Short abstract
Background
Aspirin-exacerbated respiratory disease (AERD) is a subtype of chronic sinusitis comprised of asthma, chronic rhinosinusitis with nasal polyps, and a non-IgE hypersensitivity to cyclooxygenase-1 inhibitors. AERD is typically refractory to medical and often surgical management and causes significant quality-of-life concerns for patients.
Objective
This study aimed to retrospectively assess the rhinologic disease-specific outcomes as well as quality-of-life metrics in a cohort of AERD patients who medically manage their condition with zileuton, a 5-lipoxygenase inhibitor.
Methods
Retrospective review of 45 patients at a tertiary care center with diagnosis of AERD who underwent functional endoscopic sinus surgery (FESS) with at least 6 months of pre- and postoperative clinical data were included in the study. Zileuton cohort chosen based on intention-to-treat after initial FESS. Background data collected included patient demographics, surgery information, and zileuton initiation dates. Zileuton and control cohorts were followed for 2.8 and 2.4 years, respectively. Outcomes measured were rhinosinusitis disability index (RSDI) scores, antibiotics use, corticosteroid use, otolaryngology visits, and time to revision surgery.
Results
RSDI scores, antibiotics use, corticosteroid use, otolaryngology visits, and time to revision surgery had no statistically significant difference between cohorts. However, those taking zileuton tended to undergo fewer revision surgeries during the study.
Conclusion
Zileuton therapy for AERD patients shows no statistical benefit for rhinologic quality-of-life symptoms but may be useful in diminishing frequency of surgical intervention. In addition, pulmonary symptoms may be improved by treatment but were not assessed. Future study is warranted.
Keywords: aspirin-exacerbated respiratory disease, sinusitis, functional endoscopic sinus surgery, revision surgery, zileuton, rhinosinusitis disability index, quality of life, questionnaire, rhinology, leukotriene
Background
Aspirin-exacerbated respiratory disease (AERD) is a syndrome comprised of asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and a non-IgE hypersensitivity to cyclooxygenase-1 (COX-1) inhibitors.1 Although understanding of AERD pathophysiology is incomplete, it is understood that there is dysregulation of eicosanoid formation leading to decreased inhibition of the 5-lipoxygenase pathway, which is on the leukotriene branch of the arachidonic acid metabolism.2 The importance of the biosynthesis of eicosanoids from arachidonic acid stems from their role in inflammation, allergy, and immunologic response. There is increase in the baseline levels of leukotrienes in the body, in conjunction with a reduction in synthesis of prostaglandin E2 (PGE2). The imbalance of leukotrienes and the anti-inflammatory PGE2 manifests with increased mast cell activation and a pro-inflammatory state in the airway. Furthermore, acute reactions to COX-1 inhibition occur and amplify the inflammation. These exacerbations are dose-dependent and begin 30 minutes after oral administration, ranging from nasal congestion to severe bronchospasm. AERD symptoms manifest more severely than non-AERD CRSwNP and correlate to more revision surgeries.3
Current literature describes several reported therapies for the treatment of CRS in AERD.4 First, there is evidence that supports benefits to aspirin (ASA) desensitization. This is where AERD patients are repeatedly exposed to COX-1 inhibition (ASA) until a tolerance threshold can be maintained.5 Another reported therapy is leukotriene modification. This modulates the leukotriene synthesis pathway, which has 5-lipoxygenase as a key enzyme.6 Zileuton, a 5-lipoxygenase inhibitor, has been clinically proven to improve symptoms of AERD by inhibiting leukotriene synthesis. The last involves dietary changes to limit external salicylate exposure of AERD patients, which has been shown to subjectively and objectively improve respiratory symptoms, though less evidence to support this therapy is available.7,8
This study is the first, to our knowledge, to analyze long-term outcomes of AERD patients on zileuton therapy. The aims of this study were to assess quality-of-life scores as well as more objective measurements of rhinologic health in AERD patients to quantify outcomes in patients treated with zileuton. This study did not examine pulmonary symptoms associated with AERD.
Methods
Study Design and Participants
The University of North Carolina Hospitals’ institutional review board approved this study and waived informed consent for data collection.
This study was conducted by examining medical record for patients with a diagnosis of AERD treated from 2005 to 2018. Of these patients, those evaluated by the otolaryngology department, underwent functional endoscopic sinus surgery (FESS), and had more than 3 months of postoperative follow-up were included in this study. AERD diagnoses were made clinically by otolaryngologists by the constellation of symptoms, including asthma, nasal polyps, and reports of either anaphylaxis or respiratory problems when taking NSAIDs. Forty-five patients met these criteria. All AERD patients were offered zileuton; however, some elected not to use the medication for a variety of reasons including price (although most patients qualified for free supplies from the manufacturer) or concerns for liver function monitoring. The zileuton cohort was followed with intention-to-treat analysis.
Data Sources and Measurements
Retrospective review of the electronic medical record was used to collect data for a database of AERD patients. Data were recorded in a Health Insurance Portability and Accountability Act compliant online database.
Demographic data collected included patient age, sex, race, ethnicity, and dates of previous sinus surgeries. Race and ethnicity were defined by electronic medical record categories, not by the investigator, and these data were collected and analyzed to comprehensively assess any patient factors associated with outcomes of treatment.
Multidimensional sinus measures included zileuton use, antibiotic use, steroid use, sinusitis exacerbations, and rhinosinusitis disability index (RSDI) questionnaire were collected from clinic notes in the electronic medical record. FESS at our institution was marked as start point for data collection, as an objective, comparable, symptomatic floor for all patients. All the zileuton patients had been started on therapy postoperatively. The RSDI questionnaire is a validated, patient-completed survey completed at each clinic visit comprised of 30 items rated on a 5-point scale (rating 0–4) to assess rhinologic health from physical, functional, and emotional domains.9 Each item is graded by patients at each otolaryngology office visit. A higher overall composite score (0–120) implies greater rhinologic disability. Other measures were taken from recorded patient histories and prescription of antibiotics and oral corticosteroids. The magnitude of changes of sinus measures following primary operative intervention until present or revision surgery was calculated. Comparisons of these measures were performed between AERD patients on zileuton therapy and the control group consisting of AERD patients not on zileuton, over 6-month intervals starting immediately postoperatively to 24+ months postoperative. The end point of data collection for patients was subsequent FESS, most current clinical visit, or loss to follow-up. Zileuton and control cohorts were followed for 2.8 and 2.4 years, respectively.
Qualitative Variables and Statistical Methods
Data were collected and stored in a customized REDCap AERD database hosted at the University of North Carolina and analyzed with Stata. All statistical analyses were performed using STATA/IC 15.0 software (Stata Corporation, College Station, TX).
Results
Participants
A total of 45 patients meeting inclusion criteria were identified, and postoperative sinus measures were collected. Average age of zileuton cohort was 50.2 years, with range of 28.3 to 66.7 years. Average age of control cohort was 57.5 years, with range of 27.3 to 84.8 years. Zileuton cohort included 10 females and 8 males. Control cohort included 18 females and 9 males. Preoperative RSDIs of zileuton and control cohort were 37.4 and 34.8, respectively. Implementation of zileuton therapy started on average 77.7 days postoperatively, with range of 6 to 300 days. See Table 1 for detailed patient demographic characteristics.
Table 1.
Baseline Characteristics Among AERD Patients.
| Demographic Characteristic | Zileuton | Non-Zileuton |
|---|---|---|
| Sex | ||
| Male | 8 | 9 |
| Female | 10 | 18 |
| Age, mean (SD), years | ||
| Race | ||
| White | 10 | 13 |
| African American | 4 | 10 |
| Asian | 2 | 3 |
| Unknown | 2 | 1 |
| Ethnicity | ||
| Hispanic or Latino | 0 | 2 |
| Not Hispanic or Latino | 17 | 23 |
| Unknown | 1 | 2 |
| Preoperative RSDI | 37.4 | 34.8 |
Abbreviation: RSDI, rhinosinusitis disability index.
Outcomes
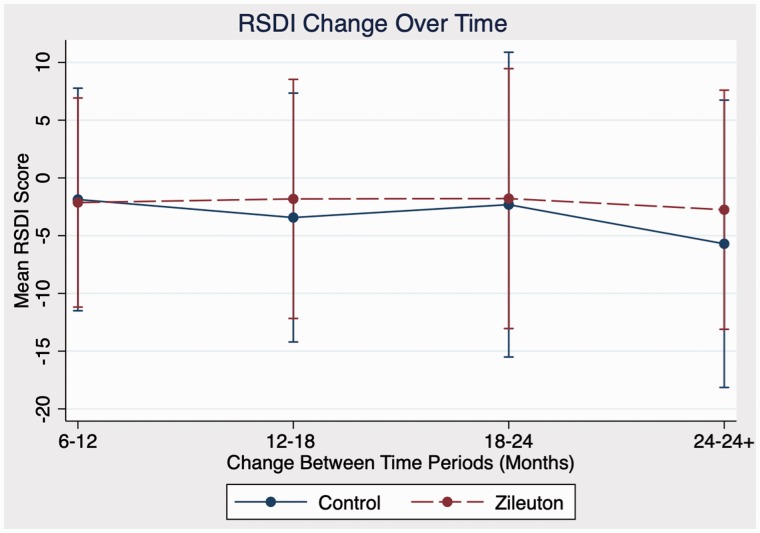
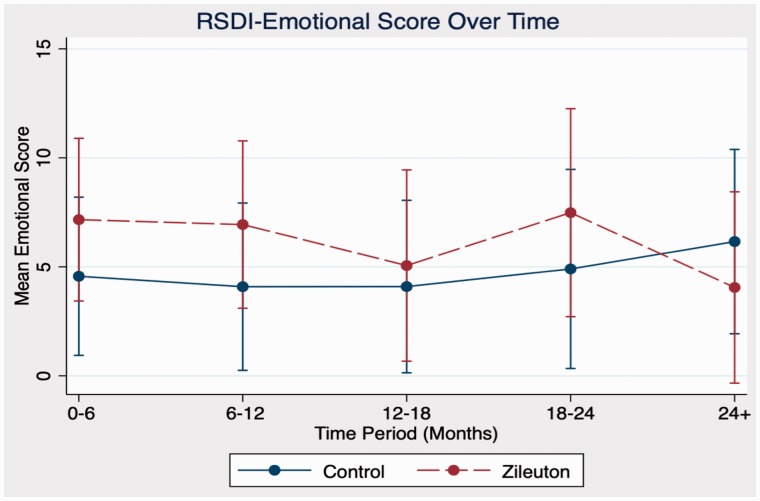
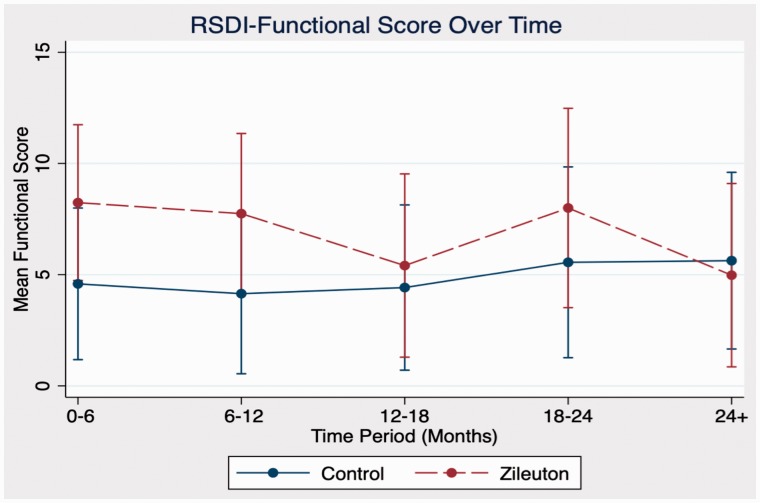
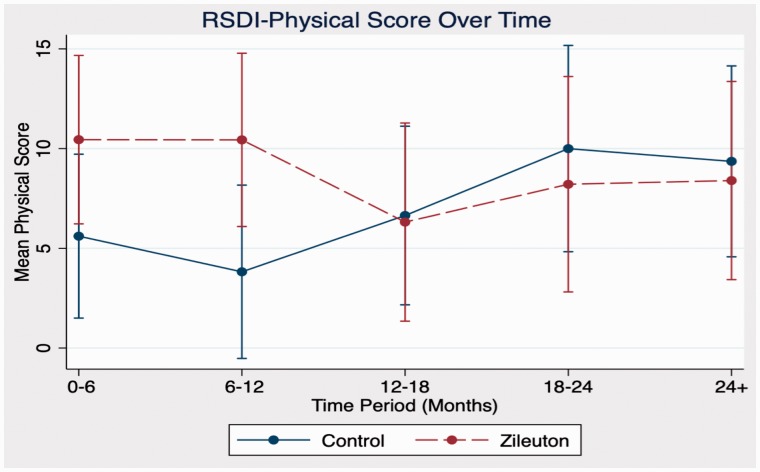
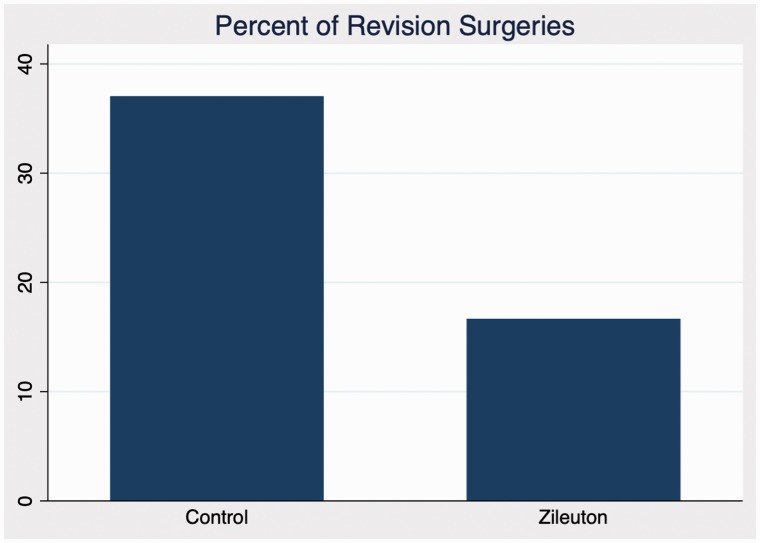
In comparison between zileuton and control cohorts in domains of antibiotic use, steroid use, sinusitis exacerbations, and RSDI, there were no statistically significant differences in outcomes at any time point. Further analysis of emotional, functional, and physical subdomains of RSDI also showed no statistical significance between cohorts (see Figures 1 to 4 for further RSDI information). A statistic of note is the number of revision surgeries. Three patients of zileuton cohort required revision FESS, while 10 patients of control cohort required revision FESS (P = .14), see Figure 5.
Figure 1.
Comparison of RSDI score change postoperatively between zileuton and control cohorts. RSDI, rhinosinusitis disability index.
Figure 2.
Comparison of RSDI emotional subdomain between cohorts. RSDI, rhinosinusitis disability index.
Figure 3.
Comparison of RSDI functional subdomain between cohorts. RSDI, rhinosinusitis disability index.
Figure 4.
Comparison of RSDI physical subdomain between cohorts. RSDI, rhinosinusitis disability index.
Figure 5.
Percentage of control and zileuton cohorts requiring revision FESS.
Discussion
AERD is a chronic condition with severe and refractory sinonasal despite medical treatment.10 Treatment remains a challenge, as there is currently no clear consensus on treatment algorithms. Zileuton is one of those treatment options for AERD. To our knowledge, there have been few studies of longitudinal effects of zileuton treatment on rhinologic health of these patients. In addition, there have not been studies for quality-of-life assessment using validated questionnaires.
Although our study demonstrates no statistically significant difference in antibiotic use, steroid use, sinusitis exacerbations, or quality-of-life metric changes between AERD patients on zileuton therapy compared to those not, any improvements may be difficult to appropriately statistically assess due to floor effect. With baseline scores being relatively low in our patient subsets, any significant improvements with zileuton management are difficult to assess. Of note, there were fewer revision surgeries noted in those taking zileuton than the non-zileuton cohort. As this was not statistically different, it may represent where zileuton therapy benefits AERD patients when not considering pulmonary symptoms.
The literature to date demonstrate the lower respiratory tract benefits of zileuton in aspirin-intolerant asthma, with improvements in expiratory volumes in pulmonary function tests.11–15 However, the evidence for zileuton’s effect on the upper airway is equivocal, with some research showing alleviation or stabilization of polyp progression, while other studies show even increased nasoocular reactivity in aspirin-sensitive asthmatics.16–18
This study was performed with interpretation of retrospective data; appropriate caution should be taken with analysis. Other limitations include the sample size. Although the data included in this study elucidates limited statistical significance, the limited patient subsets included likely are not sufficiently powered to detect small differences in quality-of-life metrics between the cohorts. This relative rarity of patients at our hospital system with AERD precludes any meaningful subgroup analysis. Therefore, further studies are warranted.
Conclusions
In summary, zileuton therapy for AERD patients showed no statistical benefit for rhinologic quality-of-life symptoms but may be useful in diminishing frequency of surgical intervention. Future study is warranted.
Authors’ Note
This study was presented at the American Rhinologic Society at Combined Otolaryngology Spring Meeting, on April 18–20, 2018, National Harbor, MD.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant support (T35-DK007386) from the National Institute of Diabetes, Digestive and Kidney diseases of the National Institutes of Health. REDCap project is used for data collection. This study received funding also from the University of North Carolina: Carolina Medical Student Research Program.
References
- 1.Samter M, Beers RF. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968; 68(5):975–983. [DOI] [PubMed] [Google Scholar]
- 2.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013; 33(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendelsohn D, Jeremic G, Wright ED, et al. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol. 2011; 120(3):162–166. [DOI] [PubMed] [Google Scholar]
- 4.Levy JM, Rudmik L, Peters AT, et al. Contemporary management of chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016; 6(12):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuler C, Baldwin JL, Baptist AP. Reaction doses in aspirin desensitization for aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016; 137(2):AB39. [Google Scholar]
- 6.Rossi A, Pergola C, Koeberle A, et al. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br J Pharmacol. 2010; 161(3):555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer DD, Rotenberg BW, Sowerby LJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. Int Forum Allergy Rhinol. 2016; 6(4):385–391. [DOI] [PubMed] [Google Scholar]
- 8.Schneider TR, Johns CB, Palumbo ML, et al. Dietary fatty acid modification for the treatment of aspirin-exacerbated respiratory disease: a prospective pilot trial. J Allergy Clin Immunol Pract. 2018; 6(3):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benninger MS, Senior BA. The development of the rhinosinusitis disability index. Arch Otolaryngol—Head Neck Surg. 1997; 123(11):1175–1179. [DOI] [PubMed] [Google Scholar]
- 10.Ta V, White AA. Survey-defined patient experiences with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2015; 3(5):711–718. [DOI] [PubMed] [Google Scholar]
- 11.Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007; 99(2):178–184. [DOI] [PubMed] [Google Scholar]
- 12.White AA, Stevenson DD, Simon RA. The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005; 95(4):330–335. [DOI] [PubMed] [Google Scholar]
- 13.Hope AP, Woessner KA, Simon RA, et al. Rational approach to aspirin dosing during oral challenges and desensitization of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2009; 123(2):406–410. [DOI] [PubMed] [Google Scholar]
- 14.Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998; 157(4):1187–1194. [DOI] [PubMed] [Google Scholar]
- 15.Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy. 2002; 32(10):1491–1496. [DOI] [PubMed] [Google Scholar]
- 16.Pauls JD, Simon RA, Daffern PJ, et al. Lack of effect of the 5-lipoxygenase inhibitor zileuton in blocking oral aspirin challenges in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol. 2000; 85(1):40–45. [DOI] [PubMed] [Google Scholar]
- 17.Cingi C, Muluk NB, Ipci K, et al. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep. 2015; 15(11):64. [DOI] [PubMed] [Google Scholar]
- 18.Parnes SM. The role of leukotriene inhibitors in patients with paranasal sinus disease. Curr Opin Otolaryngol Head Neck Surg. 2003; 11(3):184–191. [DOI] [PubMed] [Google Scholar]