Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) belong to an important therapeutic class for treatment of type 2 diabetes. Six GLP-1 RAs, each utilizing a unique drug delivery strategy, are now approved by the Food and Drug Administration (FDA) and additional, novel GLP-1 RAs are still under development, making for a crowded marketplace and fierce competition among the manufacturers of these products. As rapid elimination is a major challenge for clinical application of GLP-1 RAs, various half-life extension strategies have been successfully employed including sequential modification, attachment of fatty-acid to peptide, fusion with human serum albumin, fusion with the fragment crystallizable (Fc) region of a monoclonal antibody, sustained drug delivery systems, and PEGylation. In this review, we discuss the scientific rationale of the various half-life extension strategies used for GLP-1 RA development. By analyzing and comparing different approved GLP-1 RAs and those in development, we focus on assessing how half-life extending strategies impact the pharmacokinetics, pharmacodynamics, safety, patient usability and ultimately, the commercial success of GLP-1 RA products. We also anticipate future GLP-1 RA development trends. Since similar drug delivery strategies are also applied for developing other therapeutic peptides, we expect this case study of GLP-1 RAs will provide generalizable concepts for the rational design of therapeutic peptides products with extended duration of action.
Keywords: GLP-1 receptor agonist, exenatide, peptide delivery, half-life, pharmacokinetics, fatty acid conjugate, albumin fusion, Fc fusion
Graphical abstract
1. Introduction
Type-2 diabetes is a chronic metabolic disease characterized by hyperglycemia, reduced insulin secretion, and insulin resistance. If not properly treated, type 2 diabetes can result in serious or even fatal complications including blindness, limb amputation, kidney failure, and cardiovascular diseases. In 2017, the International Diabetes Federation estimated that, globally, 423 million people between the ages of 20 and 79 had diabetes, and that type 2 diabetes comprised about 90% of these cases [1, 2]. That same year, the global economy spent an estimated 727 billion USD for diabetes treatment [1]. As both diabetes diagnoses and the life expectancy of individuals with diabetes continue to rise, the total number of diabetic patients is expected to reach 629 million by 2045 [1], resulting in an increasing demand for anti-diabetes therapies.
Historically, metformin has remained the first-line treatment for type 2 diabetes. Other long-used therapeutic options to help manage glucose levels include insulin, sulphonylureas, and thiazolidinediones [3]. However, maintaining glucose homeostasis with these agents remains challenging for many patients [4]. Furthermore, some of these drugs—particularly insulin and sulfonylurea—lead to undesired risks including hypoglycemia and weight gain [5]. Thus, identifying new drug targets and developing more effective and safer treatments is necessary to achieve optimal management of type 2 diabetes.
Glucagon-like peptide-1 (GLP-1) emerged as a target for type-2 diabetes treatment due to its unique mechanism of action. GLP-1 is an endogenous incretin hormone produced by intestinal enteroendocrine L-cells following nutrient ingestion [6]. The initial product, GLP-1 (1-37), is quickly cleaved by enzymes, resulting two active truncated forms GLP-1 (6-37) and GLP-1 (7-37) [7]. GLP-1 exerts multiple physiological effects by activating GLP-1 receptors distributed in various organs, including the pancreas, gastrointestinal (GI) tract, brain, heart and the kidneys [7, 8]. When glucose levels are elevated, GLP-1 promotes insulin secretion in the pancreas but has minimal effect when glucose levels are normal [9]. This glucose-dependent insulinotropic effect is particularly favorable for diabetes treatment because it avoids the risks of hypoglycemia, a common side effect of some anti-diabetes drugs, including insulin [9]. GLP-1 also decreases glucagon secretion, further contributing to reduction of glucose levels [6, 10]. Studies have also suggested that GLP-1 can improve β-cell function and inhibit β-cell apoptosis, both of which could prevent or slow the progression of β-cell failure in type 2 diabetes [6, 11, 12]. GLP-1 also displays positive effects on other tissues and organs. In the GI tract, GLP-1 slows gastric emptying, leading to lower postprandial glucose levels [13, 14]. By activating GLP-1 receptors in the nervous system, GLP-1 could enhance satiety and inhibit energy intake, which may help reduce bodyweight [15, 16].
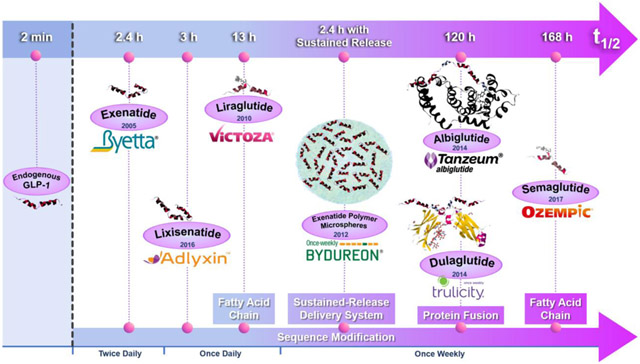
Despite its potent anti-diabetes effects, the clinical application of native GLP-1 is hindered by its rapid clearance by the dipeptidyl peptidase-4 (DPP-4) enzyme in vivo, resulting in a half-life of only 2 minutes [17, 18]. GLP-RA developers have applied various half-life extending strategies and some of them have successfully resulted in FDA-approved diabetes products (Table 1). Simple sequential modification enhances DPP-4 resistance and improves GLP-1 receptor activation potency. This strategy led to the twice-daily and once-daily products, Byetta® and Adlyxin®, respectively. Sequence modification to enhance DPP-4 resistance combined with covalent attachment of a fatty acid leads to slower absorption and mediates albumin binding in plasma, a strategy employed in the development of the once-daily and once-weekly agents, Victoza® and Ozempic®, respectively. More complicated molecular modifications that led to the approval of once-weekly products include fusing either recombinant human serum albumin (Tanzeum®) or an antibody fragment crystallizable (Fc) moiety (Trulicity®) to a GLP-1 analog. Other GLP-1 modifications have also been tested, including GLP-1 RA modified either with a recombinant peptide polymer XTEN® (VRS-859) or polyethylene glycol (LY2428757). Both molecules progressed into clinic trials, leading to the possibility of once-monthly and once-weekly products, but the development of these molecules appears to have been halted. Controlled release GLP-RA products have also been developed including the FDA-approved once-weekly poly(lactide-co-glycolide) microspheres (Bydureon®) and a once-yearly titanium implant (ITCA 650) which is currently under review by the FDA.
Table 1.
Overview of GLP-1 RAs on the market for type 2 diabetes treatment.
| Drug name | Brand name | Administration | Dosage | FDA Approval |
Company |
|---|---|---|---|---|---|
| Exenatide | Byetta® | Twice a day (SQ) |
Initiate at 5 μg/dose (equivalent to 1.2 nmol GLP-1 RA per dose). The dose can be increased to 10 μg/dose (2.4 nmol/dose) after 1 month.[19] | Apr 2005 | AstraZeneca |
| Liraglutide | Victoza® | Once a day (SQ) |
Initiate at 0.6 mg/dose (equivalent to 0.16 μmol GLP-1 RA per dose) for one week then increase to 1.2 mg/dose (0.32 μmol/dose). Dose can be increased to 1.8 mg/dose for additional glycemic control (0.48 μmol/dose).[20] | Jan 2010 | Novo Nordisk |
| Exenatide | Bydureon® | Once weekly (SQ) |
2 mg/dose (equivalent to 0.48 μmol GLP-1 RA per dose). [21] [22] | Jan 2012 | AstraZeneca |
| Bydureon® Bcise | Nov 2017 | ||||
| Albiglutide | Tanzeum® (US) Eperzan® (EU) |
Once weekly (SQ) |
Initiate at 30 mg/dose (equivalent to 0.82 μmol GLP-1 RA per dose); Dose can be increased to 50 mg/dose (1.4 μmol/dose). [23] | Apr 2014 | GlaxoSmithKline |
| Dulaglutide | Trulicity® | Once weekly (SQ) |
Initiate at 0.75 mg/dose (equivalent to 0.024 μmol GLP-1 RA per dose); Dose can be increased to 1.5 mg/dose (0.048 μmol/dose). [24] | Sep 2014 | Eli Lilly & Co |
| Lixisenatide | Adlyxin® (US) Lyxumia® (EU) |
Once a day (SQ) |
Initiate at 10 μg/dose (equivalent to 2.06 nmol GLP-1 RA per dose). On day 15, increase to 20 μg/dose (4.12 nmol/dose). [25] | Jul 2016 | Sanofi-Aventis |
| Semaglutide | Ozempic® | Once weekly (SQ) |
Initiate at 0.25 mg/dose (equivalent to 0.061 μmol GLP-1 RA per dose), increase up to 1 mg/dose (0.24 μmol/dose) after 4 weeks. [26] | Dec 2017 | Novo Nordisk |
| Liraglutide and insulin degludec | Xultophy® 100/3.6 | Once a day (SQ) |
Recommended starting dosage is 16 units of insulin degludec and 0.58 mg (0.15 μmol) of liraglutide per day. Maximum daily dosage is 50 units of insulin degludec and 1.8 mg (0.48 μmol) of liraglutide. [27] | Nov 2016 | Novo Nordisk |
| Lixisenatide and insulin glargine | SULIQUA (EU) SOLIQUA® 100/33 (US) |
Once a day (SQ) |
Initiate at 15 units insulin glargine/5 μg (1.03 nmol) lixisenatide or at 30 units insulin glargine/10 μg (2.06 nmol) lixisenatide. Maximum daily dose is 60 units insulin glargine/20 μg (4.12 nmol) of lixisenatide. [28] | Nov 2016 | Sanofi-Aventis |
The employment of different half-life extension strategies, thus, results in significant differences in the pharmacokinetic profiles, efficacy, safety, and usability of these products, which in turn largely impacts the use and market penetration of GLP-1 RA products. In this review, we discuss the structure-activity relationship, pharmacokinetics, efficacy and market dominance of approved GLP-1 RA products, GLP-1 RA molecules in development and combination therapy strategies. The implementation of various creative delivery technologies for improving circulation half-life of molecules will likely be repeated for other peptide or protein products, making this review relevant for scientists working within the drug delivery field.
2. Strategies to increase half-life
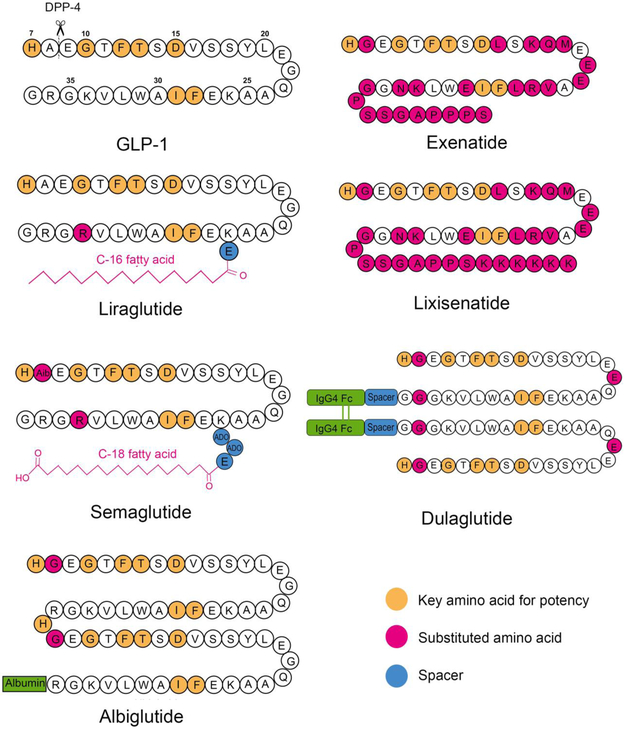
To design GLP-RAs with an increased circulation time, a broad array of half-life extension strategies has been utilized including sequential modification (Byetta® and Adlyxin®), attachment of a fatty-acid (Victoza® and Ozempic®), development of a polymer-based sustained release formulation (Bydureon®), fusion with human serum albumin (Tanzeum®), and fusion with the fragment crystallizable (Fc) region of a monoclonal antibody (Trulicity®) (Figure 1). In this section we review the structure-activity relationship behind the various half-life extending strategies in the context of GLP-1 RA development and, in turn, how these modifications impact pharmacokinetic parameters and dosing scheduling of the resulting GLP-1 RA products.
Figure 1.
Peptide sequences and molecular structures of FDA approved GLP-1 RAs.
2.1. Critical structural elements of GLP-1 sequence
Early structure-activity studies of endogenous GLP-1 revealed the key sequence domains that correlate with potency and enzymatic degradation. Studies have suggested that the N-terminal residues His7, Gly10, Phe12, Thr13 and Asp15 are essential for interaction with the GLP-1 receptor, as substituting these residues with L-Ala leads to a severe loss of affinity for its receptor [43]. Similarly, C-terminal positions Phe28 and Ile29 are also important for receptor binding due to their role in maintaining the peptide’s secondary structure [43]. Endogenous GLP-1 has a short plasma half-life because of its rapid degradation by DPP-4, which cleaves GLP-1 between the Ala8 and Glu9 amino acids [44]. In addition, neutral endopeptidase (NEP) plays a minor role in GLP-1 degradation by cleaving at six sites, Asp15-Val16, Ser18-Tyr19, Tyr19-Leu20, Glu27-Phe28, Phe28-Ile29 and Trp31-Leu32, within the central and C-terminal peptide domains [44].
Based on structure-activity studies, many GLP-1 analogs have been designed to evade enzymatic degradation while maintaining their potency. Because DPP-4 cleavage at Ala8-Glu9 is the major reason for the rapid deactivation of native GLP-1, replacing Ala8 is a commonly used strategy to reduce DPP-4 degradation. For example, replacing Ala8 with a-aminoisobutyric acid (Aib) was found to completely prevent DPP-4 degradation [45]. Similarly, in two other commercialized GLP-1 analogs, albiglutide and dulaglutide, Ala8 is substituted with Gly to prevent DPP-4 degradation (Figure 1).
2.2. Exendin-4 and its analogs
In addition to human GLP-1 analogs, a naturally-occurring peptide and a hormone found in the saliva of Gila monster (a venomous lizard), exendin-4, was identified as a GLP-1 RA (Figure 1). Although exendin-4 shares only 53% homology with human GLP-1, in vitro studies demonstrated that exendin-4 has a slightly higher potency than native GLP-1 [46], attributable to their high similarity of key N-terminal residues. Compared to native GLP-1, exendin-4 is more resistant to cleavage by DPP-4 due to a Gly to Ala substitution at the second position [47]. Furthermore, the Leu29-Ser39 segment of exendin-4 can form a tertiary fold, or “Trp cage”, which conformationally shields residues 21-39, protecting these sites from being cleaved by NEP 24.11 [48, 49]. The enzyme resistance results in a distinct metabolic pathway of exendin-4. Studies reported that exendin-4 is mainly degraded on kidney membranes and eliminated via glomerular filtration [50, 51]. In comparison, native GLP-1 is eliminated by both the kidneys and liver while also subject to metabolism in peripheral tissues.
Exenatide, a synthetic version of exendin-4, was the first GLP-1 RA to be approved by the FDA under the brand name of Byetta® (Table 1). In 2009, Byetta® was approved as a stand-alone therapy for type 2 diabetes by the FDA [19]. Byetta® is supplied in multi-dose prefilled pens consisting of 250 μg/ml synthetic exenatide solution in pH 4.5 acetate buffer with mannitol (tonicity-adjusting agent) and metacresol (preservative). It can be stored at 2-8°C for 3 years before use, and stored at room temperature below 25 °C for 30 days after first use (Table 2) [19]. Byetta® is administered as a twice daily (BID) subcutaneous injection before each main meal (Table 1). Patients begin with a 5 μg dose for one month, followed by titration up to 10 μg [19]. Pharmacokinetic parameters of exenatide are summarized in Table 3. Peak plasma concentration is achieved about 2 hours post administration. The product half-life is 2.4 hours, which allows for twice-daily administration, and is a large improvement over endogenous GLP-1 [19]. Animal studies have shown that exenatide is mainly cleared by renal filtration.
Table 2.
Formulations and storage conditions for GLP-1 RA products
| Product | Dosage form | Strength | Excipients | Shelf-life (in use stability) |
Ref |
|---|---|---|---|---|---|
| Exenatide BID Byetta® |
Solution in multi-dose prefilled pens | 300 μg in 1.2 ml 600 μg in 2.4 ml |
Mannitol (43 mg/ml), metacresol (2.2 mg/ml), glacial acetic acid and sodium acetate trihydrate. (pH 4.5) | 3 years at 2-8°C (30 days <25°C) |
[19, 29, 30] |
| Lixisenatide Adlyxin® |
Solution in multi-dose prefilled pens | 150 μg in 3 ml 300 μg in 3 ml |
Glycerol 85% (18 mg/ml), methionine (3.0 mg/ml), metacresol (2.7 mg/ml), sodium acetate trihydrate (3.5/ml mg), hydrochloric acid and sodium hydroxide (pH 4.5) | 2 years at 2-8°C (14 days < 30°C) |
[25, 31, 32] |
| Liraglutide Victoza® |
Solution in multi-dose prefilled pens | 18 mg in 3 ml | Propylene glycol (14.0 mg/ml), phenol (5.50 mg/ml), disodium phosphate dihydrate (1.42 mg/ml) (pH 8.15) | 30 months at 2-8°C (30 days at 2-8°C or 15-30°C) |
[20, 33] |
| Semaglutide Ozempic® |
Solution in multi-dose prefilled pens | 2 mg in 1.5 ml | Propylene glycol (14.0 mg/ml), phenol (5.50 mg/ml), disodium phosphate dihydrate (1.42 mg/ml), hydrochloric acid and sodium hydroxide (pH 7.4) | 3 years at 2-8°C (56 days at 2-8°C or 15-30°C) |
[34, 35] |
| Albiglutide Tanzeum® |
Lyophilized powder and solvent in single-dose prefilled dual-chamber pens | 30 mg or 50 mg in 0.5 ml after reconstitution | Mannitol (27.9 mg/ml), polysorbate 80 (0.01% w/w), sodium phosphate (1.64 mg/ml), trehalose dihydrate (44.3 mg/ml) | 3 years at 2-8°C (4 weeks < 30°C) |
[23, 36] |
| Dulaglutide Trulicity® |
Solution in single-dose prefilled syringes or pens | 0.75 mg in 0.5 ml 1.5 mg in 0.5 ml |
Mannitol (46.4 mg/ml), polysorbate 80 (0.20 mg/ml), citric acid anhydrous (0.14 mg/ml), trisodium citrate dihydrate (2.74 mg/ml) | 2 years at 2-8°C (14 days <30°C) |
[24, 37] |
| Exenatide QW Bydureon® |
Powder and diluent supplied with trays. Powder and diluent in single-dose dual-chamber pens |
2 mg in 0.65 ml after resuspension | 50:50 PLGA (57.2 mg/ml), sucrose (1.2 mg/ml), sodium chloride (6.3 mg/ml), carboxymethylcellulose sodium (29.2 mg/ml), polysorbate 20 (0.01 mg/ml), sodium phosphate monobasic monohydrate (0.9 mg/ml), sodium phosphate dibasic heptahydrate (0.78 mg/ml), sodium hydroxide | 3 years at 2-8 °C (4 weeks < 25°C) |
[21, 38] |
| Exenatide QW Bydureon® BCISE |
Suspension in single dose injector | 2 mg in 0.85 ml | 50:50 PLGA (43.7 mg/ml), sucrose (0.94 mg/ml); medium chain triglycerides (875.8 mg/ml) | 3 years at 2-8°C (4 weeks <25°C) |
[39] |
| Liraglutide and insulin degludec | Solution in multi-dose prefilled pens | 300 units insulin degludec and 10.8 mg | Glycerol 19.7 mg/ml, phenol 5.70 mg/ml, zinc 55 μg/ml, hydrochloric acid and sodium hydroxide (pH 8.15) | 2 years at 2-8°C (21 days at 2-8°C or 15-30°C) |
[27, 40] |
| Xultophy® 100/3.6 | liraglutide in 3 ml | ||||
| Lixisenatide and insulin glargine SOLIQUA® 100/33 |
Solution in multi-dose prefilled pens | 300 units insulin glargine and 100 μg lixisenatide in 3 ml | Glycerol 20 mg/ml, methionine 3 mg/ml, metacresol 2.7 mg/ml, zinc 30 μg/ml, hydrochloric acid and sodium hydroxide | 2 years at 2-8°C (28 days <25°C) |
[28, 41] |
Table 3.
GLP-1 RAs pharmacokinetics parameters
| Product name |
Dose | Tmax | Vd | T1/2 | AUC | CL | Ref |
|---|---|---|---|---|---|---|---|
| Exenatide BID Byetta® |
Single dose of 10 μg | 2.1 h | 28.3 L | 2.4 h | 1036 pg•h/ml | 9.1 L/h | [19] |
| Lixisenatide Adlyxin® |
N.A. | 1-3.5 h | 100 L | 3 h | N.A. | 35 L/h | [25] |
| Liraglutide Victoza® |
Single dose of 0.6 mg | 8-12 h | 13 L | 13 h | 960 ng•h/ml | 1.2 L/h | [20] |
| Semaglutide Ozempic® |
Single dose of 0.5 mg | 56 h | 9.4 L | 7 days | 3123 nmol•h/L | 0.039 L/h | [34] |
| Albiglutide Tanzeum® |
Single dose of 30 mg | 3-5 d | 11 L | 5 days | 465 μg•h/ml | 0.067 L/h | [23] |
| Dulaglutide Trulicity® |
Multiple doses of 1.5 mg to steady state | 24-72 h | 17.4 L | 5 days | 14 μg•h/ml | 1.07 L/h | [24] |
| Exenatide QW Bydureon® |
Multiple doses of 2 mg to steady state | Css =300 pg/ml; Tss = 6~7 weeks | [21] | ||||
| ITCA 650 | Continuous delivery of 20 μg/day | Css = 75.8 pg/ml | [42] | ||||
Abbreviations: Tmax: time to reach peak plasma concentration after administration; Vd: apparent volume of distribution; AUC: area under the curve for peptide concentration in plasma, CL: clearance; Css: average peptide concentration at the steady state; Tss: time to reach steady state after administration.
Another successful GLP-1 RA, lixisenatide, was launched in the U.S. in July 2016 under the brand name Adlyxin® (Table 1). Modified from exendin-4, lixisenatide is a 44-amino-acid peptide with the C-terminal Pro replaced by 6 Lys residues (Figure 1). In vitro studies found that lixisenatide has four times greater affinity for the GLP-1 receptor than native GLP-1 and exenatide [52]. Adlyxin® is administered once daily at 10 – 20 μg per day (Table 1). The pharmacokinetic profile of lixisenatide is similar to that of exenatide with a characteristic kidney elimination and Tmax of 2 hours post administration. The apparent volume of distribution is 100 L, with approximately 55% of lixisenatide bound to plasma proteins in circulation (Table 3) [25]. The half-life of lixisenatide is 2.6 hours, which is similar to that of exenatide [25]; however, unlike exenatide, lixisenatide is indicated as a once-daily injection. This is partly due to lixisenatide’s better ability to slow gastric emptying, yielding a better glucose control and, thus, reduced administration frequency [53]. Similar to Byetta®, Adlyxin® is supplied in multi-dose prefilled pens. The formulation of Adlyxin® contains lixisenatide 50 or 100 μg/ml in pH 4.5 acetate buffer, with glycerol 85% (cosolvent), methionine (antioxidant) and metacresol (preservative). Adlyxin® can be stored at 2-8°C for 2 years before use, and stored at room temperature below 30 °C for 14 days after first use (Table 2) [25, 31].
Although amino acid substitution is an effective strategy to reduce proteolytic degradation, modified GLP-1 analogs are still subject to rapid renal clearance, making it difficult to further prolong peptide circulation time. Thus, other drug delivery strategies were used to develop longer-acting GLP-1 RAs, as described below.
2.3. Fatty acid conjugates
Covalent attachment of a fatty acid, known as acylation, is used extensively to control the half-life of therapeutic peptides [54]. One example is the insulin detemir (Levemir®), in which myristic acid is introduced to insulin at position B29. The conjugation of myristic acid results half-life extension from 4-6 min (insulin) to 5-7 hours (insulin detemir).
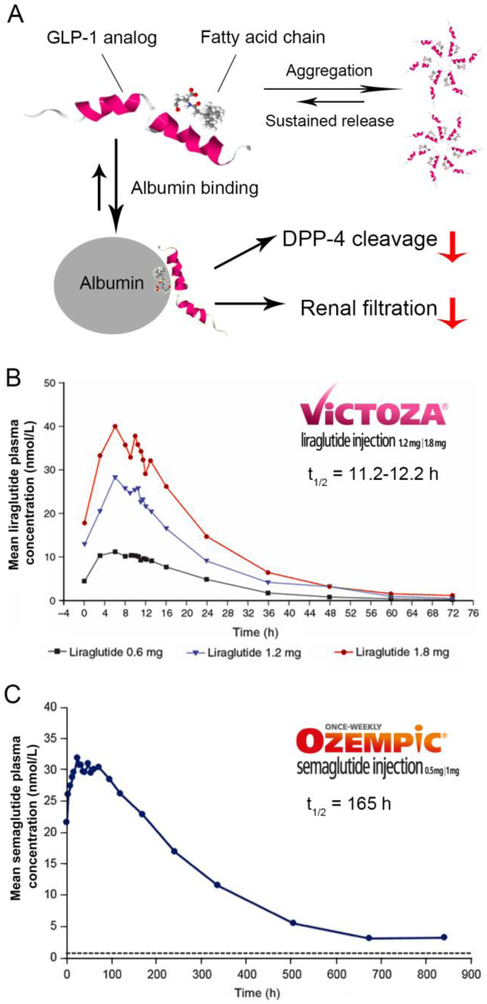
Acylation with fatty acids can increase the circulation time of therapeutic peptides by several mechanisms. First, acylated peptides could self-assemble to supramolecular structures due to their amphiphilic nature. Once subcutaneously injected, acylated peptide spontaneously forms a depo at the injection site, leading to a delayed absorption into systemic circulation [55]. In the case of acylated GLP-1 RAs, it was reported that liraglutide, a palmityolated GLP-1 RA, could self-assemble into hexa-, hepta- or octamers, depending on the pH and ionic strength [56-58]. When the acylated peptide monomer finally dissociates from the oligomer and enters blood circulation, it readily yet reversibly binds to serum albumin through hydrophobic and ionic interactions between the fatty acids and albumin [59, 60]. Albumin binding provides steric hindrance against enzymatic degradation of the acylated peptide and slows its renal clearance due to the large size of the peptide-albumin complex. The albumin-bound peptide is also hypothesized to interact with the neonatal Fc receptor (FcRn), allowing it to shuttle through the albumin recycling pathway and evade intracellular degradation, thus, significantly increasing the circulation half-life [61]. However, although the FcRn recycling is proposed as a half-life extension mechanism for albumin-bound peptides in general, to the best of our knowledge there is no existing experimental evidence that proves FcRn recycling occurs for acylated GLP-1 RAs.
The stability and potency of acylated GLP-1 RAs depends on the site of acylation, the bulkiness and length of the fatty acid chains, and the spacer used between the peptide and fatty acid [62-64], Because the N-terminus is key for receptor binding and activation, acylation in the N-terminal region leads to serious loss of potency. In contrast, C-terminal modifications have little impact on potency [63]. The spacers linking peptide segment and fatty acid chains also greatly affect the potency of acylated GLP-1 RAs [64]. While the bulkiness of fatty acid was found to negatively impact the potency of GLP-1 RAs, the length of the fatty acid had more complex effects [64]. The length of fatty acid chain is positively correlated with the binding affinity to albumin, leading to a longer circulation time [62, 64]. However, the peptide must dissociate from albumin to activate GLP-1 receptor, thus GLP-1 RAs with longer fatty acid chain exhibit lower potency in the presence of serum [63, 64]. Therefore, the acylation site and the length of fatty acid chain must be selected to achieve the right balance between potency and in vivo stability.
The most successful example of an acylated GLP-1 is liraglutide, which was approved by the FDA in 2010 under the brand name Victoza®. Liraglutide, derived from native GLP-1, has a 16-C fatty acid linked to Lys26 with a γ-Glu spacer and substituting Arg for Lys at position 34 (Figure 1, 2) [65]. Once administered, the fatty acid chain causes oligomerization of liraglutide, enabling formation of oligomers at the injection site [56, 58]. The self-association of liraglutide leads to delayed absorption into systemic circulation, which was observed in clinical studies where intravenously administered liraglutide was found to have a shorter residence time than when administered subcutaneously [66, 67]. In plasma, liraglutide monomers dissociate from the oligomers [67] and bind to albumin within the central blood compartment [68]. In vitro studies showed that while liraglutide could be metabolized by DPP-4 and NEP in a manner similar to GLP-1, its degradation was much slower [69], possibly due to the steric hindrance provided by fatty-acid-bound albumin. Albumin binding also prevents renal filtration of liraglutide, evidenced by the fact that no intact liraglutide could be detected in patients’ urine in clinical studies [69]. Combined, these factors all contribute to enhance the in vivo stability of liraglutide. Pharmacokinetic data showed that liraglutide has a longer half-life, about 9-13 hours [20], compared to the other short-acting GLP-1 Ras, such as exenatide (2.1 hours) [19]. This affords liraglutide the ease of once-daily injections. Pharmacokinetic analysis also suggests that, compared to exenatide BID, liraglutide has smaller peak-to-trough fluctuations and better continuous exposure [66]. Liraglutide (Victoza®) is approved in the U.S. as a second-line type 2 diabetes treatment (Table 1). Victoza® is supplied in multi-dose prefilled pens. The formulation of Victoza® contains liraglutide 6 mg/ml in phosphate buffer with propylene glycol (cosolvent) and phenol (preservative). With a pKa of about 9.5, the solubility of liraglutide decreases below pH 7, requiring the formulation’s pH to be adjusted to 8.15 [70]. Victoza® can be stored at 2-8°C for 30 months before use. After first use, the product can be stored at room temperature at 2-8°C or 15-30°C for 30 days (Table 2) [20, 33]. Patients initiate the treatment with a 0.6 mg/dose for one week then increase to a 1.2 mg/dose. The dose can be increased to a 1.8 mg/dose for additional glycemic control (Table 1). In addition, a higher dose liraglutide (3 mg/dose) has also been approved by the FDA for weight management under the brand name Saxenda® [16].
Figure 2.
(A) Half-life extension mechanisms of acylated GLP-1 analogs. (B) Mean concentration-time profile of liraglutide following single dose of 0.6 mg, 1.2 mg, or 1.8 mg at steady state in healthy male Chinese subjects. Adapted with permission of © John Wiley & Sons, Inc. from Jiang et al. J Clin Pharmacol. 2011;51:1620–7. [72] (C) Mean concentration-time profile of semaglutide following single dose of 1.0 mg at steady-state. Adapted with permission of © John Wiley & Sons, Inc. from Kapitza et al. J Clin Pharmacol. 2015; 55(5):497-504. [73]
Semaglutide—which was approved by the FDA in December 2017 under the brand name of Ozempic®—is a next generation GLP-1 RA based on liraglutide. Compared to liraglutide, semaglutide has an additional amino acid substitution in the 8th position from Ala to α-aminobutyric acid (Aib) to avoid degradation by DPP-4 [64]. A longer stearic (C18) di-acid fatty chain was introduced into semaglutide, whereas liraglutide has a C16 fatty acid [64]. The spacer between the stearic di-acid chain and peptide backbone has been optimized [62], and the spacers composed of γGlu or 8-amino-3,6-dioxaoctanoic acid (ADO) were found to be optimal for the potency of GLP-1 RAs, though mechanisms are not clear. For semaglutide, the fatty di-acid chain is attached to Lys26 through one glutamic acid and two 8-amino-3,6-dioxaoctanoic acid (ADO) moieties to achieve the optimal binding affinity [64]. Semaglutide shows a higher albumin binding affinity in comparison to liraglutide, which may be a result of the longer fatty acid side chain included in semaglutide [64]. The GLP-1 receptor binding affinity of semaglutide is slightly lower than that of liraglutide, which can be attributed to the longer lipid chain and presence of the terminal acid group on steric acid [64]. In clinical studies, semaglutide was shown to be degraded by enzymes prior to renal extraction. Because of its slow enzyme degradation and reduced renal extraction, semaglutide has a half-life of 7 days, allowing for once-weekly administration (Figure 2, Table 3) [71]. Despite the reduced dosing frequency, the dose of semaglutide — initially 0.25 mg/dose with the ability to increase to 1.0 mg/dose—is lower than that of liraglutide, which starts with a 0.6 mg/dose (Table 1) [26]. Similar to Victoza®, Ozempic® is supplied in multi-dose prefilled pens containing semaglutide 0.75 mg/ml in phosphate buffer (pH 7.4) with propylene glycol (cosolvent) and phenol (preservative). Ozempic® can be stored at 2-8°C for 3 years prior to use. After first use, the product can be stored at room temperature at 2-8°C or 15-30°C for 56 days. (Table 2) [26, 35].
2.4. Albumin fusion
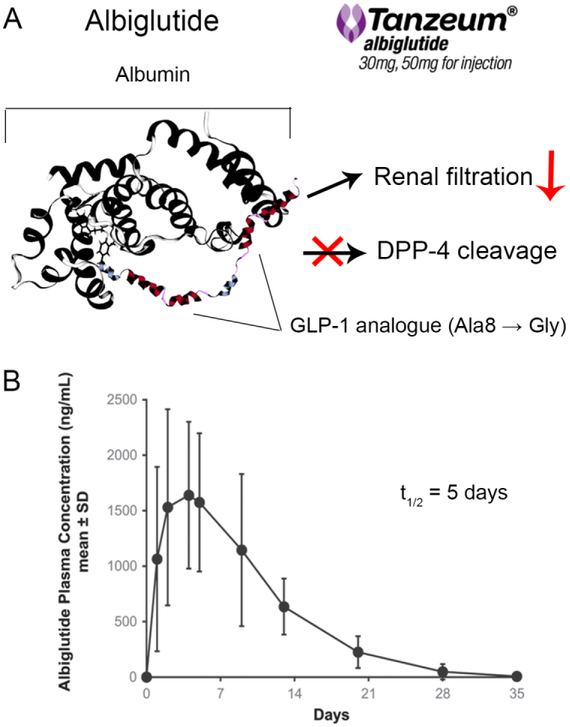
Albumin is well-recognized as a potential carrier of therapeutic peptides due to its long circulation half-life, ability to distribute broadly in the body and low immunogenicity. This strategy has been successfully used in the development of the FDA-approved drug Idelvion® (albumin fusion of coagulation factor IX) [74]. Albumin conjugation likely improves GLP-1 RA plasma half-life by several mechanisms, including providing steric hindrance from proteolysis and increasing molecular weight to reduce kidney filtration (Figure 3). In addition, covalent attachment to albumin may also prolong the peptide’s circulation time by a unique albumin recycling pathway through the neonatal Fc receptor (FcRn) [75]. Following endocytosis by endothelial cells, albumin binds to the FcRn receptor within the acidic lysosomal environment, which enables albumin rescue from degradation, transport back to the cell’s surface and return to the circulation [75, 76]. Such a recycling mechanism is believed to contribute to the prolonged half-life of endogenous albumin and, presumably, albumin-fusion proteins [77].
Figure 3.
(A) Half-life extension mechanisms of albiglutide. (B) Mean concentration-time profile of albiglutide following a single subcutaneous 30 mg injection. Adapted with permission of © 2015 Taylor & Francis Ltd. from Young et al. Postgraduate Medicine. 126:7, 84-97. www.tandfonline.com [78].
An albumin conjugation strategy was used to design albiglutide (Tanzeum®), which was approved by the FDA in 2014. Albiglutide is a recombinant protein composed of two copies of GLP-1 analogs fused to human albumin. The molecule has a Gly8 to Ala substitution in both copies of the GLP-1 analogs to improve resistance to DPP-4 degradation. Albiglutide is administered once weekly by subcutaneous injection and is thought to be absorbed through the lymphatic circulation [23]. The peak plasma concentration is achieved about 4 days after a single administration, and steady-state is obtained after the first 3-4 weekly doses (Table 3) [23]. Due to its high molecular weight, renal filtration is not expected to be an important elimination mechanism for albiglutide. Overall, albiglutide has an in vivo half-life of 6-8 days, and is approved for once-weekly administration. In Tanzeum®, albiglutide is lyophilized with mannitol (stabilizer/tonicity adjuster), trehalose dihydrate (stabilizer/tonicity adjuster), polysorbate 80 (surfactant), and sodium phosphate (pH adjuster). The lyophilized cake and solvent (water for injection) are supplied in single-dose dual-chamber pens (Table 2) [23, 36]. Reconstitution is needed prior to use to make the albiglutide solution of 60 mg/ml or 100 mg/ml for injection. The product can be stored 3 years at 2 – 8 °C or 4 weeks at room temperature below 25 °C [23, 36]. Patients are initiated at 30 mg/dose (equivalent to 0.82 μmol GLP-1 RA per dose) and can be increased to 50 mg/dose (1.37 μmol/dose) if necessary (Table 1) [23].
2.5. Fc fusion
Fc fusion is another established strategy to prolong the half-life of therapeutic peptides. Several Fc fusion products, such as etanercept (TNFR-Fc fusion protein, Enbrel®) and aflibercept (VEGFR-Fc fusion, Zaltrap®), are approved by the FDA [79]. Overall, incorporating the Fc domain of IgG prolongs a peptide’s circulation time through several mechanisms. First, the Fc domain can endow hybrid proteins with a longer in vivo circulation time through its interactions with FcRn. Similar to albumin’s recycling process, FcRn can bind with the Fc domain in an acidic endosomal environment to protect the Fc domain from degradation, while disassociating from the Fc domain in neutral pH environment outside the cell [80]. The important role played by FcRn in prolonging a peptide half-life was evidenced in Suzuki et al. [81], where the affinities of Fc-fusion proteins for FcRn were closely correlated to their half-lives. Fc fusion also reduces renal filtration of peptides by increasing their molecular weight, which is especially favorable for GLP-1 RAs that are eliminated primarily by the kidney [19]. Fc fusion also reduces DPP-4 degradation of GLP-1, likely due to steric hindrance by the bulky IgG fragment [82]. From a technological viewpoint, Fc-fusion to GLP-1 allows for an easier purification process relative to albumin fusion due to widely available protein A binding columns used for IgG purification [83].
An important concern associated with the development of GLP-1 Fc fusion products is their potential safety risk arising from the ability of the Fc fragment to trigger antibody-dependent cell-mediated (ADCC) cytotoxicity and complement-mediated cell lysis through Fc-γ receptor interactions. When compared to the IgG1 Fc domain, the IgG2 and IgG4 Fc domains may be more favorable in the development of GLP-1 RAs due to their lower affinity for Fc-γ and complement receptors [84, 85]. The potency of GLP-1 RA after Fc fusion is another concern, as linking the GLP-1 analog with the IgG1 hinge region directly results in a severe loss of potency, suggesting a suitable spacer is needed for potency retention [86].
The most successful GLP-1 product employing the Fc-fusion strategy is dulaglutide (Trulicity®), approved by the FDA in 2014 as an adjunctive therapy for type 2 diabetes. Dulaglutide is a disulfide-bonded homodimer fusion peptide with each monomer consisting of one GLP-1 analog moiety and one IgG4 Fc region (Figure. 1 and 4). Within the GLP-1 analog, Ala8 is replaced by Gly to resist DPP-4 degradation, and Gly36 is substituted for Arg to avoid a potential T-cell epitope interaction [86]. The IgG4 Fc region was selected and its sequence modified at several positions to further reduce its interaction with the Fc-y receptors to avoid ADCC immunologic side effects [86]. A spacer comprising 16 amino acids was added between the GLP-1 analog and the Fc moiety to prevent activity loss. Dulaglutide has an extended half-life of 4.7 days, allowing for once weekly dosing. Trulicity® is supplied in single-dose pens or syringes. The formulation consists of dulaglutide solution at a concentration of 1.5 mg/ml or 3 mg/ml in citrate buffer with mannitol (stabilizer/tonicity adjuster) and polysorbate 80 (surfactant) [37]. The product can be stored at 2 – 8 °C for 2 years or below 30 °C for 14 days (Table 2) [24, 37]. Patients begin therapy with a 0.75 mg/dose once weekly (corresponding to 0.024 μmol GLP-1 RA/dose), and the dose can be doubled for additional glycemic control (Table 1) [23].
Figure 4.
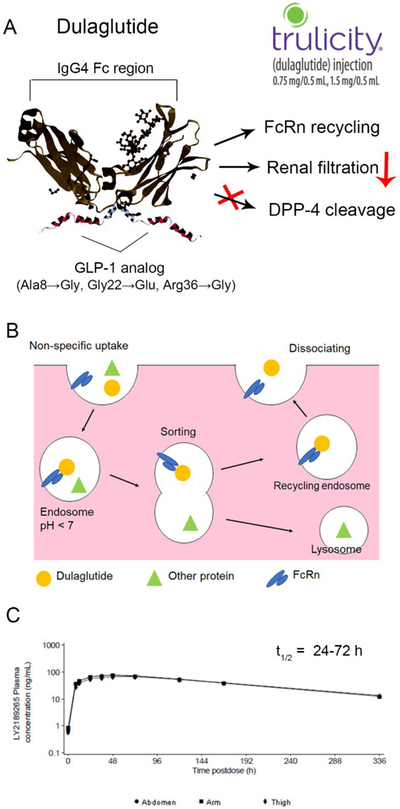
(A) Half-life extension mechanisms of dulaglutide. (B) FcRn recycling pathway of dulaglutide. (C) Mean concentration-time profile of dulaglutide following single dose of 1.5 mg subcutaneously injected in abdomen, thigh, or arm. Data obtained from an EMA assessment report of Trulicity® [87].
2.6. Sustained-release drug delivery systems
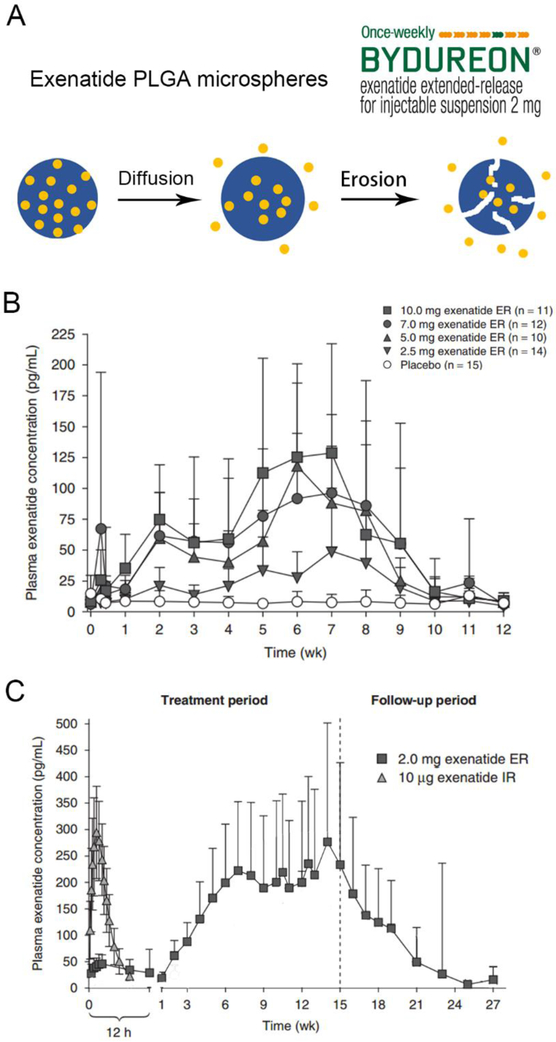
Another strategy to achieve sustained plasma levels of GLP-1 RAs is by taking advantage of drug delivery systems. Various systems including polymeric hydrogels [88], nanoparticles [89] and microparticles [90] have been tested in preclinical studies. However, only poly(lactic-co-glycolic acid) (PLGA) based implants of GLP-1 RA have been approved by the FDA thus far. PLGA is well accepted by regulatory authorities as a safe and efficacious way to deliver therapeutic peptides [91, 92]. Lupron Depot®, a microsphere formulation of leuprolide acetate and Zoladex®, a millicylinder formulation of goserelin, are examples of FDA approved PLGA-based sustained release products [93, 94]. In these products, peptides are loaded within a PLGA polymer which slowly degrades in the body, allowing for sustained release of the therapeutic peptide. For example, Lupron Depot® can release the loaded leuprolide over an extended period of 1 or 6 months, depending on the specific polymer formulation used [95]. Such an extended release profile enables maintenance of target peptide concentrations with fewer administrations, thus improving patient compliance.
The PLGA delivery approach was applied in the design of a polymer microsphere formulation of exenatide in collaboration between Eli Lilly, Amylin and Alkermes [96]. Although the degradation of PLGAs can be tuned from a few weeks to more than six months, exenatide PLGA microspheres fall short of a more desirable monthly administration, and the final product, Exenatide QW (Bydureon®), was launched as a once-a-week product [96]. Once injected subcutaneously, the 60-μm-diameter microspheres hydrate and adhere to each other, forming a matrix drug reservoir in situ. A discontinuous drug release profile with a low initial burst and two peaks of drug release at the 2nd and 7th week is observed, corresponding to different mechanisms of exenatide release from microspheres (Figure 5A and B) [79]. During the first 48-hour “burst release” stage exenatide is released from the surface and surface pores of the microspheres. This product was specifically designed to have low initial drug burst in order to avoid nausea and vomiting side effects [96]. The second stage is characterized by the slow diffusion of exenatide out of the polymer matrix with the maximum diffusion rate and corresponding plasma concentration peak achieved at about 2 weeks. The third stage of drug release results from the hydrolysis and erosion of the PLGA polymer [96]. The maximum drug-release rate at this stage is achieved at 7 weeks, which is evidenced by the second peak of the blood concentration-time curve. The overall release of exenatide can last for 11 weeks [21]. After the first 6-7 weekly administrations of Bydureon®, a stable steady state plasma level of exenatide is achieved at 300 pg/ml as peaks and valleys from weekly dosages at different injection sites begin to overlap (Figure 5C) [97]. From the formulation perspective, Bydureon® is supplied as single-dose trays and single-dose dual-chamber pens where the exenatide PLGA microparticle powders and diluent are supplied separately. Prior to use, patients need to resuspend the PLGA microparticles containing 2 mg exenatide in 0.65 ml phosphate buffer, which also contains sodium chloride (tonicity adjuster), carboxymethylcellulose sodium (suspending agent) and polysorbate 20 (surfactant) (Table 2) [21]. To achieve better usability, a new formulation, Bydureon® Boise™, was developed and approved by the FDA in 2017. In Bydureon® Boise™, the PLGA powders are suspended in medium chain triglycerides supplied in single-dose autoinjectors, which avoids the cumbersome procedures before use and enhances the patient experience [39]. Despite the different formulations, Bydureon® and Bydureon® Boise™ are both administered subcutaneously with 2 mg/dose and have similar pharmacokinetics profiles (Table 1) [21, 39]. Bydureon® can be stored at 2 – 8 °C for 3 years or at the room temperature below 25 °C for 4 weeks (Table 2) [21, 38].
Figure 5.
(A) Release process of exenatide from microspheres. (B) Mean concentration-time profile of exenatide following single dose of Bydureon®. (C) Mean concentration-time profile of exenatide following repeated weekly administrations of Bydureon® (exenatide ER same as exenatide QW) compared with a single administration of Byetta® (exenatide IR). Adapted with permission of © 2011 Adis Data Information BV from Fineman et al. Clin Pharmacokinet 2011; 50 (1).[97]
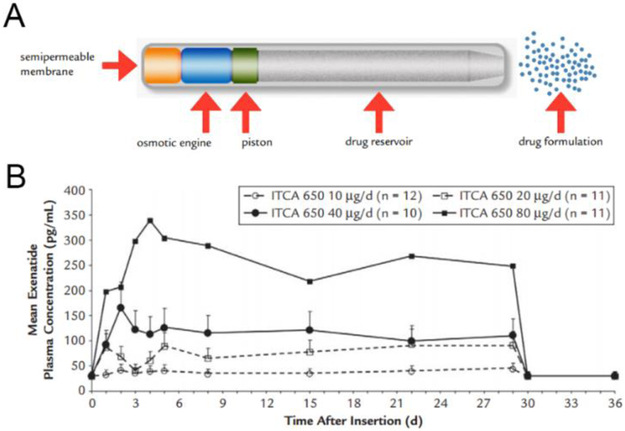
As discussed above, one of the drawbacks of PLGA microparticles in the Bydureon® formulation is that they cannot deliver GLP-1 RAs at a steady rate. To address this problem and achieve steady and continuous delivery of GLP-1 RAs, other controlled-release devices such as osmotic pumps have been developed. One representative product is ITCA 650, developed by Intarcia Therapeutics. ITCA 650 is a subcutaneously implanted matchstick-sized osmotic mini-pump, which continuously releases exenatide for 6 months in a zero-order manner, making twice-yearly administration possible [98]. The zero-order release of exenatide results in a favorable pharmacokinetic profile of ITCA 650 for several reasons. Pharmacokinetic studies showed the ITCA 650 could achieve steady-state exenatide concentration within 5 days following implantation, compared to 6-7 weeks for exenatide QW [42]. The plasma concentration of exenatide remained steady over the period of treatment [42]. Furthermore, the drug treatment can be discontinued if necessary by removal of the implant. In the clinical trials, once the implanted pump was removed, exenatide levels became undetectable by 24 hours [42]. A New Drug Application for ITCA 650 was submitted to the FDA in November 2016 [99]. If approved, it would be the first needle-free GLP-1 RA on the market.
2.6. Other approaches for half-life extension
PEGylation is another common strategy for enhancing in vivo stability of therapeutic peptides. PEGylation increases molecular size and provides steric hindrance to reduce renal filtration and protect peptide from enzymatic degradation [101]. PEGylated GLP-1 analogs have been investigated by several groups. Lee et al. [102] synthesized N-terminally PEGylated PEG2k-N-GLP-1 and Lys26- or Lys34-modified PEG2k-Lys-GLP-1s. The in vitro study showed that PEG2k-N-GLP-1 exhibited a significant reduction in potency, which is likely due to the steric hindrance of PEG shielding the GLP-1 receptor interaction [102]. PEG2k-Lys-GLP-1, however, showed a similar potency to native GLP-1 [102]. Stability studies found that PEG2k-Lys-GLP-1 was resistant to DPP-4 degradation and had a longer mean retention time in vivo [102]. One PEGylated GLP-1 analog, LY2428757, advanced to clinical testing by Eli Lilly & Co. for once-weekly administration. To the best of our knowledge, the exact structure of LY2428757 has not been disclosed. The Phase 2 clinical trial was completed in 2010 [103], however, no further information is available about this product’s development status. As an alternative to PEG, XTEN® is a series of recombinant protein polymers which are specifically designed for fusion to therapeutic peptides to achieve long circulation in vivo [104]. The XTEN-GLP-1 fusion protein, VRS-859, was tested in a Phase I clinical trial conducted by Versartis in 2010 as a once-monthly administration [105]. However, the development of this product has also likely been discontinued.
3. The effects of GLP-1 RA delivery strategies on product efficacy and safety
Different modification strategies not only affect the pharmacokinetic profiles of GLP-1 RAs but also strongly influence their efficacy, safety profile and usability. The main pharmacological efficacy biomarkers determined in Phase 3 studies for GLP-1 RAs are summarized in Table 4. Based on the pharmacokinetic properties, GLP-1 RAs could be roughly divided into short-acting agonists which are administered once or twice daily, and long-acting agonists which are administered once weekly. The two groups of GLP-1 RAs, while sharing many similarities, present different pharmacodynamic features and, thus, yield different antidiabetic efficacy.
Table 4.
Summary of GLP-1 RA initial therapy and monotherapy Phase 3 clinical trials.
| GLP-1 RAs | Dose | FPG (mg/dl) |
PPG (mg/dl) |
HbA1c (%) |
Weight loss (kg) |
References |
|---|---|---|---|---|---|---|
| Exenatide BID (Byetta®) |
10 μg BID | −18.7 | −24.7 | −0.9 | −3.1 | [133] |
| Lixisenatide (Lyxumia®/Adlyxin®) |
10 μg for 2 wks then 20 μg | −19.8 | −98.5 | −0.85 | −2 | [134] |
| 10 μg for wk 1, 15 μg for wk 2, then 20 μg | −16.2 | −81.2 | −0.73 | −2 | [134] | |
| Liraglutide (Victoza®) |
1.2 mg | −14.4 | −30.6 | −0.84 | −2.1 | [135] |
| 1.8 mg | −25.2 | −37.8 | −1.14 | −2.5 | [135] | |
| Semaglutide (Ozempic®) |
0.5 mg | −45 | N. A. | −1.45 | −3.7 | [71] |
| 1.0 mg | −41.4 | N. A. | −1.55 | −4.53 | ||
| Dulaglutide (Trulicity®) |
0.75 mg | −26 | −41.4 | −0.71 | −2.36 | [136] |
| 1.5 mg | −29 | −43.4 | −0.78 | −2.29 | [136] | |
| Albiglutide (Tanzeum®/Eperzan®) |
30 mg | −34 | N. A. | −0.84 | −0.4 | [137] |
| 50 mg | −43 | N. A. | −1.04 | −0.9 | [137] | |
| Exenatide QW (Bydureon®) |
2 mg | −41.4 | N. A. | −1.53 | −2.0 | [138] |
Abbreviations: FPG: fasting plasma glucose level; PPG: postprandial plasma glucose level; HbA1c: level of hemoglobin A1c; BID: twice a day; QW: once a week; N. A.: information is not available.
3.1. Glycemic control patterns
GLP-1 RAs can efficiently regulate plasma glucose levels through multiple mechanisms, including stimulation of insulin production, inhibition of glucagon secretion and slowing gastric emptying. Though both short-acting and long-acting GLP-1 RAs show favorable glucose control efficacy, they have different effects on fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) levels. Clinical studies have demonstrated that short-acting GLP-1 RAs have a greater efficacy in lowering PPG level, while long-acting GLP-1 RAs predominantly affect FPG levels. For example, in a head-to-head clinical trial comparing exenatide BID (Byetta®) and exenatide QW (Bydureon®), patients treated with exenatide BID had a lower PPG, while patients treated with exenatide QW had a lower FPG [106]. As exenatide was the same active ingredient in both groups, this data suggests that PK profile significantly influences pharmacological activity of short-acting versus long-acting GLP-1 RAs, resulting in differential reduction of PPG and FPG levels.
It is believed that a product’s effect on FPG level is directly related to pharmacokinetics of GLP-1 RA and GLP-1 receptor activation pattern, which, in turn, leads to the differential secretion of insulin and glucagon during a fasting state [107]. Short-acting GLP-1 RAs usually have limited drug exposure within the body due to rapid elimination. Exenatide, for example, administered one hour before the morning and evening meal, is almost completely eliminated from the plasma after 10 hours [108]. Thus, the GLP-1 plasma concentration in the middle of the day and overnight is low, leading to modest efficacy on FPG regulation. On the contrary, long-acting GLP-1 RAs that are present in the circulation between injections enables sustained GLP-1 receptor activation and continuous stimulation of insulin secretion. In the clinical trial comparing exenatide BID (Byetta®) and liraglutide (Victoza®), the fasting insulin level of the Victoza®-treated group increased on average by 12.43 pmol/L, while that of Byetta® decreased by 1.38 pmol/L [109]. Increased insulin secretion during a fasting state was also observed for other long-acting GLP-1 RAs such as dulaglutide (Trulicity®) [110] and albiglutide (Tanzeum®) [111].
The efficacy of GLP-1 RAs on PPG, however, is thought to primarily result from delayed gastric emptying rather than stimulation of insulin production [112]. An inverse relationship between gastric emptying rate and PPG has been demonstrated in clinical trials [113, 114]. Short-acting GLP-1 RAs such as exenatide BID (Byetta®) and lixisenatide (Adlyxin®) can significantly delay gastric emptying processes in vivo. The delayed gastric emptying leads to the reduced plasma glucose excursion. As a result, postprandial insulin concentrations are actually dose-dependently decreased, by exenatide and lixisenatide despite the known ability of GLP-1 RAs to stimulate insulin production [113, 114]. On the other hand, long-acting GLP-1 RAs have only a modest effect on gastric emptying, possibly caused by tachyphylaxis due to continuous activation of the GLP-1 receptor [115, 116]. For example, in a clinical study comparing exenatide BID (Byetta®) and exenatide QW (Bydureon®), it was found that after 14 weeks of administration, Byetta® still induced significant retardation of gastric emptying, while Bydureon® had little effect on gastric mobility [106]. Consequently, long-acting GLP-1 RAs typically lead to less profound reductions of PPG compared to short-acting GLP-1 RAs [112].
3.2. Impact of various GLP-1 RAs on glycated hemoglobin (HbA1c) levels
Glycated hemoglobin (HbA1c) is a biomarker extensively used by diabetologists to evaluate the ability of patients to control overall blood sugar level over periods of weeks to months. The higher the HbA1c level, the worse a patient’s glycemic control and the higher the likelihood that that individual will develop diabetes-related complications [117]. All commercialized GLP-1 RAs have proved to effectively reduce levels of HbA1c (Table 4). Though directly comparing the HbA1c reduction of different GLP-1 RAs is challenging, several head-to-head clinical studies have provided insight into differences between these products based on the pharmacodynamic and efficacy results from clinical trials [118]. Compared with short-acting products, long-acting GLP-1 RAs usually present better efficacy in reducing HbA1c, which may result from their ability to better control FPG levels. The DURATION-1 and DURATION-5 clinical trials, which directly compared exenatide BID (Byetta®) to exenatide QW (Bydureon®), reported better HbA1c level reduction for Bydureon® [119, 120]. Another clinical trial, LEAD-6, showed greater reduction of HbA1c levels in patients treated with long-acting Victoza® relative to short-acting Byetta® (−1.12% versus −0.79%, p < 0.0001) [109]. Similarly, dulaglutide (Trulicity®) reduced HbA1c levels more effectively than exenatide twice daily (Byetta®) in the AWARD-1 trial (−1.51% for dulaglutide 1.5 mg, −1.30% for dulaglutide 0.75 mg versus −0.99% for exenatide twice daily, p < 0.001 for both dulaglutide groups relative to exenatide) [121].
Differences in efficacy among GLP-1 RAs belonging to the same class seem less significant. No statistically significant differences were found between the short-acting compounds Byetta® and Adlyxin® in the head-to-head GetGoal-X trial [53]. Among long-acting GLP-1 RAs, the HbA1c-reduction efficacy of Victoza® (liraglutide) was not significantly different from that of Trulicity® (dulaglutide) [121] or Tanzeum® (albiglutide) [122]. The DURATION-6 trial demonstrated that Victoza® showed greater reduction of HbA1c levels than Bydureon® [123]. However, additional retrospective meta-analyses of various clinical data sets found no meaningful difference in HbA1c reduction between Victoza® and Bydureon® [124-126].
3.3. Weight loss
GLP-1 receptor activation may lead to significant weight loss by suppressing appetite, thus reducing calorie intake [127]. Though delayed gastric emptying and GI side effects of GLP-1 RAs are tempting explanations for the weight loss, their correlation with weight loss in diabetic patients was contradicted by several clinical trials [128, 129]. Instead, the anorectic effect of GLP-1 RAs was found to be responsible for the weight loss, which is mainly attributed to the satiating effect or rapid hunger satisfaction after the start of a meal. This effect is a result of peripheral and central GLP-1 receptor activation, and is afforded by the ability of GLP-1 RAs to cross the blood brain barrier (BBB) [130]. In one study, vagal deafferentation attenuated the anorectic effects of exendin-4 and liraglutide [130]. Another recent study showed that liraglutide can activate the GLP-1 receptor on appetite-regulating neurons in the arcuate nucleus (ARC), which may mediate its long-term weight loss effects[131]. Collectively, GLP-1 RAs show varied effects on weight loss, which may result from multiple factors. For example, unlike liraglutide, clinical studies with albiglutide resulted in little weight loss, potentially attributed to limited penetration of the large molecular weight of albumin fusion proteins into the central nervous system, the major site of action [132].
3.4. GLP-1 RA safety issues
The most frequently-observed side effects of GLP-1 RAs are gastrointestinal in nature, including nausea, diarrhea and vomiting. Nausea is the most commonly-reported adverse effect, though it usually decreases over time as a patient’s tolerance toward GLP-1 administration increases [139]. Nausea directly correlates with plasma levels of GLP-1 RAs and, thus, is more profound in short-acting GLP-1 RA products relative to long-acting GLP-1 RAs due to high peak peptide plasma concentration and large peak-to-through ratios [140]. For example, in several clinical studies, between 33% and 57% patients treated with exenatide [141] and between 24% and 76% of patients treated with lixisenatide [53, 134, 142-148] reported nausea. In contrast, only 9.9% to 16% patients treated with albiglutide [122, 137, 149-152] and 6.6% to 28% of patients treated with dulaglutide [121, 136, 153-156] experienced nausea. In a head-to-head clinical trial comparing exenatide BID (Byetta®) and exenatide QW (Bydureon®), the incidence of nausea in patients treated with Byetta® was 35% compared to 14% in those treated with Bydureon® [119]. This difference could be explained by the decreased fluctuation of plasma-drug concentrations seen with long-acting products, particularly the avoidance of the high peak concentrations seen with GLP-1 RAs [119].
Injection site reactions are another common side effect of GLP-1 RAs. The incidence of injection site reactions differs among GLP-1 RAs with different molecular structures. For short-acting GLP-1 RAs, such as Byetta®, Victoza® and Adlyxin®, the incidence of injection site reactions is relatively low (< 5%) [121, 123, 134, 142, 148, 157]. For long-acting GLP-1 RAs, 8% to 16% patients treated with Tanzeum® had injection site reactions [122, 137, 149-152]. In addition, less than 5% of patients treated with Trulicity® experienced injection site reactions [121, 136, 153-156]. Formulation factors also affect the incidence of injection site reactions. Bydureon® administration leads to a higher incidence of injection site reactions compared to Byetta® [158]. Particularly, injection of Bydureon® can cause a bump at the injection site and local inflammatory response, which may lead to discontinuation of the drug [106, 159].
Development of anti-drug antibodies is a common concern with therapeutic peptides and proteins. For GLP-1 RAs, exenatide seems to have the highest incidence of antibody formation, which be due to its relatively low homology to human GLP-1 (53%). About 37% of patients treated with Byetta® and 57% patients treated with Bydureon® tested positively for the presence of anti-drug antibodies [158]. While low titers of the antibody have little impact on the drug’s efficacy and safety profiles, high titers may reduce the efficacy of exenatide [158]. For comparison, only 8.6% of patients treated with liraglutide generated antibodies, which can be explained by the higher sequence homology with GLP-1 [160]. Incidence of anti-drug antibodies was much lower for albiglutide and dulaglutide. In Phase 3 clinical trials, anti-drug antibodies were detected in 3.0% to 7.0% of patients treated with albiglutide [122, 146, 149, 161, 162] and 2.2% of patients treated with dulaglutide [136]. In both cases, the presence of anti-drug antibodies was not correlated with relevant hypersensitivity events.
There are also concerns that long-term usage of GLP-1 RAs may cause pancreatitis or even lead to the development of pancreatic cancer. In a mouse study, exendin-4 induced pancreatic duct gland expansion in rats and exacerbated chronic pancreatitis [163]. Elashoff et al. analyzed data from the FDA database and found that patients treated with exenatide had a 6-fold higher incidence of pancreatitis compared to those treated with other diabetes medications, such as rosiglitazone, nateglinide, repaglinide and glipizide [164]. However, in the studies directly conducted by the FDA, no correlation between exenatide and pancreatic injury was found in animal models, and no compelling evidence was found to link the use of exenatide with an increased risk of pancreatitis or pancreatic cancer [165]. Because of these inconsistent results, no final conclusion has been made regarding correlations between exenatide use and serious adverse pancreatic events [165].
Liraglutide was found to be associated with thyroid cancer in rodents. Further studies found that long-term activation of GLP-1 receptors could stimulate calcitonin secretion and induce C-cell hyperplasia, which increases the incidence of medullary thyroid cancer in mice [166]. Such observations raise safety concerns about GLP-1 RAs. However, in humans, liraglutide was found to have no significant effect on calcitonin secretion [167]. In a meta-analysis, no correlation was found between liraglutide and an increased risk for thyroid cancer [168]. Thus, the correlation between GLP-1 RAs and thyroid cancer is not yet clear. However, based on these results, liraglutide is contraindicated in patients with a history of medullary thyroid cancer.
4. Combination treatments
Because of the complex pathological processes of type 2 diabetes, optimal glucose control typically requires multifaceted therapeutic strategies. The combination of GLP-1 RAs and basal insulin is frequently used because of the complementary effects of both agents on glucose control. Basal insulin consistently increases insulin levels and lowers FPG. Meanwhile, GLP-1 RAs stimulate insulin secretion in a glucose-dependent manner which provides better control over PPG. Also, adding GLP-1 RAs could counteract the hypoglycemia and weight gain frequently resulting from insulin therapy [169]. However, in clinical practice, healthcare providers may find it challenging to adjust the dose of each drug in combination to achieve optimal glucose control while avoiding side effects, hindering the practical application of combination therapy. To address this problem and to gain market advantage, pharmaceutical companies have developed various combination therapy products for type 2 diabetes. Novo Nordisk developed an insulin degludec/liraglutide combination named Xultophy® 100/3.6, or IdegLira [27]. Sanofi developed titratable lixisenatide and insulin glargine combination SOLIQUA® 100/33 (also named LixiLan or IGlarLixi) with a fixed ratio of 100 Units/mL lixisenatide to 33 mcg/mL insulin glargine [28]. Both Xultophy® and SOLIQUA® were approved by the FDA in November 2016.
It is noteworthy that although GLP-1 and insulin combination therapy may provide convenience in practice, combination therapy comes at the cost of diminished dosing flexibility of both drugs, raising concerns about whether the specific combination therapy actually achieves better therapeutic efficacy than two drugs that are titrated separately. To prove the superiority of the combination therapies, clinical trials were designed to test whether the combination has a superior efficacy over monotherapy. The Xultophy® DUAL™ Phase 3 clinical trials showed combination therapy leads to greater HbA1c decrease and weight loss compared to insulin monotherapy [170]. As for SOLIQUA®, the clinical trial showed that a larger proportion of patients reached H1A1c target levels compared to patients treated with insulin alone [171].
5. GLP-1 RA market dominance analysis
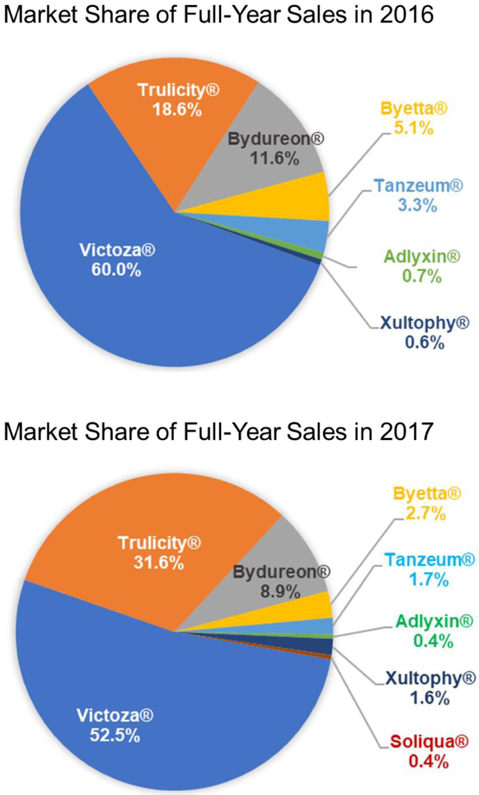
Pharmaceutical sales are affected by a variety of factors, including product efficacy, safety and the ability to fulfill an unmet need within the target therapeutic area. Other factors such as first-to-market, patent exclusivity status, marketing strategy, product pricing and government price controls, and geography of drug approval also have a substantial impact on total sales and ultimate market share. In the context of the diabetes therapeutic market, other points to consider are country specific disease treatment guidelines and pricing/reimbursement decisions [172, 173]. While it can be difficult to predict which factors will be most relevant in affecting commercial success from one product to the next, it is possible to observe sales trends and make pharmacoeconomic assessments within the current global GLP-1 market. Figure 7 and Table 5 show market shares of GLP-1 RA products in 2016 and 2017. The figure illustrates the overall market dominance of Victoza®, growing market share of Trulicity® and steady presence of Bydureon®. The rest of the market was shared by Byetta®, Tanzeum®, and Adlyxin®.
Figure 7.
Market share of GLP-1 RAs in 2016 and 2017.
Table 5.
Global sales of GLP-1 RAs in 2016 and first six months of 2017.
| Company | Brand Name |
Drug Name | 2016 Sales (in USD, millions) |
2017 Sales (in USD, millions) |
Market Share 2016 (%) |
Market Share 2017 (%) |
|---|---|---|---|---|---|---|
| AstraZeneca | Byetta® | Exenatide | 254 [191] | 176 [192] | 5.1 | 2.7 |
| AstraZeneca | Bydureon® | Exenatide (extended-release) |
578 [191] | 574 [192] | 11.6 | 8.9 |
| Novo Nordisk | Victoza® | Liraglutide | 2977 [193] | 3376 [180]* | 60.0 | 52.5 |
| Novo Nordisk | Xultophy® 100/3.6 | Liraglutide/insulin-degludec | 30 [180]* | 106 [180]* | 0.6 | 1.6 |
| GlaxoSmithKline | Tanzeum®/Eperzan® | Albiglutide | 165 [194]* | 108 [195]* | 3.3 | 1.7 |
| Eli Lilly & Co | Trulicity® | Dulaglutide | 926 [187] | 2030 [189] | 18.6 | 31.6 |
| Sanofi | Lyxumia®/Adlyxin® | Lixisenatide | 36 [196]* | 28 [197]* | 0.7 | 0.4 |
| Sanofi | Soliqua® 100/33 | Lixisenatide/Insulin | N/A | 28 [197]* | N/A | 0.4 |
All sales used to calculate market share which were not reported in USD were first converted to USD using 2016 yearly average currency exchange rates or 2017 yearly average currency exchange rates respectively. Market shares calculated using publicly-reported full-year sales for 2016 and 2017 based on proportion of USD earned in total global sales.
Byetta® was first launched in the U.S. in 2005 and was the only GLP-1 RA on the market until the approval of Victoza® in 2010. The drug reached $678.5 million peak sales in 2008. Despite the overall growth in the diabetes market, sales of Byetta® steadily declined to $254 million (5.1% market share) in 2016 and $176 (2.7% market share) in 2017 (Figure 7, Table 5) due to competition from other safer and more convenient GLP-1 RAs. Byetta® is expected to lose patent protection soon, and a generic injectable exenatide has been filed with the FDA [174]. Introduction of generic versions of Byetta® could affect the market, as generic drugs provide cheaper treatment alternatives preferable to patients, prescribers, and payers [175]. The introduction of low-price GLP-1 RA generics will certainly change sales dynamics and prescribing habits, especially within healthcare systems under financial scrutiny.
A more convenient once-daily dose product, Victoza®, was introduced into the U.S. market in 2010 by Novo Nordisk. Victoza® is supplied in an easy-to-use self-injector and offers therapeutic efficacy similar to Byetta® but with a reduction in side effects [109]. Victoza® immediately gained market share with 2011 sales of $1,289.7 million relative to $517.7 million for Byetta® [176, 177]. Over the years Victoza® has continued its growth and market dominance with global sales reaching $3376 million in 2017, capturing over 50% of the overall GLP-1 market share. Based on positive clinical trial results, Novo Nordisk recently received approval from the FDA to add a new indication to Victoza®: lowering cardiovascular risks in type 2 diabetes patients with established cardiovascular disease [178]. This addition makes Victoza® the only GLP-1 RA product approved for a cardiovascular indication. Similarly, an alternative dosage form of liraglutide, Saxenda®, was recently approved to treat obesity [179], and is currently the only GLP-1 RA approved in for this indication. Another strategy to maintain market dominance is to seek approval for combination therapies in a single injection. Thus, Xultophy®, a combination of liraglutide and insulin-degludec, was developed by Novo Nordisk. However, the sales of Xultophy® were relatively small in 2017 (just $106 million or 1.6% of GLP-1 RA market share) [180].
Amylin, a company initially developing exenatide, focused on the design of a PLGA polymer extended release formulation of exenatide in partnership with Alkermes. While for peptides like leuprolide, PLGA delivery offers once-monthly to once every six months products, the exenatide PLGA implant formulation, Bydureon®, was launched as a once- weekly product due to discontinuous release of peptide from the polymer and a narrow therapeutic index. Despite robust efficacy and being the first once-weekly formulation to reach the market, sales of Bydureon® fell short of expectation. In 2013, Bydureon® world-wide sales were a mere $151 million, significantly lower than that of twice daily injections of Byetta® ($206 million) and once-daily Victoza® ($2,035 million) [181, 182]. Inadequate sales of Bydureon® could be attributed to Bydureon®’s failure to show non-inferiority compared to Victoza® in the DURATION-6 trial [123]. Another possibility is the difficulty for some patients to create a suspension and self-administer the polymer microspheres [21]. Knowing that the multistep process for injection may hinder patient compliance, AstraZeneca designed a new, easy-to-use Bydureon® pre-filled pen, called Bydureon® BCise™ [39]. In addition, injection site side effects were reported for Bydureon® including local inflammation, abscess formation and cosmetic concerns due to retention of “bumps” under the skin that remain for extended periods of time due to the long time required for polymer degradation [159]. Despite these concerns the sales of Bydureon® remained steady at ~$570 million in 2016 and 2017 and could further improve with the launch Bydureon® BCise™ in 2018.
Great expectations were associated with the launch of easy-to-administer solutions of once-weekly GLP-1 RA products: albumin fusion (Tanzeum®) and Fc-fusion (Trulicity®). Interestingly, upon launch, Tanzeum® did not capture a significant market share. Its sales were only $63 million in 2015 as compared to $2.673 billion in sales for Victoza® that same year [183, 184]. Weak sales could be attributed to the increased immunogenicity and reduced therapeutic benefit offered by Tanzeum®, with efficacy parameters trailing behind Victoza® [122]. In addition, the relative inexperience of GSK in marketing diabetes drugs may have contributed to the poor sales. To improve sluggish sales, GSK tried to lower prices of Tanzeum®. Yet, Tanzeum® still failed to be listed as a preferred formulary by PBM Express Scripts [185]. Due to the continuous decline in sales, GSK chose to cease manufacturing and sale of Tanzeum® by July 2017 [186].
In contrast, once weekly Trulicity® launched by Eli Lilly in the same time frame did gain rapid acceptance. When compared with Victoza®, Trulicity® offered a similar therapeutic benefit and showed even better control over HbA1c levels in the AWARD-6 clinical trial [121]. Eli Lilly also had a large existing sales force in diabetes due to its insulin franchise, which likely facilitated successful product launch. Trulicity® sales in 2015 reached $248.7 million and continued to climb to $925.5 million in 2016 and to $2030 million in 2017, taking significant market share away from Victoza® (Figure 7, Table 5) [187-189]. Based on 2016 sales, Victoza® dominated the GLP-1 RAs with 60.0% market share followed by Trulicity® with 18.6%. In 2017, Trulicity® gained a spot on Express Scripts’ preferred formulary for the second year in a row, while Victoza® did not [185]. This designation was expected to further boost Trulicity® sales. In 2017, the growth of Trulicity® continued as Victoza®’s market share dropped to 52.2% and Trulicity®’s rose to 31.6%. While the main competition will likely remain between Victoza® and Trulicity®, several developments could impact the market, such as introduction of generic versions of Byetta® and approval of Ozempic®. Ozempic® is expected to be the next blockbuster on the GLP-1 market due to its excellent anti-diabetes efficacy [71], substantial reduction of cardiovascular risks [190] and once-weekly dosing frequency. Thus, it would be interesting to see how the landscape of the growing GLP-1 RA market will change over the next several years.
6. Emerging trends in GLP-1 RA development
Despite the large number of GLP-1 RAs already approved, there are numerous ongoing efforts to develop new GLP-1 RA molecules as well as explore novel routes of administration. Oral products have better patient compliance relative to subcutaneous injectable products, as some patients have a fear or injection [198, 199]. Another challenge for subcutaneous injection products is the difficulty of administration for patients suffering with visual or motor skill impairment, common ailments for patients with type 2 diabetes [200]. To address these issues, two orally available GLP-1 RAs are currently in clinical trials. A once-daily oral semaglutide is being developed by Novo Nordisk. The oral delivery of semaglutide is achieved by using the Eligen® technique developed by Emisphere Technologies. Semaglutide is co-formulated with sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC), a delivery carrier to enhance gastrointestinal absorption [201]. SNAC can non-covalently interact with peptide, forming a peptide/SNAC complex with increased lipophilicity able to permeate through the gastrointestinal epithelium via transcellular pathways [202]. The efficacy of different doses of oral semaglutide (2.5 mg, 5 mg, 10 mg, 20 mg and 40 mg once daily) were assessed and compared to subcutaneously delivered semaglutide (1 mg/dose once weekly) in Phase 2 clinical trials. Although percent semaglutide bioavailability is in single digit [203], the efficacies of either the 20 mg or 40 mg once daily oral doses of semaglutide were comparable to the 1 mg once weekly dose of drug administered subcutaneously [204]. Additionally, a dose-dependent HbA1c reduction ranging from −0.7% to −1.9% and body weight loss ranging from −2.1 kg to −6.9 kg was found [204, 205]. Oral semaglutide is currently being tested in the Phase 3 trial (PIONEER) in type 2 diabetes patients [206]. Another oral GLP-1 RA product, ORMD-0901, developed by Oramed, was tested in Phase 2 clinical trials [207]. Here, oral delivery is achieved by using POD™ technology where exenatide is encapsulated into coated capsules with protease inhibitors and absorption enhancers [208]. However, there have been no recent updates nor published efficacy data regarding this investigational drug.
Other delivery routes for GLP-1 RAs have also been investigated. Mannkind Corporation recently developed a GLP-1 inhalation powder, MKC253, for pulmonary delivery based on the Technosphere™ technique [209]. In this method, GLP-1 is absorbed onto Technosphere™ microparticles composed of fumaryl diketopiperazine (FDKP) with a size range of 2-5 μm. Once the GLP-1 loaded microspheres arrive at the lung, FDKP dissolves and GLP-1 is absorbed into systemic circulation [209]. In a phase 1 clinical study, peak concentrations of GLP-1 were achieved within 5 min, and GLP-1 was eliminated after 30 minutes with an estimated bioavailability between 0.5% to 1.6% [210]. TransPharma Medical Ltd developed a transdermal delivery system for their GLP-1 agonist ViaDor-GLP1, achieved using a hand-held microporation-based device, and have completed a Phase 1 clinical trial [211]. Based on the available data, the long-term success of these oral, inhalable or transdermal GLP-1 RAs remains unclear. Several non-invasive approaches have been developed in the past for insulin delivery [212, 213], but many of these products, particularly oral insulin products, faced challenges in clinical trials due to variable therapeutic responses owing to patient-to-patient differences in bioavailability [214-216]. Others, such as Exubera® (inhaled insulin) failed due to poor market penetration as well as safety concerns associated with drug accumulation in the lungs [217, 218].
Recently, co-agonists simultaneously activating GLP-1 and other incretin receptors have generated significant interest in the pharmaceutical industry. These dual agonists could simultaneously exert the functions of GLP-1 and other incretins such as glucagon [219, 220], gastric inhibitory polypeptide (GIP) [221] and gastrin [222], enabling better anti-diabetes efficacy. Although using glucagon as a therapy for diabetes seems counterintuitive, GLP-1/glucagon co-agonists have been reported to produce favorable effects with respect to body weight loss and glucose control in animal models [223]. For example, MEDI0382, one GLP-1/glucagon co-agonist developed by MedImmune, was reported to have glucose-lowering efficacy comparable to liraglutide and led to greater weight loss in mice [224]. Major pharmaceutical companies, including Novo Nordisk, Sanofi, Eli Lily, Janssen and Merck are currently developing their own GLP-1/glucagon co-agonist candidates, most of which are in Phase 1 or Phase 2 clinical trials [225]. Other combinations, such as GLP-1/gastrin co-agonists and GLP-1/GIP co-agonists, are also under development. One example, a GLP-1/gastrin dual agonist developed by Zealand Pharma, is reported to improve glucose control in diabetic mice by improving pancreatic β-cell function [226]. As many of these products are peptides, similar half-life extension strategies as were used for GLP-1 products will likely be employed in their design.
7. Summary
Here, we presented a case study of the various drug delivery strategies that have been applied to extend circulation the half-life of labile GLP-1 peptides. Simple amino acid substitutions or sequence modifications of the endogenous GLP-1 to avoid proteolysis lead to a modest increase in GLP-1 RA half-life from 2 min to 2-3 hours, resulting in the twice- and once-daily products Byetta® and Adlyxin®, respectively. However, side effects and reduced efficacy associated with high peak to trough ratios in their pharmacokinetic profiles resulted in only limited commercial success of these products, with 2.7 and 0.4% market share for Byetta® and Adlyxin®, respectively. When sequence modification was combined with the conjugation of fatty acid to allow for slow absorption of peptide and endogenous albumin binding, the plasma residence time increased and half-life was extended to 13 hours. Victoza® offers both convenience of dosing, robust activity, proven efficacy in cardiovascular event protection and reduced side effects relative to Byetta®. In addition, Victoza® was the first product on the market to achieve once-daily dosing and was launched by Novo Nordisk, a company experienced in diabetes product sales due to its large insulin franchise. These factors contributed strongly to development of the most commercially successful GLP-1 RA product, Victoza®, earning last year ~$3.4 billion and controlling over 50% of the overall GLP-1 RA market.
To mount competition to Victoza®, four once-weekly GLP-1 RA products were designed. Fusion of peptide to either antibody Fc fragment or albumin resulted in the development of Trulicity® and Tanzeum®, both with a plasma half-life of 5 days. To improve on Victoza® performance, Novo Nordisk developed Ozempic® by modifying the peptide with a longer steric fatty di-acid, resulting in a plasma half-life of 7 days. Finally, a polymer-controlled release formulation of exenatide, Bydureon®, was developed for once-weekly administration. Interestingly, while the clinical efficacy parameters for all weekly products are similar, variations in side effect profiles and ease of use between the products have contributed to vastly different market penetration. While Bydureon® sales hold steady at ~ 10% of the market, Trulicity® sales took off rapidly following product introduction, exceeding 30% of the market share in 2017. In contrast, Tanzeum® was unable to capture any market and was discontinued in 2017. The 2018 market introduction of both potent and easy to use once-weekly Ozempic® will likely again alter the GLP-1 RA prescribing landscape. Overall, competition between various peptide delivery technologies for capture of GLP-1 RA market represents an interesting case study broadly applicable to the development of delivery systems for other peptides and proteins. As scientists in the drug delivery field we often focus on engineering products for maximizing plasma half-life and investigating mechanisms to achieve it. In that regard, actual market dominance appears to be significantly driven by simplicity of use, dosing schedule, injection site discomfort, robust efficacy, indication and contraindication in certain subpopulations, and low side-effect profile along with non-scientific factors, such as first-to-market, size and experience of the company’s diabetes sales force, negotiated pricing and reimbursement agreement, geographic areas of regulatory approvals and patent exclusivity. Understanding the entire product landscape is thus important in order to maximize product success.
Figure 6.
Schematic of ITCA 650 (A) and mean concentration-time profile of exenatide following implantation of ITCA 650 (B). With permission of Henry et al. Clinical therapeutics 35.5 (2013): 634-645. [100]
8. Acknowledgements
This work was supported by NIH HL134569 and Amneal Pharmaceuticals grant to A. Schwendeman. Emily E. Morin was supported by NIH T32 GM008353 and T32 HL125242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].B.I.D.F. Brussels, International Diabetes Federation. IDF Diabetes Atlas, 8th edn., 2017. [Google Scholar]
- [2].Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, Global estimates of diabetes prevalence for 2013 and projections for 2035, Diabetes research and clinical practice, 103 (2014) 137–149. [DOI] [PubMed] [Google Scholar]
- [3].G. International Diabetes Federation Guideline Development, Global guideline for type 2 diabetes, Diabetes research and clinical practice, 104 (2014) 1–52. [DOI] [PubMed] [Google Scholar]
- [4].Ali MK, Bullard KM, Gregg EW, Achievement of goals in U.S. Diabetes Care, 1999–2010, The New England journal of medicine, 369 (2013) 287–288. [DOI] [PubMed] [Google Scholar]
- [5].Marathe PH, Gao HX, Close KL, American Diabetes Association Standards of Medical Care in Diabetes 2017, Journal of diabetes, 9 (2017) 320–324. [DOI] [PubMed] [Google Scholar]
- [6].Baggio LL, Drucker DJ, Biology of incretins: GLP-1 and GIP, Gastroenterology, 132 (2007) 2131–2157. [DOI] [PubMed] [Google Scholar]
- [7].Holst JJ, The physiology of glucagon-like peptide 1, Physiol Rev, 87 (2007) 1409–1439. [DOI] [PubMed] [Google Scholar]
- [8].Franek E, Gajos G, Gumprecht J, Kretowski A, Zahorska-Markiewicz B, Malecki MT, The role of glucagon-like peptide 1 in glucose homeostasis and in other aspects of human physiology, Pol Arch Med Wewn, 119 (2009) 743–751. [PubMed] [Google Scholar]
- [9].Meloni AR, DeYoung MB, Lowe C, Parkes DG, GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence, Diabetes, obesity & metabolism, 15 (2013) 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren A, Shibasaki T, Gribble F, Renstrom E, Seino S, Eliasson L, Rorsman P, GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis, Cell Metab, 11 (2010) 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ, Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis, The Journal of biological chemistry, 278 (2003) 471–478. [DOI] [PubMed] [Google Scholar]
- [12].Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ, GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress, Cell Metab, 4 (2006) 391–406. [DOI] [PubMed] [Google Scholar]
- [13].Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A, The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity, Int J Obes Relat Metab Disord, 25 (2001) 781–792. [DOI] [PubMed] [Google Scholar]
- [14].Wishart JM, Horowitz M, Morris HA, Jones KL, Nauck MA, Relation between gastric emptying of glucose and plasma concentrations of glucagon-like peptide-1, Peptides, 19 (1998) 1049–1053. [DOI] [PubMed] [Google Scholar]
- [15].Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR, A role for glucagon-like peptide-1 in the central regulation of feeding, Nature, 379 (1996) 69–72. [DOI] [PubMed] [Google Scholar]
- [16].Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rossner S, Hellstrom PM, Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men, Int J Obes Relat Metab Disord, 23 (1999) 304–311. [DOI] [PubMed] [Google Scholar]
- [17].Vilsboll T, Agerso H, Krarup T, Holst JJ, Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects, The Journal of clinical endocrinology and metabolism, 88 (2003) 220–224. [DOI] [PubMed] [Google Scholar]
- [18].Kieffer TJ, McIntosh CH, Pederson RA, Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV, Endocrinology, 136 (1995) 3585–3596. [DOI] [PubMed] [Google Scholar]
- [19].Byetta® (exenatide) injection [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. [Google Scholar]
- [20].Victoza® (liraglutide) injection, solution for subcutaneous use [prescribing information]. Denmark: Novo Nordisk A/S; 2016. [Google Scholar]
- [21].Bydureon®(exenatide sustained-release) for injectable suspension [prescribing information]. Wilmington, DE: Astrazeneca Pharmaceuticals LP; 2015. [Google Scholar]
- [22].Bydureon BCISE®(exenatide sustained-release) for injectable suspension [prescribing information]. Wilmington, DE: Astrazeneca Pharmaceuticals LP; 2017. [Google Scholar]
- [23].Tanzeum® (albiglutide) for injection, for subcutaneous use [prescribing information]. Wilmington, DE: GlaxoSmithKline LLC; 2016. [Google Scholar]
- [24].Trulicity® (dulaglutide) injection, for subcutaneous use [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2017. [Google Scholar]
- [25].Adlyxin® (lixisenatide) injection, for subcutaneous use [prescribing information]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2016. [Google Scholar]
- [26].OZEMPIC® (semaglutide) injection, for subcutaneous use [prescribing information]. Denmark: Novo Nordisk A/S; 2017. [Google Scholar]
- [27].Xultophy® 100/3.6 (insulin degludec and liraglutide injection) [prescribing information]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016. [Google Scholar]
- [28].SOLIQUA™ 100/33 (insulin glargine and lixisenatide injection), for subcutaneous use [prescribing information]. Bridgewater, NJ: Sanofi-Aventis; 2016. [Google Scholar]
- [29].European Medicines Agency, BYETTA: EPAR - Product Information - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. Accessed June 2018.
- [30].CoriolisPharma., GRAJ001 Phase 2: AF4 comparability study of generic exenatide injection and originator samples, 2017.
- [31].European Medicines Agency, Lyxumia, INN-lixisenatide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed June 2018.
- [32].FDA., Adlyxin (lixisenatide) Injection Summary Review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208471Orig1s000SumR.pdf. Accessed June 2018.
- [33].European Medicines Agency, Victoza, INN-liraglutide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed June 2018.
- [34].Jensen L, Helleberg H, Roffel A, van Lier JJ, Bjornsdottir I, Pedersen PJ, Rowe E, Derving Karsbol J, Pedersen ML, Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 104 (2017) 31–41. [DOI] [PubMed] [Google Scholar]
- [35].E.M. Agency, Ozempic, INN-semaglutide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004174/WC500244163.pdf. Accessed June 2018.
- [36].European Medicines Agency, Eperzan, INN-albiglutide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Accessed June 2018
- [37].European Medicines Agency, TRULICITY, INN-dulaglutide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002825/WC500179470.pdf. Accessed June 2018.
- [38].E.M. Agency, Bydureon- INN -exenatide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. Accessed June 2018.
- [39].Bydureon® BCise (exenatide sustained-release) for injectable suspension [prescribing information]. Wilmington, DE: Astrazeneca Pharmaceuticals LP; 2017. [Google Scholar]
- [40].European Medicines Agency, Xultophy, INN-insulin degludec/liraglutide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002647/WC500177659.pdf, Accessed June, 2018.
- [41].E.M. Agency, Suliqua, INN-insulin glargine / lixisenatide - http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004243/WC500224673.pdf. Accessed June 2018.
- [42].Henry RR, Logan D, Alessi T, Baron MA, A Randomized, Open-Label, Multicenter, 4-Week Study to Evaluate the Tolerability and Pharmacokinetics of ITCA 650 in Patients With Type 2 Diabetes, Clinical therapeutics, 35 (2013) 634–645. [DOI] [PubMed] [Google Scholar]
- [43].Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O, Structure-activity studies of glucagon-like peptide-1, The Journal of biological chemistry, 269 (1994) 6275–6278. [PubMed] [Google Scholar]
- [44].Deacon CF, Circulation and degradation of GIP and GLP-1, Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 36 (2004) 761–765. [DOI] [PubMed] [Google Scholar]
- [45].Sebokova E, Christ AD, Wang H, Sewing S, Dong JZ, Taylor J, Cawthorne MA, Culler MD, Taspoglutide, an analog of human glucagon-like Peptide-1 with enhanced stability and in vivo potency, Endocrinology, 151 (2010) 2474–2482. [DOI] [PubMed] [Google Scholar]
- [46].Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B, Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells, The Journal of biological chemistry, 268 (1993) 19650–19655. [PubMed] [Google Scholar]
- [47].Thum A, Hupe-Sodmann K, Goke R, Voigt K, Goke B, McGregor GP, Endoproteolysis by isolated membrane peptidases reveal metabolic stability of glucagon-like peptide-1 analogs, exendins-3 and −4, Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association, 110 (2002) 113–118. [DOI] [PubMed] [Google Scholar]
- [48].Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH, Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states, Biochemistry, 40 (2001) 13188–13200. [DOI] [PubMed] [Google Scholar]
- [49].Lee JG, Ryu JH, Kim SM, Park MY, Kim SH, Shin YG, Sohn JW, Kim HH, Park ZY, Seong JY, Kim JI, Replacement of the C-terminal Trp-cage of exendin-4 with a fatty acid improves therapeutic utility, Biochem Pharmacol, 151 (2018) 59–68. [DOI] [PubMed] [Google Scholar]
- [50].Simonsen L, Holst JJ, Deacon CF, Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs, Diabetologia, 49 (2006) 706–712. [DOI] [PubMed] [Google Scholar]
- [51].Copley K, McCowen K, Hiles R, Nielsen LL, Young A, Parkes DG, Investigation of exenatide elimination and its in vivo and in vitro degradation, Curr Drug Metab, 7 (2006) 367–374. [DOI] [PubMed] [Google Scholar]
- [52].Thorkildsen C, Neve S, Larsen BD, Meier E, Petersen JS, Glucagon-like peptide 1 receptor agonist ZP10A increases insulin mRNA expression and prevents diabetic progression in db/db mice, The Journal of pharmacology and experimental therapeutics, 307 (2003) 490–496. [DOI] [PubMed] [Google Scholar]
- [53].Rosenstock J, Raccah D, Koranyi L, Maffei L, Boka G, Miossec P, Gerich JE, Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X), Diabetes care, 36 (2013) 2945–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kontermann RE, Strategies for extended serum half-life of protein therapeutics, Curr Opin Biotechnol, 22 (2011) 868–876. [DOI] [PubMed] [Google Scholar]
- [55].Erak M, Bellmann-Sickert K, Els-Heindl S, Beck-Sickinger AG, Peptide chemistry toolbox - Transforming natural peptides into peptide therapeutics, Bioorganic & medicinal chemistry, (2018). [DOI] [PubMed] [Google Scholar]
- [56].Frederiksen TM, Sonderby P, Ryberg LA, Harris P, Bukrinski JT, Scharff-Poulsen AM, Elf-Lind MN, Peters GH, Oligomerization of a Glucagon-like Peptide 1 Analog: Bridging Experiment and Simulations, Biophysical journal, 109 (2015) 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Steensgaard DB, Thomsen JK, Olsen HB, Knudsen LB, The molecular basis for the delayed absorption of the once-daily human GLP-1 analoge, liraglutide, Diabetes, 57 (2008) A164–A164. [Google Scholar]
- [58].Wang Y, Lomakin A, Kanai S, Alex R, Benedek GB, Transformation of oligomers of lipidated peptide induced by change in pH, Molecular pharmaceutics, 12 (2015) 411–419. [DOI] [PubMed] [Google Scholar]
- [59].Spector AA, Fatty acid binding to plasma albumin, Journal of lipid research, 16 (1975) 165–179. [PubMed] [Google Scholar]
- [60].Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Larsen UD, Ribel U, Markussen J, Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo, The Biochemical journal, 312 ( Pt 3) (1995) 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bertucci C, Domenici E, Reversible and covalent binding of drugs to human serum albumin: methodological approaches and physiological relevance, Curr Med Chem, 9 (2002) 1463–1481. [DOI] [PubMed] [Google Scholar]
- [62].Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thogersen H, Wilken M, Johansen NL, Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness, Journal of medicinal chemistry, 50 (2007) 6126–6132. [DOI] [PubMed] [Google Scholar]
- [63].Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thogersen H, Wilken M, Agerso H, Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration, Journal of medicinal chemistry, 43 (2000) 1664–1669. [DOI] [PubMed] [Google Scholar]
- [64].Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T, Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide, Journal of medicinal chemistry, 58 (2015) 7370–7380. [DOI] [PubMed] [Google Scholar]
- [65].Russell-Jones D, Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue, Molecular and cellular endocrinology, 297 (2009) 137–140. [DOI] [PubMed] [Google Scholar]
- [66].Watson E, Jonker DM, Jacobsen LV, Ingwersen SH, Population pharmacokinetics of liraglutide, a once-daily human glucagon-like peptide-1 analog, in healthy volunteers and subjects with type 2 diabetes, and comparison to twice-daily exenatide, Journal of clinical pharmacology, 50 (2010) 886–894. [DOI] [PubMed] [Google Scholar]
- [67].Wang Y, Lomakin A, Kanai S, Alex R, Belli S, Donzelli M, Benedek GB, The molecular basis for the prolonged blood circulation of lipidated incretin peptides: Peptide oligomerization or binding to serum albumin?, Journal of controlled release : official journal of the Controlled Release Society, 241 (2016) 25–33. [DOI] [PubMed] [Google Scholar]
- [68].Plum A, Jensen LB, Kristensen JB, In vitro protein binding of liraglutide in human plasma determined by reiterated stepwise equilibrium dialysis, Journal of pharmaceutical sciences, 102 (2013) 2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Malm-Erjefalt M, Bjornsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuis B, van Lier JJ, Zdravkovic M, Olsen AK, Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase, Drug metabolism and disposition: the biological fate of chemicals, 38 (2010) 1944–1953. [DOI] [PubMed] [Google Scholar]
- [70].FDA., NDA 206321 Chemistry reviews. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206321Orig1s000ChemR.pdf. Accessed June 2018.
- [71].Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbol JD, Hansen T, Bain SC, Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial, The lancet. Diabetes & endocrinology, 5 (2017) 251–260. [DOI] [PubMed] [Google Scholar]
- [72].Jiang J, Zhang J, Jacobsen LV, Hu P, The pharmacokinetics, pharmacodynamics, and tolerability of liraglutide, a once-daily human GLP-1 analogue, after multiple subcutaneous administration in healthy Chinese male subjects, Journal of clinical pharmacology, 51 (2011) 1620–1627. [DOI] [PubMed] [Google Scholar]
- [73].Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A, Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel, Journal of clinical pharmacology, 55 (2015) 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lyseng-Williamson KA, Coagulation Factor IX (Recombinant), Albumin Fusion Protein (Albutrepenonacog Alfa; Idelvion(R)): A Review of Its Use in Haemophilia B, Drugs, 77 (2017) 97–106. [DOI] [PubMed] [Google Scholar]
- [75].Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL, The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan, The Journal of experimental medicine, 197 (2003) 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, Anderson CL, Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces, Am J Physiol Gastrointest Liver Physiol, 290 (2006) G352–360. [DOI] [PubMed] [Google Scholar]
- [77].Sleep D, Cameron J, Evans LR, Albumin as a versatile platform for drug half-life extension, Biochim Biophys Acta, 1830 (2013) 5526–5534. [DOI] [PubMed] [Google Scholar]
- [78].Young MA, Wald JA, Matthews JE, Scott R, Hodge RJ, Zhi H, Reinhardt RR, Clinical pharmacology of albiglutide, a GLP-1 receptor agonist, Postgraduate medicine, 126 (2014) 84–97. [DOI] [PubMed] [Google Scholar]
- [79].Strohl WR, Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters, BioDrugs, 29 (2015) 215–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI, The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity, J Cell Biol, 185 (2009) 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T, Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR, Journal of immunology, 184 (2010) 1968–1976. [DOI] [PubMed] [Google Scholar]
- [82].Kim DM, Chu SH, Kim S, Park YW, Kim SS, Fc fusion to Glucagon-like peptide-1 inhibits degradation by human DPP-IV, increasing its half-life in serum and inducing a potent activity for human GLP-1 receptor activation, BMB reports, 42 (2009) 212–216. [DOI] [PubMed] [Google Scholar]
- [83].Rath T, Baker K, Dumont JA, Peters RT, Jiang H, Qiao SW, Lencer WI, Pierce GF, Blumberg RS, Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics, Crit Rev Biotechnol, 35 (2015) 235–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M, Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses, Blood, 113 (2009) 3716–3725. [DOI] [PubMed] [Google Scholar]
- [85].Czajkowsky DM, Hu J, Shao Z, Pleass RJ, Fc-fusion proteins: new developments and future perspectives, EMBO molecular medicine, 4 (2012) 1015–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, Etgen G, Bumol T, Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein, Diabetes-Metab Res, 26 (2010) 287–296. [DOI] [PubMed] [Google Scholar]
- [87].European Medicines Agency, Trulicity assessment report, 2014.
- [88].Oh KS, Kim JY, Yoon BD, Lee M, Kim H, Kim M, Seo JH, Yuk SH, Sol-gel transition of nanoparticles/polymer mixtures for sustained delivery of exenatide to treat type 2 diabetes mellitus, European Journal of Pharmaceutics and Biopharmaceutics, 88 (2014) 664–669. [DOI] [PubMed] [Google Scholar]
- [89].Perez-Ortiz M, Zapata-Urzua C, Acosta GA, Alvarez-Lueje A, Albericio F, Kogan MJ, Gold nanoparticles as an efficient drug delivery system for GLP-1 peptides, Colloids Surf B Biointerfaces, 158 (2017) 25–32. [DOI] [PubMed] [Google Scholar]
- [90].Huotari A, Xu W, Monkare J, Kovalainen M, Herzig KH, Lehto VP, Jarvinen K, Effect of surface chemistry of porous silicon microparticles on glucagon-like peptide-1 (GLP-1) loading, release and biological activity, International journal of pharmaceutics, 454 (2013) 67–73. [DOI] [PubMed] [Google Scholar]
- [91].Schwendeman SP, Shah RB, Bailey BA, Schwendeman AS, Injectable controlled release depots for large molecules, Journal of controlled release : official journal of the Controlled Release Society, 190 (2014) 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhong Y, Zhang L, Ding AG, Shenderova A, Zhu G, Pei P, Chen RR, Mallery SR, Mooney DJ, Schwendeman SP, Rescue of SCID murine ischemic hindlimbs with pH-modified rhbFGF/poly(DL-lactic-co-glycolic acid) implants, Journal of controlled release : official journal of the Controlled Release Society, 122 (2007) 331–337. [DOI] [PubMed] [Google Scholar]
- [93].Kovalchuk A, Fischer W, Epple M, Controlled release of goserelin from microporous polyglycolide and polylactide, Macromolecular bioscience, 5 (2005) 289–298. [DOI] [PubMed] [Google Scholar]
- [94].Okada H, Doken Y, Ogawa Y, Toguchi H, Preparation of three-month depot injectable microspheres of leuprorelin acetate using biodegradable polymers, Pharmaceutical research, 11 (1994) 1143–1147. [DOI] [PubMed] [Google Scholar]
- [95].LUPRON DEPOT® (leuprolide acetate for depot suspension) [prescribing information].
- [96].DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P, Encapsulation of Exenatide in Poly-(D,L-Lactide-Co-Glycolide) Microspheres Produced an Investigational Long-Acting Once-Weekly Formulation for Type 2 Diabetes, Diabetes technology & therapeutics, 13 (2011) 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fineman M, Flanagan S, Taylor K, Aisporna M, Shen LZ, Mace KF, Walsh B, Diamant M, Cirincione B, Kothare P, Li WI, MacConell L, Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing, Clinical pharmacokinetics, 50 (2011) 65–74. [DOI] [PubMed] [Google Scholar]
- [98].Henry RR, Rosenstock J, Logan D, Alessi T, Luskey K, Baron MA, Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin-treated subjects with type 2 diabetes, Journal of diabetes and its complications, 28 (2014) 393–398. [DOI] [PubMed] [Google Scholar]
- [99].Intarcia Therapeutics Inc, Intarcia Announces FDA Filing Acceptance of New Drug Application (NDA) for ITCA 650 for the Treatment of Type 2 Diabetes, 2017. [Google Scholar]
- [100].Henry RR, Logan D, Alessi T, Baron MA, A randomized, open-label, multicenter, 4-week study to evaluate the tolerability and pharmacokinetics of ITCA 650 in patients with type 2 diabetes, Clinical therapeutics, 35 (2013) 634–645 e631. [DOI] [PubMed] [Google Scholar]
- [101].Shu JY, Panganiban B, Xu T, Peptide-polymer conjugates: from fundamental science to application, Annu Rev Phys Chem, 64 (2013) 631–657. [DOI] [PubMed] [Google Scholar]
- [102].Lee SH, Lee S, Youn YS, Na DH, Chae SY, Byun Y, Lee KC, Synthesis, characterization, and pharmacokinetic studies of PEGylated glucagon-like peptide-1, Bioconjug Chem, 16 (2005) 377–382. [DOI] [PubMed] [Google Scholar]
- [103].A Study for Patients With Type 2 Diabetes Mellitus.
- [104].Schellenberger V, Wang CW, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WP, A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner, Nat Biotechnol, 27 (2009) 1186–1190. [DOI] [PubMed] [Google Scholar]
- [105].Podust VN, Sim BC, Kothari D, Henthorn L, Gu C, Wang CW, McLaughlin B, Schellenberger V, Extension of in vivo half-life of biologically active peptides via chemical conjugation to XTEN protein polymer, Protein Eng Des Sel, 26 (2013) 743–753. [DOI] [PubMed] [Google Scholar]
- [106].Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L, D.-S. Group, Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study, Lancet, 372 (2008) 1240–1250. [DOI] [PubMed] [Google Scholar]
- [107].Fineman MS, Cirincione BB, Maggs D, Diamant M, GLP-1 based therapies: differential effects on fasting and postprandial glucose, Diabetes, obesity & metabolism, 14 (2012) 675–688. [DOI] [PubMed] [Google Scholar]
- [108].Calara F, Taylor K, Han J, Zabala E, Carr EM, Wintle M, Fineman M, A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin-4), Clinical therapeutics, 27 (2005) 210–215. [DOI] [PubMed] [Google Scholar]
- [109].Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, L.-S. Group, Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6), Lancet, 374 (2009) 39–47. [DOI] [PubMed] [Google Scholar]
- [110].Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK, Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes, Current opinion in molecular therapeutics, 12 (2010) 790–797. [PubMed] [Google Scholar]
- [111].Hompesch M, Jones-Leone A, Carr MC, Matthews J, Zhi H, Young M, Morrow L, Reinhardt RR, Albiglutide does not impair the counter-regulatory hormone response to hypoglycaemia: a randomized, double-blind, placebo-controlled, stepped glucose clamp study in subjects with type 2 diabetes mellitus, Diabetes, obesity & metabolism, 17 (2015) 82–90. [DOI] [PubMed] [Google Scholar]
- [112].Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH, Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans, Am J Physiol, 273 (1997) E981–988. [DOI] [PubMed] [Google Scholar]
- [113].Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, Wilding I, Nauck M, Horowitz M, Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes, Regulatory peptides, 151 (2008) 123–129. [DOI] [PubMed] [Google Scholar]
- [114].Lorenz M, Pfeiffer C, Steinstrasser A, Becker RH, Rutten H, Ruus P, Horowitz M, Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia, Regulatory peptides, 185 (2013) 1–8. [DOI] [PubMed] [Google Scholar]
- [115].Nauck MA, Kemmeries G, Holst JJ, Meier JJ, Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans, Diabetes, 60 (2011) 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Umapathysivam MM, Lee MY, Jones KL, Annink CE, Cousins CE, Trahair LG, Rayner CK, Chapman MJ, Nauck MA, Horowitz M, Deane AM, Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia, Diabetes, 63 (2014) 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK, Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients, Biomark Insights, 11 (2016) 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Trujillo JM, Nuffer W, Ellis SL, GLP-1 receptor agonists: a review of head-to-head clinical studies, Ther Adv Endocrinol Metab, 6 (2015) 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, Trautmann M, Porter L, DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes, The Journal of clinical endocrinology and metabolism, 96 (2011) 1301–1310. [DOI] [PubMed] [Google Scholar]
- [120].Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, Wilhelm K, Trautmann M, Shen LZ, Porter LE, D.-S. Group, DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks, Diabetes care, 33 (2010) 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, Fahrbach JL, Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial, Lancet, 384 (2014) 1349–1357. [DOI] [PubMed] [Google Scholar]
- [122].Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart M, Rosenstock J, H.s. group, Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study, The lancet. Diabetes & endocrinology, 2 (2014) 289–297. [DOI] [PubMed] [Google Scholar]
- [123].Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G, Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study, Lancet, 381 (2013) 117–124. [DOI] [PubMed] [Google Scholar]
- [124].Scott DA, Boye KS, Timlin L, Clark JF, Best JH, A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo, Diabetes, obesity & metabolism, 15 (2013) 213–223. [DOI] [PubMed] [Google Scholar]
- [125].Kayaniyil S, Lozano-Ortega G, Bennett HA, Johnsson K, Shaunik A, Grandy S, Kartman B, A Network Meta-analysis Comparing Exenatide Once Weekly with Other GLP-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus, Diabetes therapy : research, treatment and education of diabetes and related disorders, 7 (2016) 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Saunders WB, Nguyen H, Kalsekar I, Real-world glycemic outcomes in patients with type 2 diabetes initiating exenatide once weekly and liraglutide once daily: a retrospective cohort study, Diabetes, metabolic syndrome and obesity : targets and therapy, 9 (2016) 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ, GLP-1 and energy balance: an integrated model of short-term and long-term control, Nature reviews. Endocrinology, 7 (2011) 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH, Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults, Int J Obes (Lond), 38 (2014) 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, G. Exenatide-113 Clinical Study, Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes, Diabetes care, 27 (2004) 2628–2635. [DOI] [PubMed] [Google Scholar]
- [130].Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR, Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4, Endocrinology, 152 (2011) 3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L, The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss, The Journal of clinical investigation, 124 (2014) 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Baggio LL, Huang Q, Brown TJ, Drucker DJ, A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis, Diabetes, 53 (2004) 2492–2500. [DOI] [PubMed] [Google Scholar]
- [133].Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG, Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study, Clinical therapeutics, 30 (2008) 1448–1460. [DOI] [PubMed] [Google Scholar]
- [134].Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE, E.F.C.G.-M.S. Investigators, Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono), Diabetes care, 35 (2012) 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, L.-S. Group, Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial, Lancet, 373 (2009) 473–481. [DOI] [PubMed] [Google Scholar]
- [136].Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V, Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3), Diabetes care, 37 (2014) 2168–2176. [DOI] [PubMed] [Google Scholar]
- [137].Nauck MA, Stewart MW, Perkins C, Jones-Leone A, Yang F, Perry C, Reinhardt RR, Rendell M, Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise, Diabetologia, 59 (2016) 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, Wolka AM, Boardman MK, D.-S. Group, Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study, Diabetes care, 35 (2012) 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S, L.-S.s. group, Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU), Diabetic medicine : a journal of the British Diabetic Association, 26 (2009) 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Trujillo JM, Nuffer W, GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents, Pharmacotherapy, 34 (2014) 1174–1186. [DOI] [PubMed] [Google Scholar]
- [141].Gentilella R, Bianchi C, Rossi A, Rotella CM, Exenatide: a review from pharmacology to clinical practice, Diabetes, obesity & metabolism, 11 (2009) 544–556. [DOI] [PubMed] [Google Scholar]
- [142].Ahren B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R, Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M), Diabetes care, 36 (2013) 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, Hanefeld M, Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1), Diabetic medicine : a journal of the British Diabetic Association, 31 (2014) 176–184. [DOI] [PubMed] [Google Scholar]
- [144].Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R, Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P), Diabetes, obesity & metabolism, 15 (2013) 1000–1007. [DOI] [PubMed] [Google Scholar]
- [145].Rosenstock J, Guerci B, Hanefeld M, Gentile S, Aronson R, Tinahones FJ, Roy-Duval C, Souhami E, Wardecki M, Ye J, Perfetti R, Heller S, I. GetGoal Duo-2 Trial, Prandial Options to Advance Basal Insulin Glargine Therapy: Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either as Basal-Plus or Basal-Bolus in Type 2 Diabetes: The GetGoal Duo-2 Trial, Diabetes care, 39 (2016) 1318–1328. [DOI] [PubMed] [Google Scholar]
- [146].Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, Zhou T, Muehlen-Bartmer I, Ratner RE, Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S), Journal of diabetes and its complications, 28 (2014) 386–392. [DOI] [PubMed] [Google Scholar]
- [147].Seino Y, Min KW, Niemoeller E, Takami A, E.G.-L.A.S. Investigators, Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia), Diabetes, obesity & metabolism, 14 (2012) 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yu Pan C, Han P, Liu X, Yan S, Feng P, Zhou Z, Lv X, Tian H, Jin Kui Y, Su B, Shang S, Niemoeller E, Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia), Diabetes/metabolism research and reviews, 30 (2014) 726–735. [DOI] [PubMed] [Google Scholar]
- [149].Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN, H.S. Group, HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin, Diabetes care, 37 (2014) 2141–2148. [DOI] [PubMed] [Google Scholar]
- [150].Home PD, Shamanna P, Stewart M, Yang F, Miller M, Perry C, Carr MC, Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5, Diabetes, obesity & metabolism, 17 (2015) 179–187. [DOI] [PubMed] [Google Scholar]
- [151].Reusch J, Stewart MW, Perkins CM, Cirkel DT, Ye J, Perry CR, Reinhardt RR, Bode BW, Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin, Diabetes, obesity & metabolism, 16 (2014) 1257–1264. [DOI] [PubMed] [Google Scholar]
- [152].Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, Pratley R, HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea, Diabetologia, 57 (2014) 2475–2484. [DOI] [PubMed] [Google Scholar]
- [153].Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, Milicevic Z, Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study, Lancet, 385 (2015) 2057–2066. [DOI] [PubMed] [Google Scholar]
- [154].Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V, Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2), Diabetes care, 38 (2015) 2241–2249. [DOI] [PubMed] [Google Scholar]
- [155].Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z, Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5), Diabetes care, 37 (2014) 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, Kuhstoss D, Lakshmanan M, Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1), Diabetes care, 37 (2014) 2159–2167. [DOI] [PubMed] [Google Scholar]
- [157].Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M, A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study, Diabetologia, 50 (2007) 259–267. [DOI] [PubMed] [Google Scholar]
- [158].Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, Kinninger LA, Trautmann ME, Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment, Diabetes, obesity & metabolism, 14 (2012) 546–554. [DOI] [PubMed] [Google Scholar]
- [159].Jones SC, Ryan DL, Pratt VS, Niak A, Brinker AD, Injection-Site Nodules Associated With the Use of Exenatide Extended-Release Reported to the U.S. Food and Drug Administration Adverse Event Reporting System, Diabetes Spectr, 28 (2015) 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Blonde L, Russell-Jones D, The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies, Diabetes, obesity & metabolism, 11 Suppl 3 (2009) 26–34. [DOI] [PubMed] [Google Scholar]
- [161].Leiter LA, Carr MC, Stewart M, Jones-Leone A, Scott R, Yang F, Handelsman Y, Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study, Diabetes care, 37 (2014) 2723–2730. [DOI] [PubMed] [Google Scholar]
- [162].Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahren B, Chow FC, Yang F, Miller D, Johnson SL, Stewart MW, Leiter LA, G. Harmony 6 Study, Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro, Diabetes care, 37 (2014) 2317–2325. [DOI] [PubMed] [Google Scholar]
- [163].Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC, Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model, Diabetes, 61 (2012) 1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC, Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies, Gastroenterology, 141 (2011) 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C, Pancreatic safety of incretin-based drugs--FDA and EMA assessment, The New England journal of medicine, 370 (2014) 794–797. [DOI] [PubMed] [Google Scholar]
- [166].Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U, Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation, Endocrinology, 151 (2010) 1473–1486. [DOI] [PubMed] [Google Scholar]
- [167].Hegedus L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH, GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide, The Journal of clinical endocrinology and metabolism, 96 (2011) 853–860. [DOI] [PubMed] [Google Scholar]
- [168].Alves C, Batel-Marques F, Macedo AF, A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer, Diabetes research and clinical practice, 98 (2012) 271–284. [DOI] [PubMed] [Google Scholar]
- [169].Balena R, Hensley IE, Miller S, Barnett AH, Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature, Diabetes, obesity & metabolism, 15 (2013) 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Buse JB, Vilsboll T, Thurman J, Blevins TC, Langbakke IH, Bottcher SG, Rodbard HW, N.N.T. Investigators, Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira), Diabetes care, 37 (2014) 2926–2933. [DOI] [PubMed] [Google Scholar]
- [171].Aroda VR, Rosenstock J, Wysham C, Unger J, Bellido D, Gonzalez-Galvez G, Takami A, Guo H, Niemoeller E, Souhami E, Bergenstal RM, LixiLan LTI, Efficacy and Safety of LixiLan, a Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide in Type 2 Diabetes Inadequately Controlled on Basal Insulin and Metformin: The LixiLan-L Randomized Trial, Diabetes care, 39 (2016) 1972–1980. [DOI] [PubMed] [Google Scholar]
- [172].Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE, Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis, Diabetes, 66 (2017) 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Standards of Medical Care in Diabetes-2017: Summary of Revisions, Diabetes care, 40 (2017) S4–S5. [DOI] [PubMed] [Google Scholar]
- [174].Teva Pharmaceutical Industries Ltd, TEVA SETTLES PATENT LITIGATION WITH ASTRAZENECA ALLOWING TEVA TO COMMERCIALIZE ITS GENERIC VERSION OF BYETTA® (EXENATIDE INJECTION) IN THE UNITED STATES, 2016.
- [175].Antares Pharma, ANTARES PHARMA ANNOUNCES SETTLEMENT OF PATENT LITIGATION FOR KEY ALLIANCE BUSINESS PRODUCT, 2016.
- [176].Novo Nordisk A/S, Novo Nordisk Annual Report 2011, 2012.
- [177].Amylin Pharmaceuticals INC, Notice of Annual Stockholders’ Meeting, Proxy Statement and 2011 Annual Report, 2012.
- [178].Novo Nordisk A/S, Victoza® (liraglutide) is approved in the US as the only type 2 diabetes treatment indicated to reduce the risk of three major adverse cardiovascular events, 2017.
- [179].Saxenda® (liraglutide) injection [prescribing information]. Bagsvaerd, Denmark: Novo Nordisk; ; 2017 [Google Scholar]
- [180].Novo Nordisk A/S, Novo Nordisk Annual Report 2017, 2018.
- [181].AstraZeneca PLC, AstraZeneca Annual Report and Form 20-F Information 2013, 2014.
- [182].Novo Nordisk A/S, Novo Nordisk Annual Report 2013, 2014.
- [183].GlaxoSmithKline plc, Annual report 2015, 2016.
- [184].Novo Nordisk A/S, Novo Nordisk Annual Report 2015, 2016.
- [185].Express Scripts Holding Company, 2017 Express Scripts National Preferred Formulary, 2017.
- [186].GSK., Discontinuation of TANZEUM® (albiglutide), 2017.
- [187].Eli Lilly and Company, 2016 Financial Report, 2017.
- [188].Eli Lilly and Company, ELI LILLY AND COMPANY 2015 ANNUAL REPORT AND PROXY STATEMENT, 2016.
- [189].Eli Lilly and Company, Lilly Reports Strong Fourth-Quarter and Full-Year 2017 Revenue Growth, Increases 2018 EPS Guidance, 2018.
- [190].Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T, S.-. Investigators, Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes, The New England journal of medicine, 375 (2016) 1834–1844. [DOI] [PubMed] [Google Scholar]
- [191].AstraZeneca PLC, AstraZeneca Annual Report and Form 20-F Information 2016, 2017.
- [192].AstraZeneca PLC, Full-Year 2017 Results, 2018.
- [193].Novo Nordisk A/S, Financial report for the period 1 January 2017 to 30 June 2017, 2017.
- [194].GlaxoSmithKline plc, Annual report 2016, 2017.
- [195].GlaxoSmithKline plc, Full year and fourth quarter 2017, 2018.
- [196].Sanofi, Annual Report on Form 20-F 2016, 2017.
- [197].Sanofi, Sanofi Delivers 2017 Business EPS in line with Guidance, 2018.
- [198].Ross SA, Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes, The American journal of medicine, 126 (2013) S38–48. [DOI] [PubMed] [Google Scholar]
- [199].Fu AZ, Qiu Y, Radican L, Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes, Current medical research and opinion, 25 (2009) 1413–1420. [DOI] [PubMed] [Google Scholar]
- [200].Pfutzner J, Hellhammer J, Musholt P, Pfutzner AH, Bohnke J, Torsten H, Amann-Zalan I, Ganz M, Forst T, Pfutzner A, Evaluation of dexterity in insulin-treated patients with type 1 and type 2 diabetes mellitus, J Diabetes Sci Technol, 5 (2011) 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Baekdal TA, Thomsen M, Kupcova V, Hansen CW, Anderson TW, Pharmacokinetics, Safety, and Tolerability of Oral Semaglutide in Subjects With Hepatic Impairment, Journal of clinical pharmacology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Malkov D, Angelo R, Wang HZ, Flanders E, Tang H, Gomez-Orellana I, Oral delivery of insulin with the eligen technology: mechanistic studies, Curr Drug Deliv, 2 (2005) 191–197. [DOI] [PubMed] [Google Scholar]
- [203].Holst JJ, Madsbad S, Semaglutide seems to be more effective the other GLP-1Ras, Ann Transl Med, 5 (2017) 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J, Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial, JAMA, 318 (2017) 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A, Semaglutide, a Once-Weekly Human GLP-1 Analog, Does Not Reduce the Bioavailability of the Combined Oral Contraceptive, Ethinylestradiol/Levonorgestrel, Journal of clinical pharmacology, 55 (2015) 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Nordisk N., Novo Nordisk successfully completes the first phase 3a trial, PIONEER 1, with oral semaglutide, 2018.
- [207].Oramed Pharmaceuticals, Phase II Safety and Efficacy Study of Oral ORMD-0801 in Patients With Type 2 Diabetes Mellitus. https://clinicaltrials.gov/ct2/show/NCT02496000. Accessed April 2018.
- [208].Oramed Pharmaceuticals, Oramed technology. http://www.oramed.com/technology/. Accessed May 2018.
- [209].Leone-Bay A, Grant M, Greene S, Stowell G, Daniels S, Smithson A, Villanueva S, Cope S, Carrera K, Reyes S, Richardson P, Evaluation of novel particles as an inhalation system for GLP-1, Diabetes, obesity & metabolism, 11 (2009) 1050–1059. [DOI] [PubMed] [Google Scholar]
- [210].Marino MT, Costello D, Baughman R, Boss A, Cassidy J, Damico C, van Marle S, van Vliet A, Richardson PC, Pharmacokinetics and pharmacodynamics of inhaled GLP-1 (MKC253): proof-of-concept studies in healthy normal volunteers and in patients with type 2 diabetes, Clin Pharmacol Ther, 88 (2010) 243–250. [DOI] [PubMed] [Google Scholar]
- [211].Shahzad Y, Louw R, Gerber M, du Plessis J, Breaching the skin barrier through temperature modulations, Journal of controlled release : official journal of the Controlled Release Society, 202 (2015) 1–13. [DOI] [PubMed] [Google Scholar]
- [212].Cefalu WT, Skyler JS, Kourides IA, Landschulz WH, Balagtas CC, Cheng S, Gelfand RA, G. Inhaled Insulin Study, Inhaled human insulin treatment in patients with type 2 diabetes mellitus, Annals of internal medicine, 134 (2001) 203–207. [DOI] [PubMed] [Google Scholar]
- [213].Black C, Cummins E, Royle P, Philip S, Waugh N, The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation, Health Technol Assess, 11 (2007) 1–126. [DOI] [PubMed] [Google Scholar]
- [214].Smart AL, Gaisford S, Basit AW, Oral peptide and protein delivery: intestinal obstacles and commercial prospects, Expert Opin Drug Deliv, 11 (2014) 1323–1335. [DOI] [PubMed] [Google Scholar]
- [215].Heinemann L, Jacques Y, Oral insulin and buccal insulin: a critical reappraisal, J Diabetes Sci Technol, 3 (2009) 568–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Fonte P, Araujo F, Reis S, Sarmento B, Oral insulin delivery: how far are we?, J Diabetes Sci Technol, 7 (2013) 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zarogoulidis P, Papanas N, Kouliatsis G, Spyratos D, Zarogoulidis K, Maltezos E, Inhaled insulin: too soon to be forgotten?, Journal of aerosol medicine and pulmonary drug delivery, 24 (2011) 213–223. [DOI] [PubMed] [Google Scholar]
- [218].Mitri J, Pittas AG, Inhaled insulin--what went wrong, Nat Clin Pract Endocrinol Metab, 5 (2009) 24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Patel V, Joharapurkar A, Dhanesha N, Kshirsagar S, Patel K, Bahekar R, Shah G, Jain M, Co-agonist of glucagon and GLP-1 reduces cholesterol and improves insulin sensitivity independent of its effect on appetite and body weight in diet-induced obese C57 mice, Can J Physiol Pharmacol, 91 (2013) 1009–1015. [DOI] [PubMed] [Google Scholar]
- [220].Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R, Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice, Diabetes, 58 (2009) 2258–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Deryabina MA, Daugaard JR, Knudsen CB, Shelton PT, Fog JU, Jessen L, Noerregaard P, Pharmacokinetics and Pharmacodynamics of GLP-1-GIP Receptor Dual Agonist Peptides: From Once-Daily to Once-Weekly, Diabetes, 64 (2015) A534–A534. [Google Scholar]
- [222].Fosgerau K, Jessen L, Lind Tolborg J, Osterlund T, Schaeffer Larsen K, Rolsted K, Brorson M, Jelsing J, Skovlund Ryge Neerup T, The novel GLP-1-gastrin dual agonist, ZP3022, increases beta-cell mass and prevents diabetes in db/db mice, Diabetes, obesity & metabolism, 15 (2013) 62–71. [DOI] [PubMed] [Google Scholar]
- [223].Soni H, Peptide-based GLP-1/glucagon co-agonists: A double-edged sword to combat diabesity, Med Hypotheses, 95 (2016) 5–9. [DOI] [PubMed] [Google Scholar]
- [224].Henderson SJ, Konkar A, Hornigold DC, Trevaskis JL, Jackson R, Fritsch Fredin M, Jansson-Lofmark R, Naylor J, Rossi A, Bednarek MA, Bhagroo N, Salari H, Will S, Oldham S, Hansen G, Feigh M, Klein T, Grimsby J, Maguire S, Jermutus L, Rondinone CM, Coghlan MP, Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates, Diabetes Obesity & Metabolism, 18 (2016) 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Chakradhar S, All in one: Researchers create combination drugs for diabetes and obesity, Nat Med, 22 (2016) 694–696. [DOI] [PubMed] [Google Scholar]
- [226].Fosgerau K, Jessen L, Tolborg JL, Osterlund T, Larsen KS, Rolsted K, Brorson M, Jelsing J, Neerup TSR, The novel GLP-1-gastrin dual agonist, ZP3022, increases ss-cell mass and prevents diabetes in db/db mice, Diabetes Obesity & Metabolism, 15 (2013) 62–71. [DOI] [PubMed] [Google Scholar]