Summary
Background
A phase 1/2a dose escalation study of APC-100 (2,2,5,7,8-Pentamethyl-6-chromanol) was conducted to determine maximum tolerated dose (MTD), recommended phase 2 dose, toxicities and efficacy in men with castrate-resistant prostate cancer (CRPC).
Methods
This open label phase 1/2a study utilizes a time-to-event reassessment method (TITE-CRM) design. Patients in cohorts of 3 were treated with escalating doses of APC-100 (900 mg-2400 mg) orally once daily continuously. Cycles were 28 days.
Results
Twenty patients with CRPC were enrolled in the dose escalation cohort. One possible DLT (elevated ALT) was seen at dose level 1. No other DLTs were seen and no dose reductions were required. Most frequent AEs included nausea (grade 1 in 6 patients) and elevated transaminases (grade 1–3 in 5 patients). After enrolment of 20 patients the MTD was not reached, however the maximal feasible dose was exceeded due to the number of capsules ingested. Five of the 20 patients had stable disease as their best response. The median progression free survival (PFS) for the cohort was 2.8 months (range 1–8).
Conclusions
APC-100 is a novel agent with dual mechanism of action functioning both as potent antioxidant as well as antiandrogen. No detectable APC-100 was found in the plasma at dose level 5 (2100 mg) and it was felt that maximal feasibility was nearly reached. APC-100 is being reformulated as a tablet to allow further dose escalation. Once a recommended phase 2 dose is established, future studies in prostate cancer chemoprevention should be conducted.
Keywords: APC-100, Phase I, Prostate cancer, Oxidative stress
Introduction
Prostate cancer is the most common cancer diagnosis and the second cancer-leading cause of death in men [1]. Primary treatment with radiation therapy or surgery is offered with curative intent; however, 1/3 of these men will experience disease relapse [2]. While some men may still be cured with salvage therapies, most eventually require treatment with androgen deprivation therapy (ADT) [3].
Active surveillance has emerged as an attractive alternative for carefully selected patients at diagnosis, as well as in men with biochemically relapsed disease [4, 5]. While androgen-deprivation therapy is commonly used to delay progression, it is associated with short and long terms side effects [6]. As such, both the above categories represent a population for whom new treatments could be safely evaluated.
Normal prostate epithelium and prostate carcinoma exhibit high levels of oxidative stress [7-9]. Several anti-oxidants have been tested for the prevention of prostate cancer, with somewhat conflicting results [10, 11]. Androgen signaling pathways are directly involved in the production of this high oxidative stress [12, 13] and it has been postulated that this stress plays a role in the occurrence, recurrence and progression of prostate cancer [14]. Thus, modulating oxidative stress has emerged as an attractive strategy in the treatment of prostate cancer by restoring castration sensitivity or delaying castration resistance.
APC-100 (2,2,5,7,8-pentamethyl-6-chromanol) is the antioxidant moiety of vitamin E (α-tocopherol) [15]. It possesses only the 6-chromanol moiety associated with in vivo signal transduction activity, but has no phytyl chain, leading to a higher solubility in aqueous solution while retaining its in vitro anti-oxidant activity [15]. APC-100 has also been found to carry anti-androgenic properties, with preclinical studies demonstrating potent androgen receptor (AR) signaling modulation and anti-cancer activity against prostate cancer cell lines [15]. Furthermore, APC-100 has a strong anti-tumor effect as seen repeatedly in a prostate cancer mouse model in vivo (data not published). Based on the preclinical data and the proposed mechanism of action in prostate cancer, we conducted a multicenter phase 1/2a dose escalation study of APC-100 in patients with metastatic and non-metastatic castrate-resistant prostate cancer (CRPC).
Material and methods
Patients
Eligible patient were >18 years old with histopathologically confirmed prostate adenocarcinoma and either measurable or non-measurable progressive disease despite androgen deprivation treatment and testosterone level of <50 ng/dL. Non-surgically castrated patients were required to continue treatment with a luteinizing hormone-releasing hormone (LHRH) agonist for the duration of the study. Prior treatment with antiandrogen therapies was permitted, as long as there was continued evidence of progressive disease despite discontinuation of either flutamide for ≥4 weeks or bicalutamide or nilutamide for ≥6 weeks. All patients were required to have an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 2 and adequate renal, liver and bone marrow reserve (defined as serum creatinine <2.0 mg/dL and/or calculated creatinine clearance >40 mL/min [Cockcroft-Gault formula]; alanine aminotransferase and aspartate aminotransferase ≤1.5 x the institutional upper limit of normal [ULN] and total bilirubin within normal limits at the time of initial evaluation; absolute neutrophil count [ANC] > 1500/μL, hemoglobin >9 mg/dL and platelet count >100.000/μL). Key exclusion criteria included prior secondary hormonal therapies or other products that are known to decrease PSA levels, prior systemic chemotherapy for CRPC, prior radiotherapy completed <4 weeks from enrollment, history of coagulopathy or treatment with anticoagulation agents and intake of products containing vitamin E. The trial was approved by the Institutional Review Board and was conducted in accordance to the Declaration of Helsinki for human subject protection. All patients provided written informed consent prior to study entry.
Study design and treatment plan
This was a multicenter, open label, non-randomized, phase 1 dose-escalation study to determine the MTD and the recommended phase 2 dose of APC-100. Secondary endpoints included PK analysis, safety, tolerability and antitumor activity of APC-100 based on PSA response and RECIST 1.1 criteria for patients with measurable disease. A time-to-event continual reassessment method (TITE-CRM) design was used and patients were enrolled in cohorts of 3. TITE-CRM design was chosen due to the fact that it can better evaluate for late-onset toxicities, while reducing time to complete accrual [16]. DLTs were classified as either acute (during cycle 1) or late-onset (during cycles 2 and 3) and the MTD was defined as the highest dose of APC-100 at which less than one third of patients experienced a DLT. After the first patient completed one cycle of APC-100 treatment, subsequent patients were accrued in cohorts of 3 and dose assignment was determined using the TITE-CRM algorithm. Dose escalation was not allowed until at least one patient completed the observation period without experiencing a DLT and escalations was restricted to increments of 300 mg. If at any dose level more than 33 % of patients experienced acute DLTs, the dose was de-escalated to the next lower level or the dose level calculated by the TITE-CRM algorithm, whichever was lower.
APC-100 was provided by Adamis Pharmaceuticals Corporation (San Diego, CA) in 150 mg capsules and was administered orally once daily continuously with the requirement of fasting 1 h before and after dosing. The starting dose of APC-100 was 900 mg with a stepwise escalation as shown in Table 2. Cycle length was 28 days.
Table 2.
Dose levels and DLTs
| Dose Level | N | Dose of APC-100 | Number of patients with DLT/Description |
|---|---|---|---|
| 1 | 6 | 900 mg | 1 grade 3 ALT elevation |
| 2 | 3 | 1200 mg | 0 |
| 3 | 3 | 1500 mg | 0 |
| 4 | 3 | 1800 mg | 0 |
| 5 | 3 | 2100 mg | 0 |
| 6 | 2 | 2400 mg | 0 |
Safety and efficacy assessment
Acute and late-onset DLTs were assessed according to National Cancer Institute Common Toxicity Criteria (CTCAE) version 4.0 (effective May 17, 2010). DLTs to APC-100 were defined as any of the following: grade ≥ 3 neutropenia for >7 days duration, grade ≥2 thrombocytopenia for >7 days duration and any other grade ≥ 3 toxicity as per CTCAE (excluding nausea, vomiting and diarrhea). In addition, grade 3 and 4 nausea, vomiting and diarrhea despite maximal antiemetic or antidiarrheal therapy were considered DLTs.
All patients that received APC-100 but discontinued treatment due to toxicity were considered evaluable for toxicity regardless of the duration of treatment. However, patients who discontinued treatment before completing ≥75 % (21 days) of the planned cycle 1 for reasons other than toxicity were considered unevaluable. Dose reductions by 300 mg were permitted after adequate recovery time for any DLTs and for subjectively intolerable grade 2 toxicities with the provision that no patient would be allowed to get a dose below 900 mg/day.
All patients who received at least one dose of APC-100 were evaluable for efficacy. In addition to assessment for clinical response, each patient was assessed for biochemical and radiographic response. PSA testing was repeated on day 1 of each cycle and radiographic evaluation was repeated every 3 months or earlier as clinically indicated.
Pharmacokinetic analysis
PK analysis of APC-100 was scheduled at pre-dose, and 1, 2, 3, 4, 6, 9, 12, and 24 h after the dose of APC-100 on day 1 of cycle 1 (C1D1) and day 1 of cycle 2 (C2D2). Additionally, patients in phase 1 underwent a 24 h urine collection on C1D1 and C2D1 to assess the renal excretion of APC-100. For each additional cycle, the phase 1 patients underwent a blood draw prior to dosing on day 1. All samples were drawn into 8 mL K2EDTA tubes and centrifuged at 3000 rpm for 15 min and 4 °C to obtain plasma. Plasma was aliquoted into cryovials and stored at −80 °C until analysis. Plasma and urine samples were extracted with acetonitrile, and analysis was performed by LC/MS. The in-run accuracy and precision were reported to range from 89.2 to 107 % with corresponding coefficients of variation (%CVs) of 2.95–11.1 % for the back-calculated standards, while the interday accuracy and precision ranged from 99.4 to 105 % with %CVs of 3.06–6.66 %. The linear range was 50–10,000 ng/ml, with correlation coefficients at 0.99.
Statistical analysis
Dose escalation of APC-100 was performed using the TITE-CRM algorithm. The MTD was defined as the dose of APC-100 at which 33 % of the patients experience DLTs. The TITE-CRM method assumes a parametric model for the time to occurrence of toxic response as a function of dose and thereby allows information from all patients enrolled to be employed when assigning a dose to new patients entering the study. Specifically, the estimates of the probabilities of DLTs were continuously updated using a single-parameter logistic dose-toxicity model, logit(F(di, β) = 3 + exp.(β) × di, where di denotes the relabeled dose and β the dose-toxicity parameter. The posterior distribution of the dose-toxicity parameter β was calculated using the Gibbs sampling algorithm based on the logistic dose-toxicity model and the information obtained from all patients accrued to the trial. A Gibbs sample size of M = 10,000 was used with a burn-in period of 1000. Simulation studies were conducted before initiating the study to evaluate the operating characteristics of the TITE-CMR algorithm under various scenarios. The TITE-CRM algorithm was implemented using R software version 3.1 (http://www.r-project.org/).
Patient’s baseline characteristics were summarized using standard descriptive statistics. Toxicities were summarized by type and severity in tabular format. Progression-free survival was analyzed using the Kaplan-Meier method. Maximum percentage decrease in PSA from baseline to on-treatment assessment was displayed in graphical format using a waterfall plot. Statistical analysis was conducted using SAS software (SAS Institute Inc., Cary NC), version 9.3.
Results
Patient characteristics
Between August 2011 and March 2013, 20 patients with castrate-resistant metastatic and non-metastatic prostate cancer were enrolled in this study (Table 1). Median age was 72 years (range 54–87); 13 patients were Caucasians, 5 Blacks and 2 Asians. The median duration of treatment was 12 weeks (range 4–36) and the median number of cycles of treatment received was 3 (range 1–9). All patients were included in the safety and PK analyses.
Table 1.
Patient characteristics
| Patient Characteristics | (N = 20) |
|---|---|
| Age, median (range) | 72 (54–87) |
| Race | n (%) |
| White | 13 (65) |
| Black | 5 (25) |
| Asian | 2 (10) |
| Gleason Score | n (%) |
| 6 | 3 (15) |
| 7 | 4 (20) |
| 8 | 6 (30) |
| 9 | 5 (25) |
| 10 | 1 (5) |
| Unknown | 1 (5) |
| ECOG PS | n (%) |
| 0 | 9 (45) |
| 1 | 10 (50) |
| Unknown | 1 (5) |
| Site of Involvement | n (%) |
| Lymph nodes | 12 (60) |
| Bones | 11 (55) |
| Other | 8 (40) |
Dose escalation and toxicity
Dose escalation began at level 1 and continued to level 6. After enrollment of 20 patients the MTD was not reached. However the maximum feasibility dose was exceeded due to the high number of capsules ingested. All patients were available for assessment of DLTs. Only 1 DLT of elevated ALT was observed at dose level 1 and the patient was taken off study (Table 2). No other DLTs were observed in the escalated dose levels and no dose adjustments were required. No patients were enrolled in the phase 2a expansion cohort.
The most common adverse events at least possibly related to treatment and experienced by at least 10 % of the patients are summarized in Table 3. The most common adverse events were nausea (30 %, all grade 1), ALT/AST elevation (25 %, 2 patients with grade 1, 2 patients with grade 2 and 1 patient with grade 3), upset stomach (20 %, all grade 1), anorexia (10 %, all grade 1), bloating (10 %, all grade 1) and fatigue (10 %, all grade 1). No dose reduction was required.
Table 3.
Adverse events at least possibly related to APC-100 occurring in at least 10 % of subjects
| Adverse Event | Number of subjects (%) | ||||||
|---|---|---|---|---|---|---|---|
| APC-100, mg | |||||||
| 900 (n = 6) |
1200 (n = 3) |
1500 (n = 3) |
1800 (n = 3) |
2100 (n = 3) |
2400 (n = 2) |
Overall (n = 20) |
|
| Nausea | 3 (50) | 1 (33) | 2 (67) | 6 (30) | |||
| Elevated ALT/AST | 5 (83) | 5 (25) | |||||
| Upset stomach | 2 (33) | 1 (33) | 1 (33) | 4 (20) | |||
| Anorexia | 2 (33) | 2 (10) | |||||
| Bloating | 1 (17) | 1 (33) | 2 (10) | ||||
| Fatigue | 1 (17) | 1 (33) | 2 (10) | ||||
Antitumor effect
Five patients had stable disease as their best response (5/20, 25 %). From those 5 patients, 2 were treated at dose level 1 (900 mg/day), 1 at dose level 5 (2100 mg/day) and 2 at dose level 6 (2400 mg/day). The median PFS was 2.8 months (range 1–8) months. Figure 1 summarizes the effect of APC-100 on PSA level of the whole cohort while on treatment.
Fig. 1.
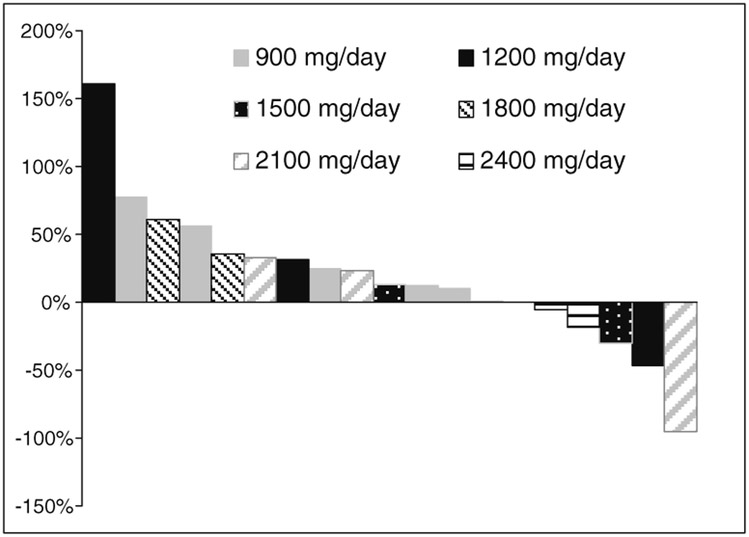
Waterfall plot of maximum percentage decrease in PSA from baseline to on-treatment assessment
Pharmacokinetics
APC-100 was undetectable at dose level 5; therefore no further PK analysis was performed.
Discussion
The effect of oxidative stress in the development and progression of prostate cancer has been studied extensively [17]. Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and antioxidant protection in favor of the former, leading to oxidative damage to DNA that is an early event in carcinogenesis [18].
Prostate cancer growth depends – at least initially - upon androgens through AR activation. In CRPC though, despite the low levels of circulating testosterone, the AR signaling pathway remains activated. Oxidative stress is believed to play a pivotal role in this activation via several different mechanisms, such as AR overexpression [19], induction of AR coregulators [20], and AR activation by growth factors and cytokines through intracellular signal-transduction pathways [21]. Thus, the rational for suppressing oxidative stress as a chemopreventive or therapeutic modality in prostate cancer is scientifically compelling.
APC-100 is an orally bioavailable derivative of vitamin E that has been shown to be an oxidative stress modulator [22]. Further, APC-100 binds to AR and acts as an androgen antagonist in prostate cancer cells [15]. Due to its dual mechanism of action, a phase 1/2a study was designed to determine the MTD and the recommended phase 2 dose of APC-100 in patients with metastatic and non-metastatic castrate-resistant prostate cancer and to assess its safety and tolerability, as well as seek preliminary evidence for antitumor activity. After enrollment of 20 patients in the phase 1 cohort the MTD was not reached. Subjects enrolled in dose level 5 received 2100 mg of APC-100 and expected plasma concentrations were anticipated to be much higher than the lower level of quantitation of the assay which was 50 ng/mL. Since APC-100 was not detected at any time after administration (samples collected 1, 2, 3, 4, 6, 9, 12, and 24 h after dosing), the most likely explanation is that APC-100 was rapidly converted to either an active or inactive metabolite. Alternative explanations include rapid and complete intracellular accumulation that resulted in no detection in the plasma or very slow absorption of the drug from the gastrointestinal tract.
As no detectable drug in the plasma was found at dose level 5, it was felt that continued dose-escalation of APC-100 was not warranted with the current drug formulation, as patients were required to take a high number of capsules daily that began to exceed what was clinically feasible. Common side effects were nausea, transaminasemia, upset stomach, anorexia, bloating and fatigue. Despite suboptimal drug exposure, there were some signs of activity as evident by PSA stabilization in 25 % of patients, thus it was elected to reformulate APC-100 into a tablet to allow further dose escalation. The dual activity of APC-100 as an antioxidant and anti-androgen suggests that future trials should focus on prostate cancer chemoprevention or non-castrate rising PSA state.
In conclusion, APC-100 was well tolerated. The maximum tolerated dose was not reached; however, the maximum feasibility dose was exceeded due to the high number of capsules ingested. Despite suboptimal drug exposure, there were signs of activity as evident by PSA stabilization in some patients. More studies are needed to explore the activity of APC-100 as a chemopreventive and therapeutic agent.
Acknowledgments
The investigators gratefully acknowledge the patients and families who participated in this study. Funding was provided by Adamis Pharmaceuticals Corporation, San Diego, CA, the National Institute of Health/National Cancer Institute Grant P30 CA014520 (University of Wisconsin Comprehensive Cancer Center Support) and the Prostate Cancer Clinical Trials Consortium Award PC131993.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281:1591–1597 [DOI] [PubMed] [Google Scholar]
- 3.Trock BJ, Han M, Freedland SJ, et al. (2008) Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299:2760–2769. doi: 10.1001/jama.299.23.2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall'Era MA, Albertsen PC, Bangma C, et al. (2012) Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 62:976–983. doi: 10.1016/j.eururo.2012.05.072 [DOI] [PubMed] [Google Scholar]
- 5.Antonarakis ES, Feng Z, Trock BJ, et al. (2012) The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int 109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwandt A, Garcia JA (2009) Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol 19:322–326. doi: 10.1097/MOU.0b013e32832a082c [DOI] [PubMed] [Google Scholar]
- 7.Cerutti PA (1985) Prooxidant states and tumor promotion. Science 227:375–381 [DOI] [PubMed] [Google Scholar]
- 8.Feig DI, Reid TM, Loeb LA (1994) Reactive oxygen species in tumorigenesis. Cancer Res 54:1890s–1894s [PubMed] [Google Scholar]
- 9.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK (2009) Oxidative stress in prostate cancer. Cancer Lett 282:125–136. doi: 10.1016/j.canlet.2008.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinonen OP, Albanes D, Virtamo J, et al. (1998) Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 90:440–446 [DOI] [PubMed] [Google Scholar]
- 11.Lippman SM, Klein EA, Goodman PJ, et al. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA 301:39–51. doi: 10.1001/jama.2008.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehraein-Ghomi F, Lee E, Church DR, Thompson TA, Basu HS, Wilding G (2008) JunD mediates androgen-induced oxidative stress in androgen dependent LNCaP human prostate cancer cells. Prostate 68:924–934. doi: 10.1002/pros.20737 [DOI] [PubMed] [Google Scholar]
- 13.Ripple MO, Henry WF, Rago RP, Wilding G (1997) Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst 89:40–48 [DOI] [PubMed] [Google Scholar]
- 14.Basu HS, Thompson TA, Church DR, et al. (2009) A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res 69:7689–7695. doi: 10.1158/0008-5472.CAN-08-2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson TA, Wilding G (2003) Androgen antagonist activity by the antioxidant moiety of vitamin E, 2,2,5,7,8-pentamethyl-6-chromanol in human prostate carcinoma cells. Mol Cancer Ther 2:797–803 [PubMed] [Google Scholar]
- 16.Cheung YK, Chappell R (2000) Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177–1182 [DOI] [PubMed] [Google Scholar]
- 17.Gupta-Elera G, Garrett AR, Robison RA, O'Neill KL (2012) The role of oxidative stress in prostate cancer. Eur J Cancer Prev 21:155–162. doi: 10.1097/CEJ.0b013e32834a8002 [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B (2006) Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiota M, Yokomizo A, Tada Y, et al. (2010) Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene 29:237–250. doi: 10.1038/onc.2009.322 [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Xie S, Jamaluddin MS, et al. (2005) Induction of androgen receptor expression by phosphatidylinositol 3-kinase/akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem 280:33558–33565 [DOI] [PubMed] [Google Scholar]
- 21.Shiota M, Yokomizo A, Naito S (2011) Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic Biol Med 51:1320–1328. doi: 10.1016/j.freeradbiomed.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 22.Koga T, Moro K, Terao J (1998) Protective effect of a vitamin E analog, phosphatidylchromanol, against oxidative hemolysis of human erythrocytes. Lipids 33:589–595 [DOI] [PubMed] [Google Scholar]


