Abstract
Pubertal development during early adolescence is modestly associated with individual differences in slowly developing inhibitory control of impulses—an aspect of self-regulation associated with reward-seeking behaviors such as the onset and frequency of sexual activity. However, this effect may be much stronger in resource-poor environments. On the basis of life-history and r/K-selection theories, we tested the hypothesis that early pubertal timing would be more strongly associated with less mature neurocognitive inhibitory control in lower-income environments. In an economically diverse Appalachian sample (N = 157; 138 with complete neuroimaging data) of 14-year-olds (52% male), inhibitory control was measured using the multisource-interference task during functional MRI. Results showed that among poor youths only, more advanced puberty for one’s age was linked with lower inhibitory control for the neural but not the behavioral measure. This finding has implications regarding poverty, neurocognitive development, and health-risk behaviors in adolescence.
Keywords: inhibitory control, risk, poverty, adolescence, puberty, medial frontal cortex
A dual-systems model of adolescent risk taking hypothesizes that the pubertal transition includes a rapid shift toward more risky decision making, including sensation and reward seeking, coupled with a far more gradual progression of cognitive regulation of impulses (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2005; Steinberg, 2008). Evidence from human neuroimaging studies suggests that pubertal hormones may influence social and affective processing, as shown by brain activation within the striatum during reward processing (Crone & Dahl, 2012; van Duijvenvoorde et al., 2014). One longitudinal study found a significant linear association between increases in self-reported pubertal development and higher nucleus accumbens activity in response to rewards (Braams, van Duijvenvoorde, Peper, & Crone, 2015). In contrast, a study involving 10- to 22-year-old participants showed a nonlinear shift in performance at the age of pubertal onset during a face–word matching task that places high demand on prefrontal-lobe circuitry by challenging working memory and decision making (McGivern, Andersen, Byrd, Mutter, & Reilly, 2002). The authors interpreted this as evidence of a proliferation of synapses at the onset of puberty, in line with structural MRI studies indicating a rapid increase in gray matter at pubertal onset (Giedd et al., 1999), which may reflect a sudden increase in the number of synapses (Blakemore & Choudhury, 2006).
Although cognitive-neuroscience researchers believe that puberty may affect social-cognitive reasoning (e.g., mentalizing, perspective taking), as reflected in the medial prefrontal cortex and temporoparietal junction during such tasks (Crone & Dahl, 2012), to date there is no clear functional neuroimaging evidence directly testing the association between pubertal development and neural indicators of cognitive regulation. Extant neuroimaging studies of decision making and cognitive regulation show age-related differences in neurobiological bases of cognitive control. Specifically, data from neuroimaging tasks that require inhibition to overcome interference from competing stimuli show that cognitive-control-related activations are initially diffuse among prefrontal regions in childhood and early adolescence. These diffuse patterns of activity become increasingly more focal at later ages in adolescence, as cognitive-control behaviors and neural-activity patterns gradually come to resemble those of adults (Casey, Giedd, & Thomas, 2000; Durston et al., 2003; Ordaz, Foran, Velanova, & Luna, 2013; Perkins, Welsh, Stern, Taylor, & Fitzgerald, 2013). However, to our knowledge, only one relevant study so far (in which inhibitory control was measured using an antisaccade task) has been designed to isolate the effect of pubertal development from age; it showed little evidence of a pubertal effect (Ordaz, Fritz, Forbes, & Luna, 2018).
Aside from the work by Ordaz et al. (2018), it remains unclear whether age-based developmental improvements are indicative of puberty-based differences (at any given age in adolescence) in neural or behavioral indicators of cognitive self-regulation. We addressed this gap in the current study. Furthermore, we tested an extension of the dual-systems model of adolescent brain development—that the link between pubertal timing and neurocognitive self-regulation operates in distinct ways in resource-poor environments, compared with environments containing adequate resources. Life-history theory posits that stable differences in environments lead to variations in biologically influenced systems of reproduction and behavior in ways that increase the likelihood of producing viable offspring within that ecological niche (Ellis et al., 2012). Subsumed within life-history theory is r/K-selection theory, which states that the ecological niche directs individuals either (a) toward earlier pubertal maturation, with production of more offspring, lower parental investment, and earlier mortality (an r-selection strategy), or (b) toward later pubertal maturation, with production of fewer offspring, higher parental investment, and later mortality (a K-selection strategy; Charles & Egan, 2005).
Although usually applied to studies examining between-species differentiation, the r/K continuum also has been applied in theories of individual differences within species, including humans (Figueredo et al., 2005). Accordingly, variation along the r/K continuum reflects, in part, the trade-off between investment in growth for the future (delayed reward) and living for today (immediate reward). This trade-off has implications for timing of the onset of puberty and sexual activity and pregnancy, the number of offspring, and the length of the life span. Accordingly, living for today is a conditional adaptation supported by low impulse control that is most likely to be found in impoverished niches that have fewer and less predictable socioeconomic and safety resources (Ellis et al., 2012; K. MacDonald, 1997). Accordingly, in such environments, earlier timing of puberty might be strongly associated with neurocognitive processes that enable impulsive, risky decision making that enhances the likelihood of earlier onset and higher frequency of sexual activity, along with other aspects of greater investment in mating effort (Charles & Egan, 2005).
Thus, in the current study, we tested the hypothesis that youths’ poverty status statistically moderates the association between pubertal timing and neurocognitive inhibitory control. Specifically, we expected that among low-income youths, earlier pubertal timing compared with age-mates would statistically predict less mature or poorer inhibitory control. We focused on inhibitory control because it is widely viewed as the most salient indicator of impulse control and, when out of synchrony developmentally (e.g., impulsive reward-seeking behavior overriding inhibitory control), yields the highest levels of risk-taking behaviors (Casey & Caudle, 2013).
Method
Participants
The sample consisted of 157 participants who were 14 years old (age: M = 14.07 years, SD = 0.54, range = 13.01–15.00), of whom 52% were male. From this sample, 138 participants provided neuroimaging data. Four adolescents declined to be scanned, and 15 who were scanned did not have usable data (11 because of sustained peak-to-peak head motion in excess of 3.75-mm translation or 2.5° rotation, 2 because of very low task-performance accuracy, 1 because of a brain abnormality, and 1 because of imaging difficulty due to braces). The sample size was determined using an a priori power analysis that was conducted as part of the grant submission that funded the project; participant enrollment was completed after the target sample size was reached. The sample size provided more than adequate power to detect a modest hypothesized effect.
Also, the sample was representative of the region. For the Appalachian-area small city and rural towns and counties where we sampled families, 2010 U.S. Census data showed the median annual household income to be in the $36,000 to $59,000 range; in the current sample, the median household income was in the $35,000 to $50,000 range. Family size (i.e., a combination of one or two caregiving adults or parents and the number of children and adolescents) varied widely from two to nine people, with a median of four people. On the basis of an income-to-needs (ITN) ratio calculation (e.g., Ursache & Noble, 2015), we deemed half of the sample to be “poor” (25% of the sample; ITN < 1) or “near poor” (25%; ITN < 2). Of the remaining “nonpoor” families (50%; ITN ≥ 2), nearly half of these (20% of the total sample) had very high discretionary income (ITN > 4). Regarding self-reported race/ethnicity, the 2010 U.S. Census data show 82% to 91% White and 4% to 13% Black for the region; in the current sample, 82% of respondents were White and 12% Black, with 6% reporting “other.”
Participants were recruited via flyers and e-mails that were distributed through schools and other community locations. Research assistants called interested individuals and described the study and then invited them to participate. Exclusion criteria included history of head injury resulting in loss of consciousness for more than 10 min, claustrophobia, orthodontia impairing image acquisition, and other contraindications to MRI. Data collection took place at the university’s offices, where adolescents provided assent and their primary caregivers provided signed consent; they were then interviewed by trained research assistants. All procedures were approved by the institutional review board of the university. Adolescents and parents were paid for their participation.
Measures
Annual household ITN
Annual household income was reported by parents using an ordinal scale (1 = none; 2 = less than $1,000; 3 = $1,000–$2,999; 4 = $3,000–$4,999; 5 = $5,000–$7,499; 6 = $7,500–$9,999; 7 = $10,000–$14,999; 8 = $15,000–$19,999; 9 = $20,000–$24,999; 10 = $25,000–$34,999; 11 = $35,000–$49,999; 12 = $50,000–$74,999; 13 = $75,000–$99,999; 14 = $100,000–$199,000; 15 = $200,000 or more). The median family income was between $35,000 and $49,999 a year. Household income is imprecise regarding specification of family- and individual-level exposure to scarcity (because of wide variations in household size and cost of living). Therefore, we used the ITN ratio, which takes into account household size and income relative to the federal poverty line. ITN is recommended for and increasingly common in brain-imaging studies (e.g., Kim et al., 2019; see Ursache & Noble, 2015).
Puberty
A five-item scale was used to assess adolescent self-reports of pubertal developmental status (Petersen, Crockett, Richards, & Boxer, 1988). A sample question is, “Would you say your growth in height . . . ?” for which there were four potential responses: (1) “has not yet begun to spurt or grow really fast,” (2) “has barely started,” (3) “has definitely started,” and (4) “seems completed.” Boys and girls answered the same three questions regarding growth spurt in height, pubic hair, and skin changes. Additionally, boys were asked about facial hair growth and voice change, and girls were asked about breast development and menarche (which, unlike other items, was coded using a binary score: 1 = premenarcheal, 4 = postmenarcheal). These five items can be used to indicate stage-normative pubertal timing. Reliability was acceptable (Cronbach’s α = .77).
Inhibitory control
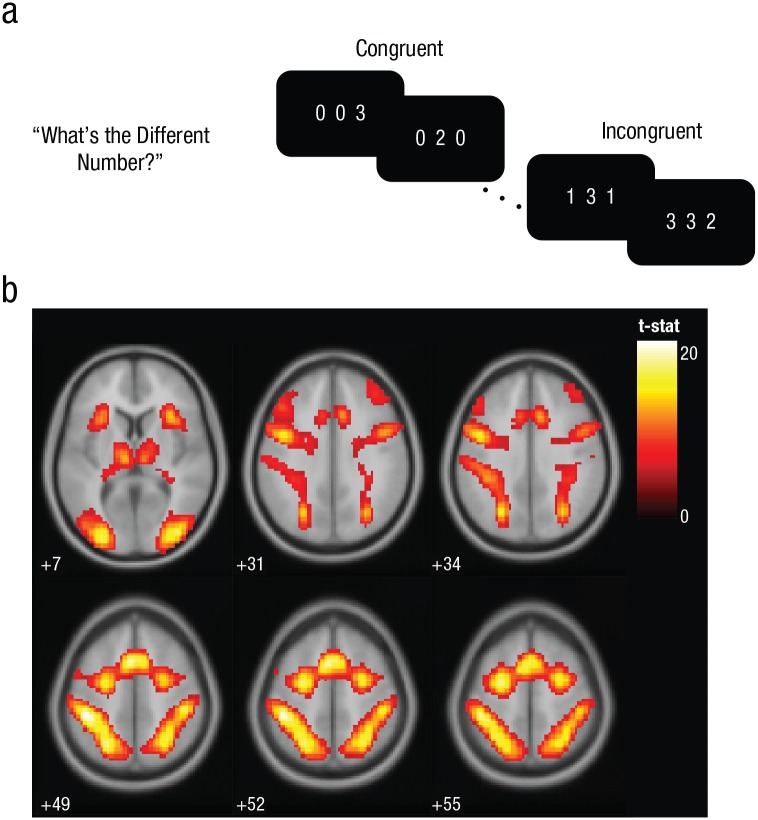
The multisource-interference task (MSIT; Bush, Shin, Holmes, Rosen, & Vogt, 2003) was used to measure detection and response to conflict associated with both flanker and spatial interference. This task requires participants to press a button on the keyboard to indicate which of three numbers is different from the other two. In control (congruent) blocks, target numbers were congruent with their left-to-right location in the number string (e.g., 3 was in the third position). In interference (incongruent) blocks, target numbers were incongruent with their location (e.g., 2 was in the third position; see Fig. 1a). Following S. W. MacDonald, Karlsson, Rieckmann, Nyberg, and Backman (2012), we also measured intraindividual variability in reaction time by computing intraindividual standard deviations across correct-response-latency trials of interference conditions. Reaction time and intraindividual-standard-deviation reaction time scores were reverse scored so that higher scores corresponded with better performance. A behavioral-inhibitory-control factor score was created using a confirmatory factor analysis in SPSS Amos (Version 22) on standardized scores of MSIT accuracy, reaction time, and intraindividual standard deviation. This model was fully saturated, χ2(0) = 0, comparative fit index (CFI) = 1.00, root-mean-square error of approximation (RMSEA) = 0. The standardized loadings were .64, .70, and .74 for MSIT reaction time, intraindividual-standard-deviation, and accuracy scores, respectively (ps < .001).
Fig. 1.
Example trial sequences and brain activations from the multisource-interference task. The multisource-interference task (a) requires participants to press a button on the keyboard to indicate which of three numbers (the target) is different from the other two. Target numbers were either congruent or incongruent with their left-to-right location in the number string. The brain images (b) show activation for the interference relative to neutral conditions in the following regions: left posterior medial frontal cortex, right and left inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus, displayed at p < .001, family-wise-error corrected (see Table 1). Whole-brain maps are shown with z-axis values (e.g., +7, +55) in the Montreal Neurological Institute coordinates system. Figure adapted from Kim-Spoon, Maciejewski, Lee, Deater-Deckard, & King-Casas (2017).
Image acquisition and analysis
While participants were performing the MSIT, functional neuroimaging data were acquired using a 3.0T Siemens TIM Trio system with the following parameters: echo-planar imaging, gradient recalled echo, repetition time (TR) = 2 s, echo time (TE) = 30 ms, flip angle = 90°, 34 axial slices, 4.0-mm slice thickness, 220-mm × 220-mm field of view (FOV), voxel size = 3.4375 mm × 3.4375 mm × 4 mm, 64 × 64 grid, and hyperangulated slices acquired at 30° from the anterior commissure-posterior commissure line. The structural scan was acquired using a high-resolution magnetization-prepared rapid-acquisition gradient-echo sequence with the following parameters: TR = 1,200 ms, TE = 2.66 ms, FOV = 245 mm × 245 mm, 1-mm slice thickness, 192 slices with spatial resolution of 1 mm × 1 mm × 1 mm. We used Statistical Parametric Mapping (SPM) software (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, England) to process and analyze the imaging data. Images were corrected for head motion using a six-parameter rigid-body transformation prior to smoothing. Then, images were realigned and normalized to the Montreal Neurological Institute template. We used parameters derived from a segmented anatomical image coregistered to the mean echo-planar image, resliced to obtain an isometric voxel of 3 mm × 3 mm × 3 mm, and spatially smoothed using a 6-mm full-width, half-maximum Gaussian kernel.
For each participant, we estimated a general linear model using SPM8. We modeled the interference and neutral conditions using a boxcar function convolved with a canonical hemodynamic response function. In addition to these, the six motion-realignment parameters were included to account for the effect of head movement. A low-pass filter was applied with a cutoff of 128 s. Each individual’s interference-minus-neutral contrast was then entered into a second-level one-sample t test to construct a whole-brain map. Individual-level eigenvariate region-of-interest (ROI) values were extracted using 6-mm spheres centered on coordinates of peak activation in the second-level map at a corrected voxel-wise threshold of p < .001 with family-wise-error correction (see Table 1 and Fig. 1b).
Table 1.
Regions of Significant Activation for the Contrast of Interference-Minus-Neutral Blocks of the Multisource-Interference Task
| Cluster No. | Cluster k | MNI coordinates | Region | Peak t | p FWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 1 | 759 | –42 | –37 | 49 | Left postcentral gyrus | 21.84 | < .001 |
| –24 | –64 | 49 | Left superior parietal lobule | 19.61 | < .001 | ||
| –30 | –55 | 52 | Left inferior parietal lobule | 18.07 | < .001 | ||
| 2 | 265 | –3 | 14 | 49 | Left posterior-medial frontal | 20.43 | < .001 |
| 3 | 506 | –39 | –85 | –2 | Left inferior occipital gyrus | 20.42 | < .001 |
| –30 | –91 | –2 | Left inferior occipital gyrus | 20.15 | < .001 | ||
| –39 | –73 | –8 | Left fusiform gyrus | 19.05 | < .001 | ||
| 4 | 654 | 42 | –64 | –8 | Right fusiform gyrus | 19.21 | < .001 |
| 42 | –82 | –2 | Right inferior occipital gyrus | 19.17 | < .001 | ||
| 33 | –91 | 1 | Right inferior occipital gyrus | 18.46 | < .001 | ||
| 39 | –67 | –23 | Right cerebellum | 15.78 | < .001 | ||
| 33 | –49 | –26 | Right cerebellum | 15.24 | < .001 | ||
| 5 | 245 | –24 | –4 | 55 | Left middle frontal gyrus | 19.06 | < .001 |
| 6 | 431 | 30 | –58 | 52 | Right inferior parietal lobule | 18.12 | < .001 |
| 45 | –31 | 49 | Right postcentral gyrus | 18.11 | < .001 | ||
| 30 | –64 | 40 | Right superior occipital gyrus | 16.52 | < .001 | ||
| 7 | 140 | 27 | –4 | 55 | Right superior frontal gyrus | 17.38 | < .001 |
| 8 | 94 | –45 | 2 | 34 | Left inferior frontal gyrus, pars opercularis | 16.89 | < .001 |
| 9 | 46 | –9 | –19 | 10 | Left thalamus | 15.01 | < .001 |
| 10 | 24 | 33 | 20 | 7 | Right insula lobe | 14.50 | < .001 |
| 11 | 7 | 6 | –73 | –20 | Cerebellar vermis | 13.47 | < .001 |
| 12 | 13 | 48 | 8 | 31 | Right inferior frontal gyrus, pars opercularis | 13.32 | < .001 |
| 13 | 12 | –30 | 17 | 10 | Left insula lobe | 13.31 | < .001 |
| 14 | 5 | 9 | –19 | 10 | Right thalamus | 12.73 | < .001 |
| 15 | 9 | –27 | –55 | –23 | Left cerebellum | 12.66 | < .001 |
| –27 | –64 | –23 | Left cerebellum | 12.60 | < .001 | ||
Note: The whole-brain t map was assessed at a corrected threshold (t) of 12, which is equivalent to a significance level (p) of 2.00 × 10−23 uncorrected. All p values are family-wise-error (FWE) corrected. MNI = Montreal Neurological Institute; k = number of voxels in each significant cluster; t = activation level in each cluster. Table adapted from Kim-Spoon, Maciejewski, Lee, Deater-Deckard, & King-Casas (2017).
In our earlier work (Kim-Spoon et al., 2016), we identified seven ROIs that were (a) significantly correlated with at least two MSIT behavioral indicators (i.e., absolute magnitude of correlation > .2) and (b) known to be engaged in interference processing and error processing (Fitzgerald et al., 2010; Koechlin, Ody, & Kouneiher, 2003; Roberts & Hall, 2008). These ROIs were left and right inferior frontal gyrus, left posterior-medial frontal cortex, left and right inferior parietal lobules, left middle frontal gyrus, and right superior frontal gyrus. Using confirmatory factor analysis, we found that all seven neural indicators significantly loaded on a common factor, χ2(11) = 12.05, p = .36, CFI = 1.00, RMSEA = 0.03; from this, we created a neural-inhibitory-control factor score. The factor loadings were .53 for left inferior frontal gyrus, .61 for left inferior parietal lobule, .64 for right inferior parietal lobule, .73 for right inferior frontal gyrus, .76 for left posterior-medial frontal cortex, .78 for right superior frontal gyrus, and .88 for left middle frontal gyrus (ps < .001). This neural-inhibitory-control factor score correlated negatively with the behavioral-inhibitory-control factor score (r = –.41), indicating that higher blood-oxygenation-level-dependent (BOLD) activation in these ROIs was associated with lower inhibitory-control behavioral performance.
Previous articles have illustrated how creating latent factor scores on the basis of confirmatory factor analysis is well suited for integrating multiple ROIs based on whole-brain analysis (e.g., Kim-Spoon et al., 2016; Moore et al., 2018). As has been shown in many functional neuroimaging studies, a single region can be involved in a broad range of tasks. Thus, it is unlikely that there is always one core region that is crucial for a particular function (Kanai & Rees, 2011). The multivariate approach to analyzing multiple ROIs related to a particular function during a behavioral task is a useful way to address correlations between ROIs for the same function because it can potentially reveal common underlying neural substrates that function together. In particular, Moore et al. (2018) showed that latent-variable analysis using structural equation modeling (SEM) is a feasible way of assessing the structural associations of functionally related brain regions and complements other functional approaches commonly used in the field to assess brain systems and networks. These advantages aside, the approach precludes consideration of ROI-specific neural-mechanism effects.
Results
Consistent with prior literature (Bush et al., 2003), our results showed a significant MSIT interference effect (i.e., main effect of congruency) in both measures of averaged task performance across the task—accuracy: t(155) = −13.29, p < .001, and reaction time: t(155) = 68.65, p < .001. Descriptive statistics and bivariate correlations are shown in Table 2. Income (ITN), pubertal development, and inhibitory-control factor scores were widely and normally distributed. Youths who were more advanced in puberty at age 14 years were slightly older (despite the restricted age range of the sample), were more often female, and had higher neural-activity scores (indicative of poorer inhibitory-control performance). For subsequent analyses, to more precisely estimate the potential effect of puberty, we included age as a covariate (Dorn & Biro, 2011).
Table 2.
Descriptive Statistics and Correlations for the Main Study Variables
| Variable | M (SD) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 1. Gender (0 = male, 1 = female) | 0.48 (0.50) | |||||
| 2. Age in years | 14.13 (0.54) | −.12 | ||||
| 3. Pubertal status score | 2.89 (0.53) | .53*** | .25** | |||
| 4. Income-to-needs ratio | 2.54 (1.90) | −.02 | −.01 | −.05 | ||
| 5. Behavioral IC factor score | 0.00 (0.86) | −.17* | .01 | −.08 | .12 | |
| 6. Neural IC factor score | 0.80 (0.33) | .12 | .09 | .21** | .03 | −.41*** |
Note: Bivariate sample sizes varied from 138 to 157. IC = inhibitory control.
p < .05. **p < .01. ***p < .001.
We began by using whole-sample SEM (in SPSS Amos Version 22) to fit a main-effects-only model in which the main effects of pubertal development and ITN, with age as a covariate, were predictors of neural and behavioral inhibitory control. (Preliminary analyses indicated that gender was not a significant predictor of inhibitory control when age and pubertal development effects were considered, so it was not included as a covariate.) Model fit was good, χ2(2) = 0.42, p = .81, CFI = 1.00, RMSEA = 0.00. Pubertal development was positively related to the neural-inhibitory-control score (indicative of poorer inhibitory control; b = 0.08, SE = 0.03, p = .01) but not behavioral inhibitory control (b = −0.07, SE = 0.07, p = .33). ITN was related to neither neural inhibitory control (b = 0.01, SE = 0.01, p = .62) nor behavioral inhibitory control (b = 0.05, SE = 0.04, p = .14).
We then used two-group SEM to test our hypothesis regarding the moderating effects of ITN in the link between pubertal development and inhibitory control (with age again included as a covariate); moderation effects could be tested by the difference in model fits using nested model comparisons. We formed high-ITN (above median) and low-ITN (below median) groups and tested whether the associations between pubertal-development and inhibitory-control variables were moderated by ITN group. To test the statistical significance of the difference between high- and low-ITN groups, we compared four nested models with the configural-invariance model, in which all parameters were freely estimated across the two groups. In the four nested models, we imposed an equality constraint to test numeric invariance between the low- and high-ITN groups with respect to the effects of pubertal development on neural and behavioral cognitive control. If the strength of the path in question significantly differed between the two groups, model fit was expected to become significantly worse by imposing the equality constraint.
SEM results are shown in Table 3, and a summary of path estimates is shown in Figure 2. We first fitted the configural-invariance model (Model 1), which is the baseline model in which all the parameters were freely estimated across groups, and the equality constraint was added on one path at a time to ask the following questions: Are the groups equivalent for the effect of age on neural inhibitory control? (Model 2), are the groups equivalent for the effect of age on behavioral inhibitory control? (Model 3), are the groups equivalent for the effect of pubertal development on neural inhibitory control? (Model 4), and are the groups equivalent for the effect of pubertal development on behavioral inhibitory control? (Model 5). In this hierarchically nested model comparison, if adding an equality constraint on a particular parameter did not degrade the model fit significantly, that equality constraint was kept. However, if the model fit degraded significantly when we added an equality constraint, then that particular parameter was left to be freely estimated in the following model.
Table 3.
Comparisons of Two-Group Structural Equation Models (Low vs. High Income-to-Needs Ratio) for Pubertal Development and Inhibitory Control
| Model | χ2 | df | p(exact) | CFI | RMSEA | p(close) | Comparison | Δχ2 | Δdf | p(d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Configural invariance | 0 | 0 | 1.00 | |||||||
| 2. Equal-age effects on neural IC | 0.02 | 1 | .90 | 1.00 | 0.00 | .91 | 1 vs. 2 | 0.02 | 1 | .90 |
| 3. Equal-age effects on behavioral IC | 0.05 | 2 | .98 | 1.00 | 0.00 | .98 | 2 vs. 3 | 0.03 | 1 | .86 |
| 4. Equal-puberty effects on neural IC | 5.81 | 3 | .12 | 0.92 | 0.08 | .24 | 3 vs. 4 | 5.76 | 1 | .02 |
| 5. Equal-puberty effects on behavioral IC | 0.07 | 3 | .96 | 1.00 | 0.00 | .98 | 3 vs. 5 | 0.02 | 1 | .88 |
Note: The best-fitting model is in boldface. p(exact) = probability of an exact fit to the data; CFI = comparative fit index; RMSEA = root-mean-square error of approximation; p(close) = probability of a close fit to the data; p(d) = probability of the difference tests; IC = inhibitory control.
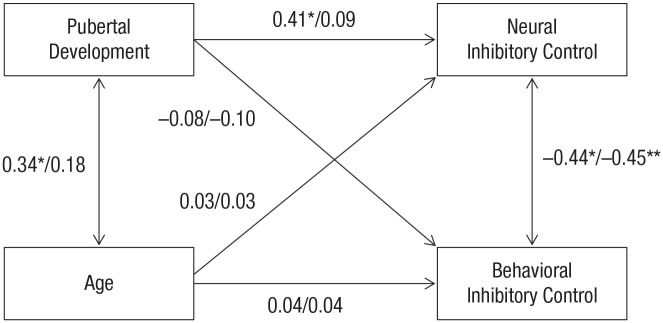
Fig. 2.
Results of the path model of relations among pubertal development, age, and neural and behavioral inhibitory control, as moderated by family income. For each path, results are shown for the low-income-to-needs-ratio group (before the slash) and the high-income-to-needs-ratio group (after the slash). All values are standardized coefficients. Asterisks indicate significant paths (*p < .05, **p < .01).
As shown in Table 3, the chi-square difference tests indicated that adding an equality constraint on the effect of age on neural and behavioral inhibitory control between the two groups (i.e., the equal-age effect on the neural-inhibitory-control model, and the equal-age effect on the behavioral-inhibitory-control model) did not significantly degrade the overall model fit. This indicated that there were no significant moderation effects of ITN in the link between age and neural and behavioral inhibitory control. In contrast, the equal-puberty effect on the neural-inhibitory-control model yielded a significantly worse fit compared with the equal-age effect on the behavioral-inhibitory-control model (which kept equality constraints for age effects on both neural and behavioral inhibitory control). This finding meant that the low- and high-ITN groups were significantly different for the effect of pubertal development on neural inhibitory control. Therefore, this particular path (pubertal development → neural inhibitory control) was freely estimated between the two groups in the subsequent model.
Next, adding equality constraints on the effects of pubertal development on behavioral inhibitory control (equal-puberty effect on the behavioral-inhibitory-control model) did not degrade the model fit significantly. Therefore, the best-fitting model (equal-puberty effect on the behavioral-inhibitory-control model in Table 3) included the path for the effect of pubertal development on neural inhibitory control freely estimated between the high- and low-ITN groups while imposing equality constraints on all other regression paths. Thus, the findings indicated that the effect of pubertal development on neural inhibitory control differed significantly between the two ITN groups. In contrast, the path linking puberty and behavioral inhibitory control, as well as age on both neural and behavioral inhibitory control, had comparable (nonsignificant) effect sizes between the ITN groups. As shown in Figure 2, more advanced pubertal development was predictive of a higher neural activity score only in the low-ITN group.
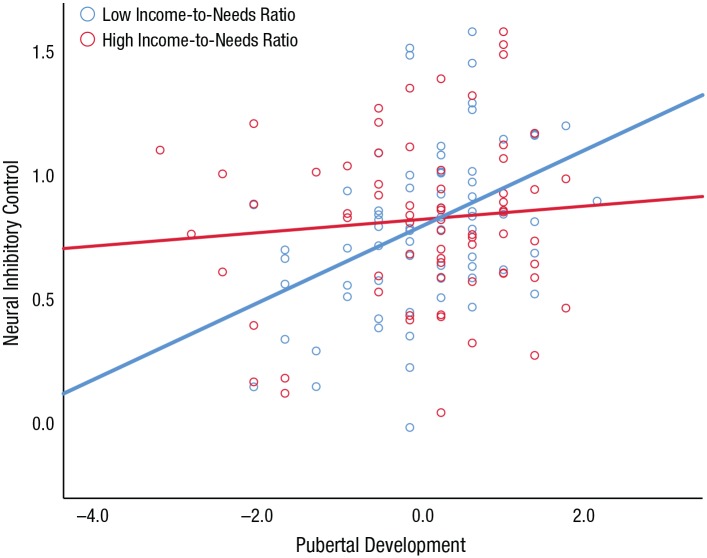
To further aid in interpreting the moderating effect of ITN, we plotted the regression line of the neural factor score regressed on pubertal development separately for the low- and high-ITN groups (see Fig. 3). Consistent with the SEM results, the regression results showed that more advanced puberty was predictive of a higher neural factor score (indicative of poorer inhibitory control) in the low-ITN group (b = 0.16, SE = 0.04, p < .001) but not in the high-ITN group (b = 0.03, SE = 0.04, p = .44).
Fig. 3.
Scatterplot (with best-fitting regression lines) showing the association between pubertal development (z scored) and neural inhibitory control, separately for the low- and high-income-to-needs-ratio groups. On the y-axis, higher scores are indicative of poorer inhibitory control.
Discussion
As children enter adolescence, a “disconnect” emerges between the neural system involved in reward-seeking and risk-taking decisions and behavior and the much slower development of the cognitive-regulation system to manage impulse control (Casey, 2015; Gluckman & Hanson, 2006). Functional MRI (fMRI) evidence shows that as development proceeds across adolescence, the brain regions involved in better cognitive regulation function with less effort, reflected in decreases in activation (Bush et al., 2003; Fitzgerald et al., 2010; Norman et al., 2011; Wetherill, Squeglia, Yang, & Tapert, 2013). These neural changes correspond with more effective behavioral and emotion self-regulation of impulses and risk-taking behaviors across adolescent development (Casey et al., 2000; Cohen et al., 2016; Durston et al., 2003; Ordaz et al., 2013; Perkins et al., 2013). In the current study, we replicated this finding—lower brain activation in ROIs covaried with better task performance.
The major aim of the current study was to address gaps in knowledge about the role of pubertal timing and resource deprivation in this developmental process. Like Ordaz et al. (2018), we found no association between pubertal development and our behavioral measure of inhibitory control. However, we did find an association with the neural measure (β = 0.21). Moving beyond the simple bivariate association, our goal was to test a hypothesis, based on life-history and r/K-selection theories pertaining to individual differences (Charles & Egan, 2005; Ellis et al., 2012; Figueredo et al., 2005), that among youths in resource-poor environments, earlier pubertal timing would come at the expense of poor cognitive regulation, measured as behavioral (i.e., MSIT performance) and neural (i.e., prefrontal activation) indicators of inhibitory control. Results provided mixed support for the hypothesis. In the sample of 14-year-olds in which half were poor or near poor (based on ITN ratios), there was a significant statistical interaction between family ITN and pubertal timing, but only in the prediction of the neural score. The association between lower inhibitory control (indicated by a higher neural factor score) and being more pubertally advanced was strongest at low levels of family ITN, and no association was observed at higher ITN levels.
The finding may reflect a trade-off between gonadal and neural development in resource-poor environments. Lower income, earlier pubertal timing, and poorer inhibitory control are predictive of more risk taking at earlier ages in adolescence, including earlier onset of sexual activity (Goldenberg, Telzer, Lieberman, Fuligni, & Galván, 2013; Graber, Nichols, & Brooks-Gunn, 2010; Qu, Galvan, Fuligni, Lieberman, & Telzer, 2015). In contrast, when income and its related resources are adequate or bountiful, there may be no trade-off between pubertal and frontal-cortex development. Overall, the neural-factor-score finding is consistent with prior structural and fMRI evidence that poverty or low socioeconomic status has large and sustained effects on brain development from early in life through adolescence and beyond (Ursache & Noble, 2015). However, it is noteworthy that there was no evidence of the hypothesized effect on the MSIT behavioral-performance score. Thus, if the puberty-by-income effect that we detected is replicated in future studies, it may be evident only in more direct measures of neural activity.
Although age-related changes in BOLD responses during inhibitory control throughout adolescence have been demonstrated in the neuroscience literature (e.g., Ordaz et al., 2013), there has been no clear evidence regarding the effects of pubertal development (when analyses controlled for age) on neural functioning related to inhibitory control. One behavioral study reported a negative association between early pubertal timing and self-control development among early adolescents (Ng-Knight et al., 2016); another behavioral study found little evidence of a link between a behavioral measure of inhibitory control and pubertal development when analyses controlled for age (Ordaz et al., 2018). Our study indicated that the associations between pubertal timing and neural indicators of inhibitory-control development varied depending on family ITN. The current finding indicates that unlike the existing literature indicating a general disadvantage of early pubertal timing in mental and behavioral problems (for a review, see Graber et al., 2010), this effect may be disproportionally large for youths growing up in resource-deprived environments. This makes apparent the need for testing effects of pubertal timing and pacing on brain development across a wide range of resource levels in adolescent environments.
There are several limitations to bear in mind. First, our correlational analyses did not allow us to infer causality in the identified relationships. Second, our measurement-modeling approach (i.e., using a factor score to represent multivariate ROIs) has the potential disadvantage of ignoring some meaningful variance in the process of aggregation; replication is important, particularly with respect to the factor-loading weights. Third, we examined only family ITN and pubertal timing. There are other important biological, environmental, and social-relationship factors (e.g., genetic factors, parenting, and peer influences) that contribute to the development of inhibitory control that we did not consider.
In sum, the current study presents the first evidence of a qualitatively different link between pubertal timing and neural development for inhibitory control, based on youths’ income scarcity. Economic disparities make a difference with respect to how pubertal timing relates to individual differences in inhibitory-control performance and related neural functioning. If replicated, this finding has implications for intervention and prevention efforts, as well as policy, supporting healthy brain and physical development in childhood and adolescence. Most immediately, this study suggests that the validity of large-scale screening for potential deficits in inhibitory control and other closely related executive functions may be maximized if family ITN and pubertal-development status are also taken into consideration. Such knowledge can be used to develop new and improved prevention and intervention tools to address the lasting effects of poverty on adolescent brain development (Johnson, Riis, & Noble, 2016).
Acknowledgments
We are grateful to the participating adolescents and their families and to the research staff, who supported the collection and management of the data.
Footnotes
Action Editor: Erika E. Forbes served as action editor for this article.
Author Contributions: K. Deater-Deckard developed the concept for the current analysis. K. Deater-Deckard, B. King-Casas, and J. Kim-Spoon contributed to the study design. J. Lee oversaw the collection and management of neuroimaging data. K. Deater-Deckard, M. Li, and J. Kim-Spoon analyzed and interpreted the data. K. Deater-Deckard, M. Li, and J. Kim-Spoon drafted the manuscript. All the authors approved the final manuscript for submission.
ORCID iD: Kirby Deater-Deckard  https://orcid.org/0000-0003-4151-2152
https://orcid.org/0000-0003-4151-2152
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported in part by grants from the National Institutes of Health (DA036017 to J. Kim-Spoon and B. King-Casas, and MH099437 to K. Deater-Deckard). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Open Practices: The current study was not preregistered. Neither the data nor the materials have been made available on a permanent third-party archive; the data and materials are available on request from the corresponding author at kdeaterdeck@umass.edu.
References
- Blakemore S. J., Choudhury S. (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology & Psychiatry, 47, 296–312. [DOI] [PubMed] [Google Scholar]
- Braams B. R., van Duijvenvoorde A. C., Peper J. S., Crone E. A. (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Shin L. M., Holmes J., Rosen B. R., Vogt B. A. (2003). The multi-source interference task: Validation study with fMRI in individual subjects. Molecular Psychiatry, 8, 60–70. [DOI] [PubMed] [Google Scholar]
- Casey B. J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Casey B. J., Caudle K. (2013). The teenage brain: Self control. Current Directions in Psychological Science, 22, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Giedd J. N., Thomas K. M. (2000). Structural and functional brain development and its relation to cognitive development. Biological Psychology, 54, 241–257. [DOI] [PubMed] [Google Scholar]
- Casey B. J., Jones R. M., Hare T. A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles K. E., Egan V. (2005). Mating effort correlates with self-reported delinquency in a normal adolescent sample. Personality & Individual Differences, 38, 1035–1045. [Google Scholar]
- Cohen A. O., Breiner K., Steinberg L., Bonnie R. J., Scott E. S., Taylor-Thompson K., . . . Silverman M. R. (2016). When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Science, 27, 549–562. [DOI] [PubMed] [Google Scholar]
- Crone E. A., Dahl R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–650. [DOI] [PubMed] [Google Scholar]
- Dorn L. D., Biro F. M. (2011). Puberty and its measurement: A decade in review. Journal of Research on Adolescence, 21, 180–195. [Google Scholar]
- Durston S., Davidson M. C., Thomas K. M., Worden M. S., Tottenham N., Martinez A., . . . Casey B. J. (2003). Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage, 20, 2135–2141. [DOI] [PubMed] [Google Scholar]
- Ellis B. J., Del Giudice M., Dishion T. J., Figueredo A. J., Gray P., Griskevicius V., . . . Wilson D. S. (2012). The evolutionary basis of risky adolescent behavior: Implications for science, policy, and practice. Developmental Psychology, 48, 598–623. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D. S., Hardin M. (2005). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo A. J., Vásquez G., Brumbach B. H., Sefcek J. A., Kirsner B. R., Jacobs W. J. (2005). The K-factor: Individual differences in life history strategy. Personality & Individual Differences, 39, 1349–1360. [Google Scholar]
- Fitzgerald K. D., Perkins S. C., Angstadt M., Johnson T., Stern E. R., Welsh R. C., Taylor S. F. (2010). The development of performance-monitoring function in the posterior medial frontal cortex. NeuroImage, 49, 3463–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Blumenthal J., Jeffries N. O., Castellanos F. X., Liu H., Zijdenbos A., . . . Rapoport J. L. (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A. (2006). Evolution, development and timing of puberty. Trends in Endocrinology & Metabolism, 17, 7–12. [DOI] [PubMed] [Google Scholar]
- Goldenberg D., Telzer E. H., Lieberman M. D., Fuligni A., Galván A. (2013). Neural mechanisms of impulse control in sexually risky adolescents. Developmental Cognitive Neuroscience, 6, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber J. A., Nichols T. R., Brooks-Gunn J. (2010). Putting pubertal timing in developmental context: Implications for prevention. Developmental Psychobiology, 52, 254–262. [DOI] [PubMed] [Google Scholar]
- Johnson S. B., Riis J. L., Noble K. G. (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Rees G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12, 231–242. [DOI] [PubMed] [Google Scholar]
- Kim D. J., Davis E. P., Sandman C. A., Glynn L., Sporns O., O’Donnell B. F., Hetrick W. P. (2019). Childhood poverty and the organization of structural brain connectome. NeuroImage, 184, 409–416. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J., Deater-Deckard K., Holmes C., Lee J., Chiu P., King-Casas B. (2016). Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia, 91, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Maciejewski D., Lee J., Deater-Deckard K., King-Casas B. (2017). Longitudinal associations among family environment, neural cognitive control, and social competence among adolescents. Developmental Cognitive Neuroscience, 26, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E., Ody C., Kouneiher F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302, 1181–1185. [DOI] [PubMed] [Google Scholar]
- MacDonald K. (1997). Life history theory and human reproductive behavior. Human Nature, 8, 327–359. [DOI] [PubMed] [Google Scholar]
- MacDonald S. W., Karlsson S., Rieckmann A., Nyberg L., Backman L. (2012). Aging-related increases in behavioral variability: Relations to losses of dopamine D1 receptors. The Journal of Neuroscience, 32, 8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern R. F., Andersen J., Byrd D., Mutter K. L., Reilly J. (2002). Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain and Cognition, 50, 73–89. [DOI] [PubMed] [Google Scholar]
- Moore M., Culpepper S., Phan K. L., Strauman T. J., Dolcos F., Dolcos S. (2018). Neurobehavioral mechanisms of resilience against emotional distress: An integrative brain-personality-symptom approach using structural equation modeling. Personality Neuroscience, 1, Article e8. doi: 10.1017/pen.2018.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng-Knight T., Shelton K. H., Riglin L., McManus I. C., Frederickson N., Rice F. (2016). A longitudinal study of self-control at the transition to secondary school: Considering the role of pubertal status and parenting. Journal of Adolescence, 50, 44–55. [DOI] [PubMed] [Google Scholar]
- Norman A. L., Pulido C., Squeglia L. M., Spadoni A. D., Paulus M. P., Tapert S. F. (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence, 119, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S. J., Foran W., Velanova K., Luna B. (2013). Longitudinal growth curves of brain function underlying inhibitory control through adolescence. The Journal of Neuroscience, 33, 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S. J., Fritz B. L., Forbes E. E., Luna B. (2018). The influence of pubertal maturation on antisaccade performance. Developmental Science, 21(3), Article e12568. doi: 10.1111/desc.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. C., Welsh R. C., Stern E. R., Taylor S. F., Fitzgerald K. D. (2013). Topographic analysis of the development of individual activation patterns during performance monitoring in medial frontal cortex. Developmental Cognitive Neuroscience, 6, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A. C., Crockett L., Richards M., Boxer A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth & Adolescence, 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Qu Y., Galvan A., Fuligni A. J., Lieberman M. D., Telzer E. H. (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. The Journal of Neuroscience, 35, 11308–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. L., Hall D. A. (2008). Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory Stroop tasks. Journal of Cognitive Neuroscience, 20, 1063–1078. [DOI] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28, 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A., Noble K. G. (2015). Neurocognitive development in socioeconomic context: Multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology, 53, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde A. C., de Macks Z. A. O., Overgaauw S., Moor B. G., Dahl R. E., Crone E. A. (2014). A cross-sectional and longitudinal analysis of reward-related brain activation: Effects of age, pubertal stage, and reward sensitivity. Brain and Cognition, 89, 3–14. [DOI] [PubMed] [Google Scholar]
- Wetherill R. R., Squeglia L. M., Yang T. T., Tapert S. F. (2013). A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]