Abstract

A composite solid polymer electrolyte (CSPE) is studied in this work to alleviate the concerns associated with poor mechanical strength of a solid polymer electrolyte (SPE) system composed of poly(ethyleneglycol)diacrylate, an electrolyte lithium bis(trifluoromethane)sulfonamide, and a plasticizer succinonitrile. CSPE is fabricated by incorporating the ingredients of SPE in the macroporous membranes of syndiotactic polystyrene to render flexibility and mechanical robustness with a 6-fold increase in tensile strength over SPE. The data from differential scanning calorimetry and wide-angle X-ray diffraction confirm the amorphous nature of the polymeric domains of SPE that produce high room-temperature ionic conductivity of ∼0.43 mS/cm. The flexible CSPE membranes are used as the electrolyte in Li-ion battery (LIB) half cells in conjunction with lithium iron phosphate as the counter electrode. The use of CSPE helps expand the electrochemical window of the cell to 5 V, indicating strong potential in the fabrication of flexible rechargeable LIBs.
Introduction
Lithium-ion batteries (LIBs) have drawn significant attention since the 1990s because of a number of valuable features such as long cycle life and high power and energy density.1 A strong demand for safe and efficient electronics presents pressing needs for high-performance batteries. However, the traditional organic carbonate-based electrolytes in commercial LIBs often lead to serious safety concerns owing to leakage and flammability. To move toward safe operation of LIBs, researchers focused their attention in the past three decades on solvent-free, solid-state electrolytes. Solid polymer electrolytes (SPEs) are ion conducting solid materials where the majority component is a polymer. The use of SPEs presents several advantages over the use of volatile liquid electrolytes, such as alleviating the concerns of internal short circuit, swelling, leakage, and fire in batteries, along with the ease of processing.2 Most SPEs are fabricated from lithium salts dissolved in ion-solvating polymers, such as polyethylene oxide (PEO), propylene oxide, poly(ethylene imine), and polyalkane sulfide.3 The ionic conductivity of SPEs are usually in the range of 0.01–1 mS/cm at room temperature.
An early discussion about the conductivity of PEO began in 1975, and the prospect of using PEO inspired further investigations on solid-state batteries.4,5 The flexible chains and ethylene oxide functional groups make PEO one of the most promising polymers for solid electrolyte system. Nevertheless, the semicrystalline structures of pristine PEO and PEO/bis(trifluoromethylsulfonyl)amine lithium (LiTFSI) complexes lead to rather low ionic conductivity (σ) at room temperature (σ ≈ 10–3 mS/cm). The low ionic conductivity, in turn, hinders direct application of PEO as an efficient electrolyte for LIBs, although the amorphous phase provides fast ion conduction.6−8 Owing to this reason, LIBs fabricated with PEO-based SPE do not function well at ambient temperatures and require a general operational temperature of above 60 °C to obtain higher values of ionic conductivity.9
The most common strategy to reduce the operating temperature and to hinder PEO crystallinity relies upon the addition of molecules that lowers the glass transition temperature (Tg).10−15 Recently, solid electrolyte systems based on plastic crystals have been reported.16−18 The organic solid plasticizers offer good compatibility with the host polymer, such as PEO, without severe phase separation. Therefore, the polymer/lithium salt/plasticizer ternary system can be tuned to specific compositions to fulfill the requirements of ionic conductivity and electrochemical stability. For example, succinonitrile is used as a solid plasticizer for various polymeric-salt systems containing PEO, poly(acrylonitrile), and poly(ethylene terephthalate).19,20 Even though the ionic conductivity is improved from 10–4 to 1 mS/cm at 30 °C,21,22 the mechanical strength of SPEs is compromised because of the use of large amounts of plasticizers in the system.11,23 In a typical ternary system consisting of LiTFSI/poly(ethyleneglycol)diacrylate (PEGDA)/succinonitrile, the highest achievable tensile strength is 0.2 MPa.24 Flexible and wearable electronics with flexible energy-storage devices are expected to gain high importance.25,26 Consequently, SPEs must not only meet the required ionic conductivity values, but also offer required mechanical strength at ambient temperature than what is available in current technologies.
The present work uses flexible macroporous membranes with >90% porosity obtained from thermoreversible gels of syndiotactic polystyrene (sPS). The pores of sPS gels are filled with ternary mixtures of LiTFSI/PEGDA/succinonitrile in the weight ratio of 35/25/40 to decouple the known negative impact of plasticizers on the mechanical strength of SPE. In this composite solid polymer network (CSPE), the ionic conductivity can be increased substantially using succinonitrile as the plasticizer, whereas the mechanical strength and dimensional stability are derived from the solid fibrillar strand networks of sPS with a typical diameter of 50 nm.27,28 Note that the solid strands of sPS have a crystalline melting temperature of ∼270 °C and a glass transition temperature of ∼100 °C. The SPE formulation in the pores serves as the ionically conductive network. The impact of high surface area sPS strands of a typical diameter of 50 nm in the gel on crystallinity of SPE and hence, on the ionic conductivity of CSPE, was investigated. The performance of LIB half-cells fabricated with the CSPE was examined.
Results and Discussion
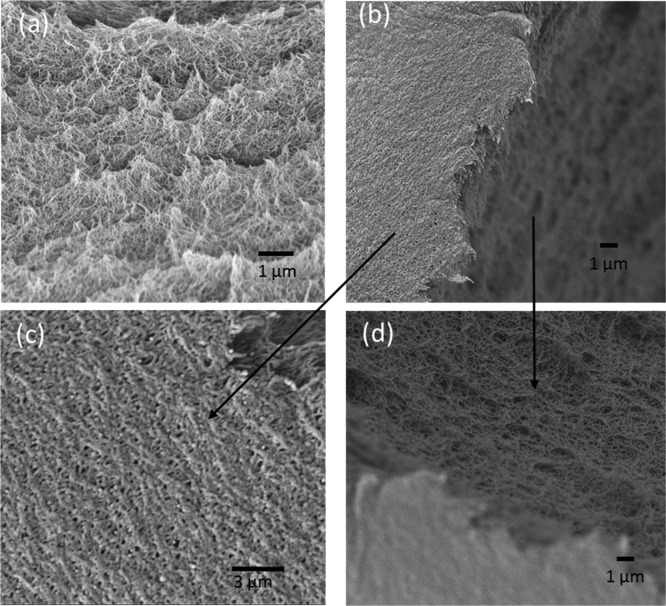
Several morphological information of the CSPE membrane were deemed important in this work—the % porosity of sPS gel membrane, the sPS polymer domain architecture, and if all of the pores of sPS gel were filled in the final SPE materials. Figure 1 shows the scanning electron microscopy images of sPS aerogel monoliths (Figure 1a) and the film (Figure 1b). Enlarged views of the solid film morphology of the surface and the cross-sectional area of the film are presented in Figure 1c,d, respectively. It is apparent that the top surface of the film (Figure 1c) was porous, giving indication that the CSPE membrane would not hinder transport of ions across the membrane. The cross-sectional area of the films in Figure 1d is highly tortuous. Such high tortuosity presents desirable attributes to deter lithium dendrite growth. The macroporous structures of sPS aerogels were used earlier in removing airborne nanoparticles of 75 nm mean diameter with close to 100% efficiency and air permeability of 10–10 m2.28,29Figure 2a,b show optical images of CSPE specimens after multiple bending and wrapping around a cylindrical wand, respectively, indicating that CSPE membranes obtained in this work were highly flexible and therefore, amenable to usage in the fabrication of energy storage devices in wearable technology installations. The corresponding SPE membranes required delicate handling because they broke easily. The porosity of sPS aerogel was 90% as determined from the values of bulk density and skeletal density, the latter obtained using helium pycnometer. Such high porosity of sPS membranes allowed higher loading of SPE in the CSPE membranes, eventually achieving higher ionic conductivity. Figure 2c presents an optical image of cross section of the fractured CSPE film, indicating that all pores on the cross-sectional plane of sPS gel were filled with SPE.
Figure 1.
Scanning electron microscope images: (a) fractured surface of bulk sPS aerogel, (b) fractured surface of sPS aerogel membrane, (c) top surface of sPS aerogel membrane, and (d) cross section of sPS aerogel membrane.
Figure 2.

Photographic images of CSPE membrane undergoing (a) bending and (b) wrapping and (c) optical image (20× magnification) of the cross section of CSPE membrane.
The thermal stability of SPEs, which is an important factor for solving the safety issues in batteries, was determined from thermogravimetric analysis (TGA). Figure 3a shows the % weight versus temperature for all of the components in SPE and CSPE obtained using TGA under nitrogen environment. On the basis of the weight loss versus temperature curves in Figure 3a, it is apparent that SPEs did not undergo thermal decomposition until about 100 °C, suggesting that the upper limit temperature for the SPEs is 100 °C. This enhanced thermal stability of SPE compared to the liquid carbonate electrolytes is especially useful for the safety of operation of the LIBs.
Figure 3.
(a) TGA traces of SPE, CSPE, porous sPS, and all of the individual components of CSPE membrane. (b) Comparison of SPE with CSPE and their derivative weight vs temperature plots.
From the data presented in Figure 3b, the first weight loss for SPE and CSPE is calculated to be approximately 40%, attributed to the loss of succinonitrile (Figure 3a) via sublimation close to 100 °C. The second and third weight loss events are, respectively, due to decomposition of PEGDA and the Li salt, as previously reported.24 The TGA trace for CSPE is similar to that of neat SPE up to the second weight loss event because of the degradation of PEGDA. The third peak in derivative weight loss versus temperature data in Figure 3b is shifted to higher temperature, to around 450 °C attributed to the higher thermal stability of sPS chains compared to the Li salt.
It is important to determine the amorphous and crystalline polymer content in CSPE. Specifically, it is important to assess the effect of sPS strands on the crystallinity of components in SPE formulation. For this purpose, differential scanning calorimetry (DSC) thermograms presented in Figure 4 are discussed. Note that SPE and CSPE specimens were dried in a vacuum oven to eliminate the amounts of water absorbed during sample fabrication. An inhomogeneous SPE formulation can produce regions of crystalline areas, resulting in spikes and peaks in the DSC traces. It is clearly seen in Figure 4 that DSC traces of neat SPE and CSPE are devoid of sharp peaks, indicating that both of these materials were amorphous in nature. A melting peak at 17 °C is observed for neat PEGDA. Succinonitrile shows sharp peaks for single plastic phase transformations at −35 and 58 °C, as reported by Alarco et al.18 The absence of peaks due to succinonitrile or PEGDA in neat SPE or CSPE indicates the amorphous nature of the fabricated membranes. The high amorphous content of CSPE, as revealed by the DSC traces, indicates reduced barrier between different phases and promises high ionic conductivity values.
Figure 4.
DSC thermograms for SPE, CSPE, and succinonitrile, porous sPS, and PEGDA.
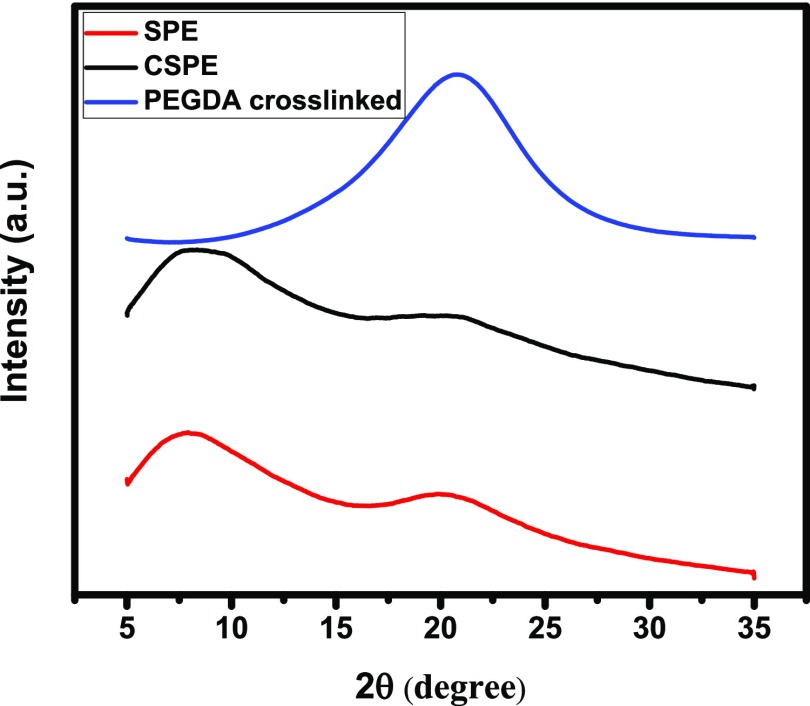
The DSC data showed two broad amorphous peaks for CSPE. The absence of crystallinity in CSPE was confirmed using X-ray diffraction (XRD) data, as presented in Figure 5. It is evident after comparing XRD patterns of SPE and CSPE that the incorporation of SPE in the pores of sPS did not alter the crystallinity of SPE; the two broad peaks observed in Figure 5 are attributed to amorphous domains of CSPE. Note that succinonitrile shows sharp crystalline 2θ peaks at ∼20° and ∼29°, whereas the peaks for crystalline PEO domains appear at ∼19° and 24°.29−31 The broad peak at around 20° is because of the cross-linked PEGDA.32 The δ-form of sPS crystals are characterized by a peak at 2θ = 8.3°,33 as shown in the traces of CSPE. The source of the peak for SPE at 2θ between 5° and 10° cannot be confirmed. The XRD data in conjunction with DSC thermograms discussed earlier confirm that SPE domains remained amorphous in CSPE membranes, and the confinement in sPS matrix had no effect on SPE crystallinity. It is anticipated that the amorphous phase of SPE in CSPE would help achieve higher ionic conductivity.34
Figure 5.
XRD patterns of SPE, CSPE, and cross-linked PEGDA.
As alluded to the earlier discussion, the mechanical strength is of prime importance for the successful implementation of CSPE membranes in flexible energy storage devices. The optical images in Figure 2 revealed that CSPE membranes were flexible and withstood several bending configurations. Figure 6 compares the stress versus strain data of neat SPE and CSPE membranes. It is apparent that the yield strength of CSPE membranes (∼1.8 MPa) is almost 6 times that of neat SPE membranes (0.3 MPa). In addition, the tensile strength of ∼2 MPa and the tensile strain at break of 35% are true reflections of the structural integrity of CSPE membranes at large strains, contributed by the sPS networks. Neat SPE shows plastic flow after the yield point, followed by necking, whereas CSPE shows stress hardening above the yield point.
Figure 6.
Stress vs strain data for SPE and CSPE.
The presence of sPS networks also augmented the storage modulus values in CSPE, as presented in Figure 7. The storage modulus of CSPE is seen to be higher than that of SPE at all temperatures, with the glassy state storage modulus approaching 1500 MPa. The drop in storage modulus values with temperature is more precipitous for SPE, for example, because of glass transition, whereas the sPS network provided rigidity to CSPE membranes and the corresponding storage modulus values of CSPE range between a factor 5–10 times that of SPE at temperatures between −20 and 80 °C. In the case of CSPE, an additional drop in storage modulus occurred at a temperature ∼90 °C because of the glass transition of sPS chains. The values of Tg of SPE and CSPE can be inferred from the peaks of tan δ versus temperature curves in Figure 7. Neat SPE shows a Tg around −45 °C due to cross-linked PEGDA, whereas CSPE shows two Tg peaks. The first peak appears at around −52 °C because of cross-linked PEGDA system, and the broad peak at around 90 °C is because of the glass transition of sPS chains. A reduction of Tg for the cross-linked PEGDA phase from −45 °C for neat SPE to −52 °C in CSPE can be attributed to lower cross-link density of PEGDA in the CSPE system. As the sPS-phase is responsible for the mechanical strength of CSPE membranes, a reduction of Tg by −7 °C may be beneficial in achieving higher electrochemical performance in CSPE membranes.35
Figure 7.
Storage modulus (solid lines) and tan δ (dotted line) of SPE and CSPE membranes and compressed sPS sheets.
The electrochemical stability of the membrane materials was evaluated using linear sweep voltammetry (LSV) and cyclic voltammetry (CV) data, as presented in Figures 8 and 9, respectively. The symmetric peak between −0.5 and 0.5 V is commonly accepted as a typical reversible lithium plating/stripping behavior. The LSV data in Figure 8a for CSPE exhibit a much wider electrochemical window compared to what is observed for general liquid electrolytes and neat SPE (Figure 8b). The electrochemical window for SPE turned unstable at ∼4.2 V because of SPE decomposition from ∼4.5 V onward. For CSPE system, however, the film remained stable until ∼5 V and started decomposing at ∼5.3 V. From the CV data in Figure 9, the stability under repeated scanning is inferred. The performance of CSPE was coherent in line with what was shown in Figure 8, and the performance remained stable up to 5 V in the first cycle. The data for second cycle do not show obvious redox/oxidation peak, suggesting the stability of CSPE when subjected to repetition of ramped voltage. LSV and CV data indicate that CSPE membranes are stable for electrochemical operations up to 5 V without any side reactions, and hence, such membranes will have the potential to be a candidate for LIBs.
Figure 8.
LSV for (a) SPE and (b) CSPE.
Figure 9.
CV data for CSPE.
Impedance spectroscopy is a commonly applied method for the evaluation of electrical performance of SPEs. The ionic conductivity (σ) at various temperatures is calculated using eq 1(36)
| 1 |
In eq 1, l is the thickness, Rb is the bulk resistance, and A is the area of SPE. The ionic conductivity describes the movement of ions inside the film; such behavior can be inferred from the data presented in Figure 10. At room temperature, the value of Rb was 230 Ω and the corresponding ionic conductivity of CSPE membranes was 0.43 mS/cm; this value meets the requirements for SPE. Ionic conductivity for neat SPE (LiTFSI/PEGDA/succinonitrile wt ratio 35/25/40) was 0.72 mS/cm at 30 °C,21 indicating that CSPE was able to maintain the same order of magnitude conductivity.
Figure 10.
Nyquist plot for the conductivity of CSPE.
The battery testing results are shown in Figure 11. The initial specific discharge capacity can reach ∼140 mA h/g at a current density of 30 mA/g; the Coulombic efficiency gradually increased and remained at 99.8% with a slight fluctuation, suggesting a reversible charge and discharge process without much loss of active materials. The specific capacity at 40 cycles remains to be 114 mA h/g, and the average fading rate from 2 to 40 cycles was only 0.5 mA h/g per cycle. The data in Figure 11 show that CSPE membranes worked well with LiFePO4 (LFP) cathode in a solid-state LIB.
Figure 11.
Charge–discharge test for LIB constructed using CSPE.
Conclusions
The CSPE membranes exhibited much higher thermal and electrochemical stability while maintaining the ionic conductivity at the level of SPE membranes. The DSC and XRD data confirmed the amorphous nature of SPE in both SPE and CSPE membranes. This helped achieve the same order of magnitude of ionic conductivity for CSPE and that of SPE. Mechanical strength of CSPE was 6 times higher than that of neat SPE because of the sPS networks that also contributed to the flexibility of membrane materials. The higher mechanical properties and thermal stability of sPS also allowed for a considerable electrochemical performance. Compared to the PEGDA/LiTFSI/plasticizer system, which remained stable until 4.7 V, the electrochemical window of CSPE was expanded to over 5 V. The ionic conductivity of CSPE can fulfill the requirements for SPEs as it reached a value of 0.43 mS/cm. The fading rate of the all-solid-state battery was less than 0.5 mA h/g per cycle, indicating that CSPE can work effectively in solid-state LIBs.
Materials and Methods
sPS (Mw = 300 000 g/mol, density = 1.05 g/mL) was purchased from Scientific Polymer Products Inc. (Ontario, NY). Tetrahydrofuran (THF) was obtained from Sigma-Aldrich and was used as received. PEGDA (Sigma-Aldrich, 99%) with a molecular weight of 700 g mol–1, succinonitrile (C4N2H4 or SCN, Alfa Aesar, 99%), LiTFSI salt (Matrix Scientific, Columbia, SC 99%), and photoinitiator bis(2,4,6-trimethylbenzoyl)-phenylphosphine oxide (Irgacure 819, Sigma-Aldrich, 97%) were used without further purification. Electrode materials including LFP (MTI Corp., Richmond, CA), carbon black (Super P, MTI Corp., Richmond, CA), and poly(vinylidene fluoride) (Sigma-Aldrich, 99.5%) were placed in the oven at 80 °C overnight to remove the residual moisture before use. Battery fabrication components included an aluminum current collector, purchased from MTI Corp., anhydrous 1-methyl-2-pyrrolidone (Alfa Aesar, 99%), and round punched lithium metal pieces (Li, MTI Corp.).
In a typical SPE fabrication process, LiTFSI salt, polymer host PEGDA, and succinonitrile plasticizer were kept and stored in an argon-filled glovebox (O2 < 0.5 ppm, H2O < 0.5 ppm). The desired weight ratio of the ternary mixture LiTFSI/PEGDA/succinonitrile was 35/25/40. The mixture was vigorously stirred for half an hour at room temperature to obtain a homogeneous solution; then, 2 wt % of photoinitiator (Irgacure 819) based on PEGDA content was introduced into the mixture, and the mixture was further stirred for another 5 min, allowing the photoinitiator to dissolve. Subsequently, the mixture was poured into a mold with a glass cover on top to obtain a smooth surface and a desired thickness. A UV light source with a wavelength of 350 nm was exposed to the sample for 1 min to cure the SPE film.
In preparation of CSPE films, sPS organogel was first synthesized, following a procedure outlined in the literature.27,28 For this purpose, 0.1 g/mL sPS pellets were dissolved in THF at 110 °C in a sealed vial. After complete dissolution of the pellets, the hot solution was cast in the form of film or poured into a mold to obtain monoliths. The THF phase of sPS organogel was later replaced with the SPE solution containing LiTFSI/PEGDA/succinonitrile in a weight ratio of 35/25/40 using a solvent exchange method. A similar process was used earlier in developing high-temperature stable LIB separators from macroporous sPS membranes filled with ionic liquids and LiTFSI.37 A complete removal of THF was confirmed from Fourier transform infrared spectra in the transmission mode. The solvent exchange steps were carried out in a glovebox under inert Ar environment.
Porous sPS organogels (films and monoliths) were dried using supercritical CO2 to obtain sPS aerogel.25,26 Supercritical drying was performed at 50 °C and 1100 KPa, which are above the critical point of CO2 (31 °C and 740 KPa).
Porosity (P) of sPS aerogel specimens was estimated from bulk density (ρb) and skeletal density (ρs) values, as in eq 2, whereas bulk density was obtained from mass and volume of the specimens, as in eq 3.
| 2 |
| 3 |
In eq 3, m is the mass of the sample, D is the diameter of bulk sPS aerogel monoliths, and h is the height of the monolith. Skeletal density was determined using helium pycnometer (AccuPyc II 1340 Series, Micromeritics Instrument Corp GA, USA).
The porous morphology of the membrane was examined using a scanning electron microscope (JEOL JSM5310) with an operating voltage of 5 kV. For this purpose, supercritically dried samples were fractured at room temperature. The fractured specimen was mounted on an aluminum stub using conductive carbon tape, and the stubs were sputter-coated under Ar atmosphere using ISA 5400 sputter coater. The CSPE membranes were examined using an optical microscope to determine the presence of obvious unfilled pores.
The crystallinity of polymeric samples was studied using XRD patterns of neat SPE and CSPE obtained using Rigaku SmartLab X-ray diffractometer equipped with HyPix 33000 detector and Cu Kα radiation (λ = 1.5604 Å). The tube voltage and current of 40 kV and 44 mA were used. Data were collected continuously at a rate of 1°/min from 2θ = 5° up to 35°.
DSC (TA Q200, TA Instruments, New Castle, DE) technique was used to study the thermal behavior of materials. The thermal properties, such as melting temperature (Tm) and glass transition temperature (Tg), were determined at a ramp rate of 10 °C/min in a sealed hermetic pan under N2 purge flow of 50 mL/min. The thermal stability of samples was investigated using TGA (Q50 TA Instruments) under nitrogen environment. Approximately, 10 mg of the sample was placed in the platinum pan and subjected to a heating rate of 10 °C under N2 flow rate of 50 mL/min.
The tensile strengths of SPE and CSPE films were measured in a tension mode. The samples were prepared in dimensions of 0.6 mm × 1 mm × 3 cm. An extension rate of 1 mm/min was used. Dynamic mechanical properties were obtained using Q800 dynamic mechanical analyzer (DMA) (TA Instruments, New Castle, Delaware). Initially, a strain sweep experiment was performed at 1 Hz to examine the linear viscoelastic regime. The temperature sweep experiments were performed at 0.1% strain and 1 Hz frequency from −90 to 150 °C. The loss tangent (tan δ) value of the CSPE was monitored as a function of temperature to obtain Tg of the composite membrane specimens. For this purpose, specimens were prepared in a mold of dimension of 0.6 mm × 1 mm × 3 cm and subsequently cured by photo-cross-linking. Neat sPS aerogel monolith specimens were compressed at 30 MPa pressure using a compression molder to obtain flat film samples for DMA testing under tension mode.
Coin cell 2032 was fabricated using LFP as the cathode material and Li foil as the anode. The composite LFP electrode with a mass loading of 2–3 mg/cm2 was put into a mold. A mixture of LiTFSI/PEGDA/succinonitrile in a weight ratio of 35/25/40 and photoinitiator was added, and the mixture was exposed to UV light for the cross-linking of the polymer. The Li foil was wetted by the electrolyte mixture on one side, overlaid on the SPE/cathode system prepared above, and the system was exposed to UV light again for curing of the polymer.
The electrochemical stability of the CSPE and the coin cell was determined from LSV and CV using SS (stainless steel)/CSPE/Li block cell on electrochemical workstation (CHI608E Electrochemical Analyzer, CH Instruments). In LSV, the voltage was changed from 0 to 10 V at a sweep rate of 0.5 mV/s. The same scan rate was applied to CV study with a voltage range of −0.5–5 V.
Ionic conductivity at room temperature was obtained from electrochemical impedance spectroscopy test using electrochemical workstation (CHI608E Electrochemical Analyzer, CH Instruments). The frequency range of the test was changed from 1 MHz to 0.1 Hz with an amplitude of 10 mV using a SS/PEM/SS cell. The temperature during measurement was controlled by using an isothermal chamber with a large thermal mass. The thickness of the films was controlled to 1 mm by using a mold.
The galvanostatic charge–discharge cycling test of the half-cells was carried out within the range of 2.5–3.9 V. The current density was 30 mA/g for the first 10 cycles; then, it was increased to 50 mA/g for later cycles.
Acknowledgments
The authors would like to thank E. Laughlin and K. Jackson of The University of Akron. The authors also thank Dr. Bojie Wang (Manager of the Microscopy Lab) of The University of Akron for guidance on electron microscopy.
The authors declare no competing financial interest.
References
- Dunn B.; Kamath H.; Tarascon J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. 10.1126/science.1212741. [DOI] [PubMed] [Google Scholar]
- Meyer W. H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. . [DOI] [PubMed] [Google Scholar]
- Marczewski M. J.; Stanje B.; Hanzu I.; Wilkening M.; Johansson P. “Ionic Liquids-in-Salt”--a Promising Electrolyte Concept for High-Temperature Lithium Batteries?. Phys. Chem. Chem. Phys. 2014, 16, 12341–12349. 10.1039/c4cp01133c. [DOI] [PubMed] [Google Scholar]
- Wright P. V. Electrical Conductivity in Ionic Complexes of Poly(Ethylene Oxide). Br. Polym. J. 1975, 7, 319–327. 10.1002/pi.4980070505. [DOI] [Google Scholar]
- Yue L.; Ma J.; Zhang J.; Zhao J.; Dong S.; Liu Z.; Cui G.; Chen L. All Solid-State Polymer Electrolytes for High-Performance Lithium Ion Batteries. Energy Storage Mater. 2016, 5, 139–164. 10.1016/j.ensm.2016.07.003. [DOI] [Google Scholar]
- Liew C.-W.; Durairaj R.; Ramesh S. Rheological Studies of PMMA-PVC Based Polymer Blend Electrolytes with LiTFSI as Doping Salt. PLoS One 2014, 9, e102815 10.1371/journal.pone.0102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam W.; Liew C.-W.; Lim J. Y.; Ramesh S. Electrical , Structural , and Thermal Studies of Antimony Trioxide-Doped Poly(Acrylic Acid ) Based Composite Polymer Electrolytes. Int. J. Pharm. 2014, 20, 665. 10.1007/s11581-013-1012-0. [DOI] [Google Scholar]
- Ngai K. S.; Ramesh S.; Ramesh K.; Juan J. C. A Review of Polymer Electrolytes: Fundamental, Approaches and Applications. Ionics 2016, 22, 1259–1279. 10.1007/s11581-016-1756-4. [DOI] [Google Scholar]
- Dias F. B.; Plomp L.; Veldhuis J. B. J. Trends in Polymer Electrolytes for Secondary Lithium Batteries. J. Power Sources 2000, 88, 169–191. 10.1016/s0378-7753(99)00529-7. [DOI] [Google Scholar]
- Long L.; Wang S.; Xiao M.; Meng Y. Polymer Electrolytes for Lithium Polymer Batteries. J. Mater. Chem. A 2016, 4, 10038–10069. 10.1039/c6ta02621d. [DOI] [Google Scholar]
- Stephan A. M. Review on Gel Polymer Electrolytes for Lithium Batteries. Eur. Polym. J. 2006, 42, 21–42. 10.1016/j.eurpolymj.2005.09.017. [DOI] [Google Scholar]
- Kolosnitsyn V. S.; Dukhanin G. P.; Dumler S. A.; Novakov I. A. Lithium-Conducting Polymer Electrolytes for Chemical Power Sources. Russ. J. Appl. Chem. 2005, 78, 1–18. 10.1007/s11167-005-0222-2. [DOI] [Google Scholar]
- Park M. J.; Choi I.; Hong J.; Kim O. Polymer Electrolytes Integrated with Ionic Liquids for Future Electrochemical Devices. J. Appl. Polym. Sci. 2013, 129, 2363–2376. 10.1002/app.39064. [DOI] [Google Scholar]
- Nan C.-W.; Fan L.; Lin Y.; Cai Q. Enhanced Ionic Conductivity of Polymer Electrolytes Containing Nanocomposite SiO2 Particles. Phys. Rev. Lett. 2003, 91, 266104. 10.1103/physrevlett.91.266104. [DOI] [PubMed] [Google Scholar]
- Agrawal R. C.; Gupta R. K. Superionic Solids: Composite Electrolyte Phase - an Overview. J. Mater. Sci. 1999, 34, 1131–1162. 10.1023/a:1004598902146. [DOI] [Google Scholar]
- Long S.; MacFarlane D. R.; Forsyth M. Fast Ion Conduction in Molecular Plastic Crystals. Solid State Ionics 2003, 161, 105–112. 10.1016/s0167-2738(03)00208-x. [DOI] [Google Scholar]
- Abouimrane a.; Davidson I. J. Solid Electrolyte Based on Succinonitrile and LiBOB. J. Electrochem. Soc. 2007, 154, A1031–A1034. 10.1149/1.2781305. [DOI] [Google Scholar]
- Alarco P.-J.; Abu-Lebdeh Y.; Abouimrane A.; Armand M. The Plastic-Crystalline Phase of Succinonitrile as a Universal Matrix for Solid-State Ionic Conductors. Nat. Mater. 2004, 3, 476–481. 10.1038/nmat1158. [DOI] [PubMed] [Google Scholar]
- Taib N. U.; Idris N. H. Plastic Crystal-Solid Biopolymer Electrolytes for Rechargeable Lithium Batteries. J. Membr. Sci. 2014, 468, 149–154. 10.1016/j.memsci.2014.06.001. [DOI] [Google Scholar]
- Muldoon J.; Bucur C. B.; Boaretto N.; Gregory T.; di Noto V. Polymers: Opening Doors to Future Batteries. Polym. Rev. 2015, 55, 208–246. 10.1080/15583724.2015.1011966. [DOI] [Google Scholar]
- Li S.; Chen Y.-M.; Liang W.; Shao Y.; Liu K.; Nikolov Z.; Zhu Y. A Superionic Conductive, Electrochemically Stable Dual-Salt Polymer Electrolyte. Joule 2018, 2, 1838–1856. 10.1016/j.joule.2018.06.008. [DOI] [Google Scholar]
- Liang W.; Shao Y.; Chen Y.-M.; Zhu Y. A 4 V Cathode Compatible, Superionic Conductive Solid Polymer Electrolyte for Solid Lithium Metal Batteries with Long Cycle Life. ACS Appl. Energy Mater. 2018, 1, 6064–6071. 10.1021/acsaem.8b01138. [DOI] [Google Scholar]
- Xue Z.; He D.; Xie X. Poly(Ethylene Oxide)-Based Electrolytes for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19218–19253. 10.1039/c5ta03471j. [DOI] [Google Scholar]
- He R.; Echeverri M.; Ward D.; Zhu Y.; Kyu T. Highly Conductive Solvent-Free Polymer Electrolyte Membrane for Lithium-Ion Batteries: Effect of Prepolymer Molecular Weight. J. Membr. Sci. 2016, 498, 208–217. 10.1016/j.memsci.2015.10.008. [DOI] [Google Scholar]
- Croce F.; Appetecchi G. B.; Persi L.; Scrosati B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. 10.1038/28818. [DOI] [Google Scholar]
- He Z.; Chen L.; Zhang B.; Liu Y.; Fan L.-Z. Flexible Poly(Ethylene Carbonate)/Garnet Composite Solid Electrolyte Reinforced by Poly(Vinylidene Fluoride-Hexafluoropropylene) for Lithium Metal Batteries. J. Power Sources 2018, 392, 232–238. 10.1016/j.jpowsour.2018.05.006. [DOI] [Google Scholar]
- Daniel C.; Dammer C.; Guenet J.-M. On the Definition of Thermoreversible Gels: The Case of Syndiotactic Polystyrene. Polymer 1994, 35, 4243–4246. 10.1016/0032-3861(94)90604-1. [DOI] [Google Scholar]
- Wang X.; Jana S. C. Tailoring of Morphology and Surface Properties of Syndiotactic Polystyrene Aerogels. Langmuir 2013, 29, 5589–5598. 10.1021/la400492m. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Fan H.; Fan L.-Z.; Shi Q. Preparation and Performance of a Non-Ionic Plastic Crystal Electrolyte with the Addition of Polymer for Lithium Ion Batteries. Electrochim. Acta 2013, 114, 720–725. 10.1016/j.electacta.2013.10.111. [DOI] [Google Scholar]
- Fan L.-Z.; Hu Y.-S.; Bhattacharyya A. J.; Maier J. Succinonitrile as a Versatile Additive for Polymer Electrolytes. Adv. Funct. Mater. 2007, 17, 2800–2807. 10.1002/adfm.200601070. [DOI] [Google Scholar]
- Michael M. S.; Jacob M. M. E.; Prabaharan S. R. S.; Radhakrishna S. Enhanced Lithium Ion Transport in PEO-Based Solid Polymer Electrolytes Employing a Novel Class of Plasticizers. Solid State Ionics 1997, 98, 167–174. 10.1016/s0167-2738(97)00117-3. [DOI] [Google Scholar]
- Paranjape N.; Mandadapu P. C.; Wu G.; Lin H. Highly-Branched Cross-Linked Poly(Ethylene Oxide) with Enhanced Ionic Conductivity. Polymer 2017, 111, 1–8. 10.1016/j.polymer.2017.01.014. [DOI] [Google Scholar]
- Daniel C.; Alfano D.; Venditto V.; Cardea S.; Reverchon E.; Larobina D.; Mensitieri G.; Guerra G. Aerogels with a MicroporousCrystalline Host Phase. Adv. Mater. 2005, 17, 1515–1518. 10.1002/adma.200401762. [DOI] [Google Scholar]
- Johansson P. First principles modelling of amorphous polymer electrolytes: Li+-PEO, Li+-PEI, and Li+-PES complexes. Polymer 2001, 42, 4367–4373. 10.1016/s0032-3861(00)00731-x. [DOI] [Google Scholar]
- Jagur-Grodzinski J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. 10.1002/pat.729. [DOI] [Google Scholar]
- Lin Y.; Li J.; Liu K.; Liu Y.; Liu J.; Wang X. Unique Starch Polymer Electrolyte for High Capacity All-Solid-State Lithium Sulfur Battery. Green Chem. 2016, 18, 3796–3803. 10.1039/c6gc00444j. [DOI] [Google Scholar]
- Raut P.; Liang W.; Chen Y.-M.; Zhu Y.; Jana S. C. Syndiotactic Polystyrene-Based Ionogel Membranes for High Temperature Electrochemical Applications. ACS Appl. Mater. Interfaces 2017, 9, 30933–30942. 10.1021/acsami.7b09155. [DOI] [PubMed] [Google Scholar]