Abstract
We propose that the influence of diet on colon cancer risk is mediated by the microbiota. To investigate how dietary fat influences risk, we compared the colonic contents of 12 adult high-risk African Americans (AAs) and 10 Caucasian Americans (CAs) who consumed a high-fat diet (123 ± 11 g/d and 129 17 g/d, respectively) to 13 native Africans (NAs) who subsisted on± a low-fat (383.0 g/d) diet, all aged 50–60 yr. The colonic bile acids were measured±by LC-MS and the short-chain fatty acids (SCFAs) by GC. The chief secondary colonic bile acids, deoxycholic acid and lithocholic acid, were correlated with fat intake and similar between AAs and CAs, but 3–4 times higher than in AAs (p < 0.05). The major SCFAs were lower in AAs (p < 0.001) and CAs (p < 0.001) compared to AAs, but conversely, the branched chain fatty acids (BFCA) were higher. Our results suggest that the higher risk of colon cancer in Americans may be partly explained by their high-fat and high-protein, low complex carbohydrate diet, which produces colonic residues that promote microbes to produce potentially carcinogenic secondary bile acids and less antineoplastic SCFAs. The role of BCFA in colonic carcinogenesis deserves further study.
INTRODUCTION
Colon cancer is the second leading cause of cancer death in Americans, and African Americans are at the highest risk of developing and dying from colon cancer (1). Based on November 2005 SEER data submission ((http://seer.cancer.gov/csr/ 1975 2003/), the age-adjusted incidence rate, based on cases diagnosed from 17 geographic areas, was 61.4 vs. 72.9 per 100,000 for White and Black males and 44.7 vs. 56.1 per 100,000 for White and Black females, respectively. Interestingly, Africans living in Africa rarely get this disease (1,2). The observation that colon cancer is more common in developed than in developing nations suggests that the Westernized way of life, and in particular the diet, may be responsible for the increased risk of the disease (3). We have, on the basis of our dietary investigations, suggested that it is the relatively high animal product and low complex carbohydrate intakes of Americans that places them at higher risk (2).
Although some dietary constituents may be directly carcinogenic, we have proposed that the diet mainly affects colonic mucosal health and cancer risk by its influence on the micro-biota to produce health-promoting or toxic metabolites (4). Thus Africans are protected by their high dietary intakes of complex carbohydrate, such as resistant starch, which increase the production of short-chain fatty acids (SCFAs) chiefly by Firmicutes of Clostridia cluster XIVa and IV (5). There is substantial evidence that SCFAs are also antiproliferative and antineoplastic (6), and one of them, butyrate, is the preferred energy source for colonocytes. On the other hand, a high meat diet stimulates the sulfate-reducing bacteria to produce hydrogen sulfide, which, experimentally, has been shown to be genotoxic (7). Furthermore, a high meat diet is usually accompanied by a high fat intake, which stimulates the synthesis and secretion of bile acids by the liver into the intestine. A proportion of these escape the enterohepatic circulation and enter the colon, where they stimulate 7α-dehydroxylating bacteria to produce secondary bile salts, which again have been shown to be carcinogenic in experimental models (8).
In support for our hypothesis, we have shown that fecal and colonic SCFAs are indeed higher in low-risk native Africans than high-risk Americans (9). We have also shown that 7α-dehydroxylating bacteria counts are higher in African Americans (2). Here we investigate the final link in the chain, fecal and colonic secondary bile acid contents between these 2 populations. In addition, we simultaneously investigate the relationship cancer risk has with the minor SCFA, including the branched chain fatty acids that are more influenced by dietary protein than carbohydrate.
METHODS
Materials
All bile acid and SCFA chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Econo-Cap EC-1000 GC capillary Column was purchased from Grace Davison Discovery Science (Deerfield, IL). Millex-GS 0.22 μm syringe filters and microconcentrators were purchased from Millipore (Bedford, MA), Luna C18 column (3 μm, 2.0 mm I.D. 150 mm; Phenomenex, Torrance, CA).
Study Population
Healthy male and female American volunteers, aged 50–60 yr, were selected because the risk of cancer increases appreciably after age 50. Subjects with a history of gastrointestinal disease, surgery, or antibiotic use during the previous 8 wk were excluded because this might influence colonic bacterial metabolism. For recruiting Caucasian and African American subjects who were from the Pittsburgh area, the protocol was reviewed and approved by the Institutional Review Boards of the University of Pittsburgh. For recruiting native Africans from urban Pretoria and the rural regions of Gauteng, South Africa, the protocol was reviewed and approved by the Medical University of Southern Africa Medical Ethics and Safety Committee.
Study Procedures
The study design, history taking, and methods have been previously described (2). Informed consent was obtained from volunteers, and then dietary histories were taken by 3-day recall and analyzed by local software programs. American subjects were admitted to the General Clinical Research Center for a 2-day stay. In the African study, participants were admitted to GaRankua Hospital, Gauteng (urban), or Tintswalo Hospital in Limpopo Province (rural) of South Africa. Subjects were studied in the morning following an overnight fast. A rapid colon evacuation was performed by the consumption of 2 L of GoLytely preparation (principal ingredient polyethylene glycol 3500) within 30 min. All stool passed over the next 3 h was then collected and weighed in a plastic container. A 10-g sample of the first (solid stool) bowel movement was separated from the collection as the fecal sample and immediately frozen at −20°C. The 3-h colonic evacuate was weighed and emulsified by shaking. Fifty-gram samples were then extracted and frozen at −20°C until analysis.
Bile Acid Assay
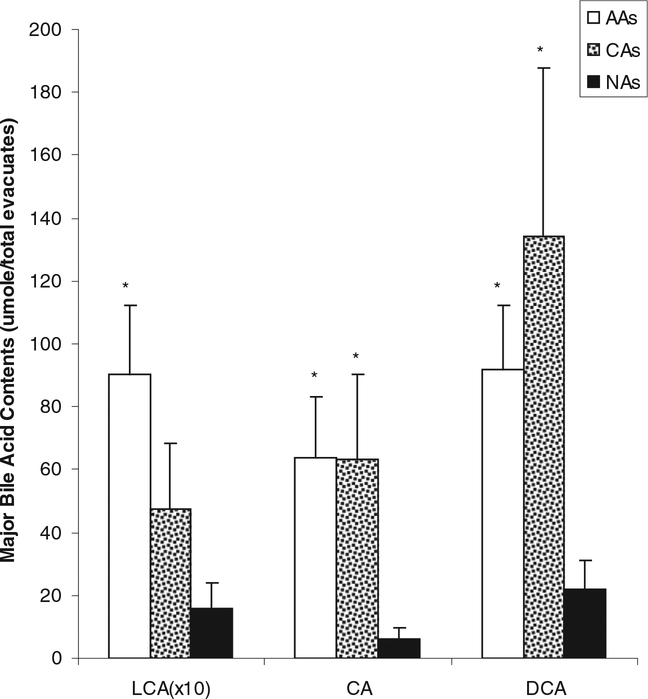
The bile acid concentrations in feces and quantities in colonic evacuates were measured based on the method described by Tagliacozzi et al. (11) except that quantitation was carried out using LC-MS as opposed to LC-tandem MS. A 125-μl colonic evacuate was mixed with 400 μl of acetonitrile, followed by 1-min vortex-mixing. After 15-min centrifugation at 13,000 g, 450 μl of the supernatant were transferred to an autosampler vial and blown to dryness with nitrogen. The residue was dissolved with 125 μl of methanol and water (1:1). Ten μl of this solution were injected into Shimadzu HPLC-MS (2010A; Columbia, MD) for quantitation using electrospray ionization in negative ion mode using selective ion monitoring of the appropriate (MH)− ion. The analytical conditions for LC-MS were as follows: Column Luna 3u, C18, 100A (2.0 mm I.D. 150 mm; Phenomenex, Torrance, CA)—Mobile phase A: 20% Acetonitrile-water containing 10-mM ammonium acetate; Mobile phase B: 80% Acetonitrile-water; gradient program—Mobile phase B: 0–6 min 15%; 20 min 30%; 30 min 60%; 40 min 80%; 45 min 15%, flow rate: 0.2 ml/min; column temperature: 40°C; Probe voltage: 4.5 kv; CDL temperature 230°C. The bile acids were calculated based on standard curves run with each sample set. Fig. 1 is a typical selective ion monitoring chromatogram of the bile acids for an African American (AA) and native African (NA) participant.
FIG. 1.
Major bile acid contents in evacuates were significantly lower in Native Africans (NAs) compared to African Americans (AAs) and Caucasian Americans (CAs). *P < 0.05 vs. NAs, Mann-Whitney testing. There was no difference between AAs and CAs. LCA data has been magnified 10 times in order to be visible on this scale. Bile acid abbreviations: LCA, lithocholic acid; CA, cholic acid; DCA, deoxycholic acid.
SCFA Assay
Evacuate samples (0.2 ml) and fecal samples (0.1 g) were transferred into plastic tubes, with the addition of 2,2-dimethylbutyric acid at 1 mM as internal standard. After mixing on a vortex and centrifugation (1,900 g, 10 min), the super-natant was filtered through a Millex-GS 0.22 μm syringe filter unit (Millipore, Bedford, MA) to remove bacterial cells. Another filtration was then done through a microconcentrator (Ultracel YM-10, Millipore, Bedford, MA) with a molecular mass cutoff of 10000 Dalton, by centrifugation (7,000 × g at 4°C for 1.5h). The filtrate was then analyzed using an Agilent Technologies 6890N Network GC System with a flame-ionization detector for SCFAs based on the method described by Scheppach et al. (12). Compounds were separated on a Grace EC-1000, 15-m length, 1.20-μm film thickness, 0.53 mm ID capillary column (Grace Davison Discovery Science, Deerfield, IL). The GC oven temperature was programmed at 5° min-1 from 80 to 175°C, which was held for 10 min and had a total running time of 25 min. The temperatures of both detector and injector were 200°C. The inlet was operated in splitless mode. High purity helium was used as carrier gas.
A mixed SCFA standard solution was prepared using high purity (>99%) reagents (Sigma, St. Louis, MO). SCFA values were computed using peak area ratio of the analyte to the internal standard. There was a good linear correlation between the peak area ratio and the corresponding standard SCFA with r values of >0.99 for all SCFAs. The interday and intraday coefficients of variation were in the range of 2.4% to 3.9%. This method cannot separate the 2-methylbutyric and isovaleric acid. The detection limits for the minor SCFA, isobutyric acid, 2-methylbutyricisovaleric acid, valeric acid, hexanoic acid, and 2-methylvaleric acid were 0.05, 0.05, 0.05, 0.1, and 0.1 μM, respectively.
Sample Size and Statistical Analysis
Earlier studies (13) had shown that the expected differences in the parameters measured between Westernized and African populations were expected to be large, justifying the small numbers of subjects included in each group. Statistical analysis was conducted using SPSS 5.0.1 (SPSS, Inc., Chicago, IL). The significance of group differences was assessed using nonparamatric Mann-Whitney test. A level of P ≤0.05 was accepted as statistically significant. Data are presented as means ± SE.
RESULTS
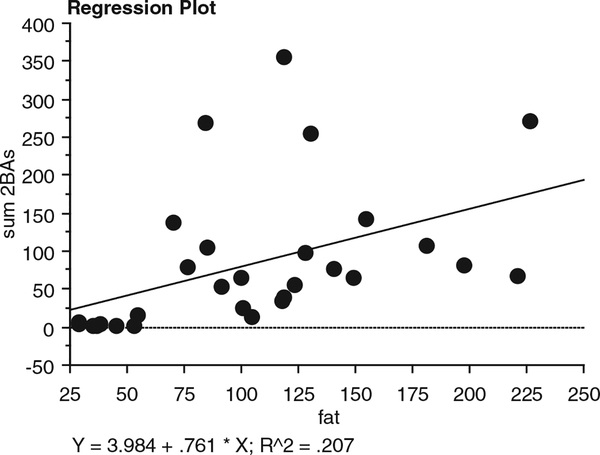
Colonic fluid concentrations of lithocholic acid, deoxycholic acid, and cholic acid were all significantly higher in AAs than NAs [6.04 ± 1.48 vs. 1.83 ± 0.74 (P = 0.018), 37.51 ± 34.25, vs. 7.30 ± 5.29 (P = 0.004), and 60.42 ± 10.63 vs. 16.83± 5.71(P 0.01), nmol/g wet weight stool, respectively]. There were no significant differences between AAs and Caucasian American (CAs), although the group mean values were higher in AAs. Fig. 1 and Table 1 show the calculations of the total colonic bile acids, corrected for evacuant mass (AAs = 1.59 ± 0.15, CAs=1.44 ± 0.13, NAs = 1.32 ± 0.15 kg). Primary bile acids (cholic and chenodeoxycholic acids), secondary (deoxycholic and lithocholic acids), tertiary (ursodeoxycholic acid), and conjugated (glycocholic, taurocholic, taurochenodeoxycholic, glycodeoxycholic, and taurodeoxycholic acids, with the exception of glycochenodeoxycholic) were all significantly higher (P < 0.01) in AAs compared to NAs. Most bile acids except glycochenodeoxycholic, taurochenodeoxycholic, and chenodeoxycholic acids were also significantly higher in CAs compared to NAs (P < 0.05). Mean bile acid levels were higher in AAs compared to CAs, but there was considerable variation, and only the difference in glycodeoxycholic acid achieved significance (Table 1). Dietary fat intake was significantly higher in both American groups (AAs: 123 ± 11 g/d, P < 0.0001; CAs: 129 ± 17 g/d, P < 0.0001) compared to native Africans (38 ±3g/d), as was total protein (AAs: 99 ± 10 g/d, P 0.002; CAs: 115 ± 11 g/d, P = 0.0001; NAs 58± 4 g/d) and animal protein intake (AAs: 67 ± 9 g/d, P < 0.0003; CAs: 88± 9 g/d, P <0.0001; NAs 28 ± 2 g/d). Dietary fat and animal protein intakes were not significantly different between th e 2 American populations. A weak, but significant, correlation was found between total dietary fat intake and total colonic bile acid content (Fig. 2: Y = 5.207 0.784 * X; R2 = 0.207; P 0.01).
TABLE 1.
The other bile acid contents in evacuates (μmole/total evacuates)
| Acid | African Americans (n = 12) | Caucasian Americans (n = 10) | Native Africans (n = 13) |
|---|---|---|---|
| GCA | 2.06(1.14)* | 0.45(0.11)* | 0.12(0.01) |
| TCA | 9.25 (7.41)** | 0.14(0.05)** | 0.004 (0.003) |
| UDCA | 7.79(1.91)** | 21.43(10.97)* | 2.56(1.05) |
| GCDCA | 6.12(5.60) | 0.17(0.16) | 0.03 (0.03) |
| TCDCA | 4.95 (4.41)** | 0.01 (0.01) | 0.03 (0.03) |
| GDCA | 5.31(2.88)** | 0.50 (0.12)** | 0.13(0.04) |
| TDCA | 5.62 (4.60)** | 0.19(0.06)** | 0.02 (0.01) |
| CDCA | 60.66 (22.01)** | 14.43 (6.66) | 5.20 (4.34) |
Results are expressed as group mean value followed by standard error in brackets.
P < 0.05.
P < 0.01 vs. NAs, P < 0.05 vs. Caucasian Americans, Mann Whitney testing.
GCA indicates glycocholic acid; TCA, taurocholic acid; UDCA, ursodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; GDCA, glycodeoxycholic acid; TDCA, taurodeocycholic acid; CDCA, chenodeoxycholic acid
FIG. 2.
Correlation between dietary fat intake calculated from 3-day recall and total colonic bile acids in all 3 population groups showing a weak but significant association (P = 0.01).
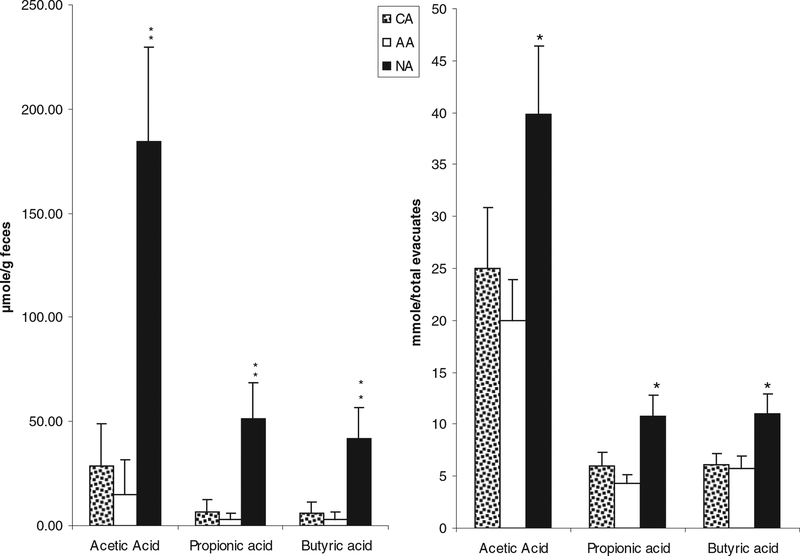
In contrast SCFA content in evacuates was significantly higher in NAs compared to AAs or CAs, with the notable exception of the branched chain SCFA, 2-methylvaleric acid, which was significantly (P < 0.05) higher in both American subgroups (Fig. 3 and Table 2).
FIG. 3.
Major short-chain fatty acid concentrations in stools (left) and in colonic evacuates [right, previously reported in O’Keefe et al. (9)] were significantly higher in Native Africans (NA) compared to African Americans (AA) and Caucasian Americans (CA). **P < 0.001 vs. CA and AA; *P < 0.05 vs. CA and AA, Mann-Whitney testing. There were no significant differences between AA and CA.
TABLE 2.
The colonic content of minor and branched short chain fatty acids (mmole/total evacuates)
| Acid | African Americans (n = 12) | Caucasian Americans (n = 10) | Native Africans (n = 13) |
|---|---|---|---|
| Isobutyric | 0.53 (0.11) | 0.63 (0.11) | 0.76 (0.16) |
| 2-Methylbutyric and Isovaleric | 1.02 (0.30) | 1.09 (0.29) | 0.89 (0.25) |
| Valeric | 0.80 (0.18) | 1.54(0.82) | 1.37 (0.25) |
| Hexanoic | 0.18 (0.07) | 0.28 (0.07) | 0.42 (0.14) |
| 2-Methylvaleric | 0.07 (0.04) | 0.20 (0.09) | 0.001 (0.001)* |
Results are expressed as group mean value followed by standard error in brackets.
P < 0.05 vs. African Americans and Caucasian Americans, Mann-Whitney testing.
In order to determine whether SCFA concentrations in fecal samples reflect the total colonic SCFA content, we compared the concentrations measured in the first (solid) stool passed to the total quantity of SCFAs measured in the colonic evacuates (Fig. 3). The same pattern of difference between Africans and Americans was found, but the significances of the differences were, if anything, greater when comparing fecal samples. NAs also had the highest level of valeric acid and the lowest level of 2-methylvaleric acid among the three groups (P < 0.05) (data not shown).
DISCUSSION
The results of the present study provide a further link in our evidence to support the suggestion that the higher risk of colon cancer in both African and Caucasian Americans than in native Africans may be explained by the interaction between the diet and the human microbiota (2). We show that the colonic content of experimentally carcinogenic secondary bile acids (lithocholic and deoxycholic acids) was, indeed, higher in high colon cancer risk AAs and CAs compared to low-risk NAs. Our earlier findings showed that dietary fat intake by the American populations was nearly 3 times higher than in NAs, and that the size of the colonic bacterial populations that contain the enzyme that converts nontoxic primary to these toxic secondary bile acids (e.g., 7-α dehydroxylating Clostridia) was higher in high-risk Americans (2). We also show that high risk was associated with not only lower colonic levels of the colonic health-promoting major SCFAs (acetate, propionate, and butyrate) but conversely with higher branched chain fatty acids. This provides evidence that the dietary residues entering the colon differed between the populations, with a predominance of carbohydrate residues in Africans and protein residues in Americans, reflecting the higher dietary protein intake in Americans (107 ±.9.3 vs. 58± 4 g/d). Our investigations do not, however, shed light on the reasons why AAs have a higher risk of colon cancer than CAs, probably because the differences in diet and colonic microbiota are very much smaller between American subpopulations. Consequently, much larger study populations would be needed to provide sufficient power to answer this question. What is clear is that the meat and fat consumption is known from national surveys to be highest in the AA population (1).
There is substantial experimental evidence linking secondary bile acids to colonic carcinogenesis. Secondary bile acid-induced mucosal proliferation may be the key step in the association between bile acids and colon carcinogenesis (14,15). Lithocholic acid, a secondary bile acid, can disrupt the integrity of the cell membrane of colonic mucosal cells, thus causing increased mucosal proliferation (16). Deoxycholic acid, another secondary bile acid, can release prostaglandin E2 from colonic tissues, enhance the release of arachidonate from colonocytes, and have a direct stimulatory effect on a subclass of protein kinase enzymes that appear to play a critical role in tumor promotion and in the action of increases in the colonic accumulation of lipoxygenase production (17). Secondary bile acids (lithocholic and deoxycholic acid) also have comutagenic effects. Among components contributing to fecal mutagenicity were reactive glyceryl ethers, known as fecapentaenes, whose biosynthesis might be stimulated by these 2 secondary bile acids (18).
There is also evidence that primary and conjugated bile acids may also exert colonic injury. For example, chenodeoxycholate, one of the primary cholic acids, has been found to have genotoxic effects on both normal and human colonic tumor cells (19), and in an in vitro study reported by Cheng et al., conjugated taurolithocholic acid, taurodeocycholic acid, and glycodeoxycholic acid were also found to stimulate the proliferation of human colon cancer cells (H508) through activating the p44/42 MAPK signaling cascade (15). Hydrophobic bile salts, such as glycochenodeoxycholic and taurochenodeoxycholic acids, have been shown experimentally to impair mitochondrial function, leading to an inhibition of oxidative phosphorylation and enhanced formation of toxic oxygen species by the mitochondrial respiratory chain in human hepatocyte (20). The authors speculated that this caused oxidative stress and ATP depletion with an increase in Ca2+ concentration with stimulation of hydro-lases, which could lead to hydrolysis of lipid membranes and structural proteins, causing cell death by necrosis (20).
The diet of the AAs and CAs was characteristically Westernized, rich in meat and saturated fats, whereas the NAs’ diet was low in meat and saturated fat and high in complex carbohydrate (21). Interestingly, our most recent studies have shown that the total fiber content of the modern African diet is not high because traditional foods are less commonly consumed and most of the diet consists of refined maize (corn) meal (22), unlike that described by Burkitt in the 1970s (23). However, the method of cooking and eating the maize meal as a porridge results in an increase in resistant starch, which acts in the same way as fiber in the colon, being a substrate for microbial fermentation and SCFA production. We have previously demonstrated, using breath hydrogen and methane production, that about 10–20% of the maize meal is resistant to small bowel digestion (13), although it was estimated that less than 5% of American starch was resistant. Furthermore, it has been recognized for many years that up to 20% of digestible starch is malabsorbed by the small intestine, and thus a high-carbohydrate diet, such as that consumed by Africans, will also contribute more colonic carbohydrate to bacterial fermentation (24–26). In keeping with this, Duncan et al. (27) demonstrated that carbohydrate restriction suppressed populations of the colonic microbiota most responsible for butyrate production. In this study, obese human subjects consumed diets with normal, reduced, or dramatically reduced carbohydrate content. Fecal concentrations of all 3 major SCFAs decreased with reduced total carbohydrate intake but, in particular, the concentration of butyrate decreased from 17.7 to 4.4 mM. In parallel, the major bacterial populations (Roseburia spp. and Eubacterium rectale) that produce butyrate decreased on average from 11.4% to 3.3% of total bacteria.
Several other studies have shown that fecal SCFA are decreased in populations with a high colon cancer risk. Clausen et al. found that the ratio of butyrate to total SCFA in feces was reduced in patients with colonic adenomas compared with healthy controls (28). Bradburn et al. (29) examined stool samples from 20 patients with familial adenomatous polyposis and 11 normal controls in the UK. They postulated that decreased butyric acid production by colonic carbohydrate fermentation in patients with familial adenomatous polyposis may predispose to colorectal cancer. Although these studies do not prove that SCFA production was lower in high-risk populations, the results of our comparison of fecal and total colonic SCFAs support this extrapolation. The use of fecal SCFA concentrations as an index of total SCFAs production can be criticized, as most of the SCFA production is in the proximal colon, and a substantial portion will have been absorbed by the time the effluent exits with feces. In the present study, fecal concentrations were predictive of total colonic evacuate SCFAs supporting the use of simple fecal analysis to assess total colonic SCFA production in normal healthy volunteers. This might not, however, hold true for patients with increased colonic transit or diarrhea (30). Finally, in vivo studies have demonstrated that increased colonic butyrate levels correlate with a decreased incidence of colon cancer (31,32) and in vitro studies have shown that butyrate inhibits cell proliferation, induces differentiation, and promotes apoptosis (33–36). In our earlier studies, we confirmed that the high SCFA fecal concentrations in Africans was associated with a reduced colonic epithelial proliferation rate [9].
The present study shows that colonic branched SCFAs (2-methlvaleric acid) were significantly higher in both American groups. This can be explained by the higher protein intake of AAs (i.e., 94 ± 9.3 vs. 58 ± 4.1 g/d) as BCFA are synthesized by microbiota from undigested protein residues (37). The role of BCFA in colonic health has not, to our knowledge, been investigated; the possibility that BCFA or the bacteria that produce them is injurious needs to be investigated.
In conclusion, our results support our hypothesis that the higher risk of colon cancer in Americans compared to Africans may be explained by the consumption of typical Westernized diets that are rich in fat and low in complex carbohydrate. Such diets produce a 2-hit insult on the colonic epithelium by stimulating the microbiota to produce toxic metabolites and suppressing them from synthesizing health-producing SCFAs. Further studies are needed to test our hypothesis that cancer risk can be modified by altering the balance between dietary carbohydrate and animal protein and fat.
Contributor Information
Junhai Ou, Department of Gastroenterology, Hepatology and Nutrition, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
James P. DeLany, Department of Medicine, Division of Endocrinology and Metabolism, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Ming Zhang, Department of Clinical Nutrition, Weifang People’s Hospital, Weifang, Shandong, China.
Sumit Sharma, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Stephen J. D. O’Keefe, Department of Gastroenterology, Hepatology and Nutrition, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1.Sharma S and O’Keefe SJD: Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J 83, 583–589, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keefe SJD, Chung D, Mahmoud N, Sepulveda AR, and Manafe M: Why do African Americans get more colon cancer than Native Africans? J Nutr 137, 175S–182S, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Doll R and Peto R: The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 66, 1191–1308, 1981. [PubMed] [Google Scholar]
- 4.Bresalier RS and Kim YS: Diet and colon cancer. New Engl J Med 313, 1413–1414, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Young VB and Schmidt TM: Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol 42, 1203–1206, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markus S, Veerle R, Dieter S, and Jurgen S, et al. : Combined treatment of Caco-2 cells with butyrate and mesalazine inhibits cell proliferation and reduces Survivin protein level. Cancer Lett 273, 98–106, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, et al. : Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J Gastroenterol 11, 3990–3997, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridlon JM, Kang D-J, and Hylemon PB: Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47, 241–259, 2006. [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe SJD, et al. : Products of the Colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr 139, 2044–2048, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J: Short chain fatty acids in the human colon. Gut 22, 763–779, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagliacozzi D, et al. : Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chemistry Lab Med 41, 1633–1641, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Scheppach W, Fabian C, and Kasper H: Fecal short-chain fatty acid (SCFA) analysis by capillary gas-liquid chromatography. Am J Clin Nutr 46, 641–646, 1987. [DOI] [PubMed] [Google Scholar]
- 13.O’Keefe SJ, Kidd M, Espitalier-Noel G, and Owira P: The rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am J Gastroenterol 94, 1373–1380, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Terpstra OT, Van Blankenstein M, Dees J, and Eilers GA: Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology 92, 704–708, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Cheng K and Raufman J-P: Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 70, 1035–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kozoni V, Tsioulias G, Shiff S, Rigas B, et al. : The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon car cinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis 21, 999–1005, 2000. [DOI] [PubMed] [Google Scholar]
- 17.DeRubertis FR, Craven PA, and Saito R: Bile salt stimulation of colonic epithelial proliferation. Evidence for involvement of lipoxygenase products. Journal Clin Invest 74, 1614–1624, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilpart M and Roberfroid M: Effects of secondary biliary acids on the mutagenicity of N-methyl-N’-nitro-N-nitrosoguanidine, 2-acetylaminofluorene and 2-nitrofluorene towards Salmonella typhimurium strains. Carcinogenesis 7, 703–706, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Jurek D, Fleckl E, Marian B: Bile acid induced gene expression in LT97 colonic adenoma cells. Food Chem Toxico 43, 87–93, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Rosser BG and Gores GJ: Liver cell necrosis: mechanisms and clinical implications. Gastroenterology 108, 252–272, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Cummings J, et al. : The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32, 2094–2101, 1979. [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe SJD: The African way of life and colon cancer risk. Am J Gastroenterol 96, 3220–3221, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Burkitt D: Diseases of the alimentary tract and western diets. Pathol Microbiol 39, 177–186, 1971. [DOI] [PubMed] [Google Scholar]
- 24.Hudson GJ and Englyst HN: Dietary intakes of starch and non-starch polysaccharides in a West African village. Br J Nutr 73, 655–666, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Miller T and Wolin M: Fermentations by saccharolytic intestinal bacteria. Am J Clin Nutr 32, 164–172, 1979. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane S and Macfarlane GT: Regulation of short-chain fatty acid production. Proc Nutr Soc 62, 67–72, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Duncan SH, et al. : Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73, 1073–1078, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clausen MR, Bonnen H, and Mortensen PB: Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut 32, 923–928, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradburn DM, et al. : Colonic fermentation of complex carbohydrates in patients with familial adenomatous polyposis. Gut 34, 630–636, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treem WR, et al. : Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Ped Gastroenterol Nutr 23, 280–286, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Avivi-Green C, Polak-Charcon S, Madar Z, and Schwartz B: Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res 12, 83–95, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Perrin P, Pierre F, Patry Y, Champ M, Berreur M, et al. : Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 48, 53–61, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariadason JM, Rickard KL, Barkla DH, Augenlicht LH, et al. : Divergent phenotypic patterns and commitment to apoptosis of Caco-2 cells during spontaneous and butyrate-induced differentiation. J Cell Physiol 183, 347–354, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Bordonaro M, et al. : Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta -catenin-tcf pathway and concordance with effects of sulindac and trichostatin a but not curcumin. Cell Growth Diff 10, 713–720, 1999. [PubMed] [Google Scholar]
- 35.Coradini D, et al. : Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif 33, 139–146, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBain JA, Eastman A, Nobel CS, Mueller GC, et al. : Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol 53, 1357–1368, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Cummings JP, Pomare EW, Branch WJ, Naylor CP and Macfarlane GT: Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]