Abstract
Metallochaperones are essential proteins that insert metal ions or metal cofactors into specific enzymes, that after maturation will become metalloenzymes. One of the most studied metallochaperones is the nickel-binding protein HypA, involved in the maturation of nickel-dependent hydrogenases and ureases. HypA was previously described in the human pathogens Escherichia coli and Helicobacter pylori and was considered a key virulence factor in the latter. However, nothing is known about this metallochaperone in the species of the emerging pathogen genus Aeromonas. These bacteria are native inhabitants of aquatic environments, often associated with cases of diarrhea and wound infections. In this study, we performed an in silico study of the hypA gene on 36 Aeromonas species genomes, which showed the presence of the gene in 69.4% (25/36) of the Aeromonas genomes. The similarity of Aeromonas HypA proteins with the H. pylori orthologous protein ranged from 21−23%, while with that of E. coli it was 41−45%. However, despite this low percentage, Aeromonas HypA displays the conserved characteristic metal-binding domains found in the other pathogens. The transcriptional analysis enabled the determination of hypA expression levels under acidic and alkaline conditions and after macrophage phagocytosis. The transcriptional regulation of hypA was found to be pH-dependent, showing upregulation at acidic pH. A higher upregulation occurred after macrophage infection. This is the first study that provided evidence that the HypA metallochaperone in Aeromonas might play a role in acid tolerance and in the defense against macrophages.
Keywords: metallochaperone, Aeromonas, macrophages, acid, alkaline, ROS, hydrogenases, ureases
1. Introduction
Metal ions are essential for the correct function of microbial biological processes [1]. In fact, many proteins contain metal ions bound directly to their amino acid chains by histidine or cysteine residues as cofactors. Particularly, metalloenzymes such as nitrogenases, ureases or hydrogenases are abundant types of metalloproteins that catalyze numerous metabolic and enzymatic reactions [2,3,4]. The synthesis of these metalloenzymes consists of complex processes that require a set of accessory proteins. In this context, metallochaperones play a key role in bacterial metal homeostasis (“metallostasis”) since they are involved in the acquisition and transfer of metals [4,5,6]. Many lines of evidence indicate an important role of metallostasis in the host-pathogen interaction [1,6,7]. For instance, during infection the host limits the availability of essential metals, inactivating metal-dependent processes of the bacterial pathogen, which compensates for this limitation by producing metallochaperones, among other proteins.
Metallochaperones act directly, inserting metal ions into specific enzymes that will become metalloenzymes after maturation [4,5,6]. One of the best-studied regulation mechanisms by metallochaperones in bacteria is the maturation of hydrogenases, which are enzymes that catalyze the interconversion of hydrogen (H2) into protons and electrons, playing a vital role in anaerobic metabolism and oxidative stress response [8]. There are three types of hydrogenase based on the metal attached to their active site: [FeFe] hydrogenase, [NiFe] hydrogenase and [Fe]-only hydrogenase [9,10]. The [NiFe]-hydrogenases are heterodimeric proteins consisting of two subunits, a small (~26 kDa) and a large (~62 kDa) which possesses in its active site the metallic cofactor NiFe(CN)2CO responsible for catalyzing the reversible production of molecular H2 [9]. Several accessory proteins encoded by genes present in the hyp operon are required for NiFe(CN)2CO biosynthesis [11,12]. In fact, the HypC, HypD, HypE and HypF proteins are considered hydrogenase maturation factors and are responsible for the synthesis and transfer of the Fe(CN)2 complex to the hydrogenase precursor. After delivery of the Fe(CN)2CO complex to the hydrogenase, the metallochaperone HypA and the GTPase HypB participate in its correct maturation by transferring a nickel ion into the large subunit of the hydrogenase [11,13].
The nickel-binding HypA metallochaperone has been well studied in the human pathogens Helicobacter pylori and Escherichia coli, in which it has been related to virulence [11,14,15,16,17]. Although the function of this metallochaperone is mainly associated with the maturation of hydrogenases, recent studies have demonstrated that HypA is also involved in urease maturation [17,18,19]. Both metalloenzymes are relevant in the adaptation to several redox conditions in pathogenic bacteria. Previous studies demonstrated that the hydrogenases participate in the defense against oxidative stress [20], as well as in acid resistance in E. coli [17,21,22]. The data showed that an E. coli K-12 hydrogenase mutant exhibits impaired acid resistance [17,21,22]. Moreover, previous works revealed that ureases facilitate the survival of H. pylori in the human gastric mucosa by protecting it from the acidic environment of the stomach [17,18,19]. The ureases neutralize the gastric acids by catalyzing the conversion of urea into ammonia and carbon dioxide. The latter studies demonstrated that the modification of the zinc- and nickel- binding sites in HypA affects its urease activity and consequently impairs acid resistance in this bacterium [17,18,19].
An earlier study investigated the redox potential of facultative aerobic and obligate anaerobic bacteria that produce H2 along the gut of earthworms by analyzing the [NiFe]- and [FeFe]-hydrogenase gene transcripts [23]. The results showed that 21% of the detected [NiFe]-hydrogenase-affiliated sequences corresponded to bacteria of the genus Aeromonas [23]. Therefore, these findings evidence the fermentative capacity of these bacteria as great hydrogen producers. Although the presence of hydrogenases has been described in Aeromonas, nothing is known about the function of the metallochaperone HypA in this genus [23]. Aeromonas species are considered opportunistic emergent pathogens producing mainly gastroenteritis and 96.5% of the clinical strains correspond to four species: Aeromonas caviae (29.9%), Aeromonas dhakensis (26.3%), Aeromonas veronii (24.8%) and Aeromonas hydrophila (15.5%). In addition, few Aeromonas species typically associated with high mortality infections in fish, such as A. salmonicida, have been reported to display strains able to produce human disease. [24,25,26]. Hence, it is of interest to investigate whether the HypA metallochaperone could be considered a new potential virulence factor of these bacteria. Therefore, the objective of this study was to search for the presence of the hypA gene in the genomes of the species of the genus Aeromonas, as well as to evaluate if HypA participates in their adaptive molecular response to acidic conditions and oxidative stress.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Aeromonas dhakensis CECT 5744T, Aeromonas caviae CECT 838T, Aeromonas veronii CECT 4257T, Aeromonas hydrophila CECT 839T, Aeromonas salmonicida CECT 894T and Aeromonas piscicola CECT 7443T type strains were used in this study and stored at −80 °C in Tryptic Soy Broth (TSB; Difco™, Bordeaux, France) with 20% of glycerol. Bacteria were grown in Tryptone Soya Agar (TSA; Difco™, Bordeaux, France) at 30 °C for 24 h. Prior to infection, bacteria were grown at 37 °C in serum and antibiotic-free Dulbecco’s Modified Eagle’s Medium (DMEM; Biowest, Nuaillé, France) for 18 h.
For evaluation of urease activity, the bacteria were grown in Urea Agar Base media (with a yellow-orange color) at 37 °C for 24 h and the change of color of the slant indicated a positive reaction. A strain of Salmonella sp. was used as a negative control and one of Proteus sp. as a positive control. The strains used in this study belong to our laboratory collection (Microbiology unit, University Rovira I Virgili)
2.2. In Silico Search of hypA and ure Genes in the Aeromonas Genomes
For identification of hypA sequences in Aeromonas species, an initial text-based search was conducted on UniProtKB from Uniprot database (https://www.uniprot.org/uniprot/). As a result, the hypA deduced amino acid sequence from Aeromonas hydrophila CECT 839T (A0KL76) was found. The corresponding nucleotide sequence of this strain (ABK37593.1) was used as query for BLAST search on 36 genomes of Aeromonas spp. type strains deposited in the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify hypA orthologues sequences. To investigate whether the presence of hypA could be a species-specific characteristic, we extended our analysis to other available genomes of non-type strains (n = 110). These genomes were verified with the Average Nucleotide Identity (ANI) using the online tool OrthoANI or with the analysis of the rpoD gene [27,28]. For identification of urease genes in the Aeromonas genomes, another BLAST search was performed using as query the sequences of the genes: ureA, ureB, ureI, ureE, ureF, ureG and ureH extracted from the genome of H. pylori strain HPAG1 (annotated in the NCBI database).
2.3. Protein Sequence Analysis and 3D-Structure Prediction
To determine sequence conservation of hypA in Aeromonas, a comparison of HypA proteins among the 25 Aeromonas species and one of E. coli (strain E24377A) and H. pylori (strain HPAG1) was assessed by multiple alignments, using the CLUSTALW algorithm via MegAlign. Phylogenetic relationships among sequences were depicted in a phylogenetic tree constructed with MEGA6 using the Neighbor-joining and Maximum-likelihood algorithms. In addition, the prediction of 3D monomeric and dimeric HypA protein structure and the comparative analyses of this protein with those of A. hydrophila (CECT 839T) and E. coli (E24377A), was done using the Swiss-model online tool (https://swissmodel.expasy.org/).
2.4. Cell Line Culture, Infection and Induction Experiments
The cell line J744A.1 from mouse BALB/C monocyte macrophages was used for the infection experiments with the six Aeromonas type strains. The macrophages cells were maintained in adhesion in Dulbecco’s Modified Eagle’s Medium (DMEM; Biowest, Nuaillé, France) (pH = 8) supplemented with 10% fetal bovine serum (FBS; Biowest, Nuaillé, France) plus 1% penicillin-streptomycin solution (P/S; Biowest, Nuaillé, France) at 37 °C and 5% CO2. Prior to infection, cells were seeded in tissue culture plates (1 × 106 cells/mL) containing serum-free DMEM without antibiotics (serum-starvation conditions) for 18 h. The macrophages J774A.1 were infected with the six Aeromonas type strains grown in serum-free DMEM without antibiotics at a multiplicity of infection (MOI) of 5. In addition, bacteria were seeded onto tissue culture plates in serum-free DMEM without antibiotics at alkaline pH (pH = 8) or acidic pH (pH = 4.5) adjusted with an HCl solution followed by filtration to remove any precipitate. Co-cultures were incubated at 37 °C and 5% CO2 up to 4 h for gene expression analyses.
2.5. RNA Extraction and Quantitative RT-PCR
Total RNA was isolated from logarithmic-phase Aeromonas cultures using TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) as previously described [29]. RNA quality and integrity were confirmed spectrophotometrically using Nanodrop 2000, calculating the 260/280 and 260/230 ratios. The cDNA was transcribed from RNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc. Hercules, CA, USA) according to the manufacturer’s instruction. Quantitative Real-Time PCR was performed in duplicate using Real-Power SYBR® green PCR Mastermix (Applied Biosystems®, Waltham, MA, USA) in 10 µL total PCR reaction mixture on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). The thermal cycling conditions were: 94 °C for 5 min, followed by 45 cycles of 30 s at 94 °C, 30 s at 60 °C, 30 s at 72 °C, and finally, 20 s at 80 °C. The threshold cycle (Ct) was automatically determined by the StepOne Software v2.0 (Applied Biosystems) to calculate the relative expression of the tested gene (hypA) using as reference the 16S rRNA housekeeping gene, as previously described [30]. Relative gene expression levels and fold change expression were estimated using 2−ΔΔCt method [31]. The specific primer pairs for the PCR amplification of hypA and 16S rRNA were designed by using consensus nucleotide sequences and Oligo Primer Analysis Software v. 7 (Table 1). Experiments were performed in triplicate using three independently prepared bacterial growth cultures obtained on three different days.
Table 1.
Oligonucleotides used in this study for transcriptional analysis.
| Primers | Sequence 5′−3′ | |
|---|---|---|
| hypA | Forward | ATGCACGAAATGTCTCTGGC |
| Reverse | TCGTAATTTGTACCCGCCAC | |
| 16S rRNA | Forward | TGTGTCCTTGAGACGTGGC |
| Reverse | ACAAAGGACAGGGGTTGCG | |
2.6. Statistical Analysis
All experiments were performed in triplicates and significant differences were determined using Student’s two-tailed t-test calculated using GraphPad Prism 6.0 (GraphPad Software, CA, USA). p values ≤ 0.05 were considered statistically significant (*).
3. Results
3.1. Identification of hypA Gene in Aeromonas Species
The results of the in silico search using the hypA sequence (339 bp) of A. hydrophila CECT 839T as template showed that hypA was present in 69% (25/36) of investigated genomes of type strains belonging to the Aeromonas species shown in Table 2. Extended analyses on 108 additional genomes from those 36 Aeromonas species available in NCBI database, allowed investigation of whether hypA is a strain or a species-specific character (Table S1). We detected hypA gene sequences in 83% (122/146) of all genomes analyzed. In most species analyzed, concordance between strains regarding the presence/absence of hypA in their genomes is observed. Although it is interesting to mention that A. caviae, A. schubertii and A. media showed a discrepancy, since some of the strains have hypA and others do not (Table S1).
Table 2.
Presence/absence of hypA gene in the genomes of 36 Aeromonas species described until now.
| Species | Strain | Source | Presence of hypA Gene | Protein Accession Number (NCBI) |
|---|---|---|---|---|
| A. aquatica | CECT 8025T | Cyanobacterial bloom | Yes | WP_033130233.1 ¥ |
| A. aquatilis π | CECT 8026T | Lake water | Yes | - |
| A. allosaccharophila | CECT 4199T | Eel | Yes | WP_042658210.1 |
| A. bestiarum | CECT 4227T | Sick fish | Yes | WP_043555398.1 |
| A. caviae | CECT 838T | Guinea pig | Yes | WP_017786826.1 |
| A. dhakensis | CECT 5744T | Children feces | Yes | WP_042008198.1 |
| A. eucrenophila | CECT 4224T | Fresh water fish | Yes | WP_042642402.1 |
| A. encheleia | CECT 4342T | Eel | Yes | WP_033130233.1 ¥ |
| A. enterica π | CECT 8981T | Human feces | Yes | - |
| A. finlandensis | CECT 8028T | Cyanobacterial bloom | Yes | WP_033136932.1 |
| A. intestinalis π | CECT 8980T | Human feces | Yes | - |
| A. jandaei | CECT 4228T | Human feces | Yes | WP_042029441.1 |
| A. hydrophila | CECT 839T | Milk | Yes | WP_005333346.1 |
| A. lacus | CECT 8024T | Cyanobacterial bloom | Yes | - |
| A. lusitana | CECT 7828T | Untreated water | Yes | WP_100861683.1 |
| A. media | CECT 4232T | Fisheries water | Yes | WP_042061779.1 |
| A. popoffi | CECT 5176T | Drinking water | Yes | WP_042034298.1 |
| A. piscicola | CECT 7443T | Sick fish | Yes | WP_021140355.1 |
| A. rivipollensis * | KN-Mc-11N1 | Wild nutria | Yes | WP_017778896.1 |
| A. salmonicida | CECT 894T | Salmon | Yes | WP_005315136.1 |
| A. sanarelli | CECT 7402T | Wound infection | Yes | WP_005301911.1 # |
| A. sobria | CECT 4245T | Fish | Yes | WP_005301911.1 # |
| A. tecta | CECT 7082T | Children feces | Yes | WP_050720085.1 |
| A. trota | CECT 4255T | Human feces | Yes | WP_026458218.1 |
| A. veronii | CECT 4257T | Sputum | Yes | WP_005351492.1 |
| A. australiensis | CECT 8023T | Irrigation water | No | - |
| A. bivalvium | CECT 7113T | Shellfish | No | - |
| A. cavernicola | CECT 7862T | Cavern creek water | No | - |
| A. crassostreae π | CECT 8982T | Shellfish | No | - |
| A. diversa | CECT 4254T | Wound infection | No | - |
| A. fluvialis | CECT 7401T | River water | No | - |
| A. molluscorum | CECT 5864T | Shellfish | No | - |
| A. rivuli | CECT 7518T | River water | No | - |
| A. schubertii | CECT 4240T | Skin abscess | No | - |
| A. simiae | IBS S-6874T | Monkey feces | No | - |
| A. taiwanensis | CECT 7403T | Wound infection | No | - |
T Type strain. * The genome of this strain was used because the genome of the type strain is not available. #,¥ Same protein accession number because their gene product display 100% sequence identity. π Species pending to be described and draft genomes not freely available.
3.2. Sequence Analyses of hypA Proteins Shows Specific Motifs Associated to Metal Binding
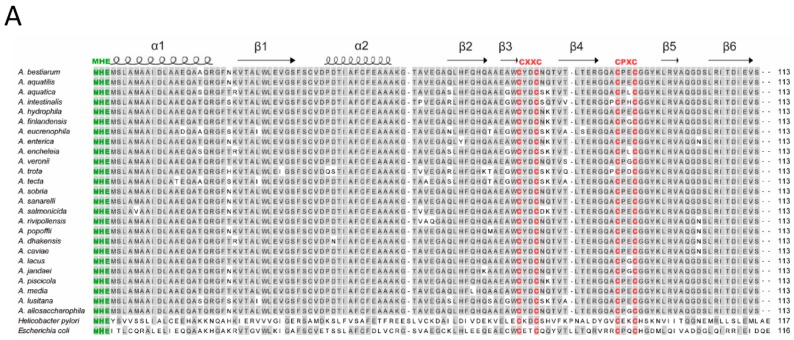
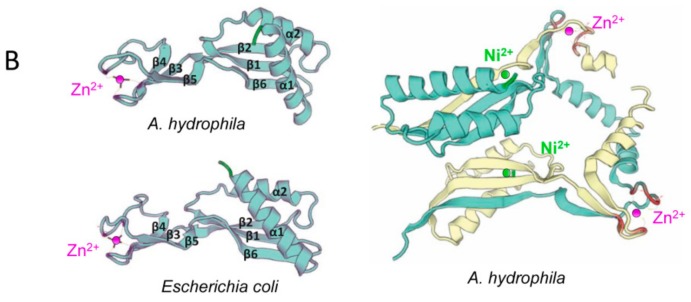
The specific metal-binding motifs consisting of N-terminal MHE motif for Ni-binding and two consecutive cysteine motifs CxxCnCPxC for Zn-binding, previously reported in the HypA proteins of E. coli and H. pylori, were also observed in the Aeromonas spp. protein sequences in Figure 1A. The three-dimensional predicted structures of HypA proteins of A. hydrophila CECT 7996T and E. coli show high similarity among them (Figure 1A). Indeed, monomeric and dimeric predicted proteins of the latter species displayed the characteristic α-helices (α1 and α2) and a β-sheet (long β1, β2, and β6 and short β3, β4, and β5) (Figure 1B).
Figure 1.
(A) Sequence alignment of the in silico-translated amino acid sequences of HypA proteins from 36 Aeromonas species, E. coli and H. pylori. The alignment was constructed with MegAlign. The MHE correspond to the motif of the Nickel binding domain (green) and CxxCnCPxP to the Zinc binding domain (red). The characteristic α-helices (wave lines) and a β-sheet (arrows) are represented. (B) Predicted monomeric and dimeric structure of HypA proteins from A. hydrophila type strain and E. coli constructed with Swiss Model online tool, the α-helices and the stranded β-sheet motifs are indicated.
3.3. Conservation and Phylogenetic Relationships of hypA in Aeromonas
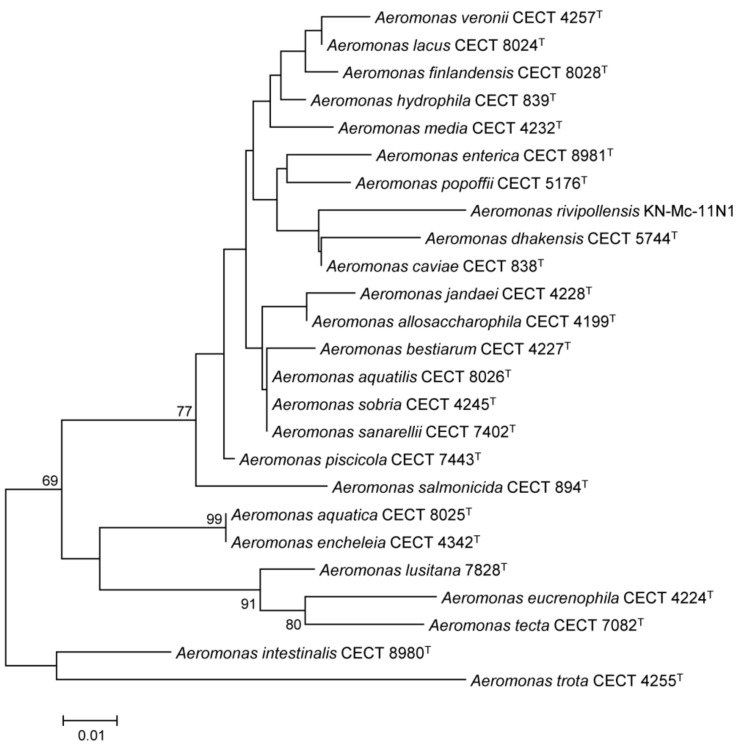
In silico HypA is the similarity of the in silico-translated amino acid sequences of HypA between the Aeromonas species ranged between 86% and 100%. As expected, when comparing the Aeromonas HypA with those from E. coli and H. pylori the similarity was significantly lower, 41–45% and 21–26% respectively (Figure 1A). As observed in the phylogenetic tree, HypA proteins were highly conserved among the 25 Aeromonas species. Two groups of species, one formed by A. aquatilis, A. sobria and A. saranellii and the other with the species A. encheleia and A. aquatica had identical hypothetical protein sequence (Figure 2 and Figure S1). Thus, in conclusion, we can state that HypA was highly conserved within the genus Aeromonas.
Figure 2.
Phylogenetic tree constructed with the in silico-translated amino acid sequences of HypA proteins (113 aa) from 36 Aeromonas species type strains (the only sequence not belonging to the type strain corresponds to A. rivipollensis KN-Mc-11N1). The phylogenetic analysis was constructed with MEGA6, using Neighbor-joining algorithm. Numbers at nodes represent bootstrap percentages (>50%) obtained by repeating analysis 1000 times.
In addition, it is important to mention that there are many identified hypA encoded proteins across different genus and bacterial species, which provide evidence of their conservation throughout evolution (Table S2). However, little is known about the biological function of HypA proteins in the vast majority of these species.
3.4. Transcriptional Regulation of hypA under Different pH-Condition and Macrophage
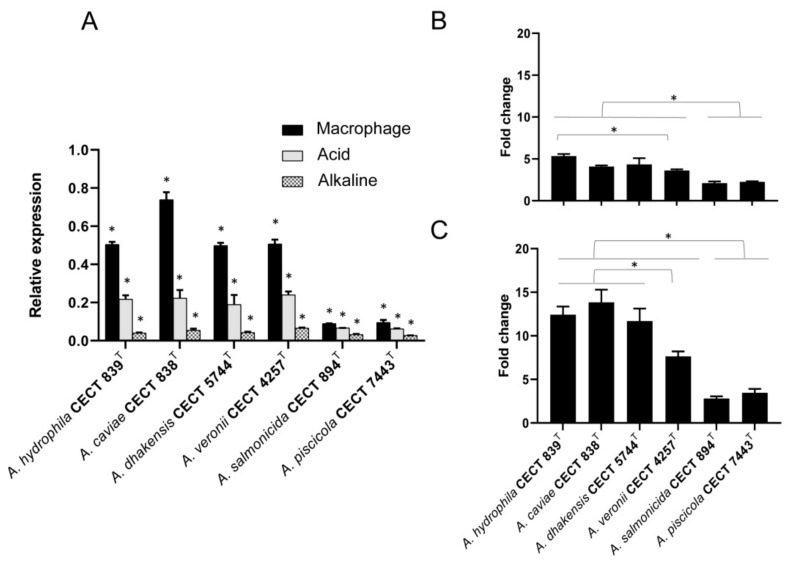
The expression patterns of hypA determined under stressful pH conditions in the most prevalent clinical species (A. hydrophila, A. caviae, A. dhakensis and A. veroni) and in two species frequently associated with fish diseases (A. salmonicida and A. piscicola) by qRT-PCR are shown in Figure 3A. All Aeromonas species displayed a similar relative expression of hypA in alkaline conditions (pH 8) (Figure 3A). Nevertheless, the expression of hypA was significantly higher (p < 0.05) under acid condition (pH 4) in comparison to alkaline condition (pH 8), displaying a greater upregulation in the most prevalent clinical species (Figure 3A,B). Furthermore, given that the phagosome of macrophages becomes acid upon phagocytosis of pathogens, we evaluated the expression of hypA during Aeromonas infection. The results showed that Aeromonas upregulates hypA in response to phagocytosis, displaying a significantly higher expression of the metallochaperone during infection than in control (alkaline media) or in vitro acid exposure (p < 0.05) (Figure 3A,C). Although transcriptional regulation of hypA seems to depend on pH or infection condition, the statistical analysis revealed strain-related differences. The most clinically prevalent species showed a significantly higher upregulation of hypA under acid exposure and during infection than species considered environmental or more related to fish disease (Figure 3B,C). Significant differences in gene induction under acid exposure were observed among the most prevalent clinical species, except between A. hydrophila and A. veronii (Figure 3B). Additionally, there were significant differences in the group of clinically prevalent species, with A. veronii showing notably lower induction of hypA when compared with A. hydrophila, A. caviae and A. dhakensis during macrophage infection (p < 0.05) (Figure 3C).
Figure 3.
Expression of hypA in Aeromonas species determined by RT-qPCR. (A) Relative expression of hypA during phagocytosis by macrophages and under acidic and alkaline culture conditions. Transcript levels of hypA were normalized to the expression of 16S rRNA. (B) Expression fold change of hypA on bacteria grown on acid media respect to the cultured in alkaline media and (C) expression fold change on bacteria phagocytized by macrophages after 4 h of infection respect to control condition on culture alkaline media, calculated using the comparative ΔΔCt method. Error bars indicate standard deviations calculated from three independent experiments. * p-value < 0.05.
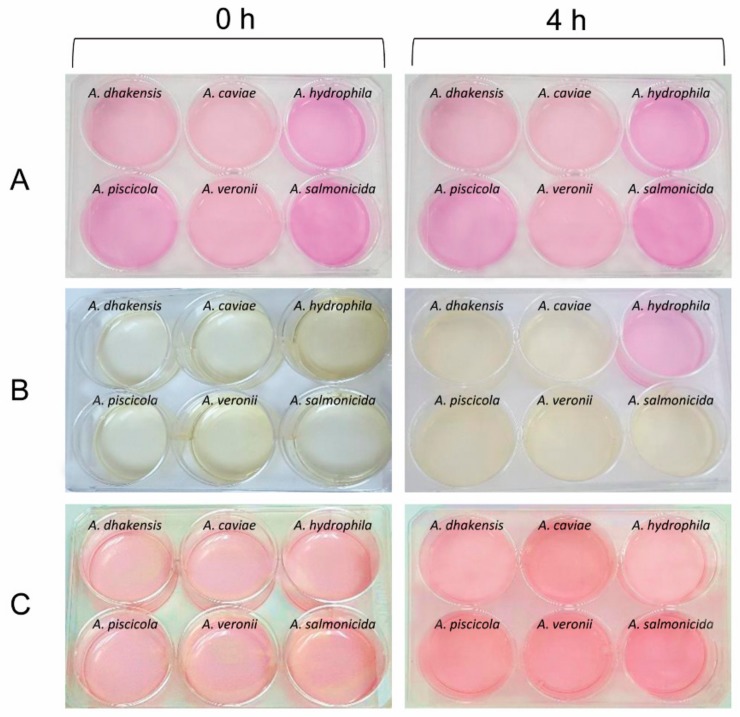
With the purpose of understanding how Aeromonas adapts to low pH environments, we determined the pH variations of the medium during in vitro growth or during infection of macrophages with the species under study. No significant changes in pH were observed when strains where grown in DMEM at pH 4 or pH 8 with the only exception of A. hydrophila (CECT 839T). When the latter strain was incubated in DMEM at pH 4 there was an increase of pH that reached up to 7.5 and that was visually evident by the changing color of the DMEM media, that functions as a pH indicator (Figure 4A,C). This basification of the medium or other pH changes were not observed for other species, neither for the infected or uninfected macrophages (Figure 4C).
Figure 4.
Culture plates with different Aeromonas species at 0 and 4 h. (A) Bacteria culture in alkaline medium DMEM. (B) Bacteria culture in acid DMEM with HCl. (C) Macrophages in alkaline DMEM infected by different Aeromonas. Yellow indicates acid pH and pink indicates alkaline pH.
3.5. Urease Activity and Urease Genes in Aeromonas Species
HypA is also involved in the urease maturation which facilitates the survival of bacteria in the human gastric mucosa neutralizing the acidic environment [17]. Therefore, we have evaluated the ability of Aeromonas to hydrolase urea by determining urease activity using a biochemical method. None of the strains assayed produced ureases because no color change in the media from light orange to magenta was observed when compared with the positive urease control (Proteus sp.). In addition, the battery of proteins associated with urease activity was not found in the genome of the six strains under study [32]. This is consistent with the absence of the urease genes in Aeromonas sp., which is inferred from our in silico search of all available genomes of the Aeromonas genus.
4. Discussion
The relevance of bacterial metal homeostasis is related to the essential role that metals play for survival in different environments, including the context of host-pathogen interaction during the infection processes [33]. In the last years, there has been significant progress in the knowledge of how metallochaperones bind metal ions, recognize the target proteins and facilitate metal transfer [4,6]. The HypA metallochaperone has been associated with [NiFe] hydrogenase and urease maturation and is considered a relevant protein for adaptation to acidic environments of pathogenic bacteria like H. pylori and E. coli [17,18,19]. Furthermore, the HypA metallochaperone participates in the defense against oxidative environments [20].
The present work is the first to address the study of the HypA metallochaperone in the genus Aeromonas. Our results suggest that hypA genes in certain species, like A. hydrophila, A. dhakensis, A. veronii and A. taiwanensis among others, are widely conserved among their strains. However, other species like A. caviae, A. schubertii and A. media showed strain-level variants, in which some strains from the same species contain hypA and others do not. Such lack of uniformity in hypA presence in well-represented species, as A. veroni, would indicate within-species microevolutionary changes that could result from environmental adaptation [34]. On the other hand, in our study some species are poorly represented, and additional analyses should be performed in order to give precise conclusions. Overall these data indicate that hypA tends to be conserved within species although cannot be considered strictly a species-specific character.
The Aeromonas HypA protein sequences showed to be moderately similar to those described in E. coli and H. pylori. However, they display the characteristic N-terminal MHE motif for Ni-binding and two consecutive cysteine motifs CXXCnCPXC for Zn-binding, also conserved in the tow human pathogenic bacteria mentioned [13,14,16,17]. In addition, the three-dimensional structure predicted for all proteins showed to be highly similar among the Aeromonas species and the other two human pathogenic bacteria, confirming that it is a metallochaperone [15,16,35].
Although the principal role of HypA has been typically associated with the maturation of hydrogenases, in the last years it has been demonstrated that HypA could play a role in the maturation of ureases. These metalloenzymes are involved in the survival of pathogenic bacteria in an acidic environment. For instance, bacterial ureases are involved in the survival of H. pylori in the human stomach at acidic pH [17,18,19]. In E. coli this function is carried out by other enzymes i.e., hydrogenases [17,21,22]. The fact that 80% of infections caused by Aeromonas are gastrointestinal diseases indicates an adaptation of these bacteria to acid environments of the gastrointestinal tract [25,26]. Additionally, a previous study hypothesized that urease activity may contribute to acid tolerance in some A. caviae strains, facilitating bacterial survival during infection, as occurs in Yersinia enterocolitica [36]. However, Aeromonas species usually have been described as urease negative [37]. Consistent with these previous data we observed in our study that all strains were urease negative. However, the expression study demonstrates that hypA is upregulated in acidic pH. A reasonable explanation could be that hydrogenases, but not ureases, would be involved in acid resistance in Aeromonas, as occurs in E. coli [18,22,23]. In addition, our results showed that A. hydrophila CECT 839T alkalinize the medium during acid exposure. One feasible explanation for this phenomenon would be that A. hydrophila has a higher tolerance to acids as a consequence of an enzymatic pH shifting, which allows a better survival. Although additional analysis including more strains should be performed to determine if this is a specific characteristic of this species. Therefore, it would seem plausible to affirm that HypA could be associated with acid tolerance in Aeromonas species.
Numerous studies provide insights into the relevance of redox signaling and reactive oxygen species (ROS) production as defense mechanisms against pathogens [38,39]. Moreover, bacterial infections also can induce oxidative stress, which contributes to increase the rates of DNA mutations in the host. For instance, H. pylori produce superoxide anion in order to counteract the toxic effect of the ROS produced in the human stomach, which contributes even more in the development of gastric cancer [40]. Considering that the immune system generates ROS as defense mechanism against pathogens after phagocytosis by macrophages [41,42], resistance to acidic environments can be of great advantage for pathogens. Indeed, some studies have emphasized a strong relationship between deficiency of ROS production and susceptibility to microbial infection [43]. In these contexts, the discovery of the chaperone Hsp33 and its role in protecting cells against the deleterious effects of reactive oxygen species [44,45,46], reinforces the hypothesis of the important role of redox regulation during bacterial colonization [47]. In our study, the results showed that the Aeromonas metallochaperone HypA was upregulated after phagocytosis of macrophages, which is in line with the previous works [44,45,46]. Therefore, considering that hydrogenases participate in oxidative stress defense [20] it is very possible that HypA also contributes to the defense against ROS produced by macrophages in the phagocytic process.
According to the literature, 96.5% of the clinical Aeromonas strains correspond to four species: Aeromonas caviae (29.9%), Aeromonas dhakensis (26.3%), Aeromonas veronii (24.8%) and Aeromonas hydrophila (15.5%). However, other species usually associated with a fish disease like A. salmonicida have been isolated from human infections [25]. Our results showed a higher upregulation of the hypA after infection with the most prevalent clinical species, independently of the condition. Specifically, one strain used in the study (A. hydrophila CECT 839T) was isolated from milk although its virulence was previously demonstrated [48]. Otherwise, the expression of the hypA after infection with the strain A. salmonicida CECT 894T, described as a pathogenic for fish, was lower. These results could be associated with that possibility of a more important role of hypA in human health compared to animals, as is the case with other human pathogens as E. coli and H. pylori. However, further studies with a higher number of strains to better corroborate this hypothesis are needed.
In conclusion, our results suggest that HypA could play a role in the survival of Aeromonas in acidic environments and in defense against macrophages, although the exact mechanism remains unclear, but the possible role of this metallochaperone in Aeromonas sp. virulence is evident.
5. Conclusions
This study reports for the first time the distribution of orthologous sequences coding for the metallochaperone HypA in bacterial genomes of the genus Aeromonas and their deduced protein structure. Interestingly, HypA was present in 69.4% of Aeromonas species showing high similarity among the species (%). Metallochaperones are relevant proteins in the host-pathogen interaction. Thus, the present study suggests a possible role of HypA in bacterial survival in acidic environments, as well as in the defense against ROS produced by macrophages. In addition, it may promote future studies to confirm and better understand the function of this metallochaperone in the survival of species from the genus Aeromonas.
Acknowledgments
The authors thank the staff in the Microbiology Unit at the University Rovira I Virgili (URV), Carme Sanmartí Solé. AFB thank URV for the Martí Franqués PhD fellowship.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/7/10/415/s1.
Author Contributions
Data curation, L.L.-F. and M.J.F.; Formal analysis, A.F.-B.; Funding acquisition, M.J.F.; Investigation, A.F.-B.; Methodology, A.F.-B. and L.L.-F.; Project administration, M.J.F.; Supervision, L.L.-F. and M.J.F.; Writing—original draft, A.F.-B.; Writing—review & editing, L.L.-F. and M.J.F.
Funding
The projects JPIW2013-095-C03-03 of MINECO (Spain) and AQUAVALENS of the Seventh Framework Program (FP7/2007-2013) grant agreement 311846 from the European Union supported the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.O’Halloran T.V., Culotta V.C. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y., Yeung N., Sieracki N., Marshall N.M. Design of functional metalloproteins. Nature. 2009;460:855–862. doi: 10.1038/nature08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschner M., Syversen T., Souza D.O., Rocha J.B. Metallothioneins: Mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp. Biol. Med. 2006;231:1468–1473. doi: 10.1177/153537020623100904. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig A.C. Metallochaperones: Bind and deliver. Chem. Biol. 2002;9:673–677. doi: 10.1016/S1074-5521(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 5.Waldron K.J., Rutherford J.C., Ford D., Robinson N.J. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila D.A., Edmonds K.A., Giedroc D.P. Metallochaperones and metalloregulation in bacteria. Essays Biochem. 2017;61:177–200. doi: 10.1042/EBC20160076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer L.D., Skaar E.P. Transition metals and virulence in bacteria. Annu. Rev. Genet. 2016;50:67–91. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan L.A., Parkin A. Electrochemical insights into the mechanism of NiFe membrane-bound hydrogenases. Biochem. Soc. Trans. 2016;44:315–328. doi: 10.1042/BST20150201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter G., Buhrke T., Lenz O., Jones A.K., Forgber M., Friedrich B. A model system for [NiFe] hydrogenase maturation studies: Purification of an active site-containing hydrogenase large subunit without small subunit. FEBS Lett. 2005;579:4292–4296. doi: 10.1016/j.febslet.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 10.Lubitz W., Ogata H., Rudiger O., Reijerse E. Hydrogenases. Chem. Rev. 2014;114:4081–4148. doi: 10.1021/cr4005814. [DOI] [PubMed] [Google Scholar]
- 11.Chan K.H., Lee K.M., Wong K.B. Interaction between hydrogenase maturation factors HypA and HypB is required for [NiFe]-hydrogenase maturation. PLoS ONE. 2012;7:e32592. doi: 10.1371/journal.pone.0032592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebgott P.P., Leroux F., Burlat B., Dementin S., Baffert C., Lautier T., Fourmond V., Ceccaldi P., Cavazza C., Meynial-Salles I., et al. Relating diffusion along the substrate tunnel and oxygen sensitivity in hydrogenase. Nat. Chem. Biol. 2010;6:63–70. doi: 10.1038/nchembio.276. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S., Kawashima T., Nishitani Y., Kanai T., Wada T., Inaba K., Atomi H., Imanaka T., Miki K. Structural basis of a Ni acquisition cycle for [NiFe] hydrogenase by Ni-metallochaperone HypA and its enhancer. Proc. Natl. Acad. Sci. USA. 2015;112:7701–7706. doi: 10.1073/pnas.1503102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacasse M.J., Douglas C.D., Zamble D.B. Mechanism of selective nickel transfer from HypB to HypA, Escherichia coli [NiFe]-hydrogenase accessory proteins. Biochemistry. 2016;55:6821–6831. doi: 10.1021/acs.biochem.6b00706. [DOI] [PubMed] [Google Scholar]
- 15.Atanassova A., Zamble D.B. Escherichia coli HypA is a zinc metalloprotein with a weak affinity for nickel. J. Bacteriol. 2005;187:4689–4697. doi: 10.1128/JB.187.14.4689-4697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia W., Li H., Sze K.H., Sun H. Structure of a nickel chaperone, HypA, from Helicobacter pylori reveals two distinct metal binding sites. J. Am. Chem. Soc. 2009;131:10031–10040. doi: 10.1021/ja900543y. [DOI] [PubMed] [Google Scholar]
- 17.Blum F.C., Hu H.Q., Servetas S.L., Benoit S.L., Maier R.J., Maroney M.J., Merrell D.S. Structure-function analyses of metal-binding sites of HypA reveal residues important for hydrogenase maturation in Helicobacter pylori. PLoS ONE. 2017;12:e0183260. doi: 10.1371/journal.pone.0183260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H.Q., Johnson R.C., Merrell D.S., Maroney M.J. Nickel ligation of the N-terminal amine of HypA is required for urease maturation in Helicobacter pylori. Biochemistry. 2017;56:1105–1116. doi: 10.1021/acs.biochem.6b00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson R.C., Hu H.Q., Merrell D.S., Maroney M.J. Dynamic HypA zinc site is essential for acid viability and proper urease maturation in Helicobacter pylori. Metallomics. 2015;7:674–682. doi: 10.1039/C4MT00306C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignais P.M., Billoud B. Occurrence, classification, and biological function of hydrogenases: An overview. Chem. Rev. 2007;107:4206–4272. doi: 10.1021/cr050196r. [DOI] [PubMed] [Google Scholar]
- 21.Hayes E.T., Wilks J.C., Sanfilippo P., Yohannes E., Tate D.P., Jones B.D., Radmacher M.D., BonDurant S.S., Slonczewski J.L. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 2006;6:89. doi: 10.1186/1471-2180-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noguchi K., Riggins D.P., Eldahan K.C., Kitko R.D., Slonczewski J.L. Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE. 2010;5:e10132. doi: 10.1371/journal.pone.0010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt O., Wust P.K., Hellmuth S., Borst K., Horn M.A., Drake H.L. Novel [NiFe]- and [FeFe]-hydrogenase gene transcripts indicative of active facultative aerobes and obligate anaerobes in earthworm gut contents. Appl. Env. Microbiol. 2011;77:5842–5850. doi: 10.1128/AEM.05432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janda J.M., Abbott S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figueras M.J., Beaz-Hidalgo R. Aeromonas infections in humans. In: Graf J., editor. Aeromonas. Caister Academic Press; Norfolk, UK: 2015. pp. 65–108. [Google Scholar]
- 26.Teunis P., Figueras M.J. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front. Microbiol. 2016;7:1395. doi: 10.3389/fmicb.2016.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee I., Ouk Kim Y., Park S.C., Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 28.Soler L., Yanez M.A., Chacon M.R., Aguilera-Arreola M.G., Catalan V., Figueras M.J., Martinez-Murcia A.J. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 2004;54:1511–1519. doi: 10.1099/ijs.0.03048-0. [DOI] [PubMed] [Google Scholar]
- 29.Canals R., Altarriba M., Vilches S., Horsburgh G., Shaw J.G., Tomas J.M., Merino S. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 2006;188:852–862. doi: 10.1128/JB.188.3.852-862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menanteau-Ledouble S., Kattlun J., Nobauer K., El-Matbouli M. Protein expression and transcription profiles of three strains of Aeromonas salmonicida ssp. salmonicida under normal and iron-limited culture conditions. Proteome Sci. 2014;12:29. doi: 10.1186/1477-5956-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich A.W., Kock R., Bielaszewska M., Zhang W., Karch H., Mathys W. Distribution of the urease gene cluster among and urease activities of enterohemorrhagic Escherichia coli O157 isolates from humans. J. Clin. Microbiol. 2005;43:546–550. doi: 10.1128/JCM.43.2.546-550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss G., Carver P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018;24:16–23. doi: 10.1016/j.cmi.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Falush D. Toward the use of genomics to study microevolutionary change in bacteria. PLoS Genet. 2009;5:e1000627. doi: 10.1371/journal.pgen.1000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N., Olson J.W., Maier R.J. Characterization of Helicobacter pylori nickel metabolism accessory proteins needed for maturation of both urease and hydrogenase. J. Bacteriol. 2003;185:726–734. doi: 10.1128/JB.185.3.726-734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbott S.L., Cheung W.K., Janda J.M. The genus Aeromonas: Biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol. 2003;41:2348–2357. doi: 10.1128/JCM.41.6.2348-2357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker J.L., Shaw J.G. Aeromonas spp. clinical microbiology and disease. J. Infect. 2011;62:109–118. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 38.James S.J., Cutler P., Melnyk S., Jernigan S., Janak L., Gaylor D.W., Neubrander J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 39.Hwang O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handa O., Naito Y., Yoshikawa T. Redox biology and gastric carcinogenesis: The role of Helicobacter pylori. Redox. Rep. 2011;16:1–7. doi: 10.1179/174329211X12968219310756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiloh M.U., Nathan C.F. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 2000;3:35–42. doi: 10.1016/S1369-5274(99)00048-X. [DOI] [PubMed] [Google Scholar]
- 43.Deffert C., Schappi M.G., Pache J.C., Cachat J., Vesin D., Bisig R., Ma Mulone X., Kelkka T., Holmdahl R., Garcia I., et al. Bacillus calmette-guerin infection in NADPH oxidase deficiency: Defective mycobacterial sequestration and granuloma formation. PLoS Pathog. 2014;10:e1004325. doi: 10.1371/journal.ppat.1004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graumann J., Lilie H., Tang X., Tucker K.A., Hoffmann J.H., Vijayalakshmi J., Saper M., Bardwell J.C., Jakob U. Activation of the redox-regulated molecular chaperone Hsp33--a two-step mechanism. Structure. 2001;9:377–387. doi: 10.1016/S0969-2126(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 45.Wholey W.Y., Jakob U. Hsp33 confers bleach resistance by protecting elongation factor Tu against oxidative degradation in Vibrio cholerae. Mol. Microbiol. 2012;83:981–991. doi: 10.1111/j.1365-2958.2012.07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang H.J., Heo D.H., Choi S.W., Kim K.N., Shim J., Kim C.W., Sung H.C., Yun C.W. Functional characterization of Hsp33 protein from Bacillus psychrosaccharolyticus; additional function of HSP33 on resistance to solvent stress. Biochem. Biophys. Res. Commun. 2007;358:743–750. doi: 10.1016/j.bbrc.2007.04.184. [DOI] [PubMed] [Google Scholar]
- 47.Kumsta C., Jakob U. Redox-regulated chaperones. Biochemistry. 2009;48:4666–4676. doi: 10.1021/bi9003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshadri R., Joseph S.W., Chopra A.K., Sha J., Shaw J., Graf J., Haft D., Wu M., Ren Q., Rosovitz M.J., et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: Jack of all trades. J. Bacteriol. 2006;188:8272–8282. doi: 10.1128/JB.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.