at each dilution was plotted against compound concentration. EC50 values were calculated by a four-parameter equation describing a sigmoidal curve, using the Sigmaplot 12.5 software (Systat Software, Inc., San Jose, CA).
Surgical procedures for rat dIoN model
Healthy Sprague-Dawley rats (200–250 g, male) were housed and monitored daily by animal care specialists at Shanghai Elixiria Biotech Co. Ltd., Shanghai, China. The care of experimental animals was in accordance with institutional guidelines. In the present study, an improved rat model of trigeminal NP was used, in which chronic constriction injury (CCI) is induced by ligation of distal segment of IoN (dIoN), which is less complicated than the ligation of other segment of IoN.10 The rats were anesthetized with pentobarbital (60 mg/kg, i.p.). The facial surface between the eye and whisker pad of the rat was gently shaved and scrubbed with iodine without damaging the whiskers on one side. At the caudal end of the third row of whisker lines toward the ipsilateral orbit, a 0.5 cm cut was made parallel to the midline. To expose the IoN trunk to its distal portion outside the orbital cavity, the superficial fascia was bluntly separated. Two chromic catgut ligatures (4–0), 2 mm apart, were loosely tied around the distal segment of the IoN. The ligatures were applied in such a way that the IoN diameter was reduced by a perceptible amount and the circulation through the superficial vasculature was retarded but not cut off. A polyester suture (4–0) was then used to close the skin incision. Rats in vehicle treatment (sham) groups underwent the same surgical procedure including skin incision and the nerve dissection with the exception of the actual nerve ligation. Naive animals were also included as a comparison group. All rats were age matched. Compared with vehicle treated rats, dIoN-CCI rats exhibited a significant decrease in threshold toward mechanical stimuli in von Frey test on the ipsilateral side, which started at one day after injury. In addition, the response toward mechanical stimulation of the contralateral IoN territory remained unchanged from naive control rats in these same rats.
Surgical procedures for mouse TIC model
Mice were age matched BALB/c males (eight weeks old, Envigo/Harlan), housed on a reversed light cycle. The trigeminal nerve injury chronic pain model was induced ipsilaterally under isoflurane anesthesia (2%) by inserting 3 mm of chromic gut suture (4.0) into the tight space between the infraorbital branch of the trigeminal nerve and the bony infraorbital fissure through a small surgical incision made orally between the cheek fold and maxillary bone. Mechanical allodynia developed within one week post-surgery as evaluated using von Frey filaments due to combined mechanical and chemical inflammatory stimulation of the nerve through nerve compression by the suture and leaching of chromate salt. The mice were monitored daily in accordance with institutional guidelines by animal care specialists at University of New Mexico, Albuquerque, NM, USA.
Behavioral assays
All behavioral tests were conducted during the animal’s active cycle (zt12-24, ie, dark phase of the dark/light cycle) during the hours of 8:00 a.m. to 6:00 p.m. During testing, either a red light or a dim lamp illuminated the room. None of the behavioral tests were conducted on the same day. The experimenter testing the mice and rats was blinded to the drug treatments given for each experiment. Many observation parameters were examined, including posture, convulsions, tremors, abnormal movements, palpebral closure, feces consistency, approach response, touch response, startle response, pupil response, eyeblink response, gait abnormalities/posture, and so on.
Detecting mechanical allodynia on the whisker pad
Pain-like behaviors were tested two days before surgery (baseline, BL). Orofacial sensitivity to mechanical stimulation was tested using von Frey filaments in an ascending order of intensity beginning with a middle fiber, with each filament applied five times at intervals of 3 s to slightly different loci within the center of the vibrissal pad. A threshold force of response (in grams) was defined as the first filament that evoked at least two reactions of five applications. The following criteria were used to determine a positive response: an immediate withdrawal reaction, attacking the filament by biting and grabbling, or asymmetric face stroke to the stimulated facial area.10 Rats included for the post-dIoN-treatment study were those which exhibited a mean response of ≤ 2.2 g. Mechanical threshold of the whisker pad area was tested before and after surgery with a modified up/down method using a graded series of von Frey fiber filaments (force: 0.008 g (size: 1.65); 0.02 g (2.36); 0.07 g (2.83); 0.16 g (3.22); 0.4 g (3.61); 1.0 g (4.08); 2.0 g (4.31); 6.0 g (4.74); 8.0 g (4.93); 10 g (5.07); 15 g (5.18); 26 g (5.46), Stoelting, Wood Dale, IL). For mice, the experimenter gently restrained the mouse in their gloved palm (2–5 min) to acclimate the animal. The experimenter then applied the von Frey filaments perpendicularly to the animal’s whisker pad as previously described.11 Bilateral mechanical head withdrawal thresholds were determined at baseline and weekly post-surgery. The efficacy of ELB00824 and carbamazepine to reduce mechanical hypersensitivity and chronic pain–induced anxiety-related behaviors was determined in weeks 3 and 6 post-surgery. The chronic six week time-point was chosen because anxiety- and depression-like behaviors can develop six to eight weeks after injury.12 For rats, between post-injury days 5 to 14, efficacy of experimental drugs to reduce mechanical hypersensitivity was determined.12 For one-time drug treatments, mechanical threshold was measured at 0, 0.5, 1, 2, 3, and 4 h after drug injection, with 5, 6, 7, and 8 h after injection evaluated to determine whether drug effect persisted. For daily injections (rats), mechanical thresholds were determined once daily at the same testing time each day. One experimenter was blinded to the drug treatments given at all times.
Detecting anxiety
Anxiety measures are only measureable in pain models persisting six weeks.1,2,12 At six weeks post model induction, mice were given vehicle, carbamazepine, or ELB00824. After the 2 h previously determined to be the peak time for pain relief, mice were placed in both an elevated zero maze and a light/dark place preference test for anxiety testing. The zero maze, divided into equal quarters, had two quarters with high walls (closed area) and two quarters with no walls (open areas). The mice were place in one of the open areas and allowed to roam freely in the maze with videorecording for a total of 5 min. Videorecords for the zero maze were analyzed for several measures using Ethovision software: the total distance traveled, the amount of time spent in either open or closed areas, the total number of area transitions, and the amount of time before the mouse initially moved to a closed area once set down in an open area on the maze (entry latency). The time spent in the closed areas of the maze is the most consistent measure of anxiety with this assay.
For the light/dark place preference test, the mice were placed in the dark side of the two chamber arena and allowed free access through a portal to either the light or the dark chamber for a total of 10 min while videorecording the behaviors. Videorecords for the light/dark anxiety test were analyzed for: the amount of time spent in the light and dark areas, the total number of area transitions, rearing behaviors, and the amount of time it took before the mouse moved to a light area once set down (entry latency). The time spent in the dark chamber is the most consistent measure of anxiety with this test.
Statistical analysis
Data were expressed as means ± standard error of the mean (SEM). The Sigmaplot 12.5 statistical program was used for data analyses (Systat Software, Inc., San Jose, CA). The homogeneity of variances between groups was determined by Levene test. Comparisons between different groups were performed with the use of one-way analysis of variance (ANOVA) and Tukey’s test; P ≤ 0.05 is considered significant.
Results
ELB00824 is highly BBB penetrable
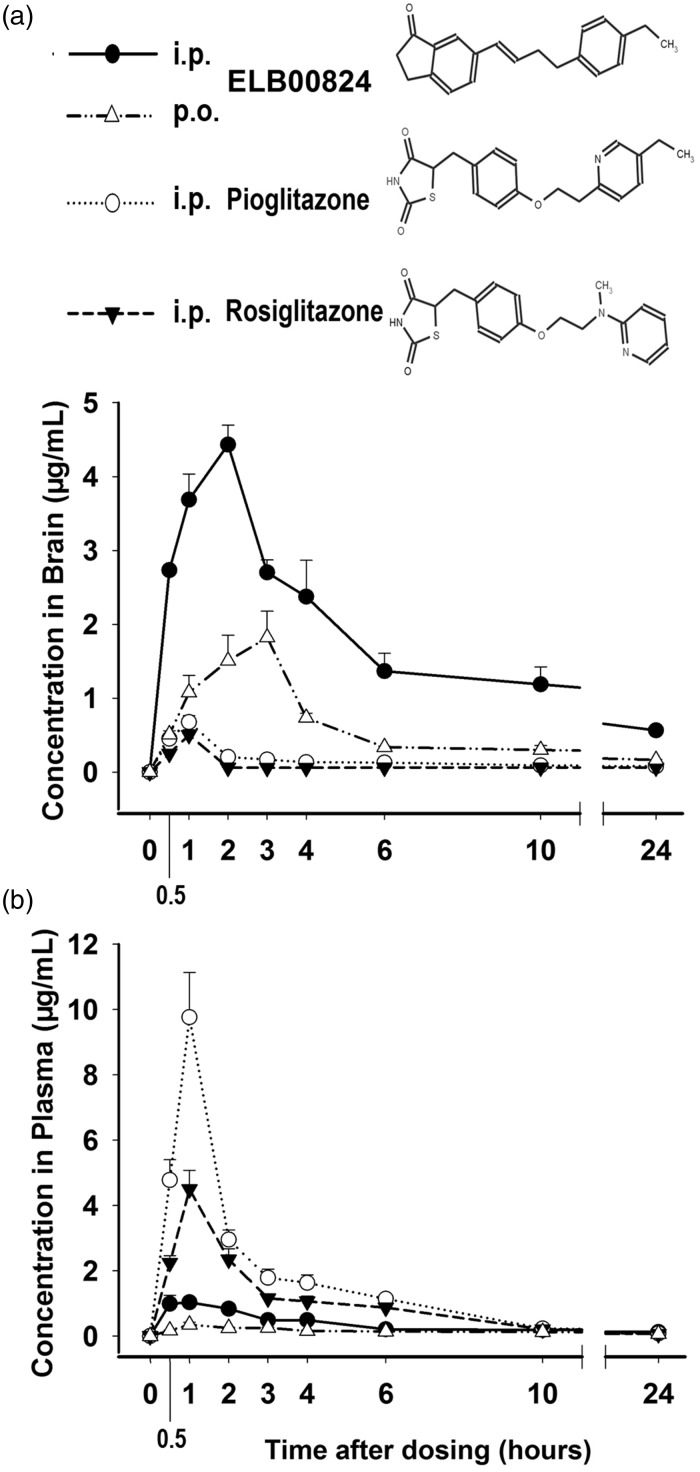
Figure 1 shows the concentration–time curve for ELB00824, pioglitazone, and rosiglitazone in both the brain and plasma of rats following a single dose (10 mg/kg, i.p or p.o.). Two routes of administration of ELB00824 were compared. In brain, the Cmax, tmax, and AUC0–24h of i.p. administration were 4.43 μg/mL, 2 h, and 33.59 μg·h/mL, respectively, while those of p.o. administration were 1.82 μg/mL, 3 h, and 10.37 μg·h/mL, respectively. Therefore, the relative brain bioavailability (AUC0–24h) of oral versus i.p. injection of ELB00824 is 31%. In plasma, the Cmax, tmax, and AUC0–24h of i.p. administration were 1.12 μg/mL, 0.5 h, and 6.55 μg·h/mL, respectively, while those of p.o. administration were 0.35 μg/mL, 1 h, and 3.03 μg·h/mL, respectively. Therefore, the relative plasma bioavailability (AUC0–24h) of oral versus i.p. injection of ELB00824 is 46%. In addition, the concentration values are linear over a dose range of 3 to 30 mg/kg for both i.p. and p.o. (data now shown, r > 0.99 for plasma and brain).
Figure 1.
Compound structure and brain permeability. Time profile of brain (a) and plasma (b) concentration of ELB00824 (i.p., solid line, solid circles; p.o., dash-dot-dot, empty triangles), rosiglitazone (i.p., dotted line, empty circles), pioglitazone (i.p., dash line, solid triangles) in rats administrated by i.p. or p.o. route (10 mg/kg). Data are presented as the mean ± SEM (n = 5).
After i.p. administration, some key pharmacokinetic parameters of ELB00824, pioglitazone, and rosiglitazone were also compared in Table 1. In brain, the Cmax, tmax, and AUC0–24h of ELB00824 were 2.73 μg/mL, 2 h, and 33.59 μg·h/mL, respectively. ELB00824 significantly increased Cmax in µM and AUC0–24h in µM·h of pioglitazone by 4.0-fold and 11-fold, respectively, and of rosiglitazone by 5.5-fold and 17-fold, respectively. On the contrary, in plasma, Cmax and AUC0–24h of the ELB00824 were less than 0.3-fold of those of pioglitazone or rosiglitazone. The brain–plasma ratio (Kp) is a widely used index of brain penetration in drug discovery. Within the 24 h after treatment, ELB00824 presented Kp of 5.13, suggesting very high brain bioavailability, which is 58-fold that of pioglitazone (5.13 vs. 0.09, P < 0.01), or 61-fold rosiglitazone (5.13 vs. 0.08, P < 0.01), respectively. Therefore, the BBB permeability of ELB00824 should be unprecedentedly higher than that of existing PPARɣ agonists, indicating its exceptionally high blood–neural barrier permeability.
Table 1.
Pharmacokinetic parameters in rat brain and plasma after an i.p. (10 mg/kg) administration.
| Compound name | Cmax |
tmax (hour) | AUC0–24h |
||
|---|---|---|---|---|---|
| (μg/mL) | (μM) | (μg·h/mL) | (μM·h) | ||
| Brain | |||||
| ELB00824 | 2.73 ± 0.05 | 9.42 ± 0.17 | 2 | 33.59 | 115.7 |
| Pioglitazone | 0.68 ± 0.14* | 1.73 ± 0.22* | 1 | 3.07 | 7.8 |
| Rosiglitazone | 0.50 ± 0.06* | 1.41 ± 0.27* | 1 | 1.93 | 5.41 |
| ELB00824/Pioglitazone | 4.0 | 5.4 | 11 | 15 | |
| ELB00824/Rosiglitazone | 5.5 | 6.7 | 17 | 21 | |
| Plasma | |||||
| ELB00824 | 1.12 ±0.17 | 3.85 ± 0.60 | 0.5 | 6.55 | 22.6 |
| Pioglitazone | 9.77 ± 1.01* | 24.86 ± 2.10* | 1 | 34.5 | 87.7 |
| Rosiglitazone | 4.50 ± 0.48* | 12.58 ± 1.81* | 1 | 22.8 | 63.7 |
| ELB00824/Pioglitazone | 0.1 | 0.2 | 0.2 | 0.3 | |
| ELB00824/Rosiglitazone | 0.2 | 0.3 | 0.3 | 0.4 | |
| Kp (Brain/Plasma) | |||||
| ELB00824 | 5.13 | 5.13 | |||
| Pioglitazone | 0.09 | 0.09 | |||
| Rosiglitazone | 0.08 | 0.08 | |||
| ELB00824/Pioglitazone | 58 | 58 | |||
| ELB00824/Rosiglitazone | 61 | 61 | |||
Note: Data are expressed as mean ± standard error of the mean (n = 5). AUC: area under the curve; Cmax: concentration maximum; Tmax: time when the maximum concentration was reached.
*P < 0.01 versus ELB00824
Preliminary 14-Day Repeat Dose Toxicity / Toxicokinetic Study in Rats
In dose range finding studies, ELB00824 was repeatedly administered orally once daily for two weeks at a dose of 30, 120, or 450 mg/kg. ELB00824 is rapidly absorbed upon oral administration as a suspension, with the peak plasma and brain concentration (Cmax) reached in 1 h and 3 h post-dose, respectively (Figure 1). Table 2 depicts 1 h post-dose concentration of ELB00824 in rat brain and plasma after a single injection of 10, 30, 120, or 450 mg/kg p.o. The vehicle is 30% DMSO, 15% Cremophor RH40, and 9% ethanol in PBS solution. These concentration values are linear over a dose range of 10 to 30 mg/kg (r = 0.999 for plasma and brain), but a rectangular hyperbola provided a better fit in the range of 10 to 450 mg/kg (r = 0.998 for plasma and brain), with saturation occurring at a dose of 450 mg/kg (Table 2).
Table 2.
ELB00824 concentrations in rat brain and plasma 1 h after p.o. administration.
| Route | (μg/mL) |
(μM) |
||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| 10 mg/kg | 0.27 ± 0.06 | 1.16 ± 0.23 | 0.94 ± 0.20 | 4.01 ± 0.79 |
| 30 mg/kg | 0.82 ± 0.15* | 4.11 ± 0.82* | 2.81 ± 0.51* | 14.15 ± 2.83* |
| 120 mg/kg | 1.51 ± 0.19* | 7.57 ± 0.22* | 5.20 ± 0.66* | 26.07 ± 0.74* |
| 450 mg/kg | 2.20 ± 0.46* | 11.03 ± 2.18* | 7.57 ± 1.60* | 38.02 ± 7.52* |
Note: Data are expressed as mean ± standard error of the mean (n = 5).
P < 0.05 versus immediately lower dose group.
All rats were removed from their cages and examined closely for clinical signs of toxicity at pre-grouping (Day -1), once weekly at approximately 1 to 3 hours after dosing. The observations included evaluation of behavior, activity, skin, fur, eyes, ears, nose,abdomen, external genitalia, anus, limbs, feet, and signs of mortality, morbidity, respiration, secretion, feces, and capability of water and food intake. All the animals given various levels of doses survived and were free of any intoxicating sign or behavioral abnormality throughout the dosing period of 14 days (no data shown), indicating no mortality and drug safety.
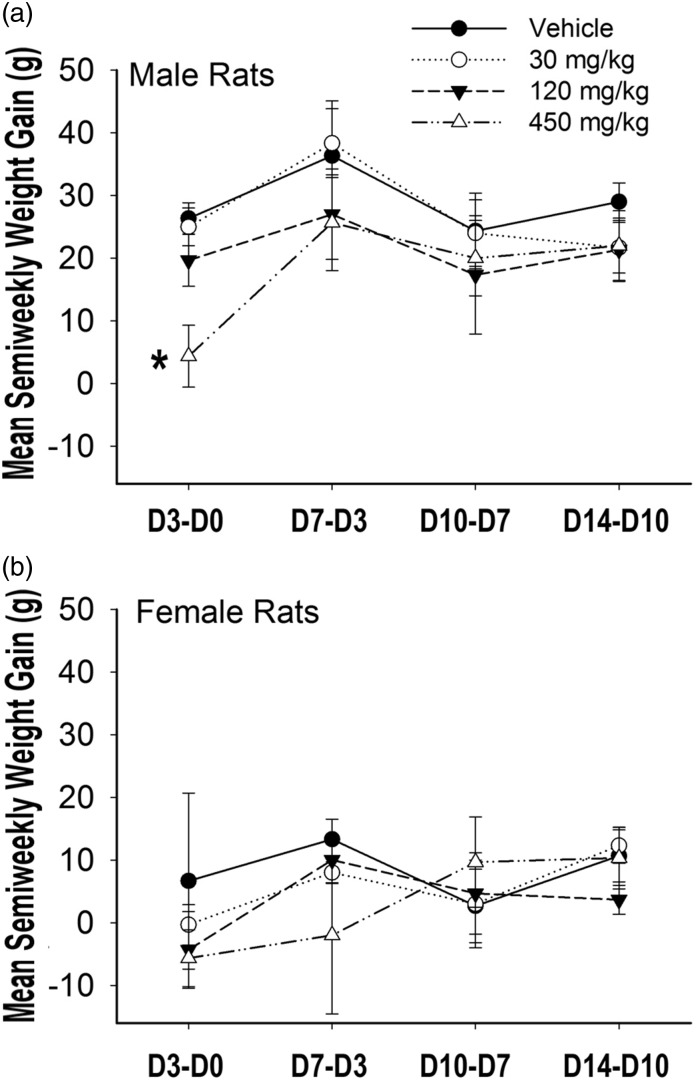
Each rat in the study was weighed semiweekly and the mass of feed consumed by each rats was recorded. The mean semiweekly weight gain (SWG) of the treatment groups was calculated and compared (Figure 2). There was no statistically significant difference in mean SWG between groups as determined by one-way ANOVA at all four semi-weeks tested, with the exception of the male 450 mg/kg group in their first semi-week (Figure 2(a)), which was consistent with their lower feed consumption during these days (data not shown).
Figure 2.
The mean semiweekly weight gain (SWG) of treatment groups. With the exception of males given the highest does, male (a) and female (b) rats of vehicle group (solid line, solid circles), 30 mg/kg group (dotted line, empty circles), 120 mg/kg group (dash line, solid triangles), or 450 mg/kg group (dash-dot-dot line, empty triangles), were gavaged with 0, 30, 120, or 450 mg/kg of ELB00824 on day 0. Weights were recorded on day 0 (before treatment, D0); day 3 (D3); day 7 (D7), day 10 (D10), and day 14 (D14). For each rat, SWG was calculated and then a mean SWG per treatment group was calculated, including D3-D0, D7-D3, D10-D7, and D14-D10. D3-D0 is the SWG between D3 and D0. D7-D3 is SWG between D7 and D3. D10-D7 is SWG between D10 and D7. D14-D10 is SWG between D14 and D10. Stars indicate significant P values compared with vehicle groups, with *P < 0.05. Data are presented as the mean ± SEM (n = 3).
In addition, the hematological, blood coagulation and serum biochemistry parameters of male (n = 3) and female (n = 3) were analyzed at termination of dosing on day 15. No significant changes were observed in any of the values of the 55 parameters studied when compared with controls, excepting slightly lower hemoglobin and hematocrit values in male 450 mg/kg group. All the values obtained were within normal biological and laboratory limits as shown in Supplementary Tables 1 and 2.
Anti-allodynic effects of ELB00824 in the rat dIoN model
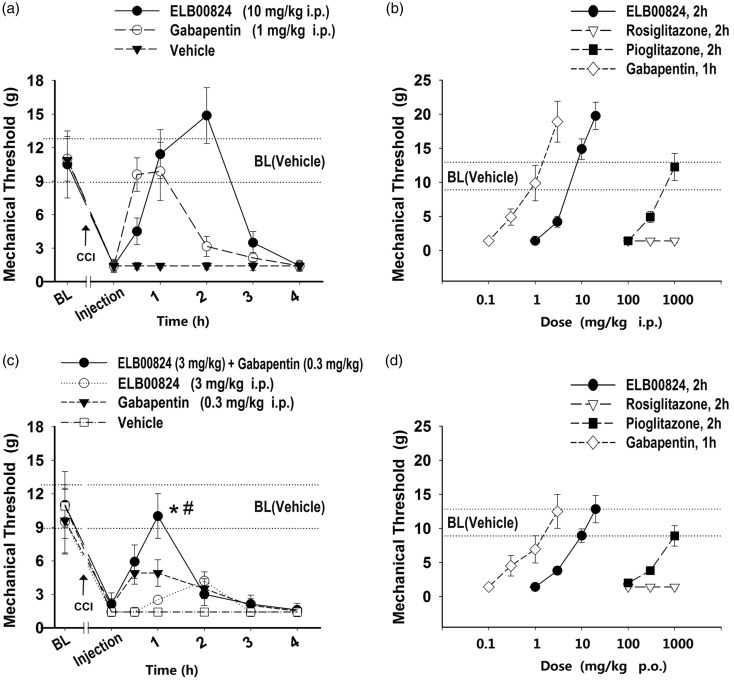
Compared with vehicle treated rats, dIoN-CCI rats exhibited a significant decrease (to 1.4 ± 0.5 g) from baseline threshold (10.9 ± 1.9 g, Figure 3(a)) in threshold toward mechanical stimuli in von Frey test on the ipsilateral side, which started at only one day after injury. In addition, the response toward mechanical stimulation of the contralateral IoN territory remained unchanged (10.9 ± 1.9 g, data not shown) in the same rats.
Figure 3.
ELB00824 attenuated ipsilateral whisker pad mechanical allodynia in the rats with trigeminal chronic constriction injury (CCI). (a and c) Time courses of the mechanical threshold increased by intraperitoneal (i.p.) injection of ELB00824 and gabapentin or both. Dose–response curves are shown for peak mechanical thresholds of gabapentin (0.1, 0.3, 1, 3 µM), ELB00824 (1, 3, 10, 20 µM), pioglitazone (100, 300, 1000 µM), and rosiglitazone (100, 300, 1000 µM) after i.p. (c) treatment or oral (p.o.) gavage (d). Values represent mean ± SEM, n = 4. BL: baseline threshold of each group; BL (Vehicle): baseline threshold of the vehicle control group (10.9 ± 1.9 g). *: P < 0.05 versus ELB00824 (3 mg/kg, 1 h). #: P < 0.05 versus gabapentin (0.3 mg/kg, 1 h).
Between 5 and 14 days post-CCI, injection of ELB00824 (10 mg/kg i.p.) effectively attenuated mechanical allodynia of the ipsilateral whisker pad (Figure 3(a)). There is a significant reversal in mechanical threshold to 11.4 ± 2.2 g at 1 h, peaked at 2 h (14.9 ± 2.5 g). For all i.p. treatments or oral (p.o.) gavage of ELB00824 (3–20 mg/kg) or pioglitazone (300–1000 mg/kg), the time courses for the mechanical thresholds had the same peak time (2 h), and produced curve shapes similar to that for ELB00824 (10 mg/kg i.p.) in Figure 3(a). Therefore, the mechanical threshold at 2 h after treatment was used as a simplified indicator for the anti-allodynic effects, and consequently dose–response curves for the peak mechanical thresholds were plotted for each compound (Figure 3(b) and (d)). At 2 h after treatment, ELB00824 and pioglitazone dose-dependently increased the mechanical threshold, but rosiglitazone did not attenuate mechanical allodynia at doses as high as 1000 mg/kg (Figure 3(b) and (d)).
For treatment by either i.p. or p.o., the minimal dose required to restore mechanical threshold to baseline was 10 mg/kg of ELB00824 or 1000 mg/kg of pioglitazone (Figure 3(b) and (d)), indicating that 10 mg/kg of ELB00824 is as potent as 1000 mg/kg of pioglitazone. Therefore, the anti-allodynic effects of ELB00824 may be equivalent to pioglitazone at approximately 100-fold higher doses. Observable neurobehavioral adverse effects of ELB00824 were not discernable to observers blinded to treatment group during an observation period one week after i.p. or p.o. treatment at doses of up to 1000 mg/kg (i.e., 100-fold the therapeutically relevant dose of 10 mg/kg). However, most rats treated with 300 mg/kg pioglitazone (i.e., approximately one-third of the therapeutically relevant dose of 1000 mg/kg; i.p. or p.o.) showed toxic signs and symptoms, for example, palpebral closure, loss of limb mobility, and reduced reactivity to external stimuli beginning one day after treatment. Therefore, pioglitazone did not have a therapeutic window in this NP model. ELB00824 may not only be far more potent, but it is also safer than existing PPARɣ agonists for NP.
We also tested other analgesics including gabapentin and anti-inflammatory drugs (acetaminophen, ibuprofen). Thirty minutes after single i.p. injection of gabapentin (1 mg/kg), rats with infraorbital nerve constriction presented a markedly increased response threshold to mechanical stimulation, which peaked at 1 h, and was reduced to previous allodynic levels at 2 h (Figure 3(a)). On the contrary, for ELB00824 treatment (10 mg/kg, i.p.), the mechanical threshold was at baseline level or higher between 1 and 2 h (Figure 3(a)). Therefore, at least in this case, ELB00824 doubled the therapeutic lifetime of gabapentin and thus provided more sustained relief from TNP.
For i.p. or p.o. treatment with gabapentin (0.1–10 mg/kg), the mechanical threshold had the same time to peak (1 h) and produced curve shapes similar to that for 1 mg/kg i.p. ELB00824 treatment (Figure 3(a)). At 1 h after treatment, gabapentin dose-dependently increased the mechanical threshold (Figure 3(b) and (d)), but neither acetaminophen nor ibuprofen attenuated mechanical allodynia at doses as high as 1000 mg/kg (data not shown). The ineffectiveness of these anti-inflammatory drugs indicated that our CCI model is specific to NP, not to inflammatory pain.
Figure 3(c) depicts the one-time drug dose combination of 3 mg/kg (i.p.) of ELB00824 and 0.3 mg/kg (i.p.) of gabapentin compared with the same doses given alone in rats with CCI injury. The mechanical threshold was increased to 10.0 ± 2.0 g in those rats when treated with the ELB00824 + gabapentin drug combination. This was a significant increase in mechanical threshold 1 h after injection compared with 3 mg/kg ELB00824 alone (10.0 ± 2.0 vs. 2.5 ± 0.3 g; P < 0.01), or 0.3 mg/kg gabapentin alone (10.0 ± 2.0 vs. 4.9 ± 1.2 g; P < 0.01). This result may indicate a synergistic or greater anti-allodynic effect of the ELB00824 in combination with gabapentin on NP.
PPARɣ-dependent mechanism of the anti-allodynic effects of ELB00824
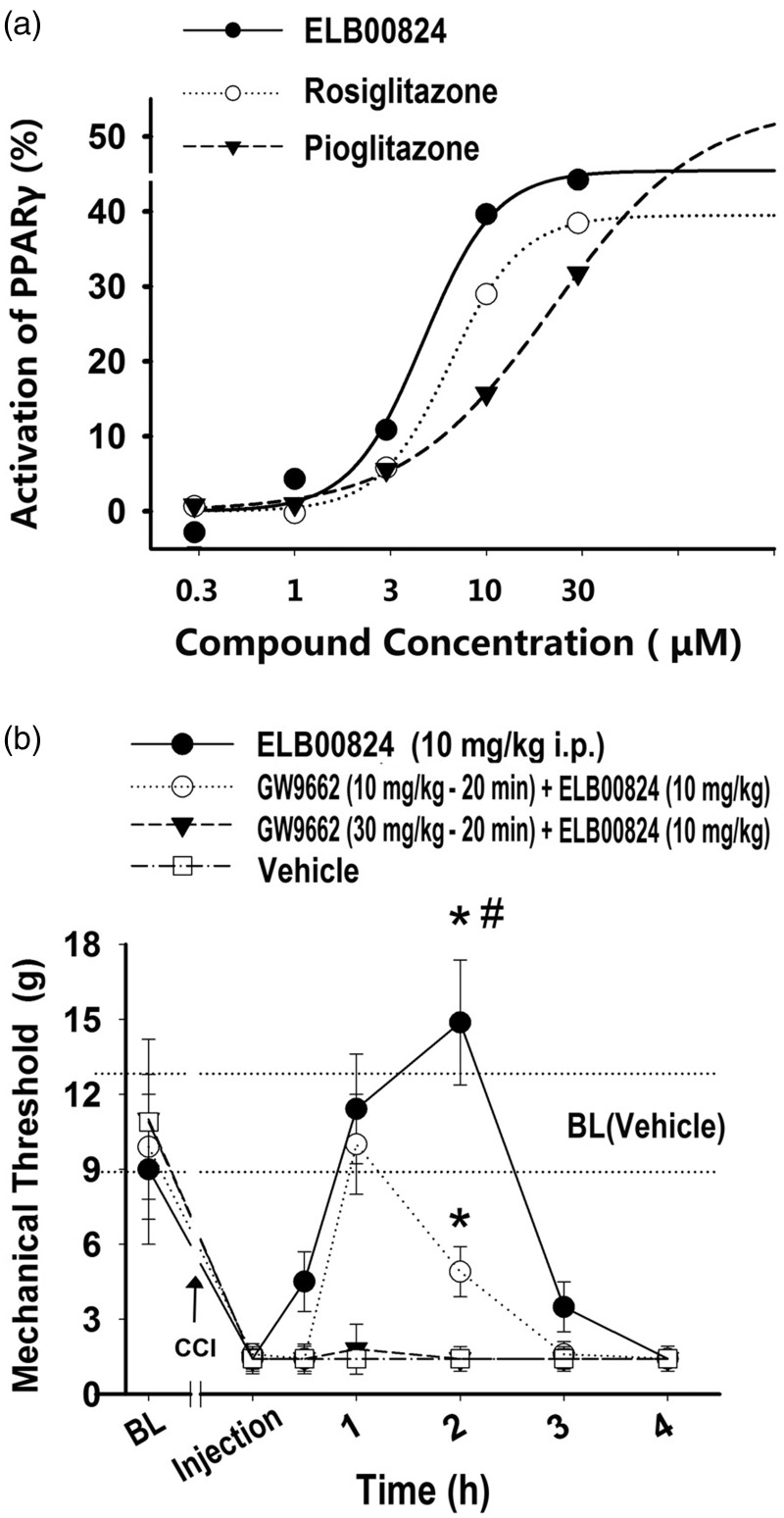
We first identified that ELB00824 is a PPARɣ agonist in in vitro experiments and then confirmed that its anti-allodynic effects are PPARɣ dependent in animal studies. Using a stable cell line, the dose–response profile for PPARɣ activation in vitro and their best fit curves for the compounds ELB00824, rosiglitazone, and pioglitazone were obtained as shown in Figure 4(a). The results indicated that the half maximal effective concentration (EC50) and maximal activities (% control) were 4.7 ± 0.4 μM and 45 ± 4% for ELB00824, 6.5 ± 0.4 μM and 39 ± 3% for rosiglitazone, as well as 22.1 ± 2.0 μM and 54 ± 5% for pioglitazone, respectively. The EC50 value of ELB00824 is significantly lower than that of rosiglitazone (4.7 ± 0.4 vs. 6.5 ± 0.4, P < 0.05), and both values are significantly lower than that of pioglitazone (4.7 ± 0.4 or 6.5 ± 0.4 vs. 22.1 ± 2.0, P < 0.01). Therefore, ELB00824 exhibited the highest in vitro PPARɣ transcriptional activity, compared to typical clinically used PPARγ agonists.
Figure 4.
PPARɣ-dependent mechanism of anti-allodynic effects of ELB00824. Representative concentration–response curves (a) for the PPARɣ activation in stable cell lines by ELB00824 (solid line, solid circles), rosiglitazone (dotted line, empty circles), pioglitazone (dash line, solid triangles). Data shown are representative of three independent experiments with similar results, normalized to control response in each case, and fitted using a sigmoidal dose–response curve (SigmaPlot). Ipsilateral whisker pad mechanical threshold (b) in the rats with trigeminal chronic constriction injury (CCI). PPARɣ antagonist, GW9662 (b), when treated i.p. 20 min before i.p. injection of ELB00824, dose-dependently blocked the anti-allodynic effect of ELB00824. Values represent mean ± SEM, n = 4. BL: baseline threshold of each group; BL (Vehicle): baseline threshold of the vehicle control group (10.9 ± 1.9 g). *P < 0.05 versus vehicle control (2 h). #P < 0.05 versus GW9662 (10 mg/kg, −20 min) + ELB00824 (10 mg/kg) at 2 h.
To demonstrate that PPARɣ has a causal role in producing the anti-allodynic effects of ELB00824, a potent specific PPARɣ antagonist GW9662 was used. Rats with CCI injury were injected i.p. with GW9662 (10 or 30 mg/kg) 20 min before i.p. injection of an efficacious dose of ELB00824 (10 mg/kg). As illustrated in Figure 4(b), GW9662 dose-dependently reduced the anti-allodynic effect of ELB00824 (2 h: GW9662 (30 mg/kg) + ELB00824 (10 mg/kg): 1.4 ± 0.5 g vs. ELB00824 (10 mg/kg) only: 14.9 ± 2.5 g, P < 0.01; GW9662 (10 mg/kg) + ELB00824 (10 mg/kg): 4.9 ± 1.0 g vs. ELB00824 (10 mg/kg) only: 14.9 ± 2.5 g, P < 0.01). In addition, GW9662 (10–30 mg/kg, i.p.) alone did not change mechanical threshold 1 to 4 h after injection (data not shown). The anti-allodynic effects were acute and occurred within 3 h, indicating the mechanism was non-genomic. These results provide evidence that ELB00824 is acting through a non-genomic PPARɣ-dependent mechanism to attenuate mechanical allodynia.
Topical treatment of ELB00824 provides the most prolonged relief from pain
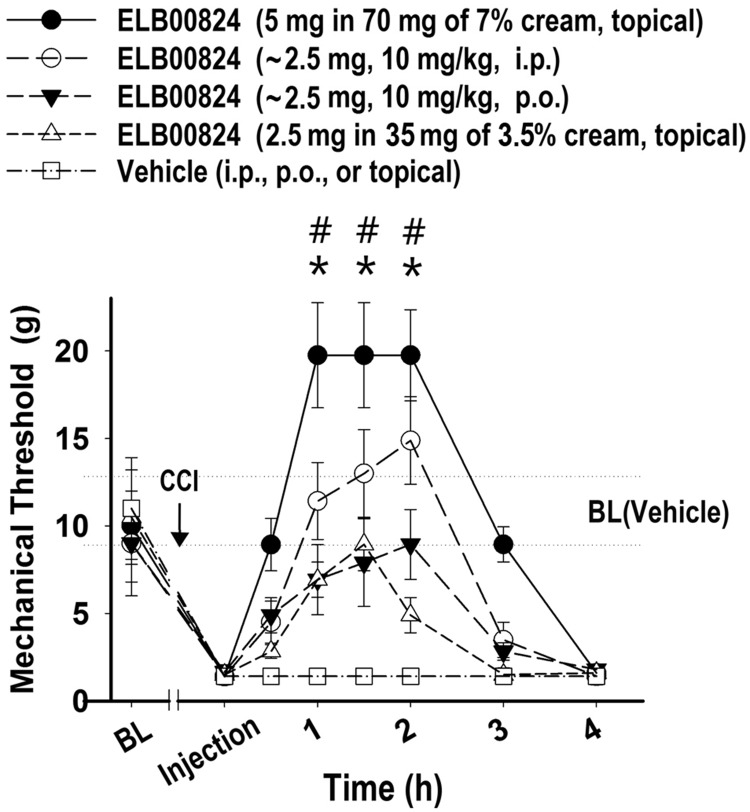
Although adverse effects of systematic treatment with ELB00824 were not observed, topical treatment is recommended for localized NP (LNP), characterized by a circumscribed and consistent area of maximum pain. A recent treatment algorithm for LNP suggests using topical therapeutics before moving to systemic medication, because the benefit/risk ratios of topical agents are far better. Most oral NP medications produce significant adverse effects that limit the doses that can be used, and with chronic use become intolerable for many patients, especially elderly, as well as patients with comorbidities or drug interactions.13 Since topical LNP therapies are designed to be applied directly near nerve endings, the effect of topical ELB00824 on NP was studied and found to provide the most prolonged effect (Figure 5).
Figure 5.
Topical treatment of ELB00824 provide provides the most prolonged relief from pain. Ipsilateral whisker pad mechanical threshold in the rats with trigeminal CCI after treated with ELB00824 by different routes, including i.p. (10 mg/kg, dash line, empty circles), p.o. (10 mg/kg, dash line, solid triangles), high-dose topical (7% cream, solid line, solid circles), and low-dose topical (3.5% cream, dash line, solid triangles), and after treatment with vehicle. Values represent mean ± SEM, n = 4. CCI: chronic constriction injury; BL: baseline threshold of each group; BL (Vehicle): baseline threshold of the vehicle control group (10.9 ± 1.9 g). *:P < 0.05 versus ELB00824 (i.p.). #:P < 0.05 versus ELB00824 (p.o.).
Thus, ELB00824 was effective by three different routes for increasing the ipsilateral whisker pad mechanical threshold in the rats with trigeminal CCI, including i.p. or p.o. (2.5 ± 0.1 mg, 10 mg/kg, body weight 250 ± 10 g), as well as a high- or low-dose topical (5 or 2.5 mg in 7% cream with 100 mg/cm2 or 50 mg/cm2, respectively). As illustrated in Figure 5, topical ELB00824 dose-dependently produced an increase in mechanical threshold in rats with CCI injury (1.5 h: 5 mg topical: 19.7 ± 3.1 g vs. 2.5 mg topical: 8.9 ± 1.5 g, P < 0.01). The higher dose topical ELB00824 provided not only a faster onset of symptom relief (onset at 0.5 h) compared to systematic treatment (onset at 1 h, either i.p. or p.o) but also a more sustained decrease in hypersensitivity. At 3 h after treatment, the anti-allodynic effect of topical treatment still persists (mechanical threshold: 8.9 ± 1.1 g), while the systematic treatment effect has diminished (i.p.: 3.5 ± 0.9 g; p.o.: 2.8 ± 0.5 g).
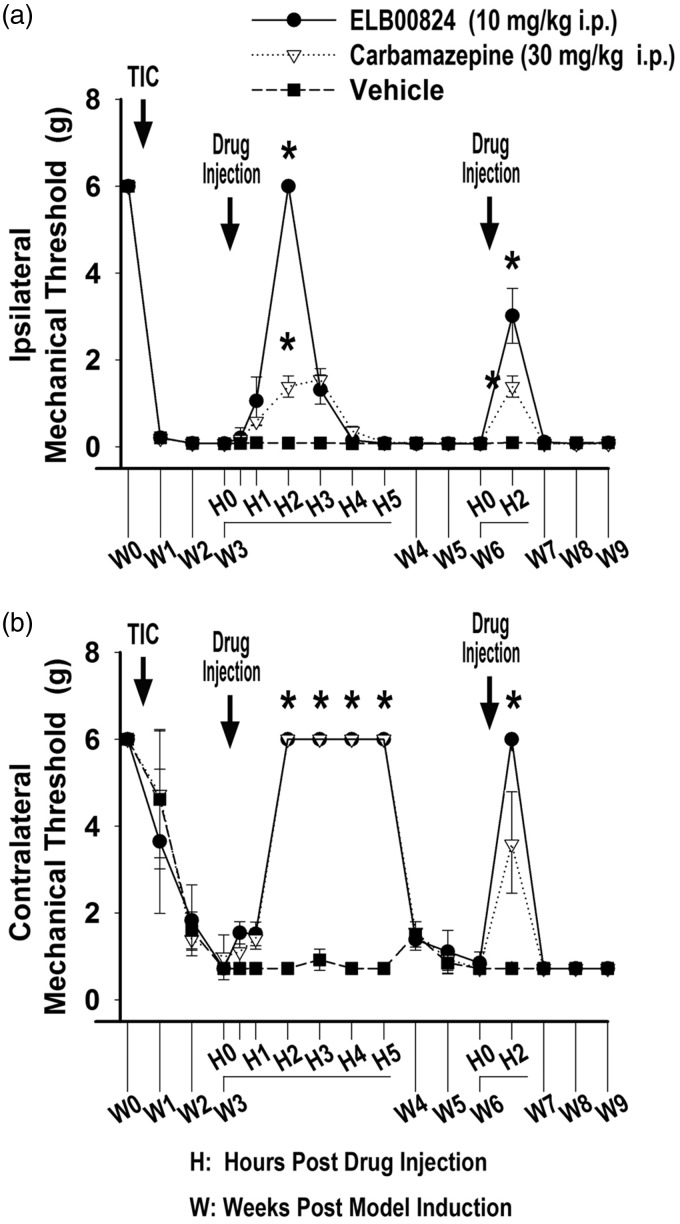
Acute and chronic anti-allodynic effects of ELB00824 in the mouse TIC model (three and six weeks)
Efficacy of ELB00824 was tested in the mouse TIC model at two time points (weeks 3 and 6). Figure 6 depicts the effect of a one-time drug dose of 10 mg/kg (i.p.) of ELB00824 compared with 30 mg/kg (i.p.) of carbamazepine in mice with TIC injury. The mechanical threshold in the ipsilateral (Figure 6(a)) and contralateral (Figure 6(b)) whisker pad was measured 1 h before treatment (baseline). In week 3, the mechanical threshold increased to 6 ± 0.0 g (4.3 fold) at 2 h post ELB00824 injection as compared to 1.4 ± 0.2 g when treated with the carbamazepine. Both treatments were significantly increased compared with the vehicle treated animals with TIC (0.01 ± 0.00 g; 2 h after injection, P < 0.001, n = 4) (Figure 6(a)), on both the ipsilateral and the contralateral side. The effect peaked at 2 h and persisted for 3 to 4 h.
Figure 6.
ELB00824 and carbamazepine attenuated whisker pad mechanical allodynia when tested at both acute and chronic time points after mouse TIC model induction. The anti-allodynic effect of ELB00824 on mechanical threshold was statistically significant for both the ipsilateral (a) and contralateral (b) whisker pads. Drug was injected i.p. on day 21 and day 42, and mechanical threshold determined 0, 0.5, 1, 2, 3, 4, and 5 h after drug injection (n = 4). The data are presented as a mean ± SEM. For all graphs, asterisk (*) indicates P < 0.05.
In week 6, treatment with both ELB00824 and carbamazepine also significantly improved allodynia compared to vehicle treatment on the ipsilateral side. ELB00824 significantly improved allodynia on the contralateral side. The allodynia test was done 15 min prior to the 2 h peak to accommodate the additional anxiety testing done after the six-week treatments. The timing of the allodynia testing to accomodate the two anxiety tests is likely responsible for the apparent decrease in the efficacy rather than tolerance to ELB00824 since the contralateral test done sequentially had maximal efficacy.
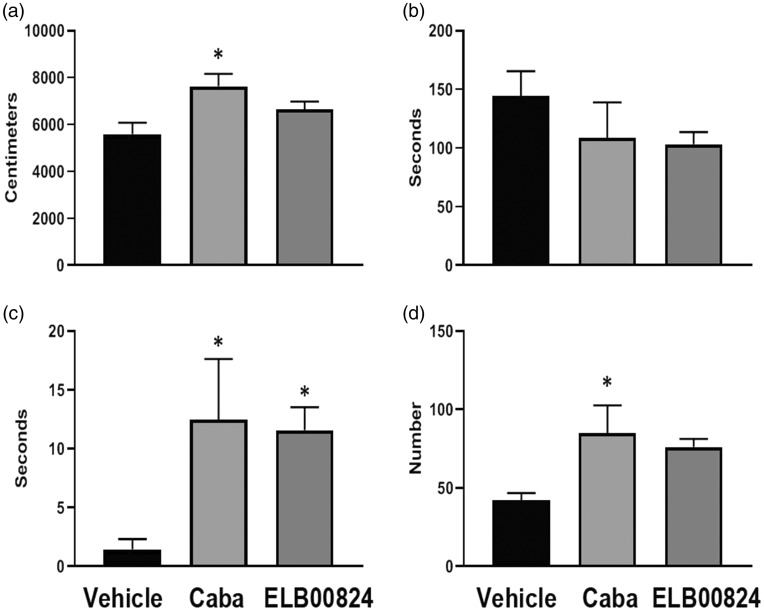
Anti-anxiety effects of ELB00824 in the mouse chronic TIC model (six weeks)
Two anxiety tests were performed at the six weeks treatment timepoint post TIC model induction in the mice given vehicle, carbamazepine, or ELB00824. At 2 h post treatment, previously determined to be the peak time for allodynia relief, anxiety effects induced by the chronic trigeminal pain model were determined with the zero maze and light/dark place preference tests. The timing to perform the three different behavioral tests likely accounted for the decrease in ipsilateral effect at the six-week treatment assessment since the von Frey test was done first just ahead of the 2 h timepoint.
For the zero maze, if mice spend more time in the closed area of the maze, it is considered anxiety behavior. Mice treated with either ELB00824 or carbamazepine did not show significant differences from those given vehicle for time spent in the closed area of the maze, but can be seen trending toward less time spent in the closed areas (ELB00824: 103 ± 21 s vs. vehicle: 144 ± 42 s; P = 0.14, Figure 7(b)). The mice with TIC did have significantly increased latency to move to the closed area if treated with ELB00824 (ELB00824: 11.6 ± 3.9s vs. vehicle: 1.5 ± 1.7s; P < 0.05) or carbamazepine (Figure 7(c)), but only mice treated with carbamazepine had significantly more time traveled (Figure 7(a)) and zone transitions (Figure 7(d)). Other indicators of anxiety assessed included less time moving between zones and less time to transition from their starting position in the open area to the closed area noted for mice with TIC given vehicle.
Figure 7.
ELB00824 (10 mg/kg) treatment was less effective than carbamazepine (Caba, 30 mg/kg) in attenuating anxiety in the mouse chronic TIC model at six weeks, using the zero maze anxiety test. Significant differences from vehicle treated mice were evident only with the latency to enter the closed area of the maze. Other measures trended toward anxiety reduction. For all graphs, asterisk (*) indicates P < 0.05 (n = 4). (a) Distance traveled; (b) Time in closed area; (c) Entry latency to closed and (d) Zone transitions.
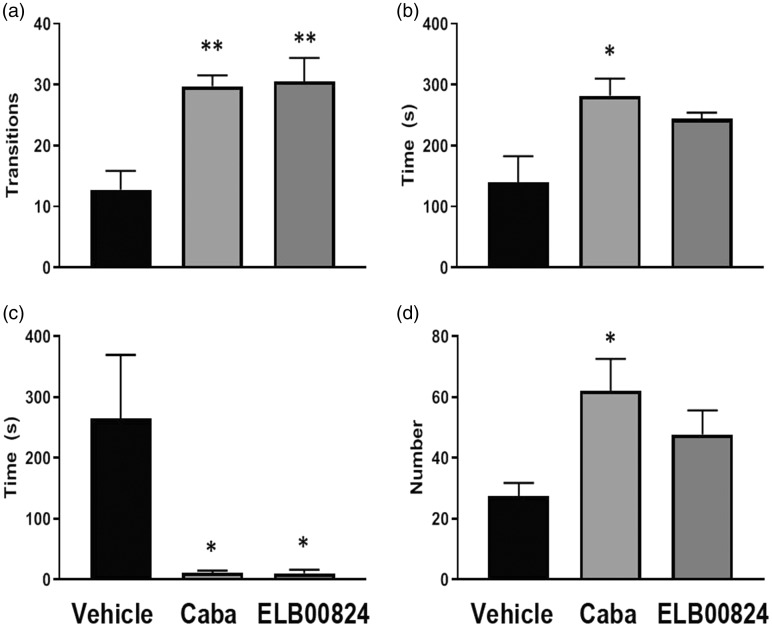
For the light/dark preference anxiety test, less time spent in the lighted area of the maze was considered an anxiety behavior. Also, less time to transition from their starting position in the dark area to the lighted area and/or less time doing rearing activities were considered anxiety behaviors. Two hours after the injection, mice treated with ELB00824 displayed significant less anxiety behavior than anti-anxiety behavior with the zone transition (ELB00824: 30.5 ± 7.8 s vs. vehicle: 12.8 ± 6.2s; P < 0.05) (Figure 8(a)). ELB00824 decreased latency to enter into the lighted chamber (ELB00824: 10 ± 12 s vs. vehicle: 265 ± 208 s; P < 0.05) (Figure 8(c)), as did carbamazepine treatment compared to vehicle in mice with TIC in this test. Occupancy time (Figure 8(b)) and number of times that rearing occurred (Figure 8(d)) were less robust anti-anxiety behaviors in mice treated with ELB00824 than those treated with carbamazepine.
Figure 8.
ELB00824 (10 mg/kg) significantly attenuated anxiety in the mice with chronic TIC at six weeks, in two of the light/dark place preference test measures. The decrease in anxiety was equivalent to reduction by carbamazepine (Caba, 30 mg/kg) for at least two of the measures. For all graphs, asterisk (*) indicates P < 0.05 and double asterisks (**) indicate P < 0.01 (n = 4). (a) Zone Transitions; (b) Total occupancy time (light); (c) Entry latency into light and (d) Number of times reared.
Other Preliminary Specific Toxicity Studies
An In vitro cardiovascular toxicity study was performed by contractor ICE bioscience Inc. in Beijing, China, for this safety evaluation in mice. Off-target effects at the hERG potassium channel have linked to cardiac liability of some drugs. It is suggested that a ratio of more than 30:1 between hERG IC50 and the Cmax at therapeutic doses could be taken as indicative of a lack of potential inducing a specific type of abnormal heart rhythm. The results showed that IC50 for ELB00824 is over 30 µM, and the ratio for ELB00824 is approximately 100:1, much larger than the required ratio, indicating that ELB00824 is very safe for the heart.
A genotoxicity study, micronucleus test, was conducted since it is one of the most successful and reliable assays used in screening for potential genotoxic compounds. Data from Elixira Biotech show that testing concentrations of ELB00824 did not induce a significant increase of chromosome damage in mouse bone marrow erythroblast, even at the highest concentration (500 mg/kg, intraperitoneal), indicating ELB00824 is potentially safe for clinical testing and not genotoxic.
Discussion
Taken together, these data demonstrate that low-dose ELB00824 provides safe and satisfactory reduction of mechanical allodynia and anxiety-like behaviors associated with the trigeminal nerve injury in mice and rats of both sexes. The study animals of either sex remained alive, and no significant alteration of body weight or neurotoxicity was noticed long term. Food consumption of the entire study group was found to be normal. Evaluation of hematological and coagulation profile was also performed, and no significant change from the reference was found. Different serum biochemistry parameters were determined and treatment groups were compared with control group as a biochemical marker, including hepatic biomarker (ALT, AST, ALP, γGT, TBIL), renal biomarker (urea, creatinine), muscle biomarker (CK), and glucose metabolism biomarker (glucose) to investigate any toxicity. The results obtained were satisfactory, with no significant alterations in any serum biochemistry parameters. The sub-chronic oral toxicity study provided promising results. In addtion, ELB00824 did not produce any significant short-term alterations in cardiac toxicity or genotoxicity.
These data demonstrate that low-dose ELB00824 had the effect of both reducing mechanical allodynia after persisting nerve injury in mice and in rats of both sexes, as well as in reversing anxiety-like behaviors tested in mice with persisting allodynia.
This study indicated that ELB00824 may be the most potent PPARɣ agonist currently known for acute reduction of NP. Although previous research has determined that a clinically used PPARɣ agonist, pioglitazone, attenuates TNP, the existing study is the first to find the lower dose of PPARɣ agonist, ELB00824 (10 mg/kg), effectively decreased neuropathic hypersensitivity in trigeminal nerve injury rodent models at both acute and chronic time points. This dose is approximately 100-fold lower than the NP effective dose (1000 mg/kg, i.p.) of pioglitazone. Note that for pioglitazone, its effective dose is unacceptably higher than its rat LD50 values (558–587 mg/kg, i.p.). In combination with another analgesic, for example, gabapentin, ELB00824 at even lower doses, 3 mg/kg, elicited a similar anti-allodynic effect. One important cause of the unprecedented anti-allodynic effects of ELB00824 among PPARɣ agonists is likely its superior blood–neural barrier permeability, evidenced by approximately two orders of magnitude increase in kp of ELB00824, compared to those of pioglitazone and rosiglitazone (Table 1).
Furthermore, this study showed that the topical treatment of ELB00824 has potential to provide not only a faster onset symptom relief but also more sustained desensitization and relief from pain than systematic treatment.
One of the proposed mechanisms for ELB00824 effects is the PPARɣ-dependent mechanism. The evidence from our non-TZD type PPARɣ agonist ELB00824 was consistent with the findings that PPARɣ plays a significant role in acute trigeminal mechanical sensitivity testing with TZD type PPARɣ agonists as shown previously in the mouse TIC model.1
Our studies do not rule out other “off-target” effects that might contribute to rapid ELB00824 anti-hyperalgesia, such as by modulating G-protein-coupled receptor 40 (GPR40), mitochondrial pyruvate carrier, mitoNEET, transient receptor potential channels, and others.2,14,15
The current acute and chronic studies are just a beginning for a wider set of explorations for future research. PPARɣ is involved in reducing tactile hypersensitivity after nerve injury, but the site(s) of action remain elusive, and warrants investigation in medulla and other brain regions. Further studies, especially in the brainstem, are needed to verify downstream effects of PPARɣ-dependent mechanism, for example, astrocyte inactivation, reduced releasing of neuron-sensitizing molecules, for example, tumor necrosis factor.
Overall, the present study not only provides the first demonstration that a new PPARɣ agonist, ELB00824, in unparalleled low doses, attenuates hypersensitivity induced in TNP models in two animal species, but also provides some evidence of reduced anxiety related behavior. Furthermore, these studies determine that activation of PPARɣ are potential mechanisms for treatment. Taken together, these studies provide information relevant to utilization of ELB00824 alone or in combination with other drugs as potential treatment for patients with orofacial or other NP conditions, for example, painful diabetic neuropathy and neuropathic cancer pain after conversion to sustained relieve formulations for clinical use.
Supplemental Material
Supplemental material, MPX884498 Supplemetal Table1 for Sustained relief of trigeminal neuropathic pain by a blood–brain barrier penetrable PPAR gamma agonist by Morgan Zhang, Min Hu, Marena A Montera and Karin N Westlund in Molecular Pain
Supplemental Material
Supplemental material, MPX884498 Supplemetal Table2 for Sustained relief of trigeminal neuropathic pain by a blood–brain barrier penetrable PPAR gamma agonist by Morgan Zhang, Min Hu, Marena A Montera and Karin N Westlund in Molecular Pain
Acknowledgments
The authors would like to thank Hui Liu, Xiaoxia Zhang, Baoqi Wang for synthesis and 1H NMR spectroscopic elucidation of ELB00824. The authors also thank Katherine Gott for laboratory management during these studies.
Author Contributions
MZ and KNW conceived the project and supervised all the studies. MZ designed the molecule structure of ELB00824 and statistical analysis of all the data. MH carried out determination of blood–brain barrier permeability of ELB00824 in rats and of in vitro effects on PPARɣ receptor. She also performed surgical procedures for rat dIoN model and all the behavioral assays using the rat model. MAM performed surgical procedures for mouse TIC and all the behavioral assays using the mouse model. All authors contributed to the preparation of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Hugh Martin (Chair, Anesthesiology and Critical Care Medicine, University of New Mexico Health Sciences Center, Albuquerque, NM) provided start-up funds to accomplish mouse TIC model related studies (to MM and KNW). This part of studies were also partially funded by NIH R21 DE028096. Morgan Zhang, Zhichao Gao, and Ximing Huang (Board of directors of USA Elixiria Inc) provided seed funds to conduct all other parts of these studies.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Lyons DN, Zhang L, Danaher RJ, Miller CS, Westlund KN. PPARγ agonists attenuate trigeminal neuropathic pain. Clin J Pain 2017; 33: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons DN, Zhang L, Pandya JD, Danaher RJ, Ma F, Miller CS, Sullivan PG, Sirbu C, Westlund KN. Combination drug therapy of pioglitazone and D-cycloserine attenuates chronic orofacial neuropathic pain and anxiety by improving mitochondrial function following trigeminal nerve injury. Clin J Pain 2018; 34: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salat K, Gryzlo B, Kulig K. Experimental drugs for neuropathic pain. Curr Neuropharmacol 2018; 16: 1193–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK. Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARγ mechanisms. Pain 2015; 156: 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014; 14: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer’s disease. Curr Alzheimer Res 2007; 4: 159–164. [DOI] [PubMed] [Google Scholar]
- 7.Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain 2008; 9: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gijsbers L, Man HY, Kloet SK, de Haan LH, Keijer J, Rietjens IM, van der Burg B, Aarts JM. Stable reporter cell lines for peroxisome proliferator-activated receptor γ (PPARγ)-mediated modulation of gene expression. Anal Biochem 2011; 414: 77–83. [DOI] [PubMed] [Google Scholar]
- 9.King GD, Chen C, Huang MM, Zeldich E, Brazee PL, Schuman ER, Robin M, Cuny GD, Glicksman MA, Abraham CR. Identification of novel small molecules that elevate Klotho expression. Biochem J 2012; 441: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding W, You Z, Shen S, Yang J, Lim G, Doheny JT, Chen L, Zhu S, Mao J. An improved rodent model of trigeminal neuropathic pain by unilateral chronic constriction injury of distal infraorbital nerve. J Pain 2017; 18: 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma F, Zhang L, Lyons D, Westlund KN. Orofacial neuropathic pain mouse model induced by trigeminal inflammatory compression (TIC) of the infraorbital nerve. Mol Brain 2012; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry 2011; 70: 946–953. [DOI] [PubMed] [Google Scholar]
- 13.Coderre TJ. Topical drug therapeutics for neuropathic pain. Expert Opin Pharmacother 2018; 19: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 14.Wright MB, Bortolini M, Tadayyon M, Bopst M. Minireview: challenges and opportunities in development of PPAR agonists. Mol Endocrinol 2014; 28: 1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK. Pioglitazone inhibits the development of hyperalgesia and sensitization of spinal nociresponsive neurons in type 2 diabetes. J Pain 2016; 17: 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MPX884498 Supplemetal Table1 for Sustained relief of trigeminal neuropathic pain by a blood–brain barrier penetrable PPAR gamma agonist by Morgan Zhang, Min Hu, Marena A Montera and Karin N Westlund in Molecular Pain
Supplemental material, MPX884498 Supplemetal Table2 for Sustained relief of trigeminal neuropathic pain by a blood–brain barrier penetrable PPAR gamma agonist by Morgan Zhang, Min Hu, Marena A Montera and Karin N Westlund in Molecular Pain