Abstract
Introduction:
Early and accurate diagnosis is paramount for improving the therapeutic efficacy of pancreatic cancers. Endoscopic ultrasonography–fine needle aspiration (EUS-FNA) cytology has come up with the advantage of an early and accurate diagnosis of pancreatic cancers. This study was conducted to analyze the spectrum of pancreatic lesions cytology, and appraise the diagnostic accuracy of EUS-FNA cytology for pancreatic solid and cystic lesions.
Materials and Methods:
This retrospective study includes 288 EUS-guided pancreatic FNA cases. Clinical data, laboratory tests, cytopathology, histopathology, and imaging reports were retrieved. The final diagnosis was based on EUS-FNA cell block and/or pathology in surgical specimens, with immunohistochemistry support. The results of EUS-guided FNA were compared with the final diagnoses to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results:
Among 288 EUS-guided pancreatic FNA cases, 175 (62.0%) were malignant. The mean age was 57.8 ± 13.5 years and 50.1 ± 13.7 years, and the mean size of the lesion was 4.1 ± 1.8 cm and 2.2 ± 1.1 cm in malignant and benign groups, respectively. Sensitivity, specificity, PPV, and NPV of EUS-FNA cytology for solid malignant lesions were 98.3%, 95.1%, 98.3%, and 95.1%, and those for cystic lesions were 88%, 92.3%, 100%, and 100%. Diagnostic accuracy of EUS-FNA cytology for solid and cystic pancreatic lesions is 97.4% and 95.0%, respectively. In conclusion of the above; diagnosis of pancreatic solid and cystic malignancy can be assigned from a composite of the EUS-FNA cytology, cell block preparation and immunohistochemistry Diagnosis of pancreatic solid and cystic malignancy can be assigned from a composite of the EUS-FNA cytology, cell block preparation, and immunohistochemistry.
Keywords: Cell block histology, endoscopic ultrasonography–fine needle aspiration, cytology, pancreatic cancers
INTRODUCTION
Pancreatic cancers are a significant cause of morbidity and mortality and have become the fourth leading cause of death among cancers.[1] This poor survival rate reflects late presentation, nonspecific signs and symptoms, and limited diagnostic and therapeutic modalities. Diagnosing pancreatic benign and malignant lesions are significant for improving the efficacy of therapeutic interventions.[2]
Aspiration cytology and histology of pancreatic lesions can provide a link toward the diagnosis. Endoscopic ultrasonography (EUS) is an indispensable tool for the evaluation of solid and cystic pancreatic lesions.[3] EUS-guided approach has come up with the advantage of high-resolution images particularly for smaller, distal and cystic lesions with fine parenchyma details and pancreatic duct changes along with identification of local metastases or invasion of adjacent structures, permits simultaneous diagnosis and staging. This approach is minimally invasive, well tolerated, and has a low rate of complications.[4]
EUS-fine needle aspiration (FNA) cytology and histology integrated with immunohistochemistry may allow definitive diagnosis of pancreatic lesions. Diagnostic efficacy of EUS-FNA for pancreatic lesions depends on sample tissue adequacy, lesion characteristics, endoscopy operator, and cytopathology expertise.[5]
Our study aims to analyze the spectrum of pancreatic lesions aspirated with EUS guidance in a hepato-pancreato-biliary super specialty center, to assess the diagnostic accuracy of EUS-FNA cytology in diagnosing pancreatic malignancies and to differentiate pancreatic solid and cystic lesions.
MATERIALS AND METHODS
This is a retrospective study, and data search was conducted to identify all EUS of pancreatic lesions performed at our hospital from January 2009 to December 2016. Among 8066 cytology specimens, EUS-guided FNA was performed for 936 specimens. FNA specimens of other abdominal lesions including peripancreatic lesions, lymph nodes, or bile duct mass lesions were excluded. A total of 293 pancreatic specimens who had undergone EUS-FNA for solid and cystic lesions were the study group. For each patient, clinical data, laboratory tests, and cytopathological and imaging reports were retrieved. Imaging reports including sonography, Imaging reports including Ultrasonography (USG), Computed Tomography (CT) and magnetic resonance imaging (MRI) were reviewed to assess location, size and characteristics of the pancreatic lesions CT, and magnetic resonance imaging (MRI) were reviewed to assess location, size, and characteristics of the pancreatic lesions. All FNAs were performed under EUS guidance and in the presence of onsite pathology team comprising a trained pathologist and a technician.
EUS for guided puncture of the lesion was conducted using Olympus EVIS Exera II CLV-180. EUS-FNA technique involves the use of endoscopic ultrasound in which echoendoscope is placed into the stomach or duodenum. By using the guidance of high-frequency ultrasound transducer on the tip of echoendoscope, a small gauge needle is passed through the wall of the gastrointestinal tract into the pancreatic lesion. FNA was done through transgastric approach if lesion involves body and tail, and through transduodenal approach for lesions in the head and uncinate process.[4]
After the aspiration needle was withdrawn from the endoscope, smears were prepared immediately and stained with diff-quick stain for onsite evaluation of adequacy by a pathologist. Aspiration needle was further washed in 70% ethanol in labeled test tubes for cell block preparation. An expert pathologist evaluated the smears and sections of the cell block for confirming the onsite diagnosis and rendering the final diagnosis.
The final diagnosis was based on EUS-FNA cell block and/or pathology in surgical specimens, with immunohistochemistry support. All the patients provided informed consent before the procedure.
SPSS 21 software SPSS;21 (IBM, Chicago, USA) was used for statistical analysis. Intergroup comparison was made using the Chi-square test or Fisher's exact test (two-tailed) where appropriate. The results of EUS-guided FNA were compared with the final diagnoses to calculate the accurate sensitivity, specificity, positive predictive value, negative predictive value, favorable likelihood ratio, and negative likelihood ratio of pancreatic adenocarcinoma. Pearson's correlation analysis evaluated the correlation between variables. Significance was defined at the level of P < 0.05.
RESULTS
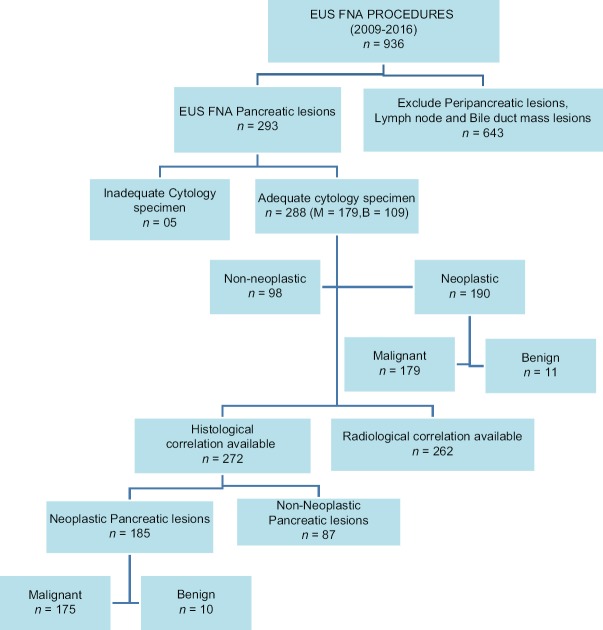
Between January 2009 and December 2016, 936 EUS-FNA procedures were carried out. Between January 2009 and December 2016, 936 EUS-FNA procedures were performed. Among them, 293 underwent EUS_FNA of a pancreatic mass lesion. Five cases were inadequate for cytological evaluation and hence excluded from the study. Abdominal pain (212; 73.6%), jaundice (63; 21.8%), and pruritus (42; 14.5%) were the most common symptoms of these patients. Among the adequate 288 cases, 219 (75.4%) were male, and 69 (24.6%) were female.
Radiological details (USG, CT, and MRI) were available for 262 cases; among them, 12 cases did not show any defined lesion to specify the location. Among 250 cases with defined solid or cystic lesion on radiology, 155 [62%] were located in the pancreatic head, 19 [7.6%] were in uncinate process, 8 [3.2] in neck, 36 [14.4%] in body, 26 [10.4%] in tail, and 6 [2.4%] patients with diffuse lesions. Solid, cystic lesions, and solid lesions with cystic component accounted for 157 [62.8%], 58 [23.2%], and 35[14.0%], respectively.
Cell block preparation was available for 272 cases, as the remaining 16 had insufficient material in cell blocks. Cell block histology confirmed that lesions include 175 malignant, 10 benign, and 87 non-neoplastic lesions [Figure 1].
Figure 1.
Flowchart depicting the frequency of EUS-FNA of pancreatic lesions
Mean age of the patients was 57.8 ± 13.5 years and 50.1 ± 13.7 years in malignant and benign groups, respectively. Mean size of the lesion in these two groups was 4.1 ± 1.8 cm and 2.2 ± 1.1 cm, respectively. Most positive diagnoses of malignancy (n = 179) were pancreatic adenocarcinomas (n = 128; 71.5%), neuroendocrine tumor (n = 19; 10.6%), mucinous neoplasm including intraductal papillary mucinous neoplasm (IPMN) (n = 3; 1.7%), mucinous cystic neoplasm (n = 6; 3.4%) and mucinous adenocarcinoma (n = 10; 5.6%), non-Hodgkin lymphoma (n = 4; 2.2%), Solid pseudopapillary epithelial neoplasm (SPEN) (n = 3; 1.7%), adenosquamous cell carcinoma (n = 3; 1.7%), acinar cell carcinoma (n = 2; 1.1%), and metastatic undifferentiated carcinoma with osteoclastic giant cells (n = 1; 0.6%) [Figure 2a–c].
Figure 2.
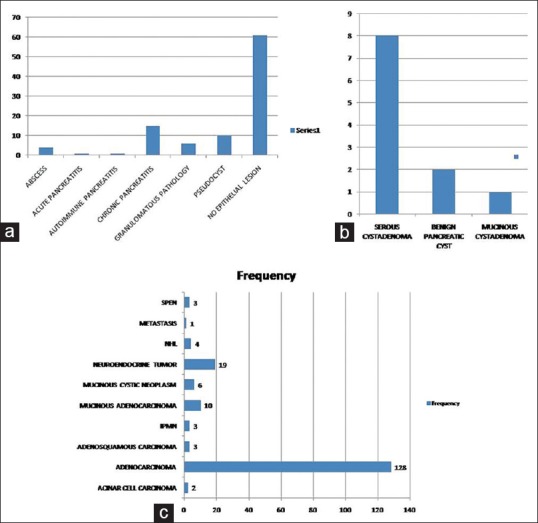
Bar diagram showing frequency of pancreatic tumors in EUS-FNA cytology: (a) non-neoplastic, (b) benign, and (c) malignant lesions
Seven cases were showing histocytological disagreement. Five cases reported as negative on EUS-FNA showed evidence of malignancy in the cell block and surgical specimen [2 neuroendocrine tumor, 1 duct adenocarcinoma, and 1 mucinous cystic neoplasm]. Two cases showing positive cytological features for a neuroendocrine tumor on aspirate were reported as normal acinar cells with the help of cell block examination and immunohistochemistry [Table 1].
Table 1.
Case with EUS-FNA cytology, Cell block histology and histocytological disagreement
| Cases | EUS-FNA Cytology (n=288) | Cell Block/Surgical Specimen Histology with or without IHC (n=272) |
|---|---|---|
| Non-Neoplastic | 98 | 87 |
| Malignant | 179 | 175 |
| Benign | 11 | 10 |
| Histo-Cytological disagreement | 7 | |
| 5 negative | 5 positive | |
| 2 positive | 2 negative | |
Sensitivity of radiology to diagnose malignancy in pancreatic lesion when compared to histology is 87.6%, Confidence Interval (CI) (83.3%–93.2%), specificity = 84.9%, CI (77.8%–92.0%), positive predictive value (PPV) = 91.4%, and negative predictive value (NPV)=79.0%. The two tests agreed 86.6% of the times. Sensitivity, specificity, PPV, and NPV of radiology for solid malignant lesion are 88.3%, 82.5%, 93.3%, and 71.7%, respectively, and the diagnostic accuracy is 86.7%. For cystic lesion, sensitivity, specificity, PPV, and NPV are 80.0%, 82.4%, 76.9%, and 84.8%, respectively, and the diagnostic accuracy is 81.3% [Table 2(a)].
Table 2a.
Diagnostic capability of EUS-FNA cytology and Radiological modalities in pancreatic malignancy
| Diagnostic modality | Cases | Tumors | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|---|---|
| EUS FNA Cytology | 288 | 179 | 97.1% | 97.9% | 98.8% | 95.0% | 97.4% |
| Radiology | 262 | 162 | 87.6% | 84.9% | 91.4% | 79.0% | 86.6% |
Sensitivity, specificity, PPV, and NPV of EUS-FNA cytology to diagnose malignancy in pancreatic lesion when compared to cell histology are 97.1%, CI (95.3%–99.9%), 97.9%, CI (89.43%–98.67%), 98.8%, and 95.0%, respectively. EUS-FNA cytology can correctly classify pancreatic lesions by 97.4%. Sensitivity, specificity, PPV, and NPV for solid malignant lesion are 98.3%, 95.1%, 98.3%, and 95.1%, respectively, with a diagnostic accuracy of 97.4%; for cystic lesion, sensitivity, specificity, PPV, and NPV are 88%, 92.3%, 100%, and 100%, respectively, and the diagnostic accuracy is 95.0%. On doing cytology and radiology (kappa) correlation, there is a kappa coefficient agreement between cytology and radiology by 67.6% though the observed agreement was 84.8% [Table 2(b)].
Table 2b.
Diagnostic capability of EUS-FNA cytology and Radiological modalities in pancreatic solid, cystic and solid cystic malignancies
| Diagnostic modality | Characterstic of lesion | Tumor | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|---|---|
| RadiologicalModalities | Cystic | 28 (16.6%) | 80.0% | 82.4% | 76.9% | 84.8% | 81.3% |
| Solid | 109 (64.5%) | 88.3% | 82.5% | 93.3% | 71.7% | 86.7% | |
| Solid with Cystic component | 32 (18.9%) | 90.9% | 80.0% | 96.8% | 57.1% | 89.4% | |
| EUS FNA Cytology | Cystic | 22 (12.3%) | 88.0% | 92.3% | 100% | 100% | 95.0% |
| Solid | 121 (67.6%) | 98.3% | 95.1% | 98.3% | 95.1% | 97.4% | |
| Solid with Cystic component | 36 (20.1%) | 100% | 100% | 100% | 100% | 100% |
DISCUSSION
With experience and deep understanding of the cytological features and incorporation of clinical, radiological, and ancillary studies, most of the solid and cystic lesions in the pancreas can be diagnosed accurately for proper patient management without the need of a Tru-Cut needle biopsy.
CT/USG-guided approach for aspiration and biopsy of the pancreatic lesion is difficult to carried out due to the retroperitoneal situation of the pancreas and have low sensitivity and specificity.[6] EUS-FNA has been shown to be reliable low-risk means of providing a tissue diagnosis of pancreatic disease process with an accuracy of biopsy. Sensitivity, specificity, PPV, and NPV of EUS-FNA procedures have been found to be 64–96%, 80–100%, 98.4–100%, and 16–86%, respectively, in different studies.[7,8] Our study also has comparable results with sensitivity, specificity, PPV, and NPV of 97.6%, 94.0%, 96.4%, and 95.9%, respectively.
Non-neoplastic lesions include acute pancreatitis, chronic pancreatitis, autoimmune pancreatitis, granulomatous inflammation, pseudocyst, and abscess. Neoplastic lesions consist of ductal adenocarcinoma, Neuroendocrine Tumor (NET),, SPEN, Acinic cell carcinoma (ACC), IPMN, mucinous cystic neoplasm, serous cystic neoplasm, and metastasis, which may be confused with mass-forming expansive fibrosis in chronic pancreatitis and Autoimmune Pancreatitis (AIP) on imaging.[9] On CT, carcinoma appear as poorly defined hypodense masses with central attenuation distorting normal pancreatic lobulations and may be associated with abrupt stricture of the main pancreatic duct.[10] Among them, IPMN, mucinous cystic neoplasm, and serous cystic neoplasm are the common cystic neoplasm, and SPEN, duct carcinoma, and NET are the uncommon cystic neoplasms [Figure 3].[9]
Figure 3.
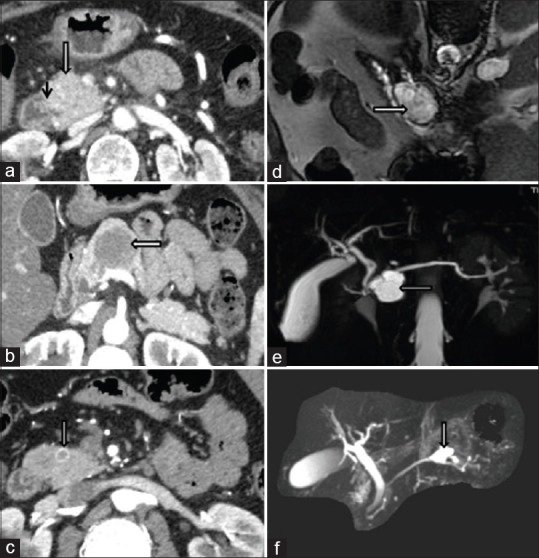
Adenocarcinoma: CT shows ill-defined heterogeneously enhancing 2.4 × 2.3 cm mass in the pancreatic head and neck (a); SPEN: well-defined hypoenhancing solid mass lesion 3.8 × 2.7 × 4.1 cm involving pancreatic neck and body (b); NET: well-defined arterial enhancing nodular lesion 0.9 × 0.9 cm in the head of the pancreas (c); Serous cystadenoma: MRCP shows lobulated T2 hyperintense lesion 3.6 × 3.1 × 2.7 cm in the midbody of the pancreas with septations (d); IPMN: well-defined T1-hypointense and T2-hyperintense septated cystic lesion 1.9 × 2.6 × 3.1 cm in pancreatic head/neck region connected with ventral pancreatic duct (e and f)
Chronic pancreatitis yields variably cellular smears composed of fibrotic stromal fragments, acinar tissue, mixed inflammation, and chalky calcific debris. Autoimmune pancreatitis shows a greater number of stromal fragments, increased lymphocytes (>30/hpf), and plasma cells. Well-formed granulomas are seen in granulomatous pancreatitis.[11] Pseudocyst FNA yields either thin and cloudy or dark and turbid fluid and is composed of granular debris, macrophages, and bile.[12]
Duct adenocarcinoma presents with high cellularity, crowded sheets of disordered ductal cells with irregular nuclear contours, anisonucleosis, vesicular chromatin, and a variable amount of cytoplasm.[13] Adenosquamous carcinoma is a variant of duct adenocarcinoma with mucin-producing glandular elements and an admixed squamous component (>30%).[14]
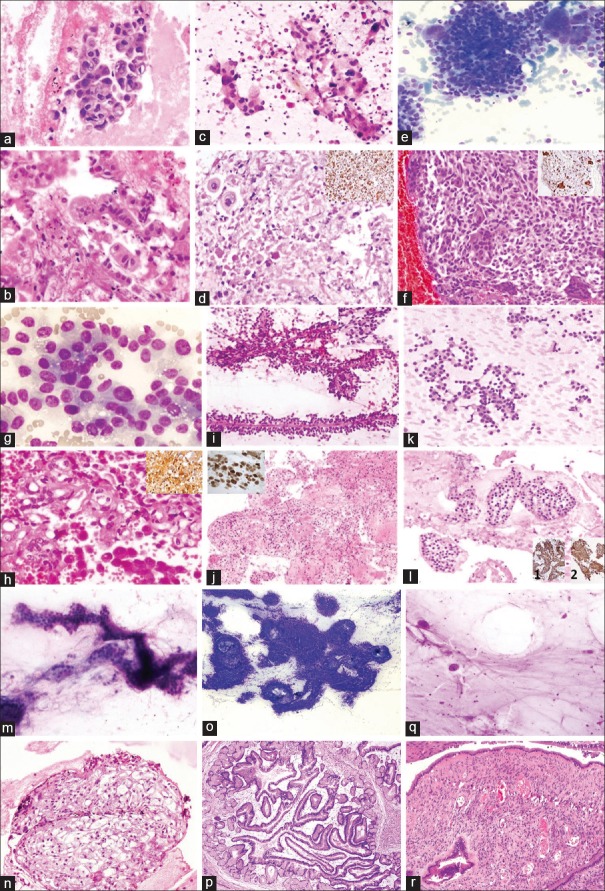
Undifferentiated carcinoma with osteoclast-like giant cells is sporadic, and scattered osteoclast-like giant cells with numerous banal-appearing nuclei are present in the background. The malignant population is poorly differentiated and is epithelioid and may react focally with antibodies to cytokeratin. Again, foci of more typical ductal adenocarcinoma may be present. Due to the presence of multinucleated giant cells, the differential diagnosis includes various infectious and inflammatory conditions; however, malignant epithelioid cell population secures the diagnosis [Figure 4].[15]
Figure 4.
Adenocarcinoma: Cytology smear [a; H and E; ×200] and CB histology [b; H and E; ×200]. Adeno-Squamous Carcinoma: Smear [c; H and E; ×200] and CB histology [d; H and E; ×200]. IHC for CK7 is negative in squamous component [Inset; ×200]. Undifferentiated Pancreatic Carcinoma with Osteoclastic Giant Cells [e: cytology smear; MGG stain; ×200], [f: CB histology; H and E; ×200], giant cells are positive for CD68 [Inset; ×200]. Acinic Cell Carcinoma: Cytology smear [g; MGG stain; ×400] and CB histology [h; H and E; ×200], IHC for chymotrypsin is positive [Inset; ×200]. Solid Pseudopapillary Epithelial Neoplasm: Cytology smear [i; H and E; ×200] and CB histology [j; H and E; ×200] IHC shows nuclear positivity for beta catenin [Inset; ×200]. Neuroendocrine Tumor: Smear [k; MGG stain; ×200] and CB histology [l; H and E; ×200]. IHC shows cytoplasmic positivity for synaptophysin [1; ×200] and chromatogranin [2; ×200]. Serous Cystadenoma: Cytology [m; MGG stain; ×200], CB histology [n; H and E; ×200]. Intraductal Papillary Mucinous Neoplasm: Cytology [o; MGG stain; 200], Histology section [p; H and E; ×200]. Mucinous Cystadenoma: Cytology [q; MGG stain; ×200] and Histology [r; H and E; ×200]
Acinar cell carcinoma cytology shows highly cellular smears, with loose cell aggregates, many isolated cells, round or oval nucleus, smooth nuclear contour, prominent nucleolus, and delicate granular cytoplasm.[16]
SPEN yields highly cellular aspirate, myxoid, or hyalinized vascular stalks lined by neoplastic cells, delicate granular cytoplasm, indistinct cell borders, round or oval nuclei, nuclear grooves, inconspicuous nucleoli, foam cells, and necrotic debris.[17] NET will show cellular aspirate, isolated cells, bare nuclei, pseudorosettes, uniform, round or oval nuclei, eccentric nuclei, finely stippled chromatin, and moderate-to-abundant cytoplasm.[18] ACC, NET, and SPEN have close cytological features, and immunohistochemistry plays an important role as synaptophysin, chromogranin, and CD56 are positive in NET; trypsin, chymotrypsin, and phospholipase A2 are positive in acinar cell carcinoma; and SPEN shows positivity for vimentin and beta-catenin [Figure 4].[14,19,20]
Among the cystic lesions, serous cystadenoma smears display sparse cellularity, clean background, flat sheets, and loose clusters of cuboidal cells, clear or granular cytoplasm with indistinct borders, bare nuclei, small round nucleus, fine chromatin, and inconspicuous nucleolus.[21]
Mucinous neoplasm of pancreas consists of IPMN and mucinous cystic neoplasm (MCN). Their distinction, based solely on cytologic features, may not be possible. The role of cytology is to distinguish mucinous from nonmucinous cysts and to evaluate for the presence or absence of epithelial dysplasia or malignancy. Diagnostic clue toward IPMN is mucin extrusion through ampulla and cyst-by-cyst appearance in EUS. Cytomorphology of MCN and IPMN consists of the hypocellular specimen, thick mucin, columnar mucinous cells (sheets, papillae, or isolated cells), and nuclear and architectural atypia (if dysplasia or malignancy present).[22] Cytological analysis of aspiration cytology material can readily differentiate between adenocarcinoma, islet cell malignancies, metastasis, inflammatory lesions, and cystadenomas [Figure 4].
FNA of cystic malignancies does not yield diagnostic material as compared to solid and solid cystic lesions; however, EUS imaging and cell block preparation along with an integration of immunohistochemistry can yield a better diagnosis and enhance the accuracy of diagnosing cystic lesions.[23] EUS-FNA has high sensitivity, specificity, PPV, and NPV for solid and cystic pancreatic tumors,[6] allowing inadvertent surgery in non-neoplastic lesions and inappropriate delay in surgical planning of malignant cases. One of our cases with an FNA diagnosis of adenocarcinoma turned out to be negative in cell block preparation as in the presence of moderately mixed inflammation smears display reactive atypia and abnormal clustering of cells. There were four negative cases that were positive in cell block preparation and finally diagnose as IPMN in three and NET in one. Acquiring representative and adequate samples in different passes may be one of the reasons for the discrepancy.
Inadequacy rates are reported to be as low as 1.5–2% for pancreatic EUS-FNA,[6] and the results are consistent with our study (1.7%). This is usually due to the difficulty in obtaining an adequate specimen because of technical problems in accessing the mass with FNA needle, exuberant inflammation, or fibrotic reaction described in the pancreatic tumors. There is a consensus opinion that onsite cytopathology with the real-time interpretation of samples is the best for optimal patient care.[24]
In conclusion, the findings in this study showed high sensitivity, specificity, PPV, and NPV of EUS-FNA in the diagnosis of pancreatic solid and cystic malignancy. Final diagnosis can be assigned from a composite of the EUS-FNA cytology, cell block preparation, and immunohistochemistry; if required, it can be adopted as an alternative approach to biopsy and may influence the treatment plans of both surgeons and oncologists.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Weston BR, Bhutani MS. optimizing diagnostic yield for EUS-guided sampling of solid pancreatic lesions: A technical review. Gastroenterol Hepatol. 2013;9:352–63. [PMC free article] [PubMed] [Google Scholar]
- 2.Luz LP, Al-Haddad MA, Sey MS, DeWitt JM. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol. 2014;20:7808–18. doi: 10.3748/wjg.v20.i24.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan MK, Hawes RH. EUS-guided FNA of solid pancreas tumors. Gastrointest Endosc Clin N Am. 2012;22:155–67, vii. doi: 10.1016/j.giec.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Chong CCN, Tang RSY, Wong JCT, Chan AWH, Teoh AYB. Endoscopic ultrasound of pancreatic lesions. J Vis Surg. 2016;2:119. doi: 10.21037/jovs.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, et al. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Inter. 2010;60:358–64. doi: 10.1111/j.1440-1827.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, Padashi M, Zali MR. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016;5:30–4. doi: 10.4103/2303-9027.175879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluen BE, Lachter J, Khamaysi I, Kamal Y, Malkin L, Keren R, et al. Accuracy and quality assessment of EUS-FNA: A single-center large cohort of biopsies. Diagn Ther Endosc. 2012;2012:139563. doi: 10.1155/2012/139563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherian PT, Mohan P, Douiri A, Taniere P, Hejmadi RK, Mahon BS. Role of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic and peripancreatic lesions: Is onsite cytopathology necessary? HPB (Oxford) 2010;12:389–95. doi: 10.1111/j.1477-2574.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellizzi AM, Stelow EB. Pancreatic cytopathology: A practical approach and review. Arch Pathol Lab Med. 2009;133:388–404. doi: 10.5858/133.3.388. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland T, Galvin A, Little AF. Part 2: CT characterisation of pancreatic neoplasm: Tumour mimics. Insights Imaging. 2011;2:389–97. doi: 10.1007/s13244-011-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Smyrk TC. Autoimmune pancreatitis and IgG4-related systemic diseases. Int J Clin Exp Pathol. 2010;3:491–504. [PMC free article] [PubMed] [Google Scholar]
- 12.Stelow EB, Bardales RH, Stanley MW. Pitfalls in endoscopic ultrasound-guided fine-needle aspiration and how to avoid them. Adv Anat Pathol. 2005;12:62–73. doi: 10.1097/01.pap.0000155053.68496.ad. [DOI] [PubMed] [Google Scholar]
- 13.Jarboe EA, Layfield LJ. Cytologic features of pancreatic intraepithelial neoplasia and pancreatitis: Potential pitfalls in the diagnosis of pancreatic ductal carcinoma. Diagn Cytopathol. 2011;39:575–81. doi: 10.1002/dc.21430. [DOI] [PubMed] [Google Scholar]
- 14.Martinez de Juan F, Reolid Escribano M, Martinez Lapiedra C, Maia de Alcantara F, Caballero Soto M, Calatrava Fons A, et al. Pancreatic adenosquamous carcinoma and intraductal papillary mucinous neoplasm in a CDKN2A germline mutation carrier. World J Gastrointest Oncol. 2017;9:390–6. doi: 10.4251/wjgo.v9.i9.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur YH, Kim HH, Seoung JS, Seo KW, Kim JW, Jeong YY, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81:146–50. doi: 10.4174/jkss.2011.81.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stelow EB, Bardales RH, Shami VM, Woon C, Presley A, Mallery S, et al. Cytology of pancreatic acinar cell carcinoma. Diagn Cytopathol. 2006;34:367–72. doi: 10.1002/dc.20450. [DOI] [PubMed] [Google Scholar]
- 17.Pailoor K, Kini H, Rau AR, Kumar Y. Cytological diagnosis of a rare case of solid pseudopapillary neoplasm of the pancreas. J Cytol. 2010;27:32–4. doi: 10.4103/0970-9371.66693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: A study of 48 cases. Cancer. 2008;114:255–62. doi: 10.1002/cncr.23637. [DOI] [PubMed] [Google Scholar]
- 19.Ohara Y, Oda T, Hashimoto S, Akashi Y, Miyamoto R, Enomoto T, et al. Pancreatic neuroendocrine tumor and solid-pseudopapillary neoplasm: Key immunohistochemical profiles for differential diagnosis. World J Gastroenterol. 2016;22:8596–604. doi: 10.3748/wjg.v22.i38.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skacel M, Ormsby AH, Petras RE, McMahon JT, Henricks WH. Immunohistochemistry in the differential diagnosis of acinar and endocrine pancreatic neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:203–9. doi: 10.1097/00129039-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Huang P, Staerkel G, Sneige N, Gong Y. Fine-needle aspiration of pancreatic serous cystadenoma: Cytologic features and diagnostic pitfalls. Cancer. 2006;108:239–49. doi: 10.1002/cncr.21911. [DOI] [PubMed] [Google Scholar]
- 22.Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: A systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–66. doi: 10.1007/s10620-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ieni A, Barresi V, Todaro P, Caruso RA, Tuccari G. Cell-block procedure in endoscopic ultrasound-guided-fine-needle-aspiration of gastrointestinal solid neoplastic lesions. World J Gastrointest Endosc. 2015;7:1014–22. doi: 10.4253/wjge.v7.i11.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyse J, Rubino M, Iglesias Garcia J, Sahai AV. Onsite evaluation of endoscopic ultrasound fine needle aspiration: The endosonographer, the cytotechnologist and the cytopathologist. Rev Esp Enferm Dig. 2017;109:279–83. doi: 10.17235/reed.2017.4473/2016. [DOI] [PubMed] [Google Scholar]