Abstract
Aim
Clinical resistance is a complex phenomenon in major human cancers involving multifactorial mechanisms, and hypoxia is one of the key components that affect the cellular expression program and lead to therapy resistance. The present study aimed to summarize the role of hypoxia in cancer therapy by regulating the tumor microenvironment (TME) and to highlight the potential of hypoxia-targeted therapy.
Methods
Relevant published studies were retrieved from PubMed, Web of Science, and Embase using keywords such as hypoxia, cancer therapy, resistance, TME, cancer, apoptosis, DNA damage, autophagy, p53, and other similar terms.
Results
Recent studies have shown that hypoxia is associated with poor prognosis in patients by regulating the TME. It confers resistance to conventional therapies through a number of signaling pathways in apoptosis, autophagy, DNA damage, mitochondrial activity, p53, and drug efflux.
Conclusion
Hypoxia targeting might be relevant to overcome hypoxia-associated resistance in cancer treatment.
Keywords: Cancer therapy, Chemotherapy, Drug resistance, Hypoxia, Tumor microenvironment
Introduction
Cancer is a major public health problem worldwide with few effective treatment choices, poor prognosis, and high mortality rates [1]. The antitumor treatments are based on chemotherapy, radiation therapy, and targeted therapy. However, acquired resistance has become a serious challenge for anticancer therapies. Cancer cells acquire resistance through a variety of mechanisms and signaling involving both intrinsic and extrinsic factors. In the majority of patients with end-stage cancer, the primary cause for treatment failure is resistance to cancer therapy. Either primary or acquired drug resistance particularly represents a significant impediment in clinical oncology. Therefore, studying the mechanisms of drug resistance is important in parallel with the development of the drug itself. Both pharmacological factors, including inadequate drug concentration at the tumor site, and cellular factors can contribute to clinical resistance [2]. The precise mechanisms of drug resistance in tumors are complex and multifactorial, but they can be grouped into three categories: insufficiency of pharmacokinetic properties, intrinsic factors of tumor cells, and external conditions of tumor cells in the tumor microenvironment (TME) [3].
Mounting studies have confirmed that the TME promotes cancer progression in many aspects, especially therapeutic resistance. The TME decreases drug penetration and confers the advantage of proliferation and antiapoptosis to surviving cells, facilitating resistance and common modifications in disease morphology [4, 5]. Soluble factors secreted by tumor or stromal cells are rich in the TME and contribute to abnormal proliferation, angiogenesis, metastasis, and drug resistance [6]. As the rapid and uncontrolled proliferation of tumors limits the availability of oxygen, insufficient blood supply, or hypoxia is a typical microenvironment feature in nearly all solid tumors [7]. Oxygen is essential for energy metabolism to drive cellular bioenergetics. The rapid proliferation of tumors outgrows their surrounding vasculature, resulting in a drop of normal oxygen levels of 2–9% to hypoxic levels of less than 2%. Regions with low oxygen levels are generally termed as hypoxic regions. Extensive reviews have been reported about the clinical significance of hypoxia in cancer therapy [8–10]. Diminished oxygen availability (hypoxia), as a hallmark of the TME, presents in the majority of tumors, arising from an imbalance between increased oxygen consumption and inadequate oxygen supply. Although the rapid proliferation of tumors can stimulate the growth of new vasculature and the tumor-induced angiogenesis leads to the unorganized growth of vasculature, the precisely distributed vasculature in normal tissues contributes to the delivery of oxygenated blood. The irregular distribution of tumor vasculature caused by persistent hypoxic conditions can result in an increase in the distance between the capillaries, exceeding the capacity of oxygen to diffuse [11, 12]. Such chronic hypoxia or diffusion-restricted hypoxia causes the necrosis of tumor cells within the 180-μm periphery of blood vessels. However, the current anticancer strategies target only tumor cells around the blood vessels rather than those in poorly perfused regions [13, 14]. The presence of hypoxic regions is one of the independent prognostic factors for human cancer. Tumor cells, while adapting to hypoxia, lead to more aggressive and therapeutically resistant tumor phenotypes. Hypoxia induces changes in gene expression and subsequent proteomic changes that have many important effects on various cellular and physiological functions, ultimately limiting patient prognosis [15]. For example, slowly dividing cells in hypoxic regions can escape most of the cytotoxic drugs that target rapidly dividing cells, and cancer stem cells may also be present in poorly hypoxic regions ensuring epithelial-to-mesenchymal transition (EMT) [16].
Hypoxia generates intratumoral oxygen gradients, contributing to the plasticity and heterogeneity of tumors and promoting a more aggressive and metastatic phenotype. In this process, the increased expression of hypoxia-inducible factor 1α (HIF-1α) is a pivotal hallmark. HIFs play a central role in cellular mechanisms triggered in response to hypoxia [17, 18]. HIF is a heterodimer composed of two basic helix-loop-helix proteins of the Per-ARNT-Sim (PAS) family: an oxygen-sensitive α-subunit and a constitutively expressed β-subunit [19]. Three HIF-α isoforms have been identified in mammals. Compared with HIF-1, a transcriptional nucleoprotein with a wide range of target genes, HIF-2 seems to be more restricted in expression in the tissue, and less is known about HIF-3 [20]. The oxygen status can regulate the stability of HIF-α family proteins. Under normoxic conditions, two critical proline residues in HIF-α subunits are subject to hydroxylation within their oxygen-dependent degradation domain by enzymes called HIF prolyl hydroxylase domain family proteins (PHDs), which use O2, ferrous iron, and α-ketoglutarate as substrates. PHDs are HIF-preserved hydroxylases found in mammals, with three subtypes PHD1, PHD2, and PHD3, as regulators of HIF-1α oxygen sensors to participate in the degradation of HIF-1α. PHD2 keeps HIF-1α at a stable low level in an anoxic environment as the main rate-limiting enzyme, and its activity is controlled mainly by the intracellular oxygen concentration. Then, the von Hippel–Lindau tumor suppressor protein (pVHL) interacts with HIF-α as a result of hydroxylation and recruits an E3 ubiquitin ligase complex, resulting in ubiquitination and subsequent proteasomal degradation of HIF-α. Under hypoxic conditions, the inhibitory hydroxylation of HIF-α is reduced, leading to the stability and translocation of HIF-α to the nucleus, where it heterodimerizes with HIF-β [21]. The HIF-α/β dimer binds with the transcriptional coactivator p300/CBP and hypoxia response element to induce the expression of the HIF target gene located in the promoter region [22, 23]. HIFs play a distinct role in tumorigenesis, and immunohistochemical analyses show that HIF-1α and HIF-2 α are overexpressed in the majority of human cancers. Especially in recent years, more attention has been paid to HIF-1 and drug resistance in a wide spectrum of neoplastic cells [24, 25].
This study aimed to focus on the cause of therapy resistance from the perspective of tumor cell adaptation to a hypoxic microenvironment, particularly discussing the capacity of oxygen-regulated transcription factor HIF-1 in modifying cancer sensitivity to therapeutic agents. Specifically, it aimed to provide an overview of the effect of hypoxia and HIFs on anticancer drug resistance, highlighting the multifaceted interaction of HIFs with apoptosis, autophagy, DNA damage, mitochondrial activity, and p53 in the failure of HIF-mediated therapy (summarized in Fig. 1 and Table 1). Finally, the study worked toward providing a comprehensive understanding of hypoxia-mediated molecular signaling pathways and a new sight for cancer therapy.
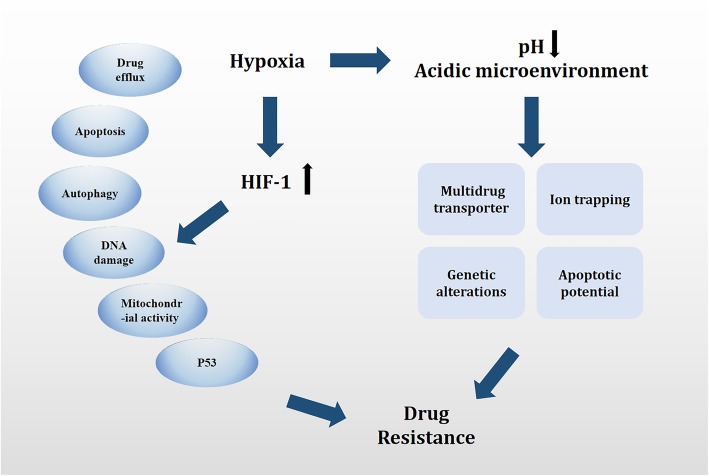
Fig. 1.
Summary of mechanisms and pathways of HIF-mediated drug therapy failure. HIF-1 confers resistance to conventional therapies through a number of signaling pathways in apoptosis, autophagy, DNA damage, mitochondrial activity, p53, and drug efflux. In addition, hypoxia results in a decrease in pH and creates an acidic TME. Mechanisms by which the tumor acidic microenvironment leads to MDR, including a decreased concentration of the drug caused by “ion trapping,” reduced apoptotic potential, genetic alterations (such as p53 mutations), and elevated activity of a multidrug transporter p-glycoprotein (P-gp)
Table 1.
Overview of HIF-1-mediated mechanisms in drug resistance
| Resistance phenotype | Cancer/Cell type | Resistant chemotherapy drug | Molecular basis | Reference |
|---|---|---|---|---|
| Overexpression of drug efflux proteins | Colon cancer cells | 5-Fluorouracil | MDR1/P-gp | [26] |
| Overexpression of drug efflux proteins | Ovarian carcinoma cells | Estramustine | ABCA2 | [27] |
| Overexpression of drug efflux proteins | Lung adenocarcinoma cells | Adriamycin | P-gp | [28] |
| Apoptosis inhibition | Breast cancer cells | Paclitaxel | Caspases 3, 8, 10, and Bak | [29] |
| Apoptosis inhibition | Colon cancer cells | Etoposide and oxaliplatin | Bid and Bax | [30] |
| Apoptosis inhibition | Gastric cancer cells | 5-Fluorouracil and cisplatin | p53 and NF-kB | [31] |
| Apoptosis inhibition | Human melanoma cells | Not mentioned | P53 and TRP2 | [88] |
| Autophagy induction | HeLa cells | N-(4-Hydroxypheny) retinamide (4-HPR) | Beclin1 | [32] |
| Autophagy induction | Gastric cancer cells | Vincristine | miR-23b-3p, ATG12, and HMGB2 | [33] |
| Autophagy induction | Colon cancer cells | Cryptotanshinone Dihydrotanshinone | p53 | [34] |
| DNA damage inhibition | Mouse embryonic fibroblasts | Etoposide | DNA–PKcs and Ku80 | [35] |
| DNA damage inhibition | Breast and liver cancer cells | Taxol and etoposide | TMEM45A | [36] |
| Mitochondrial activity | Human leukemia cell line (HL-60) human lymphoma cell line (Raji) | Doxorubicin and ara-c | BAD | [37] |
| Mitochondrial activity | Renal carcinoma cells | Not mentioned | VHL | [38] |
| Mitochondrial activity | Oral squamous cell carcinoma cells | 5-Fluorouracil and cisplatin | Cytochrome, Akt, and ERK | [39] |
| P53 | Non-small-cell lung cancer cells | Cisplatin | HIF-1α and BAX | [40] |
Hypoxia and TME
Tumor cells develop new blood vessels to adapt to low levels of oxygen and nutrients, which is called de novo angiogenesis. However, such newly formed blood vessels are leaky because of their discontinuous endothelium, along with the obstruction of lymphatic drainage, producing vascular hyperpermeability and enhanced permeation [41]. Hence, hypoxia causes vascular leakage and abnormal lymphatic drainage in the tumor, leading to an increase in interstitial fluid pressure [42]. TME refers to the local biological environment in which solid tumors are located, including cancer cells and nearby stromal cells. Cancer cells select normal host cells, such as fibroblasts, various immune cells, and blood and lymphatic cells, which are embedded in tightly packed extracellular matrices. The secondary formation of harsh metabolic and physical microenvironments results in an imbalance of positive and negative regulators of processes in activating and deregulating angiogenesis, desmoplasia, and inflammation. Most tumors have hypoxic regions. The development of an abnormal vasculature and a hypoxic microenvironment promotes abnormal angiogenesis, desmoplasia, and inflammation, all of which contribute to tumor progression and therapeutic resistance [43, 44]. HIF-1α mediates hypoxia-induced signaling, which plays a role in multiple steps of the transfer cascade [23]. Tumor metastasis is the migration of cancer cells from the primary tumor in the form of single cells or multiple cell clusters to distant sites in the body. Studies have shown that cancer-associated fibroblasts (CAFs) and myeloid cells assist in the process of tumor metastasis [45]. In a hypoxic environment, activated HIF-1α increases the activity of Snail and Twist, two transcription factors that reduce E-cadherin expression and promote EMT. More interestingly, EMT-related signaling is not required for the metastatic process, but it promotes invasion, aging, cancer stem cell–like phenotype, and resistance to chemotherapy [46]. HIF-1α can also intervene in the expression of enzymes that polymerize and regulate the alignment of collagen fibers and activity of integrins to promote cancer migration [23]. Finally, as already mentioned, hypoxia fosters leaky and compressed blood and lymphatic vessels mediated by HIFs such as angiopoietin-2, vascular endothelial growth factor (VEGF), and angiopoietin-like 4, facilitating the passage of metastatic cancer cells through the vessel wall [47].
In addition, the anoxic microenvironment is beneficial for glycolysis and lactic acid production by key enzymes of glycolysis and lactate dehydrogenase A (LDH-A); the excess production of lactic acid results in acidic pH. Moreover, HIF can reversely convert carbon dioxide and water produced by the activation of carbonic anhydrase IX or XII into HCO3−, which diffuses out of the cell membrane, resulting in excess HCO3− in the TME and a decrease in pH [9]. A large number of studies have concluded that the decreased intracellular pH of endosomes and lysosomes in tumor cells may assist in metastasis by activating proteases [42, 48]. In fact, alterations in extracellular pH induce drug resistance by inhibiting cellular and humoral immune functions because acidic pH predominates at loci of inflammation and other immunologically active sites. Investigations have shown that reduced pH inhibits mainly the chemotaxis, respiratory activity, and bactericidal ability of polymorphonuclear leukocytes. Moreover, impaired cytotoxicity and proliferation of lymphocytes at acidic pH have been reported. Similarly, the decreased lysis of various tumor cell lines by cytotoxic T lymphocytes at acidic extracellular pH has been demonstrated. The neutralization of T-cell effect or function and tumor acidity can improve the response to immunotherapy [49]. Studies on macrophages and eosinophils have suggested acid-induced activation of complement proteins and alternative complement pathway, coupled with increased binding of antibodies to leukocytes at a lower pH. The level of reactive oxygen species (ROS) has been shown to be increased in cancer cells exposed to hypoxia [50]. The reduction in oxygen utilization decreases the passage of electrons through the mitochondrial complex by the electron transport chain (ETC), allowing electrons to leak from the ETC, thus leading to the overproduction of ROS [51]. Moreover, the excessive production of ROS alters genomic stability and impairs DNA repair pathways [52]. ROS can cause cell survival or apoptosis via a mechanism referred to as oxidative stress, thus resulting in enhanced cytotoxicity and apoptosis [53]. At high concentrations (10–30 μm), ROS can damage cellular biomolecules, such as proteins, DNA, and RNA, and cause mutations that promote cancer in normal cells or multidrug resistance (MDR) in cancer cells [54]. However, most cancer cells still survive under internal oxidative stress, hence avoiding apoptosis and developing resistance to chemotherapy. Exposure to elevated levels of ROS can lead to cancer cell resistance by the activation of redox-sensitive transcription factors such as NF-κB, nuclear factor (erythroid-derived 2)-like factor 2 (Nrf2), c-Jun, and HIF-1α [55]. Subsequently, the activation of these genes enhances the activation of the antioxidant system and promotes the expression of cell survival proteins. In addition, ROS facilitate the transition from apoptosis to autophagy in methotrexate-resistant choriocarcinoma jeg-3 cells, enabling the survival of cells to methotrexate [56]. ROS can also stimulate the differentiation of cancer stem cells, thus promoting EMT and inducing metabolic reprogramming involved in the resistance of cancer cells.
Hypoxic stress causes immunosuppression by controlling angiogenesis and favoring immune suppression and tumor resistance. Macrophages constitute a principal component of the immune infiltrate in solid tumors by differentiating into tumor-associated macrophages (TAMs), which have been found to be preferentially located in tumor hypoxic areas [57]. Tumor-derived cytokines are able to convert TAMs into polarized type 2, or M2, macrophages with more immunosuppressive activities, resulting in tumor progression. Myeloid-derived suppressor cells (MDSCs) can directly promote immune tolerance [58]. In hypoxic zones, HIF-1 directly regulates the function and differentiation of MDSCs, and such tumor-derived MDSCs are more immunosuppressive compared with splenic MDSCs. A recent study showed the upregulation of the expression of programmed death-ligand 1 (PD-L1) under hypoxia [59]. Further evidence supports that HIF-1 is a major regulator of PD-L1 mRNA and protein expression. HIF-1 regulates the expression of PD-L1 by binding directly to a hypoxia response element in the PD-L1 proximal promoter [58]. The originally elevated immunosuppressive function of tumor-derived MDSCs under hypoxia was found to be abrogated following PD-L1 blockade. Along with PD-L1 blockade, the hypoxia-mediated upregulation of IL-6 and IL-10 in MDSCs was significantly attenuated [60]. At present, immunotherapeutic strategies triggering antitumor immunity are not effective because of diverse mechanisms of tumor escape from immunosurveillance. The antibody blockade of the T-cell immune checkpoint receptors PD-1 and CTLA-4 was poor in some tumors because T cells were sparse or absent in the TME; the hypoxia-driven modulation of T-cell exclusion and apoptosis help maintain this state. T cells can enter hypoxic tumors. The hypoxia-mediated acidification of the extracellular milieu blocks the capacity of T cells to expand or perform cytotoxic effector functions. Taken together, tumor hypoxia predicts poor outcomes across all cancers [61]. It is clear that hypoxia plays a prominent role in establishing and maintaining tumor immune privilege or immunotherapy. It is conceivable that given the central role of hypoxia in the regulation of tumor progression and immune suppression, it might be considered in new combined cancer therapies.
Hypoxia and cancer therapy
In recent years, the contribution of hypoxia to tumor therapy, especially drug resistance, has been observed in a wide range of neoplastic cells [62–65]. Roland Wenger and colleagues first studied the effects of hypoxia on the proliferation of mouse embryonic fibroblasts. When HIF-1 is inactivated, the inhibitory effect of carboplatin and etoposide on cell proliferation is significantly enhanced [66]. The HIF-1α protein is overexpressed in various common solid malignant tumors, including breast, colon, gastric, lung, skin, ovarian, pancreatic, prostate, and renal carcinomas compared with their respective normal tissues [67–69]. For example, the significantly increased expression level of HIF in pancreatic cancer is primarily used to evaluate the prognosis of patients clinically. A large number of studies have shown that HIF-1α can be used as a marker of the survival rate of pancreatic cancer [70–72]. The expression level of HIF-1α, VEGF, and glucose transporter protein 1 was found to be increased significantly using the immunohistochemical technique in 58 patients with pancreatic cancer and 20 normal human tissue samples. The Cox regression analysis revealed that HIF-1α was an independent marker for evaluating the prognosis and survival of patients with pancreatic cancer. Further comparison of HIF-1α levels in tissues of patients with short-term survival (< 6 months) and long-term survival (6–60 months) suggested that HIF-1α was a prognostic marker with the specificity of 87.1% and sensitivity of 55.6% [72]. It reflected that HIF-1α could be used to evaluate the survival time of patients with pancreatic cancer after diagnosis. Hence, it was concluded that the expression of HIF-1α was linked to the clinicopathological features and affected the survival of patients. Moreover, quantitative data emphasized the significance of HIF-1α in the prognostic evaluation of pancreatic cancer.
Hypoxia leads to a decreased pH in the TME. Hypoxia induced by the acidic TME leads to MDR via various mechanisms, including a decreased concentration of the drug caused by “ion trapping,” reduced apoptotic potential, genetic alterations (such as p53 mutations), and elevated activity of a multidrug transporter p-glycoprotein (P-gp). Uncharged small molecules could easily diffuse through the phospholipid portion of the cell membrane, whereas charged molecules could not, providing a condition for the acidic extracellular environment of the solid tumor to become weakly alkaline. This phenomenon is called “ion trapping [73].” However, some chemotherapeutic drugs currently used in clinical practice are pH dependent in terms of their intracellular targets. Hence, changes in the intracellular pH gradient resulted in decreased drug accumulation in tumor cells, thereby greatly reducing the efficacy of chemotherapeutic drugs and eventually leading to drug resistance. Some studies demonstrated that the efficacy of benzoate mustard in vivo was 2.3 times higher than that of doxorubicin. However, the efficacy of sodium bicarbonate in studying nitrogen mustard in vitro or in vivo drastically reduced after the alkalinization of the tumor environment. Melphalan, a slightly acidic chemotherapy drug approved for treating multiple myeloma and ovarian cancer, also showed consistent results with enhanced efficacy in preclinical and clinical trials on melanoma due to local hypoxia and a slightly acidic environment [74]. One of the main mechanisms of drug resistance was the expression of multidrug transport P-gp, which was encoded by the MDR1 gene and resulted in the pumping out of cytotoxins such as doxorubicin and paclitaxel. Although its mRNA levels remained unchanged in the acidic environment, its activity increased, and this effect was doubled in low-oxygen environments. Studies showed no statistically significant difference in the expression level of P-gp after the treatment of A549 cells in the acidic medium, but the activity of P-gp was significantly enhanced and the peak appeared after 6 h. However, the cytotoxicity of daunomycin was significantly reduced and reversed under the synergistic effect of verapamil [75]. Otherwise, the p53-mutant cells were selected, causing p53-dependent apoptosis. This loss of apoptotic potential and a string of adaptive changes were likely driven by microenvironment-induced genomic instability and inhibition of DNA repair. Subsequently, data on the importance of hypoxia to the sensitivity of cancer cells under normoxic conditions are available. For example, in the hypoxic core of advanced solid tumors, a series of chain reactions caused by the high infiltration of immune cells enhanced the expression of the original gene, promoted tumor malignancy, and resulted in the emergence of drug resistance [76]. The use of cell-based targeted nanoparticles for effective therapy has been highlighted as a dual-mode treatment strategy to combat drug resistance and improve the efficacy of chemotherapy [77]. Therefore, hypoxia has been widely recognized as an active participant in tumor progression, affecting cell expression programs and therapeutic resistance. HIF-1, as the molecular basis, is commonly overexpressed in a majority of tumors, including breast, prostate, lung, and pancreatic carcinomas, besides head and neck cancer. The following section outlines general pathways and molecular mechanisms underlying the effect of HIF-1.
Hypoxia-mediated overexpression of drug efflux proteins
The first proposed explanation is that HIF-1 can activate the multidrug resistance 1 (MDR1) gene in response to hypoxia. The quantitative microarray analysis of RNA revealed a sevenfold increase in MDR in epithelial cells exposed to hypoxia (pO2 20 Torr, 18 h); these findings were further confirmed at the mRNA and protein levels. The torr is the unit of pressure based on an absolute scale (one torr ≈133.322 Pa). MDR1 encodes for the membrane-resident P-gp, a member of ATP-binding cassette (ABC) transporter family, acting as a drug efflux pump to decrease the intracellular concentration of a series of chemotherapeutic drugs. The ABC transporters are known as a large family of integral membrane proteins, with at least 48 members in humans. Further, 12 of them have been recognized as putative drug transporters, including well-known P-gp encoded by the ABCB1 gene, MDR-associated protein 1 (MRP1, encoded by the ABCC1 gene), and ABC subfamily G member2, also known as breast cancer resistance protein, which is encoded by the ABCG2 gene [78, 79]. A study using a chemotherapeutic sensitivity assay and flow cytometry (FCM) to analyze the relationship between HIF-1 expression and sensitivity to chemotherapy revealed that HIF-1α inhibition reversed MDR in colon cancer cells via the downregulation of MDR1/P-gp [26]. Further analysis revealed that ABC2 was amplified and overexpressed in an estramustine (EM)-resistant human ovarian carcinoma cell line, and antisense-mediated downregulation of ABC2 expression sensitized the cell line to EM. Thus, the overexpression of ABC2 contributed to EM resistance by serving as an efflux pump for chemotherapeutic agents [27]. A study exploring the effects of hypoxia on the expression of P-gp and MDR protein in human lung adenocarcinoma A549 cell line showed that the expression of HIF-1α, P-gp, and MDR protein was higher and the resistance of A549 cells to adriamycin increased under hypoxia [28].
Hypoxia-mediated regulation of apoptosis
Tumor cells always alter their metabolism to ensure survival and evade host immune attack to proliferate. Defective apoptosis represents another pivotal reason for drug resistance because anticancer treatments act in part by inducing apoptosis, a process mediated by members of the caspase family of proteases (summarized in Fig. 2) [80, 81]. The caspases mediate the selective cleavage of a subset of cellular polypeptides, thereby contributing to the biochemical and morphological features of apoptotic cells [82]. Two main intracellular caspase cascades are triggered by death receptor–ligand systems and various cellular stresses: DNA damage and microtubule disruption. In regulating the activation of these protease cascades, a string of factors, including B-cell lymphoma-2 (Bcl-2) family members, inhibitors of apoptosis-related proteins, and several protein kinases, are closely related [83]. The Bcl-2 protein family impairs the cell’s ability to release apoptogenic protein cytochrome c (cyt c) from the mitochondria by binding to the proapoptotic proteins Bcl-2-associated X protein, apoptosis regulator (Bax), and Bcl-2 homologous antagonist killer, mediating the balance between cell survival and apoptosis [29]. The death-receptor pathway begins with the death-effector domain, which is a critical protein interaction domain recruiting caspases into complexes with the cell surface receptors. Cyt c and other mitochondrial polypeptides were found to be released from the mitochondrial intermembrane space in the mitochondrial pathway [84]. This process involves mitochondrial permeability transition and transfer of certain Bcl-2 family members from the cytoplasm to the outer mitochondrial membranes [85]. The overall survival threshold is probably determined by the balance of interactions between proapoptotic and antiapoptotic members of the Bcl-2 family. Under hypoxic conditions, the nonadaptive cancer cells undergo apoptosis via HIF-1- and P53-dependent mechanisms. The Bcl-2 family can be divided into two categories: anti-apoptotic proteins such as Bcl-2, Bcl-xl, Myeloid cell leukemia-1 (Mcl-1), and cell death abnormality gene 9 (CED9); and pro-apoptotic proteins including mainly BCL2-associated X protein (Bax), BCL2 antagonist/killer (Bak), Bcl-xs, Bad, and Bid. The mRNA and protein levels of proapoptotic Bid and Bad decreased in vitro and in human colon cells with oxygen deprivation. HIF-1 was dispensable for the downregulation of Bad but was required for that of Bid [30]. Antiapoptotic proteins, such as an inhibitor of apoptosis 2 (IAP-2), could be induced whereas the proapoptotic protein Bax could be downregulated in an HIF-1-dependent manner [86]. Abnormalities in the apoptosis machinery lead to a resistant phenotype of tumor cells. During isolation and characterization, several drug-resistant mutants were found to be resistant to antitumor agent–induced apoptosis. For example, a subtractive hybridization of cDNA with mRNA from human monocyte leukemia U937 and its variant UK711 was performed. Glyoxalase I was found to be selectively overexpressed in antiapoptotic UK711 cells [87]. Methylglyoxal is an active dicarboxylic compound involved in a variety of deleterious processes, including the formation of AGE with proteins or DNA modification. In tumor cells, the increased glycolysis activity of the intracellular concentration of methylglyoxal (a byproduct of glycolysis) due to the rapid dysregulation of growth leads to hypoxia in the TME. Methylglyoxal is cytotoxic, and glyoxalase I is an essential component in the detoxification of methylglyoxal. The principal pathway for the catabolism of methylglyoxal is the glyoxalase pathway, which consists of two enzymes, glyoxalase I and glyoxalase II. The mRNA expression and the activity of glyoxalase I in several drug-resistant cells (including UK711, UK110, and K562/ADM) significantly increased compared with parental cells. These mutant cell lines survived after treatment with etoposide or doxorubicin, and the emergence of drug resistance might be accompanied by the overexpression of enzymes. Experiments showed that the overexpression of glyoxalase I in human leukemia cells inhibited etoposide- and doxorubicin-induced apoptosis, indicating that the enzyme was directly involved in the inhibition of apoptosis induced by the chemotherapeutic drug. However, the mechanism by which the overexpression of glyoxalase I inhibits apoptosis has not been fully elucidated. It may be related to the upstream signaling that inhibits apoptosis, leading to the activation of caspase [88, 89]. HIF-1 protected against DNA-damage-induced germ cell apoptosis by antagonizing the function of CEP-1, the homolog of the tumor suppressor p53 [89, 90]. Further, p53+/+ cells exposed to hypoxia exhibited a transient arrest in G2/M compared with 43 isogenic p53-null cells exposed to hypoxic conditions, exhibiting a sixfold to tenfold higher level of apoptosis. Hence, it was hypothesized that p53 acted as a survival factor under limiting oxygen concentrations [91]. Thus, an understanding of cell death processes after cytotoxic damage might help in cancer treatment.
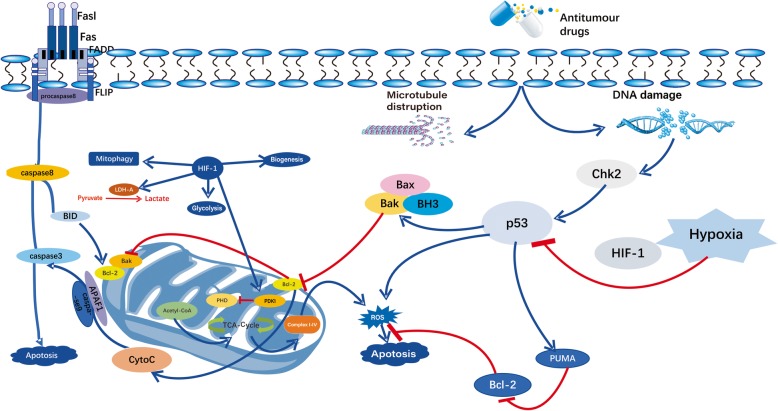
Fig. 2.
Diagram of the modification effect of HIF-1 on mitochondrial activity. HIF-1 is beneficial for glycolysis and lactic acid production by activating pyruvate dehydrogenase kinase-1 (PDK1) and hindering the activity of pyruvate dehydrogenase (PDH). In addition, HIF-1 targets PDK1, directly inhibiting pyruvate from entering the TCA cycle through the inactivation of PDH. HIF-1 also can induce mitochondrial autophagy and inhibit mitochondrial biogenesis, thus avoiding cell death and ultimately leading to HIF-1-mediated drug resistance
Hypoxia-mediated induction of autophagy
Besides apoptosis, the process of autophagy is widely recognized as a critical regulator of cellular viability [92]. Autophagy means self-digestion. It is a catabolic process of phagocytizing cytoplasmic proteins or organelles to degrade the contents of cells, thereby realizing the metabolism of cells themselves [93]. Autophagy in tumor cells is a double-edged sword. On the one hand, autophagy can remove misfolded proteins and dysfunctional organelles within tumor cells, such as mitochondria, inhibit cellular stress response, and ultimately prevent genomic damage, thereby inhibiting cancer. On the other hand, in the advanced stage of tumor growth, tumor cells can make use of autophagy to survive in the condition of nutrient deficiency or hypoxia [94]. Although the precise role of autophagy in tumor formation is controversial, the state of autophagy is closely associated with the failure of antiproliferative treatment in neoplastic cells [95]. Currently, three autophagic mechanisms have been identified: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). Autophagy-associated (ATG) proteins, upon the receipt of autophagy signals associated with autophagosome formation, trigger the activation of macroautophagy. The initiation of autophagosome formation is governed by the complex ULK-1–Atg13–FIP200, which is negatively regulated by the mammalian target of rapamycin (mTOR) by phosphorylating ULK [96]. The LKB1–AMPK axis (an energy sensor) is activated by energy starvation through phosphorylation, directly initiating ULK1–Atg13–FIP200 complex formation, or through TSC1/2-dependent or -independent mTOR inhibition. The functional inactivation of autophagy pathways results in significantly enhanced efficacy of chemotherapeutic agents [32]. Autophagy induced by hypoxia is primarily located in hypoxic tumor regions. The link between unfolded protein response (UPR) and the regulator of autophagy involving PERK-dependent transcriptional induction of MAP 1LC3B and ATG has also been identified. The data showed that UPR was a key mediator in promoting therapy resistance through its ability to facilitate autophagy [97]. Substantial evidence has shown that autophagy promotes the development of MDR. Since ABC transporters are associated with MDR, agents that modulate ABC transporters have been advocated as chemotherapeutic drugs to overcome MDR. However, the current clinical efficacy of ABC transporter modulators has not achieved the desired results. MDR is an extremely complex phenotype. An increasing number of studies have focused on clarifying the link between autophagy and MDR based on clinical data. The elevated levels of autophagy detected in patients with poor prognosis indicate that autophagy can catalyze the development of MDR. Doxorubicin and vincristine treatment upregulates the expression of S100A8, which is required for the formation of the Beclin1–III phosphatidylinositol 3-kinase (PI3KC3) complex [98]. MDR can be mediated by high-mobility box group 1 (HMGB1), which promotes protective autophagy against anticancer agents. HMGB1 is transferred from the nucleus to the cytoplasm and promotes the formation of the Beclin1–PI3KC3 complex by activating the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway [99]. Likewise, peptidylarginine deiminase IV can cause MDR in hepatocellular carcinoma cells by inducing autophagy as a protective function [100]. MiR-23b-3p inhibited autophagy mediated by ATG12 and HMGB2 and restored the sensitivity of MDR cells to chemotherapy in vivo [33]. Cumulative evidence suggested that autophagy, as a cytoprotective mechanism, mediated MDR, thereby protecting MDR cancer cells from apoptosis and promoting resistance to chemotherapy treatment. However, undoubtedly, the cells are autophagy dependent under the stress of nutrient deficiency. Chemotherapeutic agents known to cause DNA damage (temozolomide and cisplatin) or inhibition of DNA synthesis [5-fluorouracil (FU) and gemcitabine] and other agents that target signal transduction pathways due to specific gene mutation, amplification, and activation, such as erlotinib and gefitinib (EGFR mutation), imatinib (tyrosine kinase activation), and trastuzumab (HER2 amplification), induce growth inhibition and lead to autophagy-mediated cell survival. These findings indicated that autophagy inhibitors, such as chloroquine (CQ) and hydroxychloroquine (HCQ), against autolysosome formation combined with the aforementioned therapeutic agents could abrogate autophagy-dependent cell survival [34, 101–104].
Hypoxia-mediated inhibition of DNA damage
Most anticancer drugs kill tumor cells by causing DNA damage, which is considered as the basic mode of action for a majority of classical chemotherapeutic agents. However, cancer cells can activate various distinct repair mechanisms and signaling pathways as a response to overcome DNA damage. Subsequently, repaired cancer cells become more resistant to chemotherapeutic treatment [105, 106]. On exposure to hypoxia, cancer cells undergo replication stress, thereby activating DNA damage and repair pathways. Two main kinase signaling cascades exist: (1) ATM–Chk2 and (2) ATR–Chk1 pathways activated to induce cell cycle arrest. For example, the E3 ubiquitin ligase Smurf1 determines cell apoptosis rates downstream of DNA damage–induced ATR–Chk1 signaling by promoting the death of transformed cells [107, 108]. HIF-1α is associated with the increased chemoresistant phenotype of cancer cells, and downregulated HIF-1α can increase the sensitivity toward etoposide treatment. In an HIF-1α-deficient cell model, the transcript levels of DNA–PK complex members, DNA–PKcs and Ku80, were downregulated, leading to a higher susceptibility to chemotherapeutics and introducing DNA double-strand breaks [35]. The analysis of breast (MDA-MB-231) and liver (HepG2) cancer cell lines treated with taxol or etoposide under hypoxic conditions revealed that hypoxic cells showed less sensitivity to drug treatment compared with normoxic cells. The transcriptome analysis showed that the expression of transmembrane protein 45A increased in hypoxic cells, inducing cell cycle arrest and resistance to etoposide-induced DNA strand breaks [36]. In addition, the role of p53 in hypoxic drug resistance is indispensable. In cancer cells, hypoxia disrupts the p53–RPA70 complex, which in turn enhances RPA70-mediated nucleotide excision repair/nonhomologous end-joining repair whereas RPA70 binds to the p53-N-terminal domain in normal cells. Under the hypoxic stress, cancer cells activate the phosphorylation of p53 in the serine 15 region, thereby inhibiting the formation of p53–RPA70 complex [104]. However, a number of unresolved issues need to be considered when establishing a link between hypoxia and genetic instability and invasive phenotypes. Whether DNA repair defects caused by hypoxia in precancerous models and permanent alteration of genomic stability and cell transformation, rather than transient hypoxia, alter protein expression to drive cell proliferation, survival, and metastasis phenotype needs confirmation. These concepts are effectively translated into clinical needs to quantify and monitor acute and chronic hypoxia, thus providing options for individualized cancer treatment. The present model did not distinguish between the complexity of the hypoxic microenvironment and the biological effects of acute and chronic hypoxia–mediated transcriptional and translational changes. Hence, it is necessary to identify molecularly targeted drugs under different conditions, including normal oxygen levels and hypoxia (acute and chronic), equally inhibiting the targeting molecule or the pathway, helping to ensure that clinical resistance is not just due to drugs ineffective in hypoxic cells. Also, whether cancer stem cells preferentially exist in tumor hypoxic regions needs confirmation [109].
Hypoxia-mediated downregulation of mitochondrial activity
In the process of converting glucose into lactate that maintains the balance of redox homeostasis and cell survival under hypoxic conditions, HIF-1 plays a key role in the reprogramming of cancer metabolism (summarized in Fig. 2) [110]. The increase in glycolysis for ATP generation in cancer cells is frequently associated with resistance to therapeutic agents. Cells incubated under hypoxic conditions produced a significantly larger amount of lactate than those under normoxic conditions along with reduced sensitivity to doxorubicin and ara-c in both human leukemia cell line HL-60 (HCT116 cells) and human lymphoma cell line Raji (Raji cells) [111]. However, cancer cells less sensitive to chemotherapeutic agents could be effectively killed using glycolytic inhibitors. Even cells that expressed an MDR phenotype still remained sensitive to the inhibition of glycolysis. Mitochondria are indispensable for the nonapoptotic forms of cell death. Hence, the mechanism underlying the involvement of HIF in altering mitochondrial function in cancer needs to be explored [112]. In mammalian cells, HIF-1 can regulate the expression of cyt c oxidase–cytochrome c oxidase subunit IV (COX4) by activating the transcription of genes encoding COX4–2 and LON to optimize the efficiency of respiration under hypoxic conditions [113]. HIF-1 stimulates glycolysis, and the TCA cycle is suppressed by the molecular mechanism inducing PDK1 [114]. HIF-1 is required for tumorigenesis in VHL-deficient renal carcinoma cells, and these effects are mediated by HIF-1 via inhibiting C-MYC activity, which determines the transcription of the gene encoding the coactivator PGC-1b [37, 115]. The loss of PDG-1b contributes to decreased respiration in VHL-deficient renal carcinoma cells. Mitochondria and HIFs are closely connected to regulate each other during the cellular death pathways. The suppression of mitochondrial activity qualifies as a reliable mechanism of HIF-driven treatment failure [38]. Otherwise, not only drug and radiation can induce ROS production for killing tumor cells, but also mitochondria can induce death signaling via the generation of ROS. Evidence showed that cells depleted of their mitochondrial DNA were not able to elicit hypoxia-induced increase in ROS generation and HIF-1α-induced ROS protein accumulation. In human oral squamous cell carcinoma cell (OSCC) lines, the forced expression of HIF-1α suppressed the generation of ROS and increased cytosolic accumulation of cyt c, inhibiting the hypoxia-induced apoptosis of OSCC lines [39]. The model proposed for the HIF-1-dependent regulation of chemosensitivity by ROS and the experimental result showed that HIF-1-competent cells displayed a more chemoresistant phenotype. Given the central role of mitochondria, the comprehensive connection between HIF, mitochondrial activity, and chemoresistance provides a new direction of research for explaining therapy failure and proposing innovative treatment strategies [116].
Hypoxia-mediated regulation of p53
The TP53 gene is by far the most frequently mutated gene in human cancer [117, 118]. P53-deficient mice are often associated with a high incidence of spontaneous tumors, indicating strong TP53 inactivation during tumor development. HIF-1α has been proved to regulate p53 tumor suppressor protein levels to adapt to hypoxia. Conversely, p53 can also regulate HIF-1α protein levels negatively. Under mild hypoxic conditions, p53 protein levels are decreased to protect cells against apoptosis and promote cell survival. However, under severe hypoxic, even anoxic, conditions, p53 protein levels are stabilized, thereby decreasing HIF-1α transcriptional activity and inducing apoptotic cell death [119]. The HIF-1-mediated transcriptional upregulation of the secreted tyrosinase (TYR) TYR-2 (or the human homolog TRP2) was identified as the underlying p53-inhibiting mechanism. The activation of the p53 pathway upon treatment with chemotherapeutic agents was found to be markedly suppressed. Moreover, the accumulation level and activity of HIF-1α increased in p53 mutant cells, thereby allowing decreased apoptotic potential and chemoresistant properties [120, 121]. Resistance to cisplatin frequently occurs in non-small-cell lung cancer (NSCLC). Christoph et al. provided evidence that this sensitivity to cisplatin could be reversed after reoxygenation in a time-dependent manner in A549 NSCLC cells [40]. By binding with the DNA helicase Xeroderma pigmentosum group B (XPB), active p53 allows nucleotide excision repair and protects cells from chemotherapy-induced apoptosis. Studies found that in colorectal cancer (CRC) cell lines, prolyl hydroxylase domain protein PHD1 silencing could hinder p53 activation on chemotherapy treatment and reduce DNA repair to favor cell death. The simultaneous observation was that PHD1 silencing sensitized the response of CRC to 5-FU in mice [122]. Such a new posttranslational modification in the p53 field may be used in combination with chemotherapy to increase sensitivity to treatments.
Hypoxia-mediated reduced efficiency in chemotherapy
Chemotherapy drugs are still the cornerstone of cancer treatment. They have an oxygen-dependent effect on the killing of tumor cells, most of which kill cells by oxidizing free radicals and ROS in cells. The antibiotic bleomycin has a reduced efficiency in chemotherapy under hypoxic conditions, which may be related to the reduction of free radicals. Triche et al. showed that platinum-based chemotherapeutic drugs generated free radicals in cells, which captured electrons and delivered them to oxygen, thereby killing cells [123]. Wozniak et al. found that etoposide inhibited DNA strands more frequently under hypoxic conditions than under normoxic conditions due to increased levels of free radical scavenger dehydrogenase inhibitors and dehydrogenase substrates [124]. The aforementioned studies suggested that hypoxia had a significant inhibitory effect on the efficiency of chemotherapy. Hence, cancer resistance could be overcome by accurately interfering with hypoxia to improve the therapeutic effect.
Targeting hypoxia and HIFs in cancer
Hypoxia is arguably one of the most attractive therapeutic targets in cancer. Several approaches for targeting hypoxic tumor cells have been proposed, including hypoxia-activated prodrugs, gene therapy and specific targeting of HIFs, or targeting pathways important in hypoxic cells such as mTOR and UPR pathways. Otherwise, this feature can be used to target acid-induced tumors because tumor tissues have lower pH values compared with normal tissues.
A prodrug is an inactive compound that can be converted into pharmacologically active substances spontaneously or through specific metabolic pathways [125]. An anoxic prodrug activated in anoxic tissues was designed to kill anoxic tumor cells selectively using the characteristics of anoxic tumors. Hypoxic prodrugs are activated by cellular reductases, reoxidized into initial drug progenitors in anoxic cells, and converted into cytotoxic substances. The results of the recently published phase II clinical trial on the hypoxic progenitor TH-302 combined with gemcitabine for pancreatic cancer or adriamycin for soft tissue sarcoma are encouraging [126]. The efficacy of another mitomycin C derivative prodrug praziquantel (EO9) has been shown in preclinical studies. Therefore, topical praziquantel is recommended as adjunctive therapy for patients undergoing bladder cancer surgery [127].
Another strategy is to target and regulate HIF-1α in solid tumors to overcome resistance to hypoxia. For example, strategies for hypoxia can be targeted at downstream HIF signaling pathways. Monoclonal antibodies that target VEGF (bevacizumab) or small-molecule inhibitors that target VEGF receptors have achieved clinical benefits for advanced cancer. Methods for inhibiting the HIF response to hypoxia include siRNA treatment, blocking the dimerization of HIF-1α and β subunits, and direct inhibition of HIF-1α using anticancer agents known to inhibit the PI3K/AKT/HIF-1α pathway. Furthermore, drugs that trigger the activation of HIF-1α degradation pathways have the potential to clear overexpressed HIF-1α in hypoxic tumors. Treatments of hypoxic cells with rapamycin lead to the degradation of HIF-1α, which results in the increased inhibition of the expression of survivin and apoptosis in lung cancer cells. In addition, Shukla et al. found that HIF-1α mediated the resistance of pancreatic cancer cells to gemcitabine by upregulating the expression of cytidine triphosphate synthase (CTPS1) and transketolase (TKT). When digoxigenin is used to inhibit the translation of the HIF-1α subunit, pancreatic cancer cells are more sensitive to gemcitabine [128]. Potential molecular therapeutic targets based on the TME and breast cancer mechanism have been widely studied. Studies have shown that HIF-1α induces P4HA1 expression in a hypoxic microenvironment, and P4H can regulate cell metabolism and enhance the activity of tumor cells. Hence, targeting P4H is a potential strategy to improve the treatment of breast cancer [129]. Cui et al. found that HIF-1α was involved in the transcriptional regulation of the TFPI gene, and the hypoxic microenvironment in breast tumors can induce the procoagulant status in patients with breast cancer [130]. HIF-1α may be a target for the treatment of breast cancer–related coagulation and thrombosis. The oxygen sensor hypoxia-inducible factor prolyl hydroxylase 2 (PHD2) is considered to be the major regulator of HIF-1α. Kozlova et al. described a significant positive correlation between PHD2 and EGFR expression and introduced hypoxia/PHD2-mediated signaling and EGFR-induced tumor mitigation in patients with breast cancer [131]. This is of profound significance for the targeted treatment of breast cancer.
As illustrated earlier, cancer cells have higher levels of ROS and overexpressed antioxidant enzymes. Therefore, the elimination of enzymes involved in antioxidant defense results in higher oxidative stress, thus leading to the death of resistant cells. ROS modulators are effective in overcoming MDR in cancer cells through this principle of action. Moreover, target acid–induced tumors can improve the specificity of tumor therapy. In recent years, the application of proton pump inhibitors (PPIs) in treating the acidic environment is popular. Studies have shown that PPIs can increase the uptake of cisplatin cells in an acid-dependent manner and enhance the role of cytotoxic agents in chemotherapy-resistant epithelial ovarian cancer [89, 132]. Intervention strategies targeting the acidic microenvironment and novel combination therapy strategies may be future research directions.
Future perspectives
Drug resistance is a complex multifactorial phenomenon. Despite significant advancements in cancer care, especially the development of targeted anticancer therapies, the mechanisms of protecting cells from cytotoxic compounds mediated by tumor–host interactions continue to play a primary role as obstacles to cancer therapy. Evidence shows that these mechanisms determine the sensitivity to cancer treatment. Chemotherapy drugs exert an influence on biological damage by activating diverse signaling pathways of the cell death program. Defects in cell death processes controlling apoptosis (such as tolerating DNA damage) or promoting survival (such as efficient repair) lead to insensitivity to antitumor agents. In addition, in vitro toxicity screening performed at the standard air pressure is of paramount importance, but fails to recognize the impact of the TME. Fortunately, it is now widely acknowledged that hypoxia is responsible not only for pharmacokinetics but also for supporting the selection of more malignant cells or even resistance. Many cancer research laboratories are actively involved in the identification of novel therapies to target hypoxia. According to the characteristics of HIF in tumor biological behavior, the therapeutic strategies can be optimized by combining with the molecular mechanisms that drive therapy resistance, thus helping in the progress of cancer care. Therefore, a detailed understanding of the regulation of apoptosis, growth inhibition, and DNA repair, besides the identification of defects in the death pathway, may provide insights into the mechanisms of clinically relevant drug resistance. Targeting hypoxia is a potential therapy to eradicate the progression of various cancers and enable long-term survival for patients.
Conclusions
Clinical studies have demonstrated that the components in the tumor hypoxic microenvironment are associated with poor prognosis in patients and can promote apoptosis and autophagy or inhibit DNA damage and mitochondrial activity through a number of signaling pathways associated with the failure of immunotherapy, chemotherapy, or radiation therapy. Especially evident in advanced metastatic cancer, a hypoxic environment is often established, which plays an important role in cancer evolution. Further investigations proved that HIF-1α was involved in hypoxia-induced therapy resistance, and its knockdown could reverse the resistance. Hence, it was inferred that the selection pressure of tumor hypoxic environment ecology could affect the evolution of cancer cells. HIF-1 facilitates glycolysis and lactate production through the key enzymes of glycolysis and LDH-A, thereby inhibiting the entry of pyruvate into the TCA cycle. Furthermore, the HIF-1 target gene PDK1 directly inhibits the migration of pyruvate to mitochondria by inactivating pyruvate dehydrogenase (PDH). An increasing number of studies have shown that HIF-1 can induce mitochondrial autophagy and inhibit mitochondrial biosynthesis to inhibit cell death, ultimately leading to HIF-1-mediated resistance. The hypoxia-induced acidic microenvironment of tumor is very important for chemoresistance, in some cases promoting EMT and stem cell–like phenotypes. Major factors such as V-ATPase, NHE, and MCT in regulating the tumor acidic microenvironment also provide targets for tumor therapy. Therefore, understanding the regulation of these molecules is important for identifying potential therapeutic targets. A better understanding of the pathways in the hypoxic environment during tumor progression may contribute to breakthroughs in cancer immunotherapy research and provide a theoretical basis for clinical trials to help improve treatment outcomes.
Acknowledgments
None.
Abbreviations
- ABC
ATP-binding cassette
- Bak
BCL2 antagonist/killer
- BAX
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma-2
- bHLH
Basic helix-loop-helix
- CBP
CREB-binding protein
- CHK2
Checkpoint kinase 2
- CMA
Chaperone-mediated autophagy
- COX4
Cytochrome c oxidase subunit IV
- CQ
Chloroquine
- CRC
Colorectal cancer
- Cyt c
Cytochrome c
- DED
Death-effector domain
- DSBs
Double-strand break
- EM
Estramustine
- FCM
Flow cytometry
- HCQ
Hydroxychloroquine
- HIF-1α
Hypoxia-inducible factor 1α
- HIFs
Hypoxia-inducible factors
- LDH-A
Lactate dehydrogenase-A
- Mcl-1
Myeloid cell leukemia-1
- MRP1
Multidrug resistance protein 1
- mTOR
Mammalian target of rapamycin
- NER
Nucleotide excision repair
- NHEJ
Nonhomologous end-joining
- Nrf2
Nuclear factor (erythroid-derived 2)-like factor 2
- NSCLC
Non-small-cell lung cancer
- NTD
N-terminal domain
- OSCC
Oral squamous cell carcinoma cell
- PAS
Per-ARNT-Sim
- PDK1
Pyruvate dehydrogenase kinase1
- P-gp
P-glycoprotein
- PHD
Prolyl hydroxylase domain
- ROS
Reactive oxygen species
- TCA
Tricarboxylic acid cycle
- TME
Tumor microenvironment
- TMEM45A
Transmembrane protein 45A
- TYR-2
Tyrosinase-2
- ULK
Unc-51-likekinase
- UPR
Unfolded protein response
- VEGF
Vascular endothelial growth factor
- VHL
Von Hippel–Lindau
- XPB
Xeroderma pigmentosum group B
Authors’ contributions
YS, HS, and FY designed the study. XJ and FY drafted the manuscript. CS and KW critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Jiangsu Province (Grants No BK20171484), the Project of Invigorating Health Care through Science, Technology, and Education (Jiangsu Provincial Medical Youth Talent QNRC2016856), the National Natural Science Foundation of China (No. 81672896), the Summit of the Six Top Talents Program of Jiangsu Province (2017-WSN-179), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_1483), the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX18_1482), The Natural Science Foundation of China (81874230) and the Jiangsu Social Development Project (BE2018726).
Availability of data and materials
The material supporting the conclusion of this study has been included in the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinming Jing, Fengming Yang and Chuchu Shao contributed equally to this work.
Contributor Information
Hua Shen, Email: medshenhua@126.com.
Yongqian Shu, Email: shuyongqian1998@163.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods in molecular medicine. 2005;111:127. doi: 10.1385/1-59259-889-7:127. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380:205–215. doi: 10.1016/j.canlet.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17:120. doi: 10.1186/s12943-018-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 9.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 10.Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piazza M, Gatter KC, Harris AL. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001;61:1830–1832. [PubMed] [Google Scholar]
- 11.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 13.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 15.Roma-Rodrigues Catarina, Mendes Rita, Baptista Pedro, Fernandes Alexandra. Targeting Tumor Microenvironment for Cancer Therapy. International Journal of Molecular Sciences. 2019;20(4):840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- 17.Huang Y, Lin D, Taniguchi CM. Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe? Sci China Life Sci. 2017;60:1114–1124. doi: 10.1007/s11427-017-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br J Radiol. 2014;87:20130676. doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 20.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 24.Nardinocchi L, Puca R, Sacchi A, D'Orazi G. Inhibition of HIF-1alpha activity by homeodomain-interacting protein kinase-2 correlates with sensitization of chemoresistant cells to undergo apoptosis. Mol Cancer. 2009;8:1. doi: 10.1186/1476-4598-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7:1961–1973. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 26.He J, Pei L, Jiang H, Yang W, Chen J, Liang H. Chemoresistance of colorectal cancer to 5-fluorouracil is associated with silencing of the BNIP3 gene through aberrant methylation. J Cancer. 2017;8:1187–1196. doi: 10.7150/jca.18171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia S, Yu S, Yuan X. Effects of hypoxia on expression of P-gp and mutltidrug resistance protein in human lung adenocarcinoma A549 cell line. J Huazhong Univ Sci Technolog Med Sci. 2005;25:279–281. doi: 10.1007/BF02828142. [DOI] [PubMed] [Google Scholar]
- 28.Zunino F, Perego P, Pilotti S, Pratesi G, Supino R, Arcamone F. Role of apoptotic response in cellular resistance to cytotoxic agents. Pharmacol Ther. 1997;76:177–185. doi: 10.1016/S0163-7258(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 29.van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 30.Greijer AE. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465:577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP 1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu T, Wang L, Zhang L, Lu L, Shen J, Chan RLY, Li M, Wu WKK, To KKW, Cho CH. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine. 2015;22:536–544. doi: 10.1016/j.phymed.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Yan Y, Xu Z, Dai S, Qian L, Sun L, Gong Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res. 2016;35:23. doi: 10.1186/s13046-016-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flamant L, Roegiers E, Pierre M, Hayez A, Sterpin C, De Backer O, Arnould T, Poumay Y, Michiels C. TMEM45A is essential for hypoxia-induced chemoresistance in breast and liver cancer cells. BMC Cancer. 2012;12:391. doi: 10.1186/1471-2407-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madan E, Gogna R, Pati U. p53 Ser15 phosphorylation disrupts the p53-RPA70 complex and induces RPA70-mediated DNA repair in hypoxia. Biochem J. 2012;443:811–820. doi: 10.1042/BJ20111627. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA, Kim J, Yustein JT, Lee LA, Dang CV. Myc stimulates Nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasabe E, Tatemoto Y, Li D, Yamamoto T, Osaki T. Mechanism of HIF-1alpha-dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005;96:394–402. doi: 10.1111/j.1349-7006.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 40.Deschoemaeker S, Di Conza G, Lilla S, Martin-Perez R, Mennerich D, Boon L, Hendrikx S, Maddocks OD, Marx C, Radhakrishnan P, et al. PHD1 regulates p53-mediated colorectal cancer chemoresistance. Embo Mol Med. 2015;7:1350–1365. doi: 10.15252/emmm.201505492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 42.Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whatcott CJ, Han H, Von Hoff DD. Orchestrating the tumor microenvironment to improve survival for patients with pancreatic Cancer: normalization, not destruction. Cancer J. 2015;21:299–306. doi: 10.1097/PPO.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 46.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64. doi: 10.1016/j.semcancer.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mule JJ, Ibrahim-Hashim A, Gillies RJ. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76:1381–1390. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sgarbi Gianluca, Gorini Giulia, Liuzzi Francesca, Solaini Giancarlo, Baracca Alessandra. Hypoxia and IF1 Expression Promote ROS Decrease in Cancer Cells. Cells. 2018;7(7):64. doi: 10.3390/cells7070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X, Zuo L. Characterization of oxygen radical formation mechanism at early cardiac ischemia. Cell Death Dis. 2013;4:e787. doi: 10.1038/cddis.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the Pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bridge G, Rashid S, Martin SA. DNA mismatch repair and oxidative DNA damage: implications for cancer biology and treatment. Cancers (Basel) 2014;6:1597–1614. doi: 10.3390/cancers6031597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syu JP, Chi JT, Kung HN. Nrf2 is the key to chemotherapy resistance in MCF7 breast cancer cells under hypoxia. Oncotarget. 2016;7:14659–14672. doi: 10.18632/oncotarget.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y, Yang J, Zhao J, Xiao C, Xu C, Xiang Y. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: a survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp Cell Res. 2015;334:207–218. doi: 10.1016/j.yexcr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 58.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 60.Saggar JK, Yu M, Tan Q, Tannock IF. The tumor microenvironment and strategies to improve drug distribution. Front Oncol. 2013;3:154. doi: 10.3389/fonc.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to Mesenchymal transition in colorectal Cancer cells. Gastroenterology. 2017;153:505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 62.Ueda S, Saeki T, Osaki A, Yamane T, Kuji I. Bevacizumab induces acute hypoxia and Cancer progression in patients with refractory breast Cancer: multimodal functional imaging and multiplex cytokine analysis. Clin Cancer Res. 2017;23:5769–5778. doi: 10.1158/1078-0432.CCR-17-0874. [DOI] [PubMed] [Google Scholar]
- 63.Chen A, Sceneay J, Godde N, Kinwel T, Ham S, Thompson EW, Humbert PO, Moller A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene. 2018;37:4214–4225. doi: 10.1038/s41388-018-0259-3. [DOI] [PubMed] [Google Scholar]
- 64.Lin Steven H, Koong Albert C. Breathing New Life Into Hypoxia-Targeted Therapies for Non–Small Cell Lung Cancer. JNCI: Journal of the National Cancer Institute. 2017;110(1):1–2. doi: 10.1093/jnci/djx163. [DOI] [PubMed] [Google Scholar]
- 65.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 66.Daponte A, Ioannou M, Mylonis I, Simos G, Minas M, Messinis IE, Koukoulis G. Prognostic significance of hypoxia-inducible factor 1 alpha (HIF-1 alpha) expression in serous ovarian cancer: an immunohistochemical study. BMC Cancer. 2008;8:335. doi: 10.1186/1471-2407-8-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayer A, Hockel M, Wree A, Leo C, Horn LC, Vaupel P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008;68:4719–4726. doi: 10.1158/0008-5472.CAN-07-6339. [DOI] [PubMed] [Google Scholar]
- 68.Simiantonaki N, Taxeidis M, Jayasinghe C, Kurzik-Dumke U, Kirkpatrick CJ. Hypoxia-inducible factor 1 alpha expression increases during colorectal carcinogenesis and tumor progression. BMC Cancer. 2008;8:320. doi: 10.1186/1471-2407-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun HC, Qiu ZJ, Liu J, Sun J, Jiang T, Huang KJ, Yao M, Huang C. Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Oncol. 2007;30:1359–1367. [PubMed] [Google Scholar]
- 70.Zhang JJ, Wu HS, Wang L, Tian Y, Zhang JH, Wu HL. Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J Gastroenterol. 2010;16:2881–2888. doi: 10.3748/wjg.v16.i23.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M, Metzger R, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–679. doi: 10.1593/neo.08292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhitomirsky B, Assaraf YG. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Zub KA, Sousa MM, Sarno A, Sharma A, Demirovic A, Rao S, Young C, Aas PA, Ericsson I, Sundan A, et al. Modulation of cell metabolic pathways and oxidative stress signaling contribute to acquired melphalan resistance in multiple myeloma cells. PLoS One. 2015;10:e119857. doi: 10.1371/journal.pone.0119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Xiang J, Zhang SS, Liu BZ, Gong F, Peng MQ. Analysis of the impact of extracellular acidity on the expression and activity of P-glycoprotein and on the P-glycoprotein-mediated cytotoxicity of daunorubicin in cancer cell by microfluidic chip technology. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:75–81. doi: 10.3881/j.issn.1000-503X.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. J Clin Invest. 2016;126:3689–3698. doi: 10.1172/JCI84430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva VL, Al-Jamal WT. Exploiting the cancer niche: tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J Control Release. 2017;253:82–96. doi: 10.1016/j.jconrel.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 77.GILLET J, EFFERTH T, REMACLE J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2007;1775:237–262. doi: 10.1016/j.bbcan.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, Zhang Y, Liang H. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One. 2014;9:e98882. doi: 10.1371/journal.pone.0098882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 82.Mow BMF, Blajeski AL, Chandra J, Kaufmann SH. Apoptosis and the response to anticancer therapy. Curr Opin Oncol. 2001;13:453–462. doi: 10.1097/00001622-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, Lenardo MJ. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 85.Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C, Stratford IJ, Dive C. Hypoxia-mediated down-regulation of bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakamoto H, Mashima T, Kizaki A, Dan S, Hashimoto Y, Naito M, Tsuruo T. Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood. 2000;95:3214–3218. doi: 10.1182/blood.V95.10.3214. [DOI] [PubMed] [Google Scholar]
- 87.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakamoto H, Mashima T, Sato S, Hashimoto Y, Yamori T, Tsuruo T. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res. 2001;7:2513–2518. [PubMed] [Google Scholar]
- 89.Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, Cramer T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5:e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Achison M, Hupp TR. Hypoxia attenuates the p53 response to cellular damage. Oncogene. 2003;22:3431–3440. doi: 10.1038/sj.onc.1206434. [DOI] [PubMed] [Google Scholar]
- 91.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Lei Y, Yao N, Wang C, Hu N, Ye W, Zhang D, Chen Z. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36. [DOI] [PMC free article] [PubMed]
- 93.Li C, Liao W, Wu M, Chu P. New insights into the role of autophagy in tumor immune microenvironment. Int J Mol Sci. 2017;18:1566. doi: 10.3390/ijms18071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen S, Rehman SK, Zhang W, Wen A, Yao L, Zhang J. Autophagy is a therapeutic target in anticancer drug resistance. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2010;1806:220–229. doi: 10.1016/j.bbcan.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Su Y, Zhu H, Cao J, Ding W, Zhao Y, He Q, Yang B. HIF-1α-dependent autophagy protects HeLa cells from fenretinide (4-HPR)-induced apoptosis in hypoxia☆. Pharmacol Res. 2010;62:416–425. doi: 10.1016/j.phrs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Yang M, Zeng P, Kang R, Yu Y, Yang L, Tang D, Cao L. S100A8 contributes to drug resistance by promoting autophagy in leukemia cells. PLoS One. 2014;9:e97242. doi: 10.1371/journal.pone.0097242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Pan B, Chen D, Huang J, Wang R, Feng B, Song H, Chen L. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma. Mol Cancer. 2014;13:165. doi: 10.1186/1476-4598-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan T, Zhang C, Zong M, Zhao Q, Yang X, Hao C, Zhang H, Yu S, Guo J, Gong R, et al. Peptidylarginine deiminase IV promotes the development of chemoresistance through inducing autophagy in hepatocellular carcinoma. Cell Biosci. 2014;4:49. doi: 10.1186/2045-3701-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766. doi: 10.1038/cddis.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O'Donovan TR, O'Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy. 2011;7:509–524. doi: 10.4161/auto.7.5.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin WM, Li ZG. Blockage of cisplatin-induced autophagy sensitizes cervical cancer cells to cisplatin. Genet Mol Res. 2015;14:16905–16912. doi: 10.4238/2015.December.14.18. [DOI] [PubMed] [Google Scholar]
- 103.Wu HM, Shao LJ, Jiang ZF, Liu RY. Gemcitabine-induced autophagy protects human lung Cancer cells from apoptotic death. Lung. 2016;194:959–966. doi: 10.1007/s00408-016-9936-6. [DOI] [PubMed] [Google Scholar]
- 104.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 105.Olcina M, Lecane PS, Hammond EM. Targeting hypoxic cells through the DNA damage response. Clin Cancer Res. 2010;16:5624–5629. doi: 10.1158/1078-0432.CCR-10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M, Guo L, Wu Q, Zeng T, Lin Q, Qiao Y, Wang Q, Liu M, Zhang X, Ren L, et al. ATR/Chk1/Smurf1 pathway determines cell fate after DNA damage by controlling RhoB abundance. Nat Commun. 2014;5. [DOI] [PubMed]
- 107.Smith J, Tho LM, Xu N, Gillespie DA. The ATM–Chk2 and ATR–Chk1 Pathways in DNA Damage Signaling and Cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 108.Wirthner R, Wrann S, Balamurugan K, Wenger RH, Stiehl DP. Impaired DNA double-strand break repair contributes to chemoresistance in HIF-1 -deficient mouse embryonic fibroblasts. Carcinogenesis. 2008;29:2306–2316. doi: 10.1093/carcin/bgn231. [DOI] [PubMed] [Google Scholar]
- 109.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. [PubMed] [Google Scholar]
- 111.Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. Embo Mol Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 113.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Zeller KI, Zhao X, Charlie WHL, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. P Natl Acad Sci Usa. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-α to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/S1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 116.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22:1239–1249. doi: 10.1038/cdd.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2:e164. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koshikawa N, Maejima C, Miyazaki K, Nakagawara A, Takenaga K. Hypoxia selects for high-metastatic Lewis lung carcinoma cells overexpressing Mcl-1 and exhibiting reduced apoptotic potential in solid tumors. Oncogene. 2006;25:917–928. doi: 10.1038/sj.onc.1209128. [DOI] [PubMed] [Google Scholar]
- 120.Kim CY, Tsai MH, Osmanian C, Graeber TG, Lee JE, Giffard RG, DiPaolo JA, Peehl DM, Giaccia AJ. Selection of human cervical epithelial cells that possess reduced apoptotic potential to low-oxygen conditions. Cancer Res. 1997;57:4200. [PubMed] [Google Scholar]
- 121.Wohlkoenig C, Leithner K, Deutsch A, Hrzenjak A, Olschewski A, Olschewski H. Hypoxia-induced cisplatin resistance is reversible and growth rate independent in lung cancer cells. Cancer Lett. 2011;308:134–143. doi: 10.1016/j.canlet.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 122.Teicher BA, Holden SA, Jacobs JL. Approaches to defining the mechanism of enhancement by Fluosol-DA 20% with carbogen of melphalan antitumor activity. Cancer Res. 1987;47:513–518. [PubMed] [Google Scholar]
- 123.Wozniak AJ, Glisson BS, Hande KR, Ross WE. Inhibition of etoposide-induced DNA damage and cytotoxicity in L1210 cells by dehydrogenase inhibitors and other agents. Cancer Res. 1984;44:626–632. [PubMed] [Google Scholar]
- 124.Denny WA. The role of hypoxia-activated prodrugs in cancer therapy. Lancet Oncol. 2000;1:25–29. doi: 10.1016/S1470-2045(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 125.Hunter FW, Young RJ, Shalev Z, Vellanki RN, Wang J, Gu Y, Joshi N, Sreebhavan S, Weinreb I, Goldstein DP, et al. Identification of P450 Oxidoreductase as a major determinant of sensitivity to hypoxia-activated Prodrugs. Cancer Res. 2015;75:4211–4223. doi: 10.1158/0008-5472.CAN-15-1107. [DOI] [PubMed] [Google Scholar]
- 126.Phillips RM, Hendriks HR, Peters GJ. EO9 (Apaziquone): from the clinic to the laboratory and back again. Br J Pharmacol. 2013;168:11–18. doi: 10.1111/j.1476-5381.2012.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic Cancer. Cancer Cell. 2017;32:392. doi: 10.1016/j.ccell.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 128.Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM, O'Connor K, Lane AN, Xu R. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1alpha stabilization and TNBC chemoresistance. Nat Commun. 2018;9:4456. doi: 10.1038/s41467-018-06893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui XY, Tinholt M, Stavik B, Dahm AE, Kanse S, Jin Y, Seidl S, Sahlberg KK, Iversen N, Skretting G, Sandset PM. Effect of hypoxia on tissue factor pathway inhibitor expression in breast cancer. J Thromb Haemost. 2016;14:387–396. doi: 10.1111/jth.13206. [DOI] [PubMed] [Google Scholar]
- 130.Kozlova N, Wottawa M, Katschinski DM, Kristiansen G, Kietzmann T. Hypoxia-inducible factor prolyl hydroxylase 2 (PHD2) is a direct regulator of epidermal growth factor receptor (EGFR) signaling in breast cancer. Oncotarget. 2017;8:9885–9898. doi: 10.18632/oncotarget.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D'Ilio S, Lugini L, Violante N, Azzarito T, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9:e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee YY, Jeon HK, Hong JE, Cho YJ, Ryu JY, Choi JJ, Lee SH, Yoon G, Kim WY, Do IG, et al. Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget. 2015;6:35040–35050. doi: 10.18632/oncotarget.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the conclusion of this study has been included in the manuscript.