Abstract
Background
Pancreatic cancer (PC) is one of the most aggressive malignancies and has a poor prognosis despite being extensively researched. The role of serum-derived exosomes in tumorigenesis and the development of PC is still unclear.
Method
The present study employed iTRAQ-based proteomic analysis to search for differences between the serum exosomes of PC patients and those from control patients. Then, bioinformatics methods were used to analyze the functions of the identified proteins, and the possible functions were verified through cell culture experiments.
Results
A total of 611 proteins were identified from exosomes, and 141 proteins were differentially expressed, with 91 up- and 50 down regulated proteins in PC cancer compared to healthy controls. Further analysis indicated that APOE serves as an important hub in the network. In addition, CRP, VWF, APOA2, NIN, and GSK3B potentially interact with many other proteins. We then tested the effect of patient serum-derived exosomes on pancreatic cancer cells and found that patient serum-derived exosomes, but not those from healthy controls, induced cell proliferation, migration, and EMT, supporting the role of exosomes in metastasis.
Conclusion
Our data suggest that exosomes derived from PC patients may promote PC metastasis.
Keywords: proteomic analysis, pancreatic cancer, serum exosome, metastasis
Background
Pancreatic cancer (PC) is one of the most aggressive malignancies and a leading cause of cancer-related mortality.1,2 More than 80% of PC patients are diagnosed at an advanced stage and lose the opportunity for surgical resection because of distant metastasis; further, the 5-year survival rate is less than 6%.1–3 Exosomes are small extracellular vesicles that are approximately 50–150 nm in size, and they are secreted by a wide variety of cell types, including tumor cells.4–6 It has been proven that exosomes contain various biologically important molecules, such as proteins, lipids, and nucleic acids, and act as shuttles between cells by transmitting signals and mediating intercellular communication.4,5 Increasing evidence shows that exosomes are involved in many physiological and pathological processes.7,8 The discovery that exosomes participate in the pathogenesis of cancer has generated tremendous interest. Several studies have already shown that exosomes are involved in the development of pancreatic cancer.9–12 However, how these exosomes contribute to PC biology is still poorly understood.
Therefore, the present study tries to conduct a comprehensive, quantitative analysis using iTRAQ-based proteomics. The study compares the proteomes of serum-derived exosomes isolated from pancreatic cancer to proteomes of exosomes isolated from healthy volunteers. The iTRAQ-based quantification method was optimized to increase the quantification accuracy and the number of proteins that were identified and quantified.13 The study also intended to evaluate the role of serum-derived exosomes on pancreatic cancer metastasis at the cellular level.
Materials And Methods
Ethical Statement
The study was approved by the Clinical Ethics Committee of Peking University Third Hospital. The samples were collected only from patients or healthy volunteers who agreed to participate in the exam for the purpose of laboratory research. Informed consent was obtained from all patients or healthy controls. All methods were performed in accordance with the relevant guidelines and regulations.
Study Population And Design
Analyzed serum samples were obtained from two groups (patients with pancreatic cancer and healthy volunteers). The two groups were matched by age and gender. Twenty-four patients who had a curative resection as a first step toward treating PC were enrolled, including 12 males and 12 females ranging in age from 57 to 72 years. All participants were recruited from Peking University Third Hospital (Beijing, China) from December 2015 to December 2016. All patients’ diagnoses were ultimately confirmed both clinically and pathologically. The pathological stage of pancreatic cancer was determined according to the American Joint Committee on Cancer (AJCC) 7th Edition.14 Healthy volunteers were enrolled from the population who visited the Health Screening Centers of Peking University Third Hospital. Twenty-one healthy controls, including 9 males and 12 females ranging in age from 48 to 85 years, were enrolled.
Blood Sampling And Exosome Isolation
For pancreatic cancer patients, a routine fasting blood sample of 4 mL was collected before any operation; the sample was collected from patients’ forearms to obtain systemic circulation samples (peripheral blood). For the healthy control patients, a routine fasting blood sample of 4 mL was taken from the forearm to obtain systemic circulation samples (peripheral blood) and was collected at Health Screening Centers. Blood samples were transferred into 4mLethylenediaminetetraacetic acid (EDTA)-K2tubes (BD) and were maintained at room temperature immediately after collection. Plasma was harvested and centrifuged at 500xg and 10000xg for 10 min to remove intact cells and cell debris, respectively. The 25 samples from PC patients were mixed together, and the 21 healthy control samples were also mixed. The plasmaof each mixed blood sample was gently washed three times with PBS and suspended with an ExoQuick exosome precipitation solution; then, exosome isolation was performed according to the manufacturer’s instructions (RiboBio).
Electron Microscopy And Western Blot Analysis Of Exosomes
Scanning electron microscopy (SEM) was applied to examine the isolated exosomes, which wereloaded onto a carbon-coated electron microscopy grid, as described previously.15 After fixing with 2% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer at pH 7.3 for 3 hrs at room temperature, the samples were critical point dried, mounted on specimen slides, sputter-coated, and visualized using a Hitachi S3400 scanning electron microscope. Exosome proteins were subjected to electrophoresis and transferred to PVDF membranes. The membranes were probed with mouse anti-CD63 (1:200, Abcam, Cambridge, UK). The details of this experimental process and its effectiveness are shown in our previous studies.12
Sample Preparation
Exosomesamples were placed in 3kDa ultrafiltration tubes and centrifuged at 14,000 × g for 15 min; the filtrate was discarded, and 8 M urea was added. These steps were repeated three times. The samples were homogenized in extraction buffer containing 4% SDS, 1 mM DTT, and 150 mM Tris-HCl (pH 8). After a 3 min incubation in boiling water, the homogenate was sonicated on ice. The crude extract was incubated in boiling water again and clarified by centrifugation at 16,000 × g at 25°C for 10 min. Protein was quantified with a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL, USA). A 200 μg portion of protein from each sample was added to 30 μL of STD buffer (4% SDS, 100 mM DTT, and 150 mM Tris-HCl, pH 8.0). The detergent, DTT, and other low-molecular-weight components were removed through replacing the STD buffer with UA buffer (8 M urea and 150 mM Tris-HCl, pH 8.0) by repeated ultrafiltration (30 kDa Microcon; Millipore Corp., Billerica, MA, USA). Then, 100 μL of 0.05 M iodoacetamide was added to the UA buffer to block the reduction of cysteine residues, and the samples were incubated for 20 min in the dark. The filters were washed three times with 100 μL of UA buffer and twice with 100 μL of DS buffer (50 mM triethylammonium bicarbonate, pH 8.5). Based on the quantitative results, the samples were subjected to an overnight trypsin treatment using a trypsin-to-protein ratio of 1:100.
iTRAQ Labeling And Fractionation By Cation Exchange Chromatography
Protein peptides (100 μg) from each group were labeled using an 8plex iTRAQ reagents multiplex kit (ABI, Foster City, CA, USA) (isobaric tags for the PC and Congroups, or PC patients and healthy controls, respectively). The 8plex iTRAQ reagents were allowed to reach room temperature, centrifuged, and reconstituted with 50 μL of isopropyl alcohol to dissolve the iTRAQ labeling reagent. The tubes were vortexed and centrifuged. The iTRAQ labeling reagents were added to the corresponding peptide samples and incubated at room temperature for 1 h. A 100 μL aliquot of water was added to stop the labeling reaction. A 1 μL aliquot of sample was removed from each group to test the labeling and extraction efficiency, and the sample was subjected to a matrix-assisted laser desorption ionization procedure after ZipTip desalting. The six sample groups were pooled and vacuum-dried. Each pool of mixed peptides was lyophilized and dissolved in solution A (2% acetonitrile [ACN] and 20 mM ammonium formate, pH 10). Then, the samples were loaded onto a reverse-phase column (Luna C18, 4.6 × 150 mm; Phenomenex, Torrance, CA, USA) and eluted using a step-wise linear elution program: 0–10% buffer B (500 mM KCl, 10 mM KH2PO4 in 25% ACN, pH 2.7) for 10 min, 10–20% buffer B for 25 min, 20–45% buffer B for 5 min, and 50–100% buffer B for 5 min at a flow rate of 0.8 mL/min. The samples were collected each min and centrifuged for 5–45 min. The collected fractions (approximately 40) were finally combined into 10 pools and desalted with C18 Cartridges (Empore™ standard density SPE C18 Cartridges, bed I.D. 7 mm, 3 mL volume; Sigma, St. Louis, MO, USA).
LC-Electrospray Ionization-MS/MS Analysis
Reconstituted peptides were analyzed with a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled with a nano high-performance liquid chromatography (UltiMate 3000 LC Dionex; Thermo Fisher Scientific) system. The peptides were loaded onto a C18-reversed-phase column (3 μm-C18 resin, 75 μm × 15 cm) and separated on an analytical column (5 μm C18 resin, 150 μm × 2 cm; Dr. Maisch GmbH, Ammerbuch, Germany) using mobile phase A: 0.5% formic acid [FA]/H2O and B: 0.5% FA/ACN at a flow rate of 300 nL/min, using a 150 min gradient. Spectra were acquired in a data-dependent mode. The 10 most intense ions selected for MS scanning (300–1800 m/z, 60,000 resolution at m/z 400, accumulation of 1 × 106 ions for a maximum of 500 ms per 1 microscan). The isolation window was 1.3 m/z, and the MS/MS spectra were accumulated for 150 ms using an Orbitrap.MS/MS spectra were measured at a resolution of 15,000 at m/z 400. Dynamic precursor exclusion was allowed for 120 s after each MS/MS spectrum measurement and was set to 17,500 at m/z 200. The normalized collision energy was 30 eV, and the underfill ratio, which specifies the minimum percentage of the target value likely to be reached at the maximum fill time, was defined as 0.1%. The instrument was run with the peptide recognition mode enabled.
Sequence Database Search And Data Analysis
The raw mass data were processed for the peptide data analysis using Proteome Discoverer 1.4 with a false discovery rate (FDR = N(decoy)*2/((N(decoy)+ N(target))) < 1% and an expected cutoff or ion score < 0.05 (with 95% confidence) for searching the UniProt Human Proteome database. The following options were used to identify the proteins: peptide mass tolerance = ±10 ppm, MS/MS tolerance = 0.6 Da, enzyme = trypsin, missed cleavage = 2, fixed modification: iTRAQ8plex (K) and iTRAQ8plex (N-term), variable modification: oxidation (M), database pattern = decoy. The differential expression threshold was defined as a 1.5-fold change for up- and down regulated proteins. The mass spectrometry proteomics data have been deposited to the ProteomeX change Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository according to the data set identifier.
Bioinformatics Analyses
Gene Ontology (GO) analysis was conducted by FunRich (version 3.0).KEGG pathway enrichment analysis was conducted by DAVID (https://david.ncifcrf.gov/). The protein-protein interaction network was generated by STRING (http://string-db.org/) and visualized using Cytoscape.
Cell Culture
Human pancreatic cancer cell lines, MiaPaCa-2 and AsPC-1(American Type Culture Collection, ATCC, Manassas, VA, 1×105), were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum in 24-well culture plates (Costar, Corning, NY, USA) for 24 hat 37°C in a humidified incubator with 5% CO2.
Cell Proliferation Assay
Proliferation analysis was performed using CCK8 assays (Dojindo). Cells were plated in 96-well plates in triplicate at approximately 1000 cells per well and cultured in growth medium. Cells were then treated with or without exosomes, and the number of cells per well was measured by the absorbance (450 nm) of reduced water-soluble tetrazolium salt (WST) at the indicated time points. Cell growth curves with the cell number as the X-axis and the OD value as the Y-axis were produced.
In Vitro Wound-Healing Assay
Cancercells (8×105/mL) were plated into 6-well plates and grown overnight to near confluence. Cells in the experimental group were treated with exosomes from different sources. Using a pipette tip, a cell-free area was scratched into each well, and culture was continued. Images were taken at different time points, and the number of cells migrating into the cell free space was quantified.
Observation Under A Laser Confocal Microscope
After culture for 24 hrs, the cell slides were fixed in 3.7% PFA and permeabilized by treatment with 0.2% Triton x-100 for 10 mins. Two secondary antibodies, goat anti-mouse IgG1 heavy chain (FITC-labeled) (ab97239) and DyLight649 goat anti-rabbit IgG(H+L)(GAR6492, MultiSciences), were added to the media after incubating with 5 mg/mL of anti-E-cadherin or 10 mg/mL of anti-Vimentin antibodies overnight; 30 mins after adding the secondary antibodies, cells were stained with Hoechst (Sigma) for 30 mins and were then visualized by epifluorescence microscopy.
Transwell Invasion Assay
Eight-millimeter Matrigel-coated transwell supports from Becton Dickson Canada (BD) were used to evaluate cell invasion. Fifty thousand cancer cells were suspended in 500 mL of 10% FBS/DMEM and seeded in the upper chamber. The bottom chamber was filled with 1 mL of 10% FBS/DMEM. Membranes were equilibrated at room temperature for 10 mins before cells were added, and the cells were allowed to invade for 24 hrs and were then fixed with 3.7% PFA. Cell invasion was calculated as the average cell coverage area in µm2 on the underside of the membrane compared with the numbers from the cancer cell control. The results were calculated based on the analysis of 20 fields (×20) in five independent experiments.
Results
Exosomal Protein Profiles
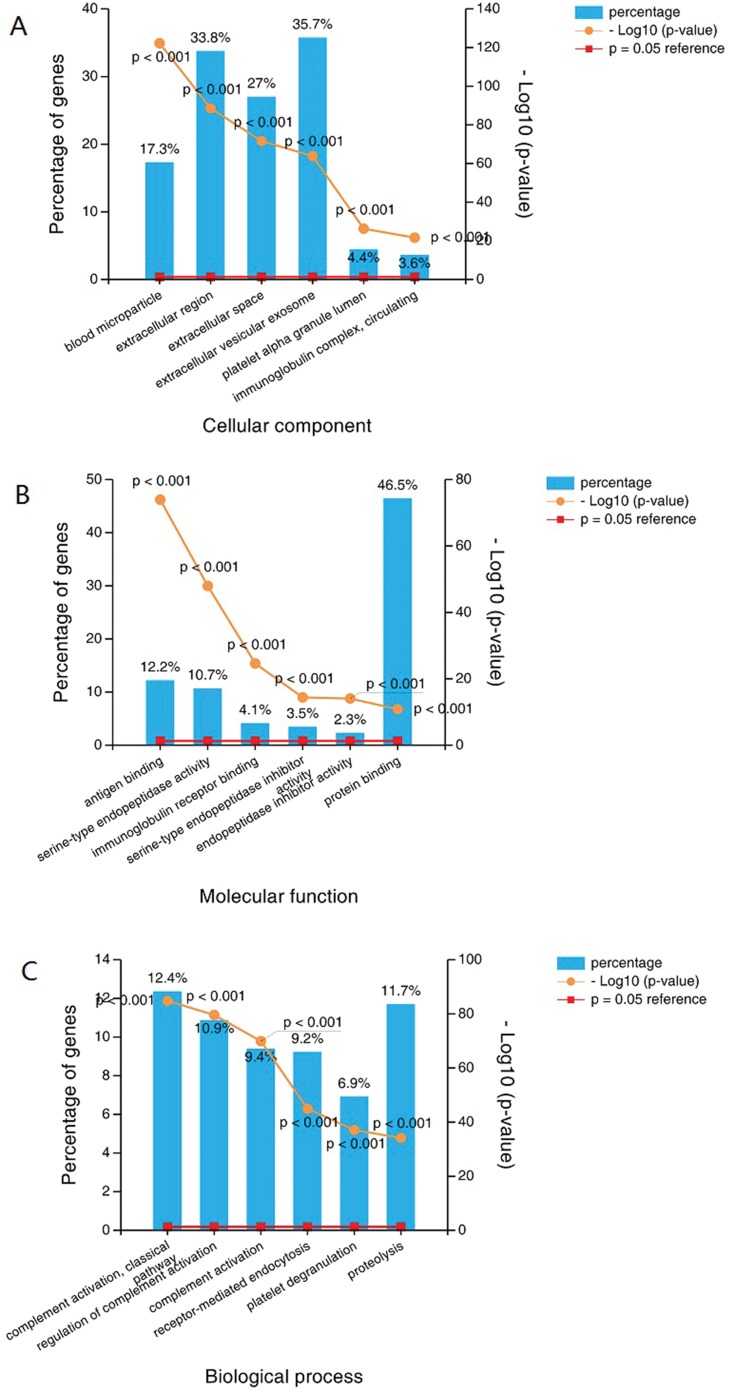
We compared protein abundance in exosomes purified from the serum of patients with PC and healthy volunteers using iTRAQ labeling quantitative MS experiments. In total, 611 proteins were identified (Supplementary Table S1). There was an overlap of 306 proteins in the Exocarta protein list (http://www.exocarta.org, Figure 1). Fortheseproteins, 554 proteins had GO information. For the category for assessing the location of proteins, or cellular components, the proteins were mainly categorized as being found in extracellular vesicular exosomes, extracellular regions and extracellular space (Figure 2A). Inmolecular function, these proteins were found mostly to be responsible for protein binding (Figure 2B). Complement activation, a classical mechanism, was highly represented in biological processes, followed by proteolysis and regulation of complement activation (Figure 2C). Pathway analysis showed that 192 proteins were involved in at least one KEGG pathway, and complement and coagulation cascades were the most overrepresented pathways associated with those exosomal proteins.
Figure 1.

The relationship between identified proteins and Exocarta database.
Figure 2.
GO classification of proteomic data for downregulated and upregulated proteins. Differently expressed proteins were submitted to the GO classification system.
Differential Expression Protein Profiles
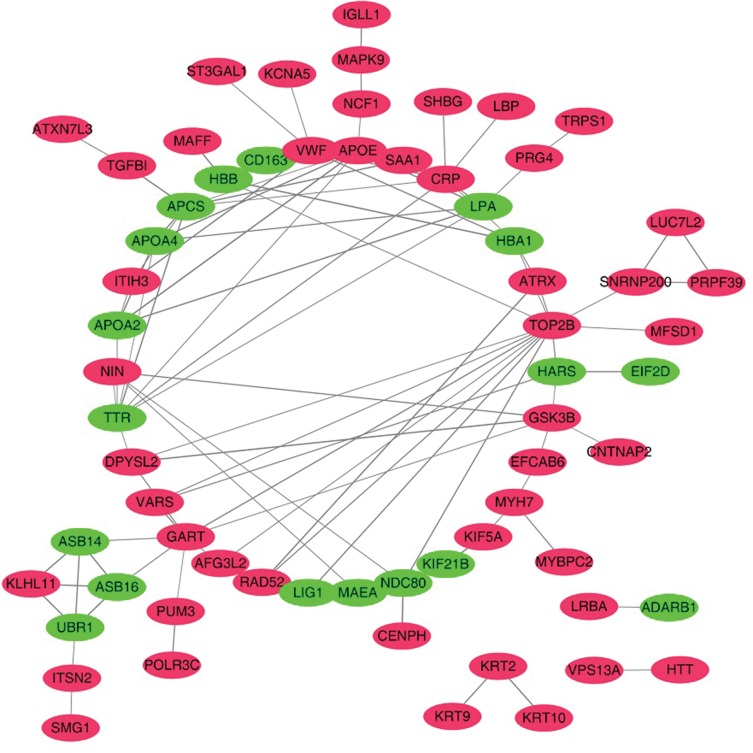
We compared the exosomal proteins from patients with those from healthy volunteers and found that 141 proteins were differentially expressed;91 were up- and 50 were downregulated (Supplementary Table S2). GO analysis showed that blood microparticles, antigen binding and receptor-mediated endocytosis were the most enriched terms inthe cellular component, molecular function and biological process categories, respectively(Figure 3A–C).To reveal the potential relationships among the differentially expressed proteins, interaction networks were reconstructed according to the STRING database, and they were illustrated using Cytoscape. As shown in Figure 4, APOE (apolipoprotein E) serves as an important hub in the network. In addition, CRP (C-reactive protein), VWF (von Willebrand factor), APOA2 (apolipoprotein A2), and GSK3B (glycogen synthase kinase 3 beta) also potentially interact with many other proteins.
Figure 3.
GO analysis shows that blood microparticle, antigen binding and receptor-mediated endocytosis were the most enriched term incellular component, molecular function and biological process.
Figure 4.
Network of proteins identified in overall differentially expressed proteins. Up- or downregulation is indicated by the color of nodes (upregulated in red and downregulated in green).
Effects Of Exosomes On Proliferation And Migration Of PC Cells
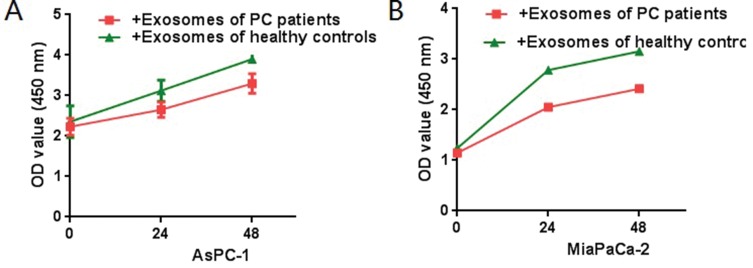
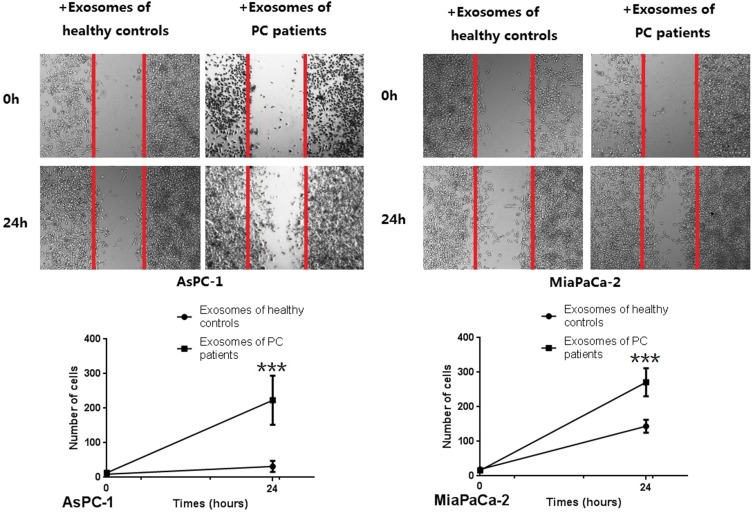
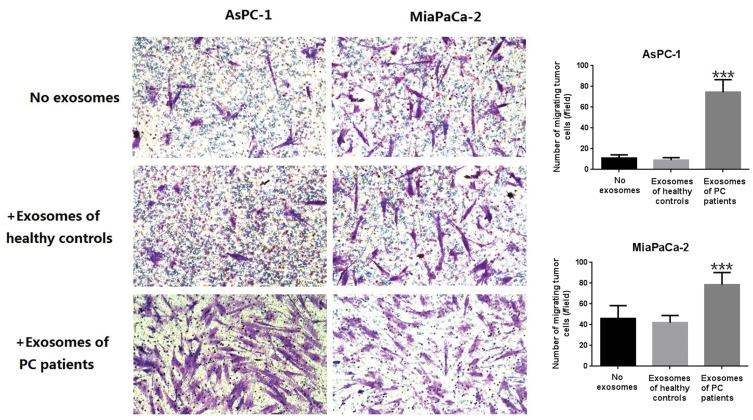
CCK8 assays were used to detect the effect of exosomes on PC cell lines (AsPC-1 and MiaPaCa-2), and it was found that exosomes from PC patientscould promote the proliferation of tumor cells (Figure 5). Further, in vitro wound-healing assays indicated that treatment with exosomes from PC patients could promote the migration of PC cell lines (Miapaca-2 and Aspc-1) (Figure 6). By comparing with untreated groups of cancer cells, transwell invasion experiments indicated that treatment with the exosomes from PC patients significantly increased the invasion of PC cell lines (Miapaca-2 and Aspc-1) (Figure 7). To further detect the effect of exosomes from PC patients on the EMT of cancer cells and the mode of action, we used laser confocal microscopy to detect EMT proteins (E-cadherin and Vimentin) in tumor cells. PC cell lines (MiaPaCa-2 and AsPC-1) were cultured in three groups: no exosome treatment, treatment with exosomes from healthy controls, and treatment with exosomes from PC patients. The results showed that when cultured with exosomes from PC patients, E-cadherin expression was significantly decreased, and Vimentin expression was significantly increased (Figure 8). Such a change is a common characteristic of EMT.
Figure 5.
CCK8 test. PC cells (MiaPaCa-2, AsPC-1, 4×106) were treated with serum exosomes derived from PC patients or healthy controls after 12 h of seeding (equal PDAC cells seeded first, and the OD value showed a significantly difference at 24h and 48h after treated with the exosomes, P<0.05). Compared with the control group, exosomes derived from PC patients can promote the proliferation of PC cells.
Figure 6.
Wound-healing assay in vitro of PC cells (MiaPaCa-2, AsPC-1) added serum exosomes derived from PC patients. As can be seen from the figure, exosomes derived from PC patients can promote the migration of PDAC cells (***P < 0.001).
Figure 7.
Transwell invasion experiment of Miapaca-2 and Aspc-1 treated with or without exosomes derived from PC patients. As can be seen from the figure, exosomes derived from PC patients can increased the trans magration of PDAC cell lines significantly (***P < 0.001).
Figure 8.
The laser confocal imaging of EMT related proteins. Vimentin was up expressed, while the E-cadherin was down expressed when treated with exosomes derived from PC patients. In order to make each group comparable, the fluorescence brightness of the nucleus was set at the same level in the laser confocal imaging of the three groups (***P < 0.001).
Discussion
The present study examined the differences in protein levels between serum-purified exosomes from PC patients and those purified from healthy volunteers. iTRAQ-based proteomic analysis was used in the study, and it possesses the advantages of maximum protein coverage and precise quantification. In this study, we found a series of exosomal proteins that were significantly differentially expressed between healthy controls and pancreatic cancer patients. To avoid the damage of exosomes that leads to material loss, we did not use an ultracentrifuge to collect exosomes. Instead, exosomes were harvested using a special kit under low speed centrifugation. A total of 611 proteins were identified, and 306 proteins overlapped with the Exocarta protein list. Further analysis indicated that most of the proteins come from cellular component categories that are responsible for binding and participating in biological processes. All of these findings indicate that exosomal proteins have a very important role in pancreatic cancer.
The differentially expressed proteins in the PC group had varied molecular functions. The upregulated proteins from serum-purified exosomes of PC patients were located in the extracellular region and extracellular space, and they were involved in protein binding; these findings are relevant to the classical methods highly represented in biological processes for these proteins: proteolysis and regulation of complement activation. GO analysis of the 141 differentially expressed genes (91 up- and 50 downregulated) revealed that blood microparticles, antigen binding and receptor-mediated endocytosis were the most enriched terms inthe cellular component, molecular function and biological process categories, respectively. A previous study found 99 differentially expressed proteins, which is significantly different from what was found in the present study.13 This can be explained by the different protein sources and different detection condition settings. However, a number of typical proteins implicated in processes related to metastasis were involved, supporting the previous observations that tumor-derived exosomes play a crucial role in the development of metastasis.13 In the network generated from the PC differentially expressed proteins, Apolipoprotein E, which was upregulated in the serum-purified exosomes of PC patients, serves as hubs at which protein interactions converge. Apolipoprotein E (ApoE) is a factor involved in Alzheimer’s disease, and ApoE has recently attracted great attention as an important protein related to tumorigenesis and metastasis.16,17 It has also been proven to be an effective biomarker for pancreatic cancer.18–20 Other proteins, such as CRP,21,22 VWF,23,24 APOA225 and GSK3B,26,27 were all shown to be involved in cancer metastasis. Moreover, the present study also indicated that the iTRAQ method may provide a clinically useful method for monitoring treatment response and classify pancreatic cancer patients based on exosome content. This method also has a wide application because it can improve the mass-spectrometry detection limit of low-abundance proteins. Among a total of 1559 proteins quantified, 8 proteins show global treatment-specific changes in all of the patients and may be potential biomarkers of treatment response or metastasis for pancreatic cancer. Two of these, namely, OBSL1 and PLF4, display the highest treatment response in our data set, and we believe that the proteins are strong candidates for development as biomarker.
Consistent with a previous study,28–30 our study further evaluated the impact of serum exosomes from PC patients on the proliferation, migration, invasion and EMT ability of PC cells. The proliferation ability of PC cells (MiaPaCa-2 and AsPC-1) was enhanced by treating them with serum-purified exosomes from PC patients, and this resultwas confirmed by CCK8 experiments. The promotion of metastasis by serum exosomes from PC patients was demonstrated in wound-healing assays and transwell invasion assays. EMT refers to the phenomenon of epithelial cells transforming into mesenchymal cells under normal physiological or specific pathological conditions, and EMT is thought to be a morphologic change before tumor infiltration and metastasis. Such transformation is evidenced by the detection of EMT proteins.The results here indicated that serum exosomes of PC patients could significantly promote the metastasis of PDAC cells.
Although our study has a relatively small sample size and the age gap between patients is large, our results showed that the levels of some differentially expressed proteins from serum-purified exosomes varied significantly between pancreatic cancer patients and healthy controls. Further, given that this is a major finding of this study, the sample size is probably adequate. Moreover, there are several strengths worth emphasizing. First, given that all PC diagnoses were confirmed pathologically and clinically, the present study avoided introducing an ascertainment bias through misdiagnosis. Second, the collection of blood specimens was highly qualitative for all participants starved prior to venesection. Finally, patients who accepted preoperative chemotherapy were excluded from the present study, and no patients used pain killers or other special drugs before the operation.
Conclusion
The present study employed iTRAQ-based proteomic analysis to search for differences in the serum exosomes of PC patients and healthy controls. Bioinformatics methods were used to analyze the functions of differentially expressed proteins, and the possible functions were analyzed through cell culture experiments. The results show that exosomes in the circulation of patients with PC play pivotal roles in PC metastasis, which may provide novel insights into the treatment of PC. However, further studies are necessary to identify key proteins and unveil potential therapeutic targets for serum-purified exosomes.
Acknowledgments
This study was supported by a grant from the Capital Characteristic Clinical Application Research and Achievement Promotion Project (No. Z171100001017121), the Doctoral Venture Capital fund of Henan Provincial People’s Hospital (No. ZC20180077), the Special Project of Henan Provincial Key Research, Development and Promotion (Science and Technology) (No. 192102310119).
Abbreviations
PC, pancreatic cancer; iTRAQ, isobaric tags for relative and absolute quantitation; APOE, apolipoprotein E; CRP, C-reactive protein; VWF, von Willebrand factor; APOA2. apolipoprotein A2; GSK3B, glycogen synthase kinase 3 beta; SEM, scanning electron microscopy; LC–MS, liquid chromatography–mass spectrometry; MS/MS, Tandem mass spectrometry.
Ethics Statement
The study was approved by Clinical Ethics Committee of Peking University Third Hospital, and thewritten informed consent was obtained from all patients.
Data Sharing Statement
All information is available through contacting the corresponding authors.
Author Contributions
Tang Puxian, Tao Lianyuan, and XiuDianrong conceived and designed this study; Tang Puxian and Tao Lianyuan performed exosomes isolation and proteomic identification; Tang Puxian, Tao Lianyuan, andXiu Dianrong performed the analysis and interpretation of data; Tang Puxian, Tao Lianyuan, Yuan Chunhui and Zhang Lingfu performed samples collection; Tao Tang Puxian, Tao Lianyuan, and Xiu Dianrong wrote the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Zeng L, Chen Y, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016;2016:8962321. doi: 10.1155/2016/8962321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimastromatteo J, Houghton JL, Lewis JS, et al. Challenges of pancreatic cancer. Cancer J. 2015;21:188–193. doi: 10.1097/PPO.0000000000000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Quah B, O’Neill HC. Review: the application of dendritic cell-derived exosomes in tumour immunotherapy. Cancer Biother Radiopharm. 2000;15:185–194. doi: 10.1089/cbr.2000.15.185 [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Yuan X, Shi H, et al. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greening DW, Gopal SK, Xu R, et al. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Tai YL, Chen KC, Hsieh JT, et al. Exosomes in cancer development and clinical applications. Cancer Sci. 2018. doi: 10.1111/cas.13697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S, Huo Z, Nguyen K. et al. The proteome of pancreatic cancer-derived exosomes reveals signatures rich in key signaling pathways. Proteomics. 2019; e1800394. doi: 10.1002/pmic.201800394 [DOI] [PubMed] [Google Scholar]
- 10.Jiao YJ, Jin DD, Jiang F, et al. Characterization and proteomic profiling of pancreatic cancer-derived serum exosomes. J Cell Biochem. 2019;120:988–999. doi: 10.1002/jcb.27465 [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Yamamoto H, Harada T, et al. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin Cancer Res. 2019;25:1936–1947. doi: 10.1158/1078-0432.CCR-18-2124 [DOI] [PubMed] [Google Scholar]
- 12.Tao L, Zhou J, Yuan C, et al. Metabolomics identifies serum and exosomes metabolite markers of pancreatic cancer. Metabolomics. 2019;15:86. doi: 10.1007/s11306-019-1550-1 [DOI] [PubMed] [Google Scholar]
- 13.An M, Lohse I, Tan Z, et al. Quantitative proteomic analysis of serum exosomes from patients with locally advanced pancreatic cancer undergoing chemoradiotherapy. J Proteome Res. 2017;16:1763–1772. doi: 10.1021/acs.jproteome.7b00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 15.Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J, Song J, Feng P, et al. Elevated serum apolipoprotein E is associated with metastasis and poor prognosis of non-small cell lung cancer. Tumour Biol. 2016;37:10715–10721. doi: 10.1007/s13277-016-4975-4 [DOI] [PubMed] [Google Scholar]
- 17.Shi H, Huang H, Pu J, et al. Decreased pretherapy serum apolipoprotein A-I is associated with extent of metastasis and poor prognosis of non-small-cell lung cancer. Onco Targets Ther. 2018;11:6995–7003. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Wu W, Zhen C, et al. Expression and clinical significance of complement C3, complement C4b1 and apolipoprotein E in pancreatic cancer. Oncol Lett. 2013;6:43–48. doi: 10.3892/ol.2013.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda K, Okusaka T, Felix K, et al. Altered plasma apolipoprotein modifications in patients with pancreatic cancer: protein characterization and multi-institutional validation. PLoS One. 2012;7:e46908. doi: 10.1371/journal.pone.0046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zheng W, Wang W, et al. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. Br J Cancer. 2018;118:e15. doi: 10.1038/bjc.2018.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozoe T, Kono M, Hashimoto N, et al. Definition of prognosis based on lymph node metastasis and elevation of serum c-reactive protein for patients with gastric carcinoma treated with curative resection. J Med Invest. 2018;65:191–194. doi: 10.2152/jmi.65.191 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Yang L, Xia L, et al. High C-reactive protein/albumin ratio predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2018;10:371–381. doi: 10.2147/CMAR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkassery V, Winterhalter S, Nick AC, et al. Vascular-associated Muc4/Vwf co-localization in human conjunctival malignant melanoma specimens-tumor metastasis by migration? Curr Eye Res. 2017;42:1382–1388. doi: 10.1080/02713683.2017.1324630 [DOI] [PubMed] [Google Scholar]
- 24.Qi Y, Chen W, Liang X, et al. Novel antibodies against GPIbalpha inhibit pulmonary metastasis by affecting vWF-GPIbalpha interaction. J Hematol Oncol. 2018;11:117. doi: 10.1186/s13045-018-0659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermaat JS, van der Tweel I, Mehra N, et al. Two-protein signature of novel serological markers apolipoprotein-A2 and serum amyloid alpha predicts prognosis in patients with metastatic renal cell cancer and improves the currently used prognostic survival models. Ann Oncol. 2010;21:1472–1481. doi: 10.1093/annonc/mdp559 [DOI] [PubMed] [Google Scholar]
- 26.Edderkaoui M, Chheda C, Soufi B, et al. An Inhibitor of GSK3B and HDACs kills pancreatic cancer cells and slows pancreatic tumor growth and metastasis in mice. Gastroenterology. 2018;155:1985–1998 e5. doi: 10.1053/j.gastro.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Lu XX, Wang JR, et al. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/beta-catenin degradation and is targeted for GSK3B/GSK3beta-mediated phosphorylation and degradation. Autophagy. 2019. doi: 10.1080/15548627.2019.1586250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Li Z, Jiang P, et al. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37:177. doi: 10.1186/s13046-018-0822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray K. Pancreatic cancer: pancreatic cancer exosomes prime the liver for metastasis. Nat Rev Gastroenterol Hepatol. 2015;12:371. doi: 10.1038/nrgastro.2015.93 [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Zhao S, Ren L, et al. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017;8:63461–63483. doi: 10.18632/oncotarget.18831 [DOI] [PMC free article] [PubMed] [Google Scholar]