Abstract
Aim:
Recently, major attention has been paid to the role of hypoglycemia as a cardiovascular risk factor. While EURODIAB-investigators concluded that severe hypoglycemia is not a cardiovascular risk factor in type 1 diabetes, other investigators found the opposite. The primary purpose of this study was to investigate the role of severe hypoglycemia in atherosclerosis during the DCCT- and EDIC-years with special attention to overall glycemic levels.
Research design and methods:
The effect of severe hypoglycemic rates on coronary artery calcification (CAC) was evaluated for the entire cohort (n = 1205) and glycemic stratified cohorts (HbA1C < 7.5% [58 mmol/mol], HbA1C ≥ 7.5%).
Results:
The association between CAC and mean DCCT-hypoglycemia rate was stronger than the association between CAC and mean EDIC-hypoglycemia rate. Although the DCCT-severe hypoglycemia rate without HbA1C-stratification was not significantly associated with a CAC-score ≥ 100 Agatston units (p = 0.093), the interaction between above glycemic ranges and DCCT-hypoglycemic rate was significant (p < 0.05). A sub-analysis of patients belonging to the lower glycemic range (HbA1C < 7.5%), adjusted for baseline age, gender, baseline diabetes duration, baseline neuropathy, baseline albumin excretion rate, systolic blood pressure, LDL-cholesterol, smoking status, body mass index and DCCT-A1 C, indicated significant (p = 0.02) associations between DCCT-severe hypoglycemia rate and CAC-score ≥ 100. One unit increase in the natural logarithm transformed DCCT-severe hypoglycemia rate increased the risk of having a CAC ≥ 100 by 30%.
Conclusions:
Our results suggest a cumulative effect of hypoglycemic events on cardiovascular risk. They provide a possible link between above mentioned contradictory reports. Our findings support the relevance of personalizing glycemic goals in diabetes management beyond HbA1C.
Keywords: Type 1 diabetes, DCCT/EDIC-study, Severe hypoglycemia, Cardiovascular disease, Coronary artery calcification
1. Introduction
Patients with type 1 diabetes have a several-fold higher risk of developing CVD compared to an age-matched population with no diabetes [1–3]. The DCCT/EDIC-study has shown strong evidence for the importance of hyperglycemia on micro- and macro-vascular complications. The long-term benefits of prior intensive treatment were still apparent years later. This observation led to the metabolic memory concept [4–6]. However, one of the most adverse effects of tight glycemic control during the DCCT-period was an increased rate of severe hypoglycemia [7,8]. The implications of this fact on micro- and macro-vascular complications are still being debated.
Based on numerous publications, mainly regarding type 2 diabetes [9–16], it appears that hypoglycemia as a proximate or long-term cause of CVD-events might become accepted. This beginning acceptance has not yet included hypoglycemia as a chronic CVD-risk factor in type 1 diabetes [16,17] – Why would a few episodes of severe hypoglycemia increase the long-term CVD-risk? The data of the EURODIAB-investigators [17] appear to support the viewpoint that severe hypoglycemia in patients with type 1 diabetes does not increase the risk of CVD. However, two recent reports by Giménez et al. [18,19] challenged this viewpoint. Gruden et al. [20] and Giménez and Conget [21] agreed that more studies are needed. They suggested that results from datasets (severe and non-severe hypoglycemia) collected from previous landmark studies may provide relevant information.
Both hyperglycemia and hypoglycemia have effects which may exert a pathological role in CVD. Chronic hyperglycemia by itself potentiates collagen-induced platelet activation and promotes plaque formation and plaque instability [22–24] Hypoglycemia induces abnormalities in platelet function and activation of the fibrinolytic system [25,26]. Wright et al. [27] showed that markers of both inflammation and endothelial dysfunction increased after hypoglycemia was provoked. Additional evidence regarding the pathological role of hypoglycemia in CVD is highlighted by Dandona et al. [28] and Desouza et al. [29]. Given these studies, both hypoglycemia and hyperglycemia seem to be involved in processes that lead to endothelial dysfunction. Endothelial dysfunction is seen as a precursor for the development of de novo atherosclerosis [30,31].
The notion that hypoglycemia, in addition to hyperglycemia, may influence CVD-risk poses an interesting dilemma. The DCCT- and EDIC-study demonstrated the relevance of hyperglycemia on CVD-risk and the benefits of tight glycemic control. Yet, tight glycemic control can result in increased episodes of hypoglycemia, which in turn may serve as a potential CVD-risk factor. The primary purpose of this study was to investigate the impact of severe hypoglycemia during the DCCT/EDIC-period on atherosclerosis in the context of glycemic levels. CAC-score, an established atherosclerosis marker [32–35], was used to investigate the relationship between atherosclerosis and severe hypoglycemia. Cleary et al. [36] have already shown that prior intensive treatment during DCCT was associated with less CAC, largely due to better glycemic control during DCCT. Considering the prolonged duration of chronic hyperglycemia compared to the relatively few and brief episodes of severe hypoglycemia, we hypothesize that any effect of severe hypoglycemia will be more prominent in the lower glycemic range. In this range the effect of chronic hyperglycemia on atherosclerosis and hence on CVD, is reduced. Due to the design of the DCCT/EDIC-study, the cumulative effect of severe hypoglycemia over the follow-up years (and not only at a few time points) could be evaluated.
2. Material and methods
2.1. Participants
We analyzed all participants from the DCCT/EDIC-cohort on whom computed tomography 7–9 years after the end of the DCCT-study was performed (n = 1205). Data access was granted by the NIDDK repository. This study was approved by the Marshall University Institutional Review Board. The DCCT was conducted from 1983 until 1993 with a mean follow-up of 6.5 years. The study consisted of a primary (726 patients) and a secondary intervention cohort (715 patients). All patients were in good general health at entrance into the study, although patients belonging to the secondary cohort showed early signs of retinopathy ± nephropathy. At baseline, patients of the secondary cohort had diabetes for 1–15 years vs. 1–5 years of diabetes for primary cohort patients. Patients of both cohorts were randomly assigned to conventional or intensive treatments. Ages at entry ranged from 13 to 39 years. After the end of the DCCT, all patients were offered the intensive treatment regimen, and the EDIC-study commenced.
2.2. DCCT/EDIC-data
During DCCT, patient evaluations were conducted quarterly. In contrast, EDIC-evaluations were either performed annually or in alternate years. All definitions used below were taken from the DCCT (or EDIC) Manual of Operations [37,38].
2.3. Severe hypoglycemia
Severe hypoglycemia was defined as hypoglycemic events requiring assistance. Additional information can be found [37,38].
DCCT-severe hypoglycemia events were either reported immediately or at the quarterly visits. EDIC-severe hypoglycemia events were reported at the annual visits. They account for the events during the three months prior to the visit. Baseline severe hypoglycemia events were reported at the initial DCCT-visit.
2.4. Additional information
Hypertension was defined as either use of medications or documented hypertension, having systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Hypercholesterolemia was defined as either using lipid-lowering medications or having a LDL≥ 130 mg/dl [30,31]. The mean HbA1C during the combined DCCT/EDIC-follow-up until the visit prior or at the time of the CAC-measurement (A1CDCCT/EDIC) was calculated according to the approach Cleary et al. used [36]. Since Cleary et al. have shown that both mean HbA1C during DCCT (DCCT-A1C) and mean HbA1C during EDIC (EDIC-A1C) were associated with increased calcification scores, our sub-analyses stratification was based on mean HbA1C during the combined DCCT/EDIC, i.e., patients with mean A1CDCCT/EDIC < 7.5% [58 mmol/mol] and other patients with mean A1CDCCT/EDIC ≥ 7.5%.
The cut-off point was chosen a priori. Regarding a cut-off point, we defer to researchers in the past [39–41] who used the cut-off of 7.5% in the context of glycemic control. In addition, Snell-Bergeon [42] found a nonlinear relationship between HbA1C and progression of CAC in type 1 diabetes. As a result, Snell-Bergeon categorized HbA1C into good (HbA1c < 7.5%) or poor (HbA1c > 7.5%) glycemic control. They found that an HbA1C > 7.5% was a strong risk factor for increasing atherosclerotic plaque burden among CAD asymptomatic type 1 diabetes patients.
Data for all variables (covariates) of interest were included only up to the time of CAC-measurement.
2.5. CAC-measurements
CAC-measurements were made between November 2000 and March 2003. More details, especially on method, standardization and validity have been reported [36].
2.6. Statistical procedures
The dataset included 1205 patients. The outcomes of interest were severe hypoglycemia rates during DCCT and EDIC and their association with CAC-scores. Due to the variation of enrollment time by several years among the participants and the different reporting methods used during DCCT- and EDIC-study, we analyzed rates instead of the actual number of hypoglycemic events. The hypoglycemic rate was calculated separately for DCCT and EDIC, according to the following formula:
This approach has been substantiated by Lachin [43,44]. The rate reflects the overall amount of hypoglycemia events well; the baseline age and gender adjusted correlation between hypoglycemia rate and actual number of events was greater than 99% (Spearman), p < 0.001 and greater than 96% (Pearson), p < 0.001.
In accordance with Lopes-Virella [45], we focused on a CAC-score ≥ 100 as the CVD-risk marker, partly due to the lack of baseline CAC-scores. Since all patients were generally in good health initially, it appears unlikely that a significant number of them had an initial CAC-score ≥ 100 [46]. As mentioned above, sub-analyses were done by stratification according to glycemic control during the follow-up time until the visit prior or at the time of CAC-measurement.
For a comparison of clinical characteristics, Wilcoxon’s rank-sum/Kruskal-Wallis tests were employed for continuous variables and Chi-Square/Cochran-Mantel-Haenszel tests were utilized for categorical variables. Results are presented as means ± SD or percentages. Variables with highly skewed distributions were natural logarithm transformed (if the variable contained zeroes as in case of severe hypoglycemia rate: log(1 + variable)). Baseline age, gender and DCCT-A1C adjusted Spearman correlations were used to assess whether significant correlations between CAC-scores and severe hypoglycemia rates exist.
Multivariate relative risk models with robust error variances [47] were used to estimate risk ratios associated with the prevalence of CAC ≥ 100 Agatston units with one unit increase in LOG(DCCT-hypo). This approach has been used elsewhere [45,48]. Analyses included baseline age, either Eligibility-HbA1C or DCCT-A1C or EDIC-A1C, gender, baseline diabetes duration, baseline retinopathy, natural log-transformed baseline albumin excretion rate (baseline-AER), baseline severe hypoglycemia (yes/no), and baseline neuropathy. Additional covariates at the visit prior to or at the time of the CAC-scan included: systolic blood pressure, smoking, scanning site, body mass index and LDL-cholesterol.
In addition to the main effects of severe hypoglycemia rate and glycemia on calcification, the interaction effect of the defined A1CDCCT/EDIC-subgroups and severe hypoglycemia rate with regard to calcification was evaluated. This interaction describes the situation in which the simultaneous influence of the A1CDCCT/EDIC-subgroups and hypoglycemic rate on CAC ≥ 100 is not additive. In other words, if the variables A1CDCCT/EDIC-subgroups and severe hypoglycemia interact, the relationship between each of the interacting variables and CAC ≥ 100 depends on the value of the other interacting variable. All statistical analyses were performed using SAS-version 9.3 (SAS Institute Inc., Cary, NC) and R-version 3.0.2 (www.r-project.org). Significance was defined at p-values < 0.05 (two-sided) for all analyses.
3. Results
3.1. Clinical characteristics
Important clinical characteristics at DCCT-baseline, and at the exam immediately before, or at the time of, the CAC-scan for the entire cohort and for patients belonging to the sub-analysis cohorts are listed in Table 1. Additional information can be found [36].
Table 1 –
Selected clinical characteristics of participants belonging to subgroups (A1CDCCT/EDIC < 7.5%, A1CDCCT/EDIC ≥ 7.5%) and for the entire cohort.
| Clinical covariates | A1CDCCT/EDIC < 7.5% N = 367 | A1CDCCT/EDIC ≥ 7.5% N = 838 | Entire cohort N = 1205 |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 27 ± 7 | 27 ± 7 | 27 ± 7 |
| Gender-male (%) | 53 | 53 | 53 |
| Group treatment-intensive (%) | 74 | 39 | 50 |
| Diabetes duration (months) | 72 ± 53 | 65 ± 48 | 67 ± 50 |
| Retinopathy (%) | 51 | 49 | 50 |
| Eligibility-HbA1C (%) | 8.4 ± 1.3c (68 ± 14) | 9.3 ± 1.6 (78 ± 17)e | 9.0 ± 1.6 (75 ± 17)e |
| Severe hypoglycemia (%)d | 18 | 20 | 19 |
| Clinical neuropathy (%) | 4 | 7 | 6 |
| Baseline-AER (mg/24 h) | 17 ± 25 | 15 ± 16 | 16 ± 19 |
| Clinical characteristics prior to or at the time of calcification-measure | |||
| Smoking (%) | 9c | 18 | 15 |
| Hyperlipidemia (%) | 29c | 39 | 36 |
| LDL (mg/dl) | 105 ± 25c | 115 ± 30 | 112 ± 29 |
| HDL (mg/dl) | 57 ± 16c | 56 ± 14 | 56 ± 15 |
| Triglycerides (mg/dl) | 80 ± 56c | 94 ± 63 | 90 ± 61 |
| Total cholesterol (mg/dl) | 178 ± 31c | 190 ± 35 | 186 ± 34 |
| Hypertension (%) | 28c | 41 | 37 |
| Systolic blood pressure (mm/Hg) | 120 ± 13c | 123 ± 15 | 122 ± 14 |
| Diastolic blood pressure (mm/Hg) | 75 ± 9b | 77 ± 9 | 76 ± 9 |
| Body Mass Index (kg/m2) | 27 ± 4c | 28 ± 4 | 28 ± 4 |
| AER (mg/24 h) | 20 ± 106c | 162 ± 736 | 119 ± 620 |
| DCCT-follow-up time (years) | 6.5 ± 1.6 | 6.4 ± 1.7 | 6.4 ± 1.7 |
| EDIC-follow-up time (years) | 9.0 ± 0.5 | 9.0 ± 0.5 | 9.0 ± 0.5 |
| Glycemic control and severe hypoglycemia | |||
| Mean DCCT-A1C (%) | 6.9 ± 0.6c (52 ± 7)e | 8.7 ± 1.3 (72 ± 14)f | 8.2 ± 1.4 (66 ± 15)f |
| Mean EDIC-A1C (%) | 7.0 ± 0.6b (53 ± 7)f | 8.5 ± 1.0 (69 ± 11)f | 8.1 ± 1.2 (65 ± 13)f |
| Mean A1CDCCT/EDIC (%) | 7.0 ± 0.4b (53 ± 4)f | 8.6 ± 0.9 (70 ± 10)f | 8.1 ± 1.1 (65 ± 12)f |
| DCCT-severe hypoglycemia rate | 5.0 ± 8.0c | 3.6 ± 8.0 | 4.0 ± 8.0 |
| (#events/10 patient-years)e | |||
| DCCT-RR | 1.74(95%CI:1.36–2.23)c | NA | |
| A1CDCCT/EDIC < vs. A1CDCCT/EDIC ≥ 7.5% | |||
| EDIC-severe hypoglycemia rate | 5.0 ± 9.9a | 3.8 ± 8.3 | 4.2 ± 8.8 |
| (#events/10 patient-years) | |||
| EDIC-RR | 1.33(95%CI:1.03–1.72)a | NA | |
| A1CDCCT/EDIC < vs. A1CDCCT/EDIC ≥ 7.5% | |||
Data are means ± SD unless otherwise indicated.
p < 0.05.
p < 0.01.
p < 0.001 (comparison patient cohorts A1CDCCT/EDIC < 7.5% vs. A1CDCCT/EDIC ≥ 7.5%).
Baseline severe hypoglycemia events reported at initial DCCT-visit for the previous year.
DCCT-rates are based on a follow-up time which represents the time from randomization until last DCCT-visit or HbA1C-closeout visit collection date (whatever date was later). EDIC-rates or events are based on EDIC-follow-up days which mirror: [(days of 3 months) × (number of annual visits)], since hypoglycemia events were only recorded for the 3 months prior to the annual EDIC-visits. The events were confirmed by additional documentation. The mean event rate was calculated according to the following formula: Mean event rate per 10 patient-years = 10 × 365.25 × (Number of events)/(Number of recorded follow-up days).
For the EDIC it represents a projected rate.
SI, IFCC-recommended mmol/mol.
Table 1 provides a perspective on how the patients of the sub-analysis cohorts fared in comparison with the entire cohort. These data mirror the DCCT-results well; e.g., microvascular complications (AER) and cardiovascular risk markers such as hypertension, BMI, and hyperlipidemia correlated with suboptimal glycemic control. Significant differences in smoking status, glycemic control, and hypoglycemic rates were found between the patient group with A1CDCCT/EDIC < 7.5% vs. the patient group with A1CDCCT/EDIC ≥ 7.5%. The difference in A1CDCCT/EDIC between the two cohorts was 1.6%. Although the risk for hypoglycemia was reduced during EDIC compared to DCCT, it was still 33% higher in the lower A1CDCCT/EDIC-cohort (A1CDCCT/EDIC < 7.5%) compared to the higher A1CDCCT/EDIC-cohort. In terms of baseline characteristics, significant differences between the sub-analysis cohorts existed in Eligibility-HbA1C. There was a trend toward patients belonging to the higher A1CDCCT/EDIC-range group (≥7.5%) having a higher percentage of clinical neuropathy at baseline (p = 0.07). Fewer patients experienced severe hypoglycemia during EDIC compared to DCCT (entire cohort: 33% vs. 50%). The different reporting method of hypoglycemic episodes during EDIC compared to DCCT affects the calculated hypoglycemia rate. Consequently, DCCT- and EDIC-rates should not be compared directly.
Comparing CAC-participants with non-participants revealed no significant differences for gender, race, treatment, DCCT-follow-up time, DCCT-hypoglycemic rates, and percentage of patients having DCCT-hypoglycemic events, nor baseline severe hypoglycemia, baseline retinopathy and baseline diabetes duration. However, non-participants were younger, had higher DCCT-A1C and Eligibility-HbA1C (9.4% vs. 9.0%, p = 0.0194). Data on gender, race, treatment, baseline age and DCCT-A1C were already reported [36] and are not presented here.
3.2. Analyses for hypoglycemia during DCCT
3.2.1. Correlations between CAC and DCCT-hypoglycemic rate (nonparametric)
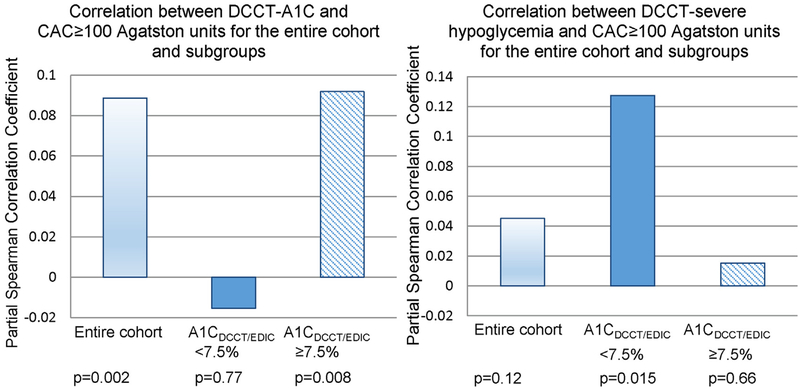
Since we hypothesized that any effect of severe hypoglycemia on CAC will be more prominent in the lower glycemic range (where the effect of hyperglycemia on CVD is reduced), we evaluated the correlations between DCCT-glycemia, severe DCCT-hypoglycemia rate and CAC 100 (Fig. 1). Significant positive correlations between DCCT-A1C and CAC≥100 were found for the entire DCCT-cohort and for subjects with A1CDCCT/EDIC ≥ 7.5%. The correlation between DCCT-A1C and CAC ≥ 100 was non-significant for patients with A1CDCCT/EDIC < 7.5%. By reducing the impact of DCCT-glycemia on CAC (patients with A1CDCCT/EDIC < 7.5%), the correlation between DCCT-hypoglycemia rate and CAC ≥ 100 reached significance. We note that the corresponding p-value for the entire cohort was roughly 0.12. For patients belonging to the A1CDCCT/EDIC ≥ 7.5%-cohort, the correlation between DCCT-severe hypoglycemia and CAC ≥ 100 was non-significant (p = 0.66).
Figure 1 –
(a) Correlation (non-parametric) between DCCT-A1C and CAC ≥ 100 (baseline age, gender adjusted) (b) Correlation between severe-DCCT hypoglycemia rate and CAC ≥ 100 (baseline age, gender, DCCT-A1C adjusted) for the entire cohort and the stratified cohorts.
3.2.2. Risk assessment for the entire cohort during DCCT (parametric)
Cleary et al. [36] have already shown that prior intensive treatment during DCCT was associated with less CAC, largely due to better glycemic control during DCCT. Since we used the Cleary et al. NIDDK archived data set, we concentrated on DCCT-A1C. Table 2 shows the role of DCCT-severe hypoglycemia in calcification. Model 1 included baseline age, gender, DCCT-glycemic control, and DCCT-severe hypoglycemia rate. The prevalence of CAC ≥ 100 was 12%. There was a significant positive association between CAC ≥ 100 and DCCT-A1 C. The p-value for group treatment when included in the model was 0.7. There was a positive trend between CAC and DCCT-hypoglycemia (p = 0.093). This positive trend remained in model 2, which includes group treatment, systolic blood pressure, LDL-cholesterol, BMI, smoking, baseline clinical neuropathy, baseline diabetes duration, baseline hypoglycemia, scanning site, baseline retinopathy, and baseline-AER. The p-values for the group treatment, DCCT-A1C and DCCT-severe hypoglycemia were 0.71, 0.02 and 0.11, respectively.
Table 2 –
The roles of DCCT-severe hypoglycemia and DCCT-A1C in atherosclerosis (entire cohort).
| clinical factor of interest in model | Risk ratio for an association with CAC ≥ 100 model 1a | p-value | Risk ratio for an association with CAC ≥ 100 model 2b | p-value |
|---|---|---|---|---|
| DCCT-severe hypoglycemia rate | 1.12 (0.99–1.29) | 0.093 | 1.13 (0.97–1.32) | 0.11 |
| DCCT-AIC | 1.21 (1.09–1.35) | <0.001 | 1.21 (1.03–1.42) | <0.05 |
Model 1: Adjusted for gender, baseline age; The p-value for group treatment when included in the model was 0.7.
Model 2: Adjusted for group treatment, systolic blood pressure, LDL-cholesterol, BMI, smoking, baseline clinical neuropathy, baseline diabetes duration, baseline hypoglycemia, scanning site, baseline retinopathy, and baseline-AER. p-value for group treatment was 0.71).
Further analyses showed that the interaction (see Section 2) between DCCT-severe hypoglycemia rate and A1CDCCT/EDIC-groups (<7.5% and ≥7.5%), adjusted for age and gender, was significant (p = 0.018). This interaction remained significant (p < 0.05) even after adjustment for additional clinical factors: systolic blood pressure, LDL-cholesterol, BMI, smoking, baseline clinical neuropathy, baseline diabetes duration, baseline hypoglycemia, scanning site, baseline retinopathy, baseline-AER, and group treatment. The observed significant interaction suggests differences in the relationship for the two groups of patients (A1CDCCT/EDIC < 7.5% and A1CDCCT/EDIC ≥ 7.5%).
3.3. Sub-analyses
Table 3 shows the results for the sub-analyses, the risk ratios for DCCT-severe hypoglycemia rate and CAC ≥ 100 for the patients with A1CDCCT/EDIC < 7.5% and patients with A1CDCCT/EDIC ≥ 7.5%. The prevalence of CAC ≥ 100 was 9.2% in the patient group with A1CDCCT/EDIC < 7.5% and 13.2% for the patient group with A1CDCCT/EDIC ≥ 7.5%. In agreement with the Spearman correlation results, a significant association between CAC ≥ 100 and DCCT-severe hypoglycemia rate, but not DCCT-A1C, existed for patients with A1CDCCT/EDIC < 7.5%. Adjusting for additional clinically relevant factors (Table 3) did not change this observation. One unit increase in LOG(DCCT-hypo) increased the risk of having a CAC ≥ 100 by 30% in the patient group with A1CDCCT/EDIC < 7.5% when adjustment was made for baseline age, gender, baseline diabetes duration, baseline neuropathy, baseline-AER, smoking status, LDL-cholesterol, systolic blood pressure and DCCT-A1C. Adjustment for Eligibility-HbA1C instead of DCCT-A1C did not significantly modify the relationship between severe hypoglycemia and CAC. In contrast, there was a significant association between DCCT-A1C and CAC ≥ 100 for patients with A1CDCCT/EDIC ≥ 7.5%, but the association between severe hypoglycemia rate and CAC ≥ 100 was non-significant (p = 0.76).
Table 3 –
The role of DCCT-severe hypoglycemia in atherosclerosis for patients with A1CDCCT/EDIC < 7.5% and A1CDCCT/EDIC ≥ 7.5% (CAC ≥ 100).
| Clinical factor of interest in model | A1CDCCT/EDIC < 7.5% | DCCT/EDIC ≥ 7.5% | ||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | ||||
| RR(95%CI) | p | RR(95%CI) | p | RR(95%CI) | p | |
| DCCT-severe hypoglycemia rate | 1.34(1.07–1.68) | 0.009 | 1.30(1.04–1.64)c | 0.02 | 1.03(0.86–1.23) | 0.76 |
| DCCT-ACI | 0.93(0.51–1.68) | 0.79 | 1.08(0.58–2.01) | 0.82 | 1.18(1.03–1.36) | 0.02 |
Model 1: baseline age, gender, DCCT-A1C, DCCT-severe hypoglycemia rate.
Model 2: baseline age, gender, DCCT-A1C, DCCT-severe hypoglycemia rate, baseline diabetes duration, baseline neuropathy, baseline-AER, smoking status, LDL-cholesterol, systolic blood pressure, BMI and DCCT-A1C. Since p-values for baseline hypoglycemia, group treatment and baseline retinopathy were large, these factors were not included in model.
Scanning site or Eligibility-HbA1C instead of DCCT-A1C did not significantly modify the relationship between severe hypoglycemia and CAC.
3.4. Analyses for hypoglycemia during EDIC
3.4.1. Risk assessment during EDIC
Table 4 shows the risk ratios for EDIC-A1C and EDIC-severe hypoglycemia rate associated with CAC ≥ 100 for the risk models, which also included baseline age and gender, for the entire cohort and the subgroups. Considering the entire cohort, hyperglycemia during EDIC was a significant risk factor for CAC ≥ 100, but severe hypoglycemia was not. As previously observed, the association between CAC ≥ 100 and severe hypoglycemia was non-significant (p = 0.51) for patients with A1CDCCT/EDIC ≥ 7.5%, and between CAC and EDIC-A1 C for patients with A1CDCCT/EDIC < 7.5% (p = 0.88). Unlike the DCCT-results, the association between EDIC-severe hypoglycemia rate and CAC ≥ 100 for patients with A1CDCCT/EDIC < 7.5% did not reach significance.
Table 4 –
The role of severe hypoglycemia during EDIC in atherosclerosis (CAC≥100).
| Clinical factor of interest in model | Entire cohorta | A1CDCCT/EDIC < 7.5%a | A1CDCCT/EDIC ≥ 7.5%a | |||
|---|---|---|---|---|---|---|
| RR(95%CI) | p | RR(95%CI) | p | RR(95%CI) | p | |
| EDIC-severe hypoglycemia rate | 1.08(0.95–1.23) | 0.213 | 1.19(0.95–1.50) | 0.12 | 1.05(0.90–1.23) | 0.51 |
| EDIC-AIC | 1.16(1.02–1.33) | 0.03 | 0.96(0.54–1.69) | 0.88 | 1.12(0.93–1.33) | 0.22 |
Model with baseline age, gender, EDIC-A1C, EDIC-severe hypoglycemia rate.
4. Discussion
This study has several important strengths. We evaluated the effect of severe hypoglycemia on CAC using longitudinal data of one of the landmark trials. Our results inject fuel into the discussion about the role of hypoglycemia in CVD in type 1 diabetes. We are adding new perspectives:
Evaluating the effect of severe hypoglycemia on the risk of CVD, taking the level of hyperglycemia into consideration and accounting for the long-term cumulative effect of hyperglycemia.
Evaluating the long-term cumulative effect of severe hypoglycemia rather than baseline hypoglycemia or hypoglycemia reported at a couple of specific time points.
Although the association between severe hypoglycemia and CAC ≥ 100 for the entire cohort (without stratification) was not quite significant, the interaction between glycemic ranges (A1CDCCT/EDIC < 7.5%, A1CDCCT/EDIC ≥ 7.5%) and severe hypoglycemia on calcification was significant. For patients who had a more optimal glycemic control during DCCT/EDIC (A1CDCCT/EDIC < 7.5%), the association between DCCT-severe hypoglycemia and CAC ≥ 100 differed compared to patients with A1CDCCT/EDIC ≥ 7.5%. The existence of significant interactions justifies our stratification. Sub-analyses, which evaluated the two patient groups separately, confirmed the above mentioned finding.
Significant positive associations between DCCT-severe hypoglycemia and CAC ≥ 100 were found for patients with A1CDCCT/EDIC < 7.5%. Significance was not dependent on whether the model was only adjusted for baseline age, gender, and DCCT-A1C, or for additional clinically relevant risk factors for CAC. The association between EDIC-hypoglycemia and CAC ≥ 100 for patients with A1CDCCT/EDIC < 7.5% did not reach significance. However, in an expanded population with CAC > 50 the association reached significance. The weaker association between CAC and EDIC-hypoglycemia compared to DCCT-hypoglycemia may be partially explained by different levels of glycemia during the two study periods; of the 367 patients with A1CDCCT/EDIC < 7.5%, 13% had a DCCT A1C > 7.5% and 22% had an EDIC-A1C > 7.5%. Additionally, fewer patients experienced severe hypoglycemia during EDIC than during DCCT (38% vs. 60%). The reporting method of severe hypoglycemia during EDIC might have added noise. We note that Cleary et al. found that DCCT-A1C played a stronger role in calcification than the EDIC-A1C, similar to our findings regarding the role of hypoglycemia during DCCT in CAC.
Our findings provide a link between the studies of Gruden et al. [17] and Giménez et al. [18,19]. Gruden did not detect a significant association between severe hypoglycemia and CVD. However, it is important to note that differences in the incidence of nonfatal CVD among hypoglycemia categories were nearly significant. The authors did not stratify their analyses by levels of glycemia. This may explain why an independent effect of severe hypoglycemia on CVD was not found. Gruden’s results are in agreement with our analysis if we do not stratify by levels of glycemia. When looking at the entire cohort we found a non-significant trend for the association between DCCT-severe hypoglycemia and CAC. By evaluating the glycemic stratified cohort of patients with A1CDCCT/EDIC < 7.5% our results became significant and remained significant even after adjustment for several additional important clinical factors such as duration of diabetes and systolic blood pressure. The effect of severe hypoglycemia might be missed if one does not stratify by the level of hyperglycemia.
In their case study [18], Giménez et al. evaluated patients with a relatively tight glycemic control (control group:6.7 ± 0.7%, hypoglycemia group: 6.6 ± 1.0%), close to the range of our patient group with more optimal glycemic control. They observed increased intima-media thickness for both carotid and femoral sites and higher inflammatory marker in the hypoglycemia group compared to the control group. These findings support our observed association between severe hypoglycemia and subclinical atherosclerosis. Severe hypoglycemia as a risk factor for CVD is more prominent when patients are evaluated who have a tighter glycemic control.
In their retrospective study, Giménez et al. [19] compared patients with a history of severe hypoglycemia vs. patients without severe hypoglycemia the year before entering the Catalan National Health registry. The prevalence of CVD was greater in patients with a history of repeated episodes of severe hypoglycemia. However, by including age and duration of diabetes in their logistic regression analysis model, severe hypoglycemia was not independently associated with nonfatal cardiovascular disease anymore. The authors did not stratify their analysis by levels of glycemia.
We are aware that we are not looking at “hard points” as Gruden and Giménez did, but we believe that our findings provide a reasonable partial explanation why Gruden et al. did not find any effect of severe hypoglycemia on CVD and why Giménez’ results from their retrospective study lost significance when the logistic model was adjusted for age and diabetes duration. We note that both authors did not investigate a possible interaction between hypoglycemia and hyperglycemia.
In contrast to Gruden et al. and Giménez et al., due to the DCCT/EDIC-study design, we were able to evaluate the cumulative effect of hyperglycemia and hypoglycemia on atherosclerosis. Although Gruden found a relatively strong association between baseline hypoglycemia and severe hypoglycemia at the follow-up visit, the correlation was “only” roughly 30%. Gruden and Giménez evaluated severe hypoglycemia reported for the year prior to a visit. This method increases the risk of recall bias. The authors subsequently categorized the hypoglycemia events into 0, 1–2, and 3 and more events, or yes/no-groups, respectively. The drawback is that valuable information is lost, and noise is added. Due to the design of the DCCT/EDIC-study the risk of recall bias is greatly reduced. We were able to draw a more precise picture of hypoglycemia events. This fact, besides the consideration of hyperglycemia, might have contributed to finding an effect of severe hypoglycemia on CAC-score and so atherosclerosis.
The significance of the associations between severe hypoglycemia during DCCT and CAC-score lends credibility to the idea of a hypoglycemic memory. CAC does not seem to be a reversible process. More studies are needed since this thought has important clinical implications, especially for the treatment of young adults.
Our results definitely warrant further investigation. They suggest that patients, especially with a glycemic range at the lower end of the glycemic spectrum and at high risk for severe hypoglycemia, might need to be monitored more carefully for CVD. Measures to avoid severe hypoglycemia such as continuous glucose monitoring might need to be considered. In agreement with the DCCT-authors suggestion in their article on hypoglycemia [8], a purposeful moderation of glycemic targets as a treatment option should be considered. In their study, although on type 2 diabetes, Currie et al. [49] found a U-shape curve describing the relationship between survival and glycemia. Just recently Schoenaker et al. [50] found a similar relationship for type 1 diabetes. Although it seems speculative, the results reported here might aid in explaining these findings. We would expect a reduction in CVD with improving glycemic control. However, due to an effect of severe hypoglycemia on CVD, we predict an upturn in the lowest glycemic range, producing a U-shape or J-shape. The kind of relationship will depend on the extent to which chronic hyperglycemia overshadows hypoglycemia.
We do not question the benefits of glycemic control at all. It is our contention that it is not the low HbA1C but severe hypoglycemia that needs to be avoided. If patients with severe hypoglycemia were excluded from the analyses, there was a general trend that the risk increase for having a CAC > 100 per one unit increase in DCCT-A1C was greater (roughly 10%). While these are clearly not independent data sets, so one cannot readily draw rigorous conclusions, the results are at least consistent with the idea that severe hypoglycemia contributes to a ‘washout’ effect regarding the effect of HbA1C and, hence, the group treatment effect on CAC.
Our results do not imply that severe hypoglycemia is unimportant in the higher HbA1C-ranges. Rather in those patients with suboptimal glycemic control, the impact of hyperglycemia on CAC makes it difficult to detect any effect of severe hypoglycemia on CAC. Additionally, patients with suboptimal glycemic control experienced fewer hypoglycemic events. This observation might contribute to our findings that hypoglycemia was significantly associated with CAC for patients with A1CDCCT/EDIC < 7.5% but not for patients with A1CDCCT/EDIC ≥ 7.5%.
The mechanism(s) by which hypoglycemia causes atherosclerosis is not well understood. Hypoglycemia seems to involve inflammation and endothelial dysfunction. Due to these processes even a limited number of episodes of severe hypoglycemia might lead to de novo calcification. It does seem that calcification is not readily reversible, explaining a cumulative effect of hypoglycemia on calcification.
Several limitations to this study exist. Some of them have been delineated [36], including several scanning sites and the fact that a CAC-score is a marker of atherosclerosis but is not equivalent to hard end-points. We concentrated on CAC ≥ 100, which is a relatively good CVD-predictor [32–36]. Due to the fact that the DCCT started out with a relatively healthy study population, a CAC ≥ 100 is not very likely at the entry point of the DCCT but rather mirrors the progression of the disease. As in other studies, we evaluated self-reported hypoglycemia events with relatively infrequent validation of suspected hypoglycemic episodes through self-measurement of blood glucose levels. However, additional information (about requiring assistance and the manifestations of severe hypoglycemia) was reported, thus increasing the reliability of the hypoglycemic data. During EDIC hypoglycemic events were reported annually reflecting three months prior to the visit. This leads to over-and under-estimating of hypoglycemic events. However, the longer the follow-up time period becomes, the better the hypoglycemia events for the patient are mirrored. The enrollment period extended over 6 years, which complicates evaluations. Analyzing subgroups resulted in reduced sample size. Unknown residual confounding factors could have led to bias; we have controlled for important clinical factors. We also stratified by levels of glycemic control during the combined DCCT/EDIC-period. We are aware that a stratification of both DCCT-A1C < 7.5% and EDICA1C < 7.5% would have been the most clean approach. However, in this case the sample size became small (236 vs. 367). The stratification by an HbA1C for the combined DCCT/EDIC-period seemed to be reasonable and a good compromise between sample size and rigor.
So far, we have not investigated the effect of mild hypoglycemia on CVD nor the correlation between mild hypoglycemia and severe hypoglycemia. This is a work in progress. Despite all limitations it is important to note that all different models assessing DCCT-hypoglycemia as a CV-risk factor were consistent (Spearman correlations, risk ratio models, Tobit models (results not shown)).
Since this study population was in good general health, we can eliminate comorbid severe illness as a factor in our analysis, which strongly supports the notion that hypoglycemia itself is a CV-risk factor.
In summary, this investigation strongly suggests that severe hypoglycemia has a cumulative effect on CAC and so might pose a risk factor in CVD. The risk magnitude of 30% is clinically relevant. We also note that even today, years after completion of the DCCT/EDIC-study, hypoglycemia events may still be relatively frequent, and the risk of hypoglycemia might be underestimated. For example, Lipska et al. [48] found that 10.8% of the study cohort patients with type 2 diabetes experienced severe hypoglycemia during the past year. Of these patients, 76.3% reported 1–3 events, 15.4% reported 4–6 events, 4.5% reported 7–11 events and 3.8% reported 12 and more events. These percentages may be even higher for patients with type 1 diabetes.
The association between severe hypoglycemia and CVD is complex. Our work supports the hypothesis that the effect of severe hypoglycemia on CVD should be seen in the context of glycemic control. Our results support personalized medicine with individualized glycemic goals beyond HbA1C.
Acknowledgements
The Diabetes Control and Complications Trial (DCCT) and its follow-up the Epidemiology of Diabetes Interventions and Complications (EDIC) study were conducted by the DCCT/EDIC-Research Group and supported by National Institute of Health grants and contracts and by the General Clinical Research Center Program, NCRR. The data from the DCCT/EDIC-study were supplied by the NIDDK Central Repositories. This manuscript was not prepared under the auspices of the DCCT/EDIC-study and does not represent analyses or conclusions of the DCCT/EDIC-study group, the NIDDK Central Repositories, or the NIH. Special thanks to Amanda Flynn, Stacie White, Drs. Rasooly, Egers, Turner and Tan (all NIDDK). J. Denvir was supported in part by NIH grants 2P20RR016477 and 8P20GM103434. We also thank Drs. J. Fährmann, Wu, and Pewen for reading the manuscript and their discussion. We acknowledge Drs. Yaqub and Leidy for their discussion and Dr. Gress for advising and encouraging us to use publicly available NIDDK-data (all Marshall University Huntington, WV).
Abbreviations:
- AER
albumin excretion rate
- A1CDCCT/EDIC
mean glycated hemoglobin during combined DCCT/EDIC-study
- BMI
body mass index
- CAC
coronary artery calcification measured in Agatston units
- CI
confidence interval
- CVD
cardiovascular disease
- CV-risk
cardiovascular risk
- DCCT-A1C
mean glycated hemoglobin during DCCT
- EDIC-A1C
mean glycated hemoglobin during EDIC
- HbA1C
glycated hemoglobin
- LDL
low density lipoprotein
- LOG(hypo)
natural logarithm (severe hypoglycemia rate + 1)
- HDL
high density lipoprotein
- RR
risk ratio
Footnotes
Conflicts of Interest
None declared.
References
- [1].Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006;29:798–804. [DOI] [PubMed] [Google Scholar]
- [2].Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–5. [DOI] [PubMed] [Google Scholar]
- [3].Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–38. [DOI] [PubMed] [Google Scholar]
- [4].The DCCT Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986. [DOI] [PubMed] [Google Scholar]
- [5].Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, et al. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].The DCCT Research Group, Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003; 290:2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].The DCCT Research Group, Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care 1995; 18:1415–1427. [DOI] [PubMed] [Google Scholar]
- [8].The DCCT Research Group, Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46:271–286. [PubMed] [Google Scholar]
- [9].Ahrén B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag 2013;9:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Holt P. Taking hypoglycaemia seriously: diabetes, dementia and heart disease. Br J Community Nurs 2011;16:246–9. [DOI] [PubMed] [Google Scholar]
- [11].Castaldo E, Sabato D, Lauro D, Sesti G, Marini MA. Hypoglycemia assessed by continuous glucose monitoring is associated with preclinical atherosclerosis in individuals with impaired glucose tolerance. PLoS One 2011;6:e28312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–63. [DOI] [PubMed] [Google Scholar]
- [13].Opie LH, Yellon DM, Gersh BJ. Controversies in the cardiovascular management of type 2 diabetes. Heart 2011;97:6–14. [DOI] [PubMed] [Google Scholar]
- [14].Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther 2012;14(Suppl 1):S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vigersky R. Mad about “U”. J Clin Endocrinol Metab 2014;99:771–3. [DOI] [PubMed] [Google Scholar]
- [17].Gruden G, Barutta F, Chaturvedi N, Schalkwijk C, Stehouwer CD, Witte DR, et al. Severe hypoglycemia and cardiovascular disease incidence in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2012;35:1598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giménez M, Gilabert R, Monteagudo J, Alonso A, Casamitjana R, Pare C, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care 2011;34:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giménez M, López JJ, Castell C, Conget I. Hypoglycaemia and cardiovascular disease in type 1 diabetes. Results from the Catalan National Public Health registry on insulin pump therapy. Diabetes Res Clin Pract 2012;96:e23–5. [DOI] [PubMed] [Google Scholar]
- [20].Gruden G, Barutta F, Perin P, Bruno G. Response to Comment on: Gruden et al. Severe Hypoglycemia and Cardiovascular Disease Incidence in Type 1 Diabetes: The EURODIAB Prospective Complications Study. Diabetes Prospective Complications Study. Diabetes Care 2012; 35:1598–1604 (letter). Diabetes Care 2012;35:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giménez M, Conget I. Comment on: Gruden et al. Severe hypoglycemia and cardiovascular disease incidence in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2012;35:1598–1604 (letter). Diabetes Care 2012;35:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 2001;50:1491–4. [DOI] [PubMed] [Google Scholar]
- [23].Funk SD, Yurdagul A Jr, Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med 2012;569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- [25].Dalsgaard-Nielsen J, Madsbad S, Hilsted J. Changes in platelet function, blood coagulation and fibrinolysis during insulin-induced hypoglycaemia in juvenile diabetics and normal subjects. Thromb Haemost 1982;47:254–8. [PubMed] [Google Scholar]
- [26].Fisher BM, Hepburn DA, Smith JG, Frier BM. Responses of peripheral blood cells to acute insulin-induced hypoglycaemia in humans: effect of alpha-adrenergic blockade. Horm Metab Res Suppl 1992;26:109–10. [PubMed] [Google Scholar]
- [27].Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010;33:1591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dandona P, Chaudhuri A, Dhindsa S. Proinflammatory and Prothrombotic Effects of Hypoglycemia. Diabetes Care 2010;33:1686–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010;33: 1389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev 2005;1:59–63. [DOI] [PubMed] [Google Scholar]
- [31].Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(suppl III):III-27–32. [DOI] [PubMed] [Google Scholar]
- [32].Agarwal S, Cox AJ, Herrington DM, Jorgensen NW, Xu J, Freedman BI, et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care 2013;36:972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ, et al. Coronary artery calcium score prediction of all-cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ 2013;346:f1654. [DOI] [PubMed] [Google Scholar]
- [34].Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378–402. [DOI] [PubMed] [Google Scholar]
- [35].Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation 2003;108:e50–3. [DOI] [PubMed] [Google Scholar]
- [36].Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55: 3556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].DCCT Research Group. Manual of Operations for the Diabetes Control and Complications Trial. Washington, DC, US: Department of Commerce; 1987. [Google Scholar]
- [38].EDIC Data Coordinating Center. Epidemiology of Diabetes Interventions and Complications Protocol and Manual of Operations. Washington, DC: Biostatistics Center, The George Washington University; 2010, amended December 2010. [Google Scholar]
- [39].National Institute for Clinical Excellence. 2002. Available at: http://www.nice.org.uk.
- [40].Morioka T, Emoto M, Tabata T, Shoji T, Tahara H, Kishimoto H, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care 2001;24:909–13. [DOI] [PubMed] [Google Scholar]
- [41].Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. ISPAD Clinical Practice Consensus Guidelines, 2006–2007. Pediatr Diabet 2007;8:408–18. [DOI] [PubMed] [Google Scholar]
- [42].Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care 2003;26:2923–8. [DOI] [PubMed] [Google Scholar]
- [43].Lachin J. The Assessment of Relative Risks. Biostatistical Methods. John Wiley and Sons; 2000. [Google Scholar]
- [44].White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–12. [DOI] [PubMed] [Google Scholar]
- [45].Lopes-Virella MF, Baker NL, Hunt KJ, Lachin J, Nathan D, Virella G, Group DER. Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis 2011;214:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Aguilera E, Serra E, Granada M, Alonso N, Pellitero S, Pizarro E, Reverter J, et al. Low prevalence of subclinical atherosclerosis in asymptomatic patients with Type 1 Diabetes in a European Mediterranean population diabetes care 2014;37:814–20. [DOI] [PubMed] [Google Scholar]
- [47].Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- [48].Lipska KJ, Warton EM, Huang ES, Moffet HH, Inzucchi SE, Krumholz H, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging study. Diabetes Care 2013;36:3535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481–9. [DOI] [PubMed] [Google Scholar]
- [50].Schoenaker DA, Simon D, Chaturvedi N, Fuller JH, Soedamah-Muha SS. Glycemic control and all-cause mortality risk in type 1 diabetes patients: the EURODIAB Prospective Complications Study. J Clin Endocrinol Metab 2014;99:800–7. [DOI] [PubMed] [Google Scholar]