Abstract
This study aimed to prepare Annona squamosa leaf extract-loaded chitosan nanoparticles (nano-ASLE) against human colon cancer (WiDr) cells. Nano-ASLE was made with ionic gelation method. Four concentrations of the nano-ASLE (50, 100, 200, and 400 μg/mL) in dimethyl sulfoxide were prepared on WiDr cells to determine the IC50 value using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Then, it was divided into three groups of concentration of IC50, 2IC50, and 4IC50 and continued with analysis of caspase-3 expression and cell cycle arrest. The results of particles size were obtained 535.1 nm and showed potent cytotoxicity with IC50 292.39 μg/mL. The expression of caspase-3 increased significantly and caused cell cycle arrest at the G2/M phase and induced apoptosis on WiDr cells. Further studies are needed to obtain the loading efficiency, release of drug concentration, and in vivo study of nano-ASLE to suppress WiDr cells.
Key words: Annona squamosa, apoptosis, cell cycle, cytotoxicity
INTRODUCTION
Cancer has emerged as one of the strongest diseases of deaths since many years ago. It is caused by many factorials by uncontrolled division cellular and metastasis.[1] Approximately 7.6 million people die from cancer annually and expected to increase around 13.1 million in 2030.[2] Colorectal cancer is one of the cancers which have high mortality in both males and females.[3]
Chemopreventive is a strategy to eliminate cancer selectively to block carcinogenesis process in stages of promotion and progression.[4] Many studies assessed chemopreventive agent use botanical products because of the capacity as a bioactive compound.[5] Annona squamosa is considered drug plant for cardiac disease, diabetes, and cancer. A. squamosa is called as custard apple in English and belonging to Annonaceae family. Phytochemical screening of leaf extract showed the presence of flavonoids, alkaloids, saponins, tannins, glycosides, phenolic, terpenoids, and steroids.[6] A. squamosa has been reported in the discovery of new Annonaceous acetogenins, called as (2,4-cis and trans)-squamolinone, (2,4-cis and trans)-9 oxoasimicinone, and bullacin B. Acetogenins have been isolated from the fruit pulp, seeds, twigs, roots, stems, leaves, and bark of many plants in the Annonaceae family. Acetogenins isolated from A. squamosa seeds reported cytotoxic activity through inhibited proliferation on HL-60 cells and induced apoptosis by the activation of caspase-3 and also against 9KB nasopharynx cells, A549 lung cells, and HT-29 colon cells.[7] In the past, studies showed that green biosynthesis of silver nanoparticles of A. squamosa leaf extract was reported to have cytotoxic effect and induction of apoptosis on MCF-7 cells.[8]
Nanotechnology is a promising approach to enhance the bioavailability of herbal medicine. Nontoxic biopolymer material is one of the nanoparticles which are expected to protect bioactive compound. A carrier material like the combination of chitosan and sodium tripolyphosphate (Na-TPP) is used to protect, stabilize, and control the release of the core. Chitosan can easily cross-link with Na-TPP as polyanions to control the release of the drug.[9] Therefore, this study designed to prepare nano-A. Squamosa leaf extract (ASLE) to enhance their bioactivities as an anticancer agent on WiDr cells due to induced apoptosis and cell cycle arrest.
MATERIALS AND METHODS
Ethical clearance
All treatment procedures have been tested through The Medical and Health Research Ethics Committee, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia (Approval Reference Number: KE/FK/0106/EC/2018).
Preparation of Annona squamosa leaf extract
A. squamosa leaves were collected from Lumajang Regency, East Java, Indonesia. The plant material was identified at Purwodadi Botanical Garden, Indonesian Institute of Sciences, Pasuruan, Indonesia, and approved with the reference number 003/IPH.06.HM/VII/2017. The leaves were cleaned and chopped into small pieces and shade dried. They were powdered using mechanical blender and passed through the coarse sieve (0.2 mm). The powder was macerated with ethanol 96% for 72 h at 37°C. The extract was evaporated in the water bath at the temperature of 60°C. The residue was then stored in a refrigerator at the temperature of 0–4°C.
Preparation of Annona squamosa leaf extract-loaded chitosan nanoparticles
A. squamosa leaf extract-loaded chitosan nanoparticles (nano-ASLE) were prepared by ionic gelation of chitosan (0.1% w/v) and sodium tripolyphosphate (0.84% w/v) anions. One gram of A. squamosa was dissolved in 50 mL distilled water and added 100 mL chitosan in glacial acetic (0.25% v/v) and added 350 mL sodium tripolyphosphate in dropwise into the solution stirring condition at room temperature. The pH solution was adjusted by adding 0.1 M NaOH solution to the chitosan-A. squamosa complex and stirred for 2 h on magnetic stirrer. Chitosan-A. squamosa was centrifuged at 6000 rpm for 30 min and decanted. The supernatant was collected and dried for onward usage as nano-ASLE.[10]
Particle size analysis
Dynamic light-scattering method was performed to measure the particle size distribution of nano-ASLE using Zetasizer Nano ZS (Malvern Instruments Ltd., UK).
Cell culture of WiDr cells
WiDr cells were collected from the Parasitology Laboratory, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia. Cells were cultured in DMEM and supplemented with 10% (v/v) fetal bovine serum, 3% streptomycin-penicillin, and 1% fungizone in 5% CO2 incubator at 37°C.
Determination of IC50value
The IC50 value of the nano-ASLE was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. WiDr cells were seeded in 96-well plates at a density of 1 × 104 cells/well with 100 μL of volume and incubated at 37°C and 5% CO2 for overnight. WiDr cells were added with various concentration of the nano-ASLE for 24 h. Then, the media were removed. Next, it was added with 100 μL of DMEM and 10 μL MTT (5 mg MTT/mL solution) to every single well. The plates were incubated for 4 h. Control cells just received only the media without the nano-ASLE samples. The crystal of formazan which formed in viable cells was solubilized with 100 μL of SDS-stopper HCl 0.1 N. The absorbance 595 nm was measured by ELISA reader by Benchmark Microplate Reader (Bio-Rad, USA) and calculated to determine the IC50 value with linear regression analysis using Microsoft Excel 2016.
Immunocytochemistry staining
WiDr cells were seeded in 24-well plates at a density of 2 × 105 cells/well and incubated for 24 h. WiDr cells were treated with IC50, 2IC50, and 4IC50 (292.39, 584.78, and 1169.56 μg/mL, respectively) of the nano-ASLE for 24 h. WiDr cells were plated in poly-L-lysine slides and fixed with methanol for 3 min, permeabilized for 3 min in PBS, blocked in 2% BSA for an hour and stained with monoclonal antibody caspase-3 for an hour, and then washed with PBS for 3 × 3 min and stained with biotinylated universal secondary antibody for an hour. WiDr cells were incubated with HRP-streptavidin for 10 min, added with DAB for 5 min, washed again with aquadest, then counterstained with hematoxylin for 20 s, and at last, mounted on glass slides. The expression of caspase-3 was evaluated semi-quantitatively based on H-score method.[11]
Apoptosis observation on WiDr cells
WiDr cells were cultured in 24-well plates at a density of 2 × 105 cells/well and incubated for 24 h. The cells were treated with IC50, 2IC50, and 4IC50 (292.39, 584.78, and 1169.56 μg/mL, respectively) of nano-ASLE for 24 h. The medium was removed and washed using FBS. Coverslips were taken and put on the glass slide before added with 10 μL acridine orange-ethidium bromide and left for 15 min. It was then observed under the fluorescence microscope.
Cell cycle arrest analysis
WiDr cells were cultured in 6-well plates at a density of 1 × 106 cells/well and added with IC50, 2IC50, and 4IC50 (292.39, 584.78, and 1169.56 μg/mL, respectively) of nano-ASLE for 24 h. The distribution of cell cycle phase was determined of treated and untreated cells. They were collected and washed twice with PBS (pH 7.4) then centrifuged at 2500 rpm for 10 min. Cells pellet was washed twice with PBS and resuspended with 400 μL of PI/RNAse detection kit then incubated at 37°C for 30 min. The distribution of cell cycle phases was determined using BD FACSCalibur Flow Cytometer (BD Biosciences, USA).
Statistical analysis
This project was analyzed using SPSS 21 (SPSS Inc., Chicago, IL, USA) employing one-way analysis of variant (P < 0.05), followed by Tukey's honestly significant difference (P < 0.05). Tabulated data were presented as the mean ± standard deviation values.
RESULTS
Particle size analysis of Nano-Annona squamosa leaf extract
The result of particle size analyzer shows that the average of nano-ASLE was obtained at 535.1 nm with a good polydispersity index, 0.365, as shown in Figure 1.
Figure 1.
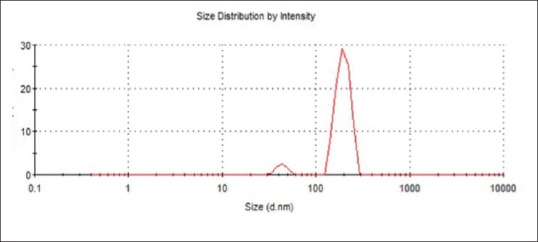
Particle size distribution of nano-Annona squamosa leaf extract
IC50 value of Nano-Annona squamosa leaf extract on WiDr cells
The highest reduction of WiDr cells found at the concentration of 400 μg/mL is 58.07 μg/mL. Meanwhile, the lowest reduction obtained at the concentration of 50 μg/mL is 29 μg/mL. The reductions of cell growth from the two other concentrations, which are 100 μg/mL and 200 μg/mL, are 32.18 μg/mL and 45 μg/mL, respectively. This study shows that the IC50 value of the nano-ASLE on WiDr cells is 292.39 μg/mL. The result of cytotoxic responses of the nano-ASLE on WiDr cells is presented in Figure 2.
Figure 2.
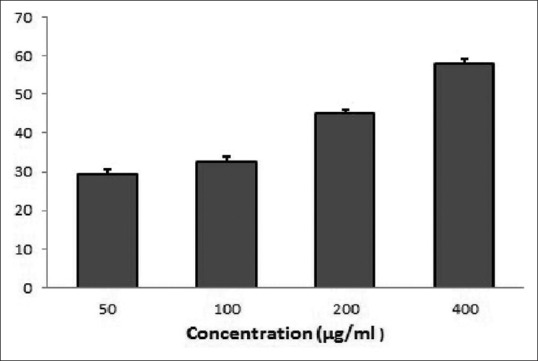
Cytotoxic responses of nano-Annona squamosa leaf extract on WiDr cells
Nano-Annona squamosa leaf extract-induced caspase-3expression on WiDr cells
The highest expression of caspase-3 expression is found at the concentration of 1169.56 μg/mL and has the mean score of 59.57 cell expression, and the lowest score shows at the concentration of 292.39 μg/mL and has the mean score of 43.15 cell expression. While the concentration of 584.78 μg/mL shows the mean of 54.23 cell expression. Furthermore, in control media, there is some little bit expression mean score of 1.2 cell expression. The result of cytotoxic responses of the nano-ASLE on WiDr cells is presented in Figure 3.
Figure 3.
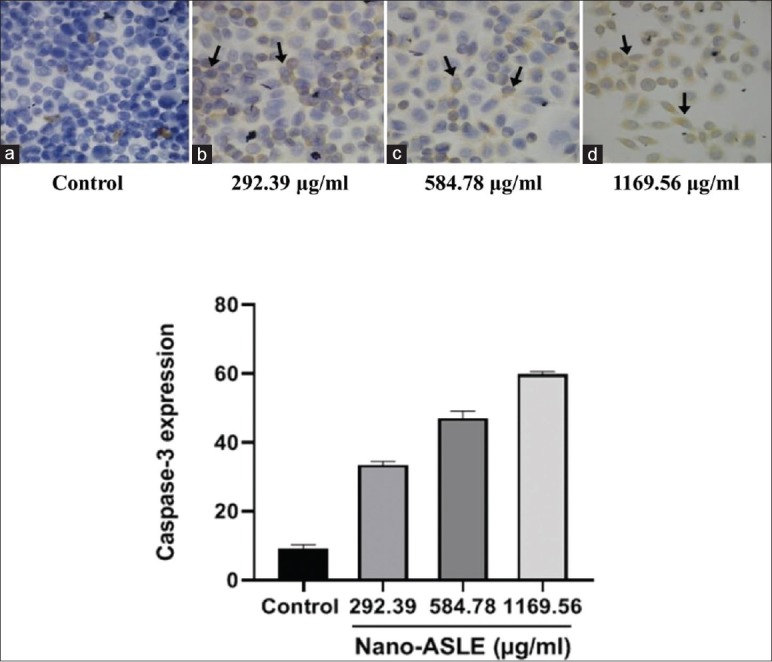
Effect the nano-Annona squamosa leaf extract on caspase-3 on WiDr cells (×400), untreated group (a), caspase-3 expression (black arrow) shown in brown color in cytoplasm of nuclei of the group treated 292.39 μg/ml (b), 584.78 μg/ml (c), 1169.56 μg/ml (d) bar represents mean ± standard deviation of caspase-3 expression score treated Nano-Annona squamosa leaf extract on WiDr cells
Nano-Annona squamosa leaf extract-induced apoptosis on WiDr cells
The result due to IC50 value and caspase-3 expression was related to this method. All cells in the untreated group showed green fluorescence meant as viable cells. Orange fluorescence cell meant as apoptotic cells that loss cell membrane permeability and then turn on apoptotic bodies, and several nuclear cells were fragmented and are shown in Figure 4.
Figure 4.
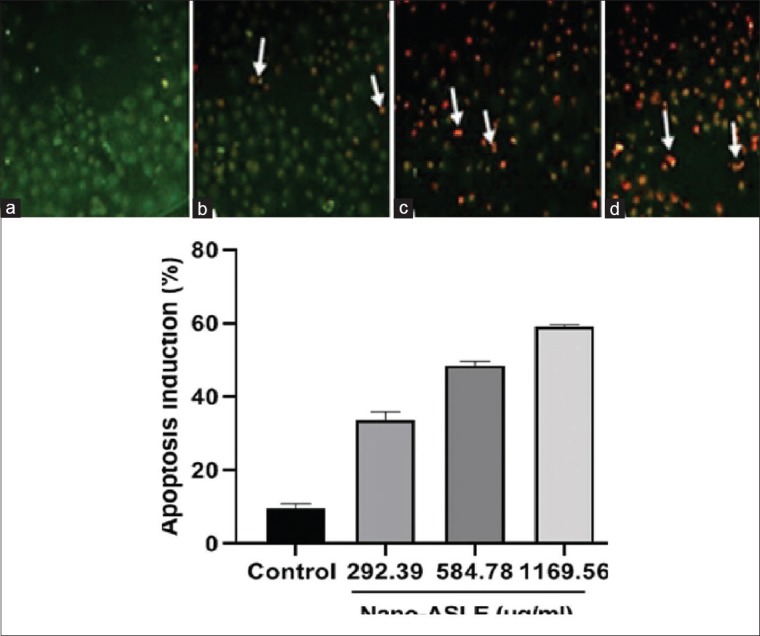
Effect the nano-Annona squamosa leaf extract on apoptosis induction on WiDr cells (×400), untreated group showed green color (a), apoptotic cells showed orange-red color (white arrow) of the group treated 292.39 μg/ml (b), 584.78 μg/ml (c), 1169.56 μg/ml (d), bar represents mean ± standard deviation of apoptosis induction score treated Nano-Annona squamosa leaf extracts on WiDr cells
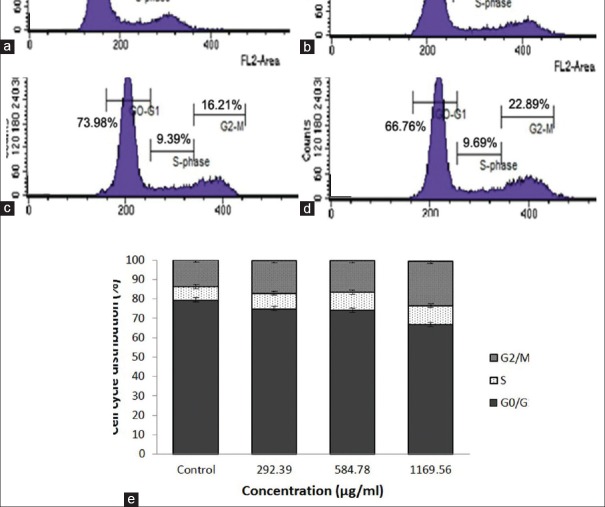
Nano-Annona squamosa leaf extract-induced G2/M phase on WiDr cells
Cell cycle arrest may mediate induction of apoptosis and shows the inhibition of cell proliferation. As shown in Figure 5 showed that nano-ASLE arrested G2/M phases and increased from 13.72% (control) to 22.89% (1169.56 μg/mL). The growth inhibition was associated with a significant cell cycle arrest in G2/M phase. Arrest in G2/M was associated with a concomitant decrease in the cell percentage in G0/G1 phase while in the S phase cells remained at almost at the same levels. When exposed to 584.78 μg/mL, the cell population of WiDr cells both in the S and G2/M phases was 9.69% and 22.89%, respectively; they increased compared with the control group.
Figure 5.
Effect the nano-Annona squamosa leaf extract on cell cycle distribution on WiDr cells. Flow cytometry histogram of cell cycle untreated group (a), group treated 292.39 μg/ml (b), 584.78 μg/ml (c), 1169.56 μg/ml (d), graph of analysis of cells number (%) each cell cycle phase relative to total phase (e), for examples, G0/G1 is calculated as percentage of the number cells in the G0/G1 population relative to the number of total cells
DISCUSSION
In this study, the cytotoxic effect of the nano-ASLE against WiDr cells is investigated. It reveals that the nano-ASLE has an IC50 value of 292.39 μg/mL. It might be caused by the particle size in nanoparticle form for optimizing activity of in vitro and increase the bioavailability of uncontrollable molecules. Releasing the bioactive compound with nanoparticles actually gives alternative therapeutic ways dropping the drug to specific target and decreases the toxicity effects.[12]
Acetogenin effect of A. squamosa also can inhibit the proliferation of cancer cells. Acetogenin selectivity for tumor cells can be explained both by the higher activities of NADH oxidase and the ATP demand that are peculiar to tumor cells and inherent due to their uncontrolled growth.[13] The previous studies reported that there are two main compounds of annonaceous acetogenins using HPLC analysis; they are 12, 15-cis-squamostatin-A and bullatacin.[14] They also showed anticancer properties against various tumor cell lines in evaluation in vitro.[15] Another acetogenin is annonacin. It is a mono-tetrahydrofuran acetogenin isolated from Annonaceae family. The past studies showed that annonacin has a cytotoxic effect on T24 cancer cells.[16]
The result of caspase-3 expression were increased in dose-dependent manner. It is caused by damage of mitochondrial membrane potential after the treatment. The pathway is activated by a variety of extra- and intracellular stress, including oxidative stress and cytotoxic drugs. The pathway is dominated by a bcl-2 family members that regulate the release of cytochrome-c from the mitochondria then activate caspase protease that dismantle cells to make apoptosis formation and signal efficient phagocytosis of cell corspses.[17,18] It is consistent with the previous study which showed that annonacin from Annonaceae family induces the apoptotic cell death in a caspase-3 related to the pathway on endometrial cancer cells.[19]
The checkpoint of cell cycle is an important control mechanism to execute of cell cycle activity. Several chemopreventive phytochemicals could restore and alter regulatory checkpoints through inducing cell cycle arrest. Recent investigations suggest that anticancer agents arrest the cell cycle at the G0/G1, S, or G2/M phase.[20] In this study showed that nano-ASLE arrest G2/M phase on WiDr cells. In the past, studies showed that annonacin isolated from the seed of Annona reticulata has a cytotoxic effect to T24 bladder cancer cells by arresting in G0/G1 phase. It induced apoptotic cell death by upregulating bax and bad expression and increasing caspase-3 activities and procaspase-3 cleavage, but annonacin of Annona muricata also induced apoptotic cell death in a caspase-3-related pathway and arrest cell cycle in G2/M phase on endometrial cancer cells.[16,19] Interestingly, it related to this study that nano-ASLE have a cytotoxic effect, induced apoptosis in caspase-3, and arrest cell cycle in G2/M phase. It could be attention that a key regulator of the G2/M transition of the cell cycle is a complex of CDC1/CDK2 and the cyclin B. If CDC1/CDK-2 was inhibited, it would be expected as an arrest at the G2/M transition.[21] However, apoptosis induction on WiDr cells may occur via upregulation of pro-apoptotic proteins and a decrease in membrane potential caused by releasing cytochrome-c of mitochondrial and activation of caspase-3.[22]
CONCLUSION
The research demonstrated that nano-ASLE can inhibit the proliferation of WiDr cells with the IC50 value of 292.39 μg/mL via a mechanism involving the induction of apoptosis and intracellular mitochondrial pathway on caspase-3 expression. It also related to cell cycle arrest at G2/M phase. Further studies are needed to obtain the loading efficiency, release of drug concentration, and in vivo study of nano-ASLE to suppress WiDr cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by PMDSU Grant Funds 1341/UN3.14/LT/2018 from the Ministry of Research, Technology and Higher Education (KEMENRISTEKDIKTI) of The Republic of Indonesia.
REFERENCES
- 1.Gautam N, Mantha AK, Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. Biomed Res Int. 2014;2014:154106. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact Sheet No. 297. World Health Organization. 2017. [Last accessed on 2018 Jun 19]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/index.html .
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Rosas C, Sinning M, Ferreira A, Fuenzalida M, Lemus D. Celecoxib decreases growth and angiogenesis and promotes apoptosis in a tumor cell line resistant to chemotherapy. Biol Res. 2014;47:27. doi: 10.1186/0717-6287-47-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol. 2014;20:4618–25. doi: 10.3748/wjg.v20.i16.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajalakshmi S, Divya R, Divya V, Deepika Mythili S, Sathiavelu A. Pharmacological activities of Annona squamosa: A review. Int J Pharm Sci Rev Res. 2011;10:24–9. [Google Scholar]
- 7.Rupprecht JK, Chang CJ, Cassady JL, McLaughlin JL, Mikolajczak KL, Weisleder D. Asimicin, a new cyttoxic and pesticidal acetogenin from the paw-paw, Asimina triloba. Heterocycles. 1986;24:1197–201. [Google Scholar]
- 8.Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405–10. [Google Scholar]
- 9.Chingunpituk J. Nanosuspension technology for drug delivery. Walailak J Sci Technol. 2011;4:139–53. [Google Scholar]
- 10.Sudjarwo SA, Wardani G, Eraiko K, Koerniasari The potency of nanoparticle of Pinus merkusii as immunostimulatory on male wistar albino rat. Int J Nutr Pharmacol Neurol Dis. 2018;8:10–5. [Google Scholar]
- 11.Mazières J, Brugger W, Cappuzzo F, Middel P, Frosch A, Bara I, et al. Evaluation of EGFR protein expression by immunohistochemistry using H-score and the magnification rule: Re-analysis of the SATURN study. Lung Cancer. 2013;82:231–7. doi: 10.1016/j.lungcan.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Ballesta M, Gil-Izquierdo Á, García-Viguera C, Domínguez-Perles R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “Smart-foods” for health. Foods. 2018;7:pii: E72. doi: 10.3390/foods7050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar N, Slar RK, Prasad M, Rnjan K. Synthesis, characterization, and anticancer activity of vincristine loaded folic acid chitosan conjugated nanoparticles on NCL-H460 non-small cell lung cancer cell line. Egypt J Basic Appl Sci. 2018;5:87–99. [Google Scholar]
- 14.McLaughlin JL. Paw paw and cancer: Annonaceous acetogenins from discovery to commercial products. J Nat Prod. 2008;71:1311–21. doi: 10.1021/np800191t. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Chen JW, Li X. Cytotoxic bistetrahydrofuran annonaceous acetogenins from the seeds of Annona squamosa. J Nat Prod. 2011;74:2477–81. doi: 10.1021/np200708q. [DOI] [PubMed] [Google Scholar]
- 16.Yuan SS, Chang HL, Chen HW, Yeh YT, Kao YH, Lin KH. Annonacin exert antitumor activity through induction of apoptosis and extracellular signal-regulated kinase inhibition. Pharmacogn Res. 2017;9:378–83. doi: 10.4103/pr.pr_19_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakeri R, Kheirollahi A, Davoodi J. Apaf-1: Regulation and function in cell death. Biochimie. 2017;135:111–25. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Günther A, Luczak V, Abel T, Baumann A. Caspase-3 and GFAP as early markers for apoptosis and astrogliosis in shRNA-induced hippocampal cytotoxicity. J Exp Biol. 2017;220:1400–4. doi: 10.1242/jeb.154583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap CV, Subramaniam KS, Khor SW, Chung I. Annonacin exerts antitumor activity through induction of apoptosis and extracellular signal-regulated kinase inhibition. Pharmacognosy Res. 2017;9:378–83. doi: 10.4103/pr.pr_19_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu RM, Li YB, Zhong JJ. Cytotoxic and pro-apoptotic effects of novel ganoderic acid derivatives on human cervical cancer cells in vitro. Eur J Pharmacol. 2012;681:23–33. doi: 10.1016/j.ejphar.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Nigg EA. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–80. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 22.Chuang JY, Huang YF, Lu HF, Ho HC, Yang JS, Li TM, et al. Coumarin induces cell cycle arrest and apoptosis in human cervical cancer HeLa cells through a mitochondria- and caspase-3 dependent mechanism and NF-kappaB down-regulation. In Vivo. 2007;21:1003–9. [PubMed] [Google Scholar]