Short abstract
Background
Commercially available activity monitors are a promising approach for tracking and changing physical activity in multiple sclerosis.
Objective
This study reports on the rate and pattern of activity monitor use in persons with multiple sclerosis, and compares self-reported physical activity levels between persons who do wear and those who do not wear activity monitors.
Methods
Participants completed a cross-sectional survey that included a demographic and clinical characteristics scale, activity monitor use questionnaire, and Godin Leisure-Time Exercise Questionnaire (GLTEQ) for measuring total and health-promoting physical activity.
Results
Of the 629 participants who completed the full survey, 249 (40%) reported using an activity monitor. The most common activity monitors were Fitbit, Apple watch, iPhone, and Garmin. There was a significant (p < 0.05), moderate difference (d = 0.5) in GLTEQ total scores between activity monitor users (36.6 ± 23.9) and non-users (25.0 ± 22.2), and in GLTEQ Health Contribution Score between activity monitor users (25.6 ± 22.3) and non-users (14.6 ± 18.9) (p < 0.05, d = 0.5). Self-reported steps from the activity monitor were significantly correlated with GLTEQ total score (ρ = 0.45; r = 0.36) and GLTEQ Health Contribution Score (ρ = 0.41; r = 0.35).
Conclusion
Activity monitor use is common among persons with multiple sclerosis, and activity monitor users report more total and health-promoting physical activity; this warrants further research investigating how devices may be used as a behavioral intervention tool.
Keywords: Exercise, leisure activities, multiple sclerosis, fitness trackers, physical fitness, movement
Introduction
Multiple sclerosis (MS) is a common, immune-mediated disease of the central nervous system that results in physical and cognitive dysfunction, symptoms such as fatigue and depression, and compromised quality of life.1–3 Such manifestations of MS can be managed through participation in health-promoting physical activity, yet persons with MS are substantially less physically active than the general population4 and fewer than 20% of persons with MS engage in sufficient amounts of health-promoting physical activity.5 This problem of low participation requires examination of strategies and approaches for promoting physical activity among persons living with MS.
Wearable technologies, including commercially available activity monitors, have been identified as a top worldwide fitness trend for 2019 and further recognized as a promising approach for increasing physical activity in the general population,6–8 despite some controversy regarding the accuracy of the comercially available activity monitors.9,10 Commercially available activity monitors range from simple spring-loaded pedometers through complex, triaxial accelerometers that further track heart rate and sleep patterns. An estimated one in 10 adults in the United States (US) regularly use an activity monitor and these devices are becoming common practice for wellness programs and insurance incentives among adults.6 The health-promoting properties of activity monitors are inherent in strategies such as self-monitoring, goal-setting, and potential for social support and social comparison.11
There is some evidence regarding the accuracy and precision of commercially available activity monitors in controlled environments among people with MS,12 yet little is known about the naturally-occurring rate and pattern of activity monitor use among persons with mobility limitations such as MS. This observation is important as there is growing interest in using wearable devices in MS for measuring physical activity and other outcomes in MS,13 and such use would benefit from an understanding of the rate and pattern of device use in MS and the association with outcomes such as physical activity levels.
This study reports on the rate and pattern of self-reported activity monitor use in a large sample of persons with MS, and compares self-reported physical activity levels between persons who wear (users) and do not wear (non-users) activity monitors. We further explore the association between self-reported steps from the activity monitors and physical activity levels among persons with MS who use these devices.
Materials and methods
Participants
Participants across the U.S. were recruited for a cross-sectional study examining correlates of physical activity in MS,14 and this article provides a secondary analysis of data from that study. The study was advertised through an e-mail distribution from the National MS Society (NMSS) with a link to complete the survey online. The inclusion criteria were self-reported (a) age of 18 years or older and (b) diagnosis of MS. We included data from 629 persons across 47 states who provided complete data on physical activity and activity monitor use.
Measures
Demographics and clinical characteristics
Self-reported demographic variables included sex, marital status, age, employment status, race, education, and annual household income. Clinical characteristics included self-report year of MS diagnosis, MS clinical course, and disability status using the Patient-Determined Disease Steps (PDDS) score.15,16
Physical activity
Self-reported physical activity was measured using the Godin Leisure Time Exercise Questionnaire (GLTEQ).17 The GLTEQ specifically inquires about frequency of mild, moderate, and strenuous physical activity in bouts of 15 min or more per day during the previous week. The total score was calculated by multiplying the frequency of strenuous, moderate, and mild physical activity by nine, five, and three metabolic equivalents, respectively, and summing the weighted scores. GLTEQ Health Contribution Score (HCS) provides a measure of health-promoting physical activity based on the frequency of strenuous and moderate physical activity multiplied by nine and five metabolic equivalents, respectively, and summing the weighted scores.18
Activity monitor use
Activity monitor use was assessed using four items that were created for this study. Item 1 inquired about activity monitor use, “Do you currently use an activity monitor or pedometer?” (“Yes” or “No”). Item 2 inquired about type of activity monitor used, “What kind of activity monitor do you use? (i.e. brand and model)” (open-ended). Items 3 and 4 inquired about step counts for weekdays and weekend days from the activity monitor. “One average how many steps do you take on a weekday/weekend day?” (open-ended). The Principal Invesigator (PI) coded Item 2 to classify activity monitors into categories for descriptive purposes.
Procedures
Study procedures were approved by the University of Alabama at Birmingham Institutional Review Board. The questionnaire was delivered online using Qualtrics survey software; this software is widely used in research and Health Insurance Portability and Accountability Act (HIPAA) compliant. An e-mail was distributed among the NMSS list serve that included the study flyer with a link to complete the questionnaire. The active link began with a consent form requiring participants to select an option prior to enrollment (“I consent” or “do not consent”). Eligibility was then assessed using self-reported questions to confirm being 18 years of age or older (“Yes” or “No”) and MS diagnosis (“Yes” or “No”). Participants were then allowed to access the full survey, which prompted completion of all items, but there were no forced responses. Participants were asked to provide a mailing address at the end of the survey for remuneration (US$10 visa gift card).
Data analysis
All analyses were performed using SPSS Statistics 24 (IBM, Inc., Armonk, New York, USA). Baseline descriptive characteristics are reported as n (%) or mean ± standard deviation unless otherwise specified. The differences in demographic and clinical characteristics between activity monitor use groups (user vs non-user) were examined using independent samples t-tests or chi-square as appropriate. Independent samples t-tests were conducted to examine differences in physical activity between activity monitor users and non-users, and the magnitude of differences was expressed as Cohen’s d and interpreted using guidelines of ≥0.20 for small difference, ≥0.50 for moderate difference, and ≥0.80 for large difference.19 Pearson’s and Spearman’s rank order correlations were used to examine associations between self-reported step count (averaged between weekday and weekend days) and GLTEQ total score and HCS in activity monitor users. We interpreted the magnitude of correlation coefficients based on Cohen’s guidelines for small ≥0.10, moderate ≥0.30, and large ≥0.50.19
Results
Of the 629 participants (Table 1), 249 (40%) reported using an activity monitor, whereas 380 reported not using an activity monitor (60%). The most popular activity monitor was Fitbit (n = 107; 43%), followed by Apple watch (n = 43; 17%), iPhone application (app) (n = 31; 12%), and Garmin (n = 20; 8%); other activity monitors included phone apps, pedometers, and smart watches.
Table 1.
Sample demographics and clinical characteristics.
| Variable, units (n) | Full samplen = 629 | Activity monitor usern = 249 | Non-usern = 380 | p-Value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age, years (626)a | 51.4 ± 12.1 | 47.1 ± 11.9 | 54.2 ± 11.3 | 0.001 |
| MS duration, years (627)a | 13.8 ± 10.0 | 11.2 ± 9.1 | 15.6 ± 10.2 | 0.001 |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| PDDS (629)a | 2.0 (0–4.0) | 1.0 (0–3.0) | 3.0 (1.0–5.0) | 0.001 |
| n (%) | n (%) | n (%) | ||
| MS clinical course (629)a | 0.001 | |||
| RRMS | 501 (79.7) | 219 (88.0) | 282 (74.2) | |
| Primary progressive | 42 (6.7) | 14 (5.6) | 28 (7.4) | |
| Secondary progressive | 86 (13.7) | 16 (6.4) | 70 (18.4) | |
| Gender (628) | 0.17 | |||
| Female | 526 (83.8) | 214 (86.3) | 312 (82.1) | |
| Male | 102 (16.2) | 34 (13.7) | 68 (17.9) | |
| Marital status (627) | 0.18 | |||
| Married | 410 (65.4) | 163 (65.6) | 248 (65.3) | |
| Single | 105 (16.7) | 49 (19.8) | 56 (14.7) | |
| Divorced/separated | 92 (14.7) | 29 (11.7) | 63 (16.6) | |
| Widow/widower | 20 (3.2) | 7 (2.8) | 13 (3.4) | |
| Employed (628)a | 0.001 | |||
| Yes | 317 (50.5) | 163 (65.7) | 154 (40.5) | |
| No | 311 (49.5) | 85 (34.3) | 226 (59.5) | |
| Race (628) | 0.11 | |||
| Caucasian | 571 (90.9) | 221 (88.4) | 351 (92.6) | |
| African American | 24 (3.8) | 8 (3.2) | 16 (4.2) | |
| Latino/a | 13 (2.1) | 9 (3.6) | 4 (1.1) | |
| Other | 20 (3.2) | 12 (4.8) | 8 (2.1) | |
| Education (628) | 0.08 | |||
| High school | 47 (7.5) | 11 (4.4) | 36 (9.5) | |
| 1–3 Years college | 151 (24.0) | 64 (25.8) | 87 (22.9) | |
| College graduate | 234 (37.3) | 94 (37.9) | 140 (36.8) | |
| Masters degree | 152 (24.2) | 60 (24.2) | 92 (24.2) | |
| PhD or equivalent | 44 (7.0) | 19 (7.7) | 25 (6.6) | |
| Annual household income, US$ (589)a | 0.001 | |||
| Less than $15,000 | 40 (6.8) | 11 (4.4) | 29 (7.4) | |
| $15,000–24,000 | 48 (8.1) | 7 (2.8) | 41 (10.5) | |
| $25,000–49,000 | 108 (18.3) | 37 (14.8) | 73 (18.6) | |
| $50,000–74,000 | 111 (18.8) | 45 (18.0) | 68 (17.3) | |
| $75,000–99,000 | 99 (16.8) | 47 (18.8) | 54 (13.8) | |
| $100,000 or greater | 183 (31.1) | 87 (34.8) | 100 (25.5) | |
IQR: interquartile range; MS: multiple sclerosis; PDDS: Patient-Determined Disease Steps; RRMS: relapsing–remitting multiple sclerosis; SD: standard deviation.
ap<0.001.
Activity monitor use differed by demographic and clinical characteristics. Activity monitor users were significantly younger, t(611) = 7.43, p = 0.001, had higher income, X2 (5, n = 589) = 23.10, p = 0.001, and had higher rates of employment, X2 (1, n = 628) = 38.12, p = 0.001. Activity monitor users had significantly lower PDDS scores, X2 (8, n = 629) = 63.66, p = 0.001, shorter MS duration, t(625) = 5.51, p = 0.001, and mostly relapsing–remitting MS, X2 (2, n = 629) = 21.96, p = 0.001.
The mean GLTEQ total score was 29.6 ± 23.5 and the mean GLTEQ HCS was 18.9 ± 21.0. There was a significant and moderate magnitude difference (d = 0.5) in GLTEQ total scores between activity monitor users (36.6 ± 23.9) and non-users (25.0 ± 22.1); t(620) = –6.32, p = 0.001. There was a significant and moderate magnitude difference (d = 0.5) in GLTEQ HCS between activity monitor users (25.5 ± 22.3) and non-users (14.6 ± 18.9); t(622) = –6.70, p = 0.001 (Table 2).
Table 2.
Self-reported physical activity levels and steps per day.
| Activity monitor user n = 249 | Non-usern = 380 | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| GLTEQ totala | 36.6 ± 23.9 | 24.9 ± 22.0 |
| GLTEQ HCSa | 25.5 ± 22.3 | 14.5 ± 18.8 |
| Steps device (n)b | ||
| FitBit (107) | 7537.8 ± 4900.3 | Not applicable |
| Apple watch (43) | 6899.9 ± 3048.9 | |
| iPhone (31) | 6432.3 ± 3402.4 | |
| Garmin (20) | 11362.1 ± 7808.3 | |
| Other (46) | 4980.1 ± 3733.2 |
GLTEQ: Godin Leisure Time Questionnaire; HCS: Health Contribution Score; SD: standard deviation.
ap = 0.001; bn = 2 removed, reported 0 steps.
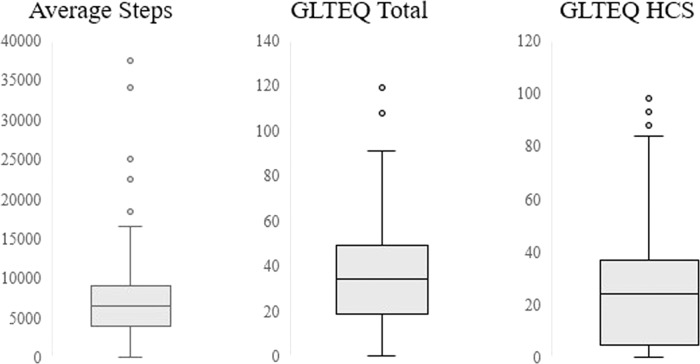
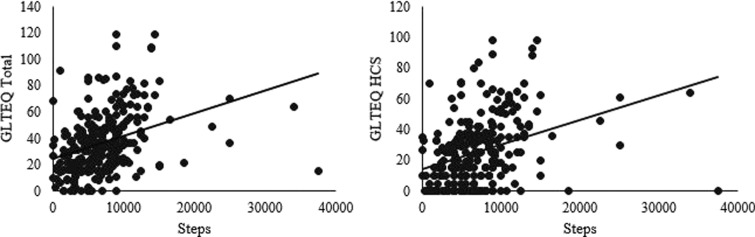
Activity monitor users reported an average of 7072 ± 4827 steps per day (Figure 1). The correlation analyses, among activity monitor users only, indicated that the GLTEQ total score was moderately associated with step counts (ρ = 0.45, r = 0.36). The GLTEQ HCS score was moderately associated with step counts (ρ = 0.41, r = 0.35). Scatterplots for correlation analyses are presented in Figure 2. Those correlations were unchanged in follow-up analyses wherein we removed the outliers identified in Figure 1.
Figure 1.
Box plots of physical activity among activity monitor users.
GLTEQ: Godin Leisure-Time Exercise Questionnaire; HCS: Health Contribution Score.
Figure 2.
Scatterplots of correlation analyses examining the relationship between self-reported physical activity and average steps.
GLTEQ: Godin Leisure-Time Exercise Questionnaire; HCS: Health Contribution Score.
Discussion
This article provides the first report of naturally-occurring activity monitor use in persons with MS across the U.S. The rate of commercially available activity monitor use in this sample of people with MS was higher than reported in the general population of adults,6 and activity monitor use varied by age, socioeconomic status, MS disease duration and disease severity. Persons with MS reported using a variety of activity monitors, and the most common were FitBit and Apple watch or iPhone apps. Activity monitor use was significantly associated with overall and health-promoting physical activity, and participant-reported steps per day from the activity monitors were associated with self-report total and health-promoting physical activity. Overall, persons with MS frequently wear activity monitors, and this use might serve as a powerful tool for integrating self-monitoring and goal-setting as strategies for promoting and sustaining physical activity behavior change in MS.
Persons with MS are interested in exercise as an effective and safe second-line therapy for improving MS symptoms and disease progression.20,21 Such a priority requires tools for initiating, monitoring, and supporting exercise and physical activity behavior. This is the first study reporting naturally-occurring activity monitor use in people with MS. Activity monitor users in this study reported mean GLTEQ HCS scores surpassing the threshold for physical activity levels that yield substantial health benefits.18 The direction of the association between activity monitor use and physical activity levels is not clear; however, several factors differentiated activity monitor users including age, income, employment, and MS clinical characteristics. Strategies for promoting activity monitor use among a wider scope of persons with MS are warranted given this association between activity monitor use and health-promoting physical activity. Such strategies may include grants or subsidies for purchasing activity monitors and health promotion campaigns that provide information regarding (a) the safety and benefits of physical activity and (b) the application of activity monitors for changing physical activity in persons with MS.
Activity monitors have been associated with increased physical activity and fitness levels in diverse populations and there is growing interest in the use of activity monitors as a tool in behavioral interventions.6,22 Activity monitors serve as a powerful self-monitoring tool to promote engagement in a sufficient amount of physical activity. Self-monitoring is one of the driving tenets of social cognitive theory (SCT), which includes both measurement and evaluation.23 Self-monitoring has been used in SCT-based physical activity interventions for persons with MS as a behavior change strategy in conjunction with goal setting, action-planning, and instruction.24 The majority of these interventions have used pedometers,25–27 but the advanced technology now available in commercially available activity monitors warrants consideration of such devices as a self-monitoring strategy to increase physical activity in MS.
Results from this study bolster the necessity for research validating commercially available activity monitors in persons with MS. We provide preliminary validity of free-living activity monitor steps in participants using a diversity of commercially available activity monitors. One laboratory-based study has examined the accuracy of five commercially available activity monitors and fitness apps for measuring 500 steps in a controlled environment involving treadmill walking.12 That study reported that the FitBit One exhibited the best absolute and relative accuracy during a 500-step walking trial.10 Other studies have compared output from activity monitors with other “device” measures such as multi-axial accelerometers in persons with stroke28,29 and Parkinson’s disease,30,31 and reported inaccuracy among persons with more severe disability (i.e. assistive device use) and during measurement of activities of daily living. Future research is necessary externally validating commercially available activity monitors in MS using both device- and self-report measures of physical activity and a variety of activity monitors (i.e. cost, brand, waist/hip worn) during free-living conditions. Such research will provide critical outcomes and self-monitoring tools for assessing and promoting free-living physical activity during clinical monitoring and behavioral interventions in persons with MS.
Limitations of this study include the cross-sectional design and self-report nature of measures, especially activity monitor steps. Researchers may address this limitation by exploring the feasibility of collaborating with manufacturers of commercial activity monitors or utilizing novel resources such as Fitabase for downloading step counts directly for reducing self-report bias, but this might be complicated by the number of devices on the market. Further, commercially available activity monitor device output may not provide accurate reflections regarding intensity of activity as this requires a metric of steps per minute, and these devices often do not provide such granular level data for users. We conducted sub-group analyses of physical activity levels between self-identified activity monitor users and non-users, but acknowledge that there may be inherent bias in physical activity levels among persons who purchase activity monitors. The majority of this sample was female, Caucasian, and higher income and therefore rate of activity monitor use may not be representative of the total MS population. Participants in this study self-identified as having MS and this might result in false reporting. We anticipate there were very few cases, if any, given that the study was advertised by the NMSS among those who have MS, participants were required to confirm a diagnosis of MS prior to initiating the study, and participants were asked to provide details regarding MS clinical characteristics that were checked for consistency by the research team. Lastly, this study involved a secondary analysis within the context of a larger study that examined environmental correlates of physical activity in persons with MS.14
Innovative strategies for promoting and sustaining engagement in physical activity are critical given the evidence regarding benefits of physical activity in persons with MS. Results from this study indicate that activity monitor use is common among persons with MS and persons who use activity monitors engage in more total and health-promoting physical activity. Commercially available activity monitors in conjunction with evidence-based behavioral strategies may be a worthwhile and economical approach for promoting naturally-occurring physical activity in MS.
Conflict of interests
The author(s) declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financialsupport for the research, authorship, and/or publicationofthis article: This work was supported by a Mentor-Based Postdoctoral Fellowship in Rehabilitation Research from the National Multiple Sclerosis Society (MB 0029).
References
- 1.Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch Phys Med Rehabil 2013; 94: 1800–1828. [DOI] [PubMed] [Google Scholar]
- 2.LaRocca NG. Impact of walking impairment in multiple sclerosis. Patient 2011; 4: 189–201. [DOI] [PubMed] [Google Scholar]
- 3.Motl RW, McAuley E, Snook EM, et al. Physical activity and quality of life in multiple sclerosis: Intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med 2009; 14: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: A meta-analysis. Mult Scler 2005; 11: 459–463. [DOI] [PubMed] [Google Scholar]
- 5.Klaren RE, Motl RW, Dlugonski D, et al. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2013; 94: 2342–2348. [DOI] [PubMed] [Google Scholar]
- 6.Wright SP, Hall Brown TS, Collier SR, et al. How consumer physical activity monitors could transform human physiology research. Am J Physiol Regul Integr Comp Physiol 2017; 312: R358–R367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WR. Worldwide survey of fitness trends for 2019. ACSMs Health Fit J 2018; 22: 10–17. [Google Scholar]
- 8.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: A systematic review. JAMA 2007; 298: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 9.Wong CK, Mentis HM, Kuber R. The bit doesn’t fit: Evaluation of a commercial activity-tracker at slower walking speeds. Gait Posture 2018; 59: 177–181. [DOI] [PubMed] [Google Scholar]
- 10.Feehan LM, Geldman J, Sayre EC, et al. Accuracy of Fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth 2018; 6: e10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons EJ, Lewis ZH, Mayrsohn BG, et al. Behavior change techniques implemented in electronic lifestyle activity monitors: A systematic content analysis. J Med Internet Res 2014; 16: e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balto JM, Kinnett-Hopkins DL, Motl RW. Accuracy and precision of smartphone applications and commercially available motion sensors in multiple sclerosis. Mult Scler J Exp Transl Clin 2016; 2: 2055217316634754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon Y, McGinnis RS, Seagers K, et al. Monitoring gait in multiple sclerosis with novel wearable motion sensors. PLoS One 2017; 12: e0171346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silveira SL, Motl RW. Environmental correlates of health-promoting leisure physical activity in persons with multiple sclerosis using a social cognitive perspective embedded within social ecological model. Prev Med Rep. Epub ahead of print 18 June 2019. DOI: 10.1016/j.pmedr.2019.100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohol M, Orav E, Weiner H. Disease steps in multiple sclerosis: A simple approach to evaluate disease progression. Neurology 1995; 45: 251–255. [DOI] [PubMed] [Google Scholar]
- 16.Marrie R, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 2007; 13: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 17.Godin G, Shephard R. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985; 10: 141–146. [PubMed] [Google Scholar]
- 18.Motl RW, Bollaert RE, Sandroff BM. Validation of the Godin Leisure-Time Exercise Questionnaire classification coding system using accelerometry in multiple sclerosis. Rehabil Psychol 2018; 63: 77–82. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Academic Press, 2013. [Google Scholar]
- 20.Pilutti LA, Platta ME, Motl RW, et al. The safety of exercise training in multiple sclerosis: A systematic review. J Neurol Sci 2014; 343: 3–7. [DOI] [PubMed] [Google Scholar]
- 21.Learmonth YC, Adamson BC, Balto JM, et al. Multiple sclerosis patients need and want information on exercise promotion from healthcare providers: A qualitative study. Health Expect 2017; 20: 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evenson KR, Wen F, Furberg RD. Assessing validity of the Fitbit indicators for U.S. public health surveillance. Am J Prev Med 2017; 53: 931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004; 31: 143–164. [DOI] [PubMed] [Google Scholar]
- 24.Motl RW, Pekmezi D, Wingo BC. Promotion of physical activity and exercise in multiple sclerosis: Importance of behavioral science and theory. Mult Scler J Exp Transl Clin 2018; 4: 2055217318786745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motl RW, Backus D, Neal WN, et al. Rationale and design of the STEP for MS Trial: Comparative effectiveness of supervised versus telerehabilitation exercise programs for multiple sclerosis. Contemp Clin Trials 2019; 81: 110–122. [DOI] [PubMed] [Google Scholar]
- 26.Motl RW, Dlugonski D, Wójcicki TR, et al. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler 2011; 17: 116–128. [DOI] [PubMed] [Google Scholar]
- 27.Motl RW, Hubbard EA, Bollaert RE, et al. Randomized controlled trial of an e-learning designed behavioral intervention for increasing physical activity behavior in multiple sclerosis. Mult Scler J Exp Transl Clin 2017; 3: 2055217317734886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui J, Heyden R, Bao T, et al. Validity of the Fitbit One for measuring activity in community-dwelling stroke survivors. Physiother Can 2018; 70: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozanski GM, Aqui A, Sivakumaran S, et al. Consumer wearable devices for activity monitoring among individuals after a stroke: A prospective comparison. JMIR Cardio 2018; 2: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendel N, Macpherson CE, Webber K, et al. Accuracy of activity trackers in Parkinson disease: Should we prescribe them? Phys Ther 2018; 98: 705–714. [DOI] [PubMed] [Google Scholar]
- 31.Lamont RM, Daniel HL, Payne CL, et al. Accuracy of wearable physical activity trackers in people with Parkinson’s disease. Gait Posture 2018; 63: 104–108. [DOI] [PubMed] [Google Scholar]