Abstract
With the purpose to explore the relationship between deoxynivalenol (DON)-induced apoptosis and autophagy and provide mechanistic explanations for the toxic effects of DON on IPEC-J2 cells, we determined the cell viability, cell morphology, apoptosis, and autophagy by using autophagy inhibitor 3-methyladenine (3-MA), PI3K pathway inhibitor LY294002, and activator 740Y-P. It turned out that 3-MA was able to attenuate the reduction of cell viability induced by DON. Moreover, 3-MA was capable of upregulating the expression of DON-induced autophagic protein p62 and downregulating the expressions of DON-induced autophagic protein LC3-II and apoptotic protein Bax, suggesting that autophagy is a driving mechanism for this apoptotic induction. The results of Annexin V-FITC/PI double staining indicated that DON could induce apoptosis by inhibiting the PI3K-AKT-mTOR signaling pathway. Subsequently, it was further confirmed by Western blot analysis that DON significantly decreased expressions of P-AKT/AKT, p-mTOR/mTOR, and autophagic protein p62, and increased expression of autophagy-related protein LC3-II, suggesting that DON triggered autophagy by inhibiting the PI3K-AKT-mTOR signaling pathway. To conclude, these data reveal that DON may induce cytotoxicity and apoptosis through the activation of autophagy by suppressing the PI3K-AKT-mTOR signaling pathway. This study provides new insights into the mechanisms by which DON incurs cytotoxic effects.
Introduction
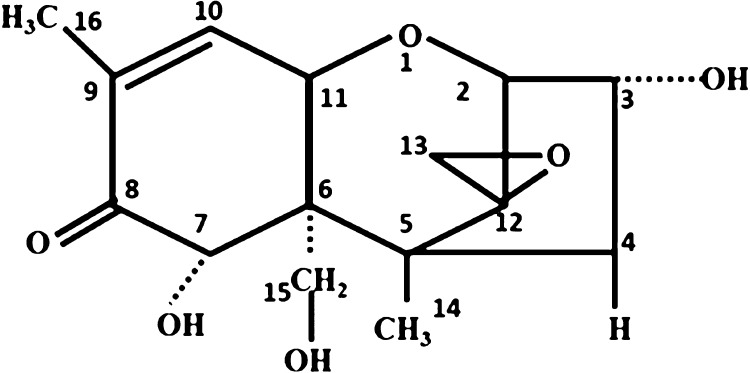
The issue of deoxynivalenol (DON) contamination of food has received considerable critical attention. DON, commonly known as vomitoxin, is a secondary metabolite of Fusarium contaminating various crops and feeds.1 In the molecule of DON (Figure 1), the C9/C10 double bond, epoxy group on C12/C13, and the free hydroxyl group are the main reasons for DON toxicity.2,3 The contamination of food by DON can be carried out before the processing, production, storage, transportation, sale of food, or through the food chain.
Figure 1.
Structural formula of DON.
The gastrointestinal tract is the first barrier against external pollutants and pathogens.4,5 The intact mucosal barrier of the small intestine is an essential basis for ensuring intestinal health. Functional tight junctions between small intestinal epithelial cells (IECs) are prerequisites for maintaining the normal barrier function and absorption function of the small intestinal mucosa.6 IECs would quickly absorb DON of high concentrations when exposed to contaminated feed or food.7−9 Many in vivo and in vitro research studies have demonstrated that DON obstructs IECs function through regulating cell proliferation and activity and impairing intestinal barrier function.7,10,11 Tang et al. found that DON reduced the activity of IPEC-J2 cells in a time- and dose-dependent manner.12 At the molecular level, DON causes toxic ribosome stress, induces MAPK phosphorylation, promotes apoptosis, induces changes in oxidative stress, and inflammatory responses, and reduces the expression of cell adhesion proteins.13,14 Moreover, our previous study found that DON can induce autophagy in addition to inducing apoptosis in IPEC-J2 cells.15
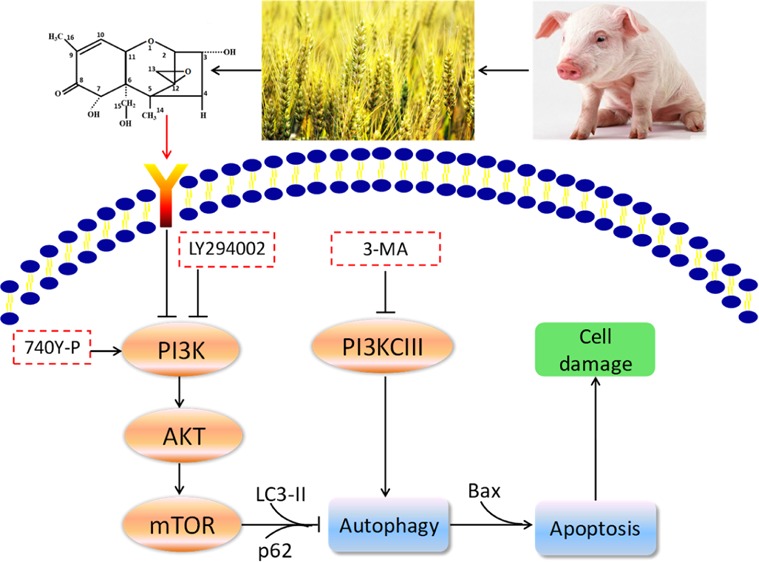
Autophagy manifests a duality with respect to the regulation of cell death: under normal physiological conditions, to some degree, autophagy protects cells from harmful conditions to improve cell survival.16 On the other hand, excessive autophagy could cause programmed cell death, which is named as autophagy-mediated cell death.17 Apoptosis and autophagy are crucial to maintaining the homeostasis of the internal environment and healthy growth.18,19 Numerous studies have found that autophagy and apoptosis were regulated by PI3K-AKT,20 p38 MAPK,21 JNK,22 and AMPK.23,24 In a recent study, DON induced autophagy and apoptosis in porcine oocytes.25
Prior studies have noted the importance of PI3K-AKT signaling pathway, which belongs to one of the classical pathways of negative regulation on autophagy.26 PI3K exists in the cytoplasm, including type I (PI3KCI), type II (PI3KCII), and type III (PI3KCIII). Currently, it is found that PI3KCI and PI3KCIII are involved in the regulation of autophagy. PI3KCI is phosphorylated by external stimulation, afterward activating AKT.27,28 PI3KCI can be inhibited and activated by LY294002 and 740Y-P, respectively, playing an important role in the PI3K-AKT signaling pathway.29,30 PI3KCIII is capable of positively regulating autophagy but can be inhibited by 3-methyladenine (3-MA), thus suppressing the formation of pre-autophagosome.31 Zhang et al. confirmed the association of JAK/STAT, p38 MAPK, and PI3K/AKT pathways with DON-induced enteric toxicity.5
As mentioned above, DON can induce autophagy and apoptosis in different cell lines, but the questions remain open as to figuring out the mechanism of DON-induced autophagy and whether DON-induced apoptosis and autophagy may be related to the PI3K-AKT-mTOR signaling pathway in IPEC-J2 cells. To this end, the objective of the present study was to explore the relationship between apoptosis and autophagy induced by DON in IPEC-J2 cells, which were employed as an in vitro model of toxicity assay. We also wanted to confirm whether DON may activate autophagy by inhibiting the PI3K-AKT-mTOR pathway in IPEC-J2 cells. This report will give a new perception to DON-induced cytotoxicity.
Materials and Methods
Chemicals and Cell Culture
Dimethylsulfoxide (DMSO) and DON were kindly purchased from Sigma-Aldrich Corp (St. Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was obtained from Promega (Madison, WI, USA). Annexin V-FITC Apoptosis Detection Kit was bought from DOJINDO (Shanghai, China). The main antibodies against Bax, p62, LC3, AKT, mTOR, and GAPDH and HRP-linked antibody were acquired from Servicebio (Wuhan, China). Primary antibodies against p-AKT and p-mTOR were obtained from Nanjing Jiancheng Bioengineering Institute (Jiangsu province, China). 740Y-P was purchased from APEXBIO (Shanghai, China). LY294002 was purchased from TargetMol (Shanghai, China). 3-MA was purchased from Selleckchem (Houston, TX). IPEC-J2 cells were procured from Biochemy (Wuhan, China) and preserved in Dulbecco’s modified Eagle medium (DMEM) incorporating 50 μg/mL streptomycin (Gibco Invitrogen), 50 μg/mL penicillin and 10% fetal bovine serum.
Design of Experiments
After being inoculated in a 96-well plate at a density of 1 × 104 cells/well, the IPEC-J2 cells were incubated at 37 °C for 24 h under a humidified circumstance containing 5% CO2. The untreated fresh cells were designated as the control group, and the cells being exposed to DON of different concentrations (1, 2, 4 and 8 μM) for a period of time (4–48 h) were referred to as the DON group. The 3-MA group was the cells treated with a combination (DON + 3-MA) of 1 μM DON and 3-MA (5 mM). The 740Y-P group was the cells treated with a combination (DON + 740Y-P) of 1 μM DON and 740Y-P (20 μM). The LY294002 group was the cells treated with a combination (DON + LY294002) of 1 μM DON and LY294002 (10 μM).
The DMSO stored at −20 °C was used to prepare stock solutions of 10 mM DON, 740Y-P, and LY294002, separately. A 100 mM 3-MA stock solution was prepared using DMEM stored at −20 °C. For each experiment, DMEM medium was used to further dilute 10 mM DON stock solutions to acquire various required concentrations of DON. All of the cells underwent 4, 8, 12, 24, and 48 h of treatment with DON.
Cell Viability Assay
After treatment with DON, 3-MA, LY294002, and 740Y-P at 37 °C for different durations (4–48 h) in a CO2 incubator, the MTS method15 was used to determine cell viability in accordance with the manufacturer’s instructions (Biochemy). Afterward, absorbance was examined via a microplate reader at 490 nm (Thermomax).
Observation of Cell Morphology by the Inverted Microscope
After IPEC-J2 cells were treated by DON, 3-MA, LY294002, and 740Y-P for 12 h, changes in cell morphology were observed at 100-fold magnification with an inverted microscope (Wuhan, China), and then the obtained images were taken with a digital camera.
Detection of Apoptosis by the Fluorescent Microscope
IPEC-J2 cells were double-stained with Annexin V-FITC (DOJINDO, Shanghai, China) and PI for detection of early apoptotic and late apoptotic/dead cells, respectively. In brief, after IPEC-J2 cells were treated by DON, 3-MA, LY294002, and 740Y-P, the cells of equal amount were collected and stained with Annexin V-FITC and PI at room temperature in the dark for 15 min, followed by observation of fluorescence intensity with a fluorescent microscope (Olympus, Japan). The specific experimental procedure was carried out following the instructions of the Annexin V-FITC Apoptosis Detection Kit.
Western Blot Assay
Western blot was carried out as described previously.15,32 Briefly, the mixtures of 3-MA (5 mM), 740Y-P (20 μM) and LY294002 (10 μM) and DON were used to treat IPEC-J2 cells for 8, 12, and 24 h, respectively, in culture dishes with an area of 25 cm2. Then, complete protease inhibitor cocktail was applied to extract the total protein in ice-cold lysis buffer after 10 min lysis for all samples. As described previously, immunoblotting assays were adopted to determine GAPDH, the polyubiquitin-binding protein p62, the autophagy marker light chain 3 (LC3), p-AKT, p-mTOR, AKT, and mTOR. An imaging system (Bio-Rad, USA) was used to scan the obtained blots. After that, ImageJ software (Image Processing and Analysis in Java; NIH, Bethesda, MD, USA) was utilized to quantify the intensity of protein by integrating densities.
Statistical Analysis
All of the data in this study were expressed as mean ± standard deviation. Comparisons of statistical differences among groups were analyzed via the T-test (Graph-Pad Prism 7 software). P < 0.05 means the differences were significant. P < 0.01 means the differences were highly significant. Each experiment was conducted in triplicate (n = 3).
Results
Cytotoxicity of DON
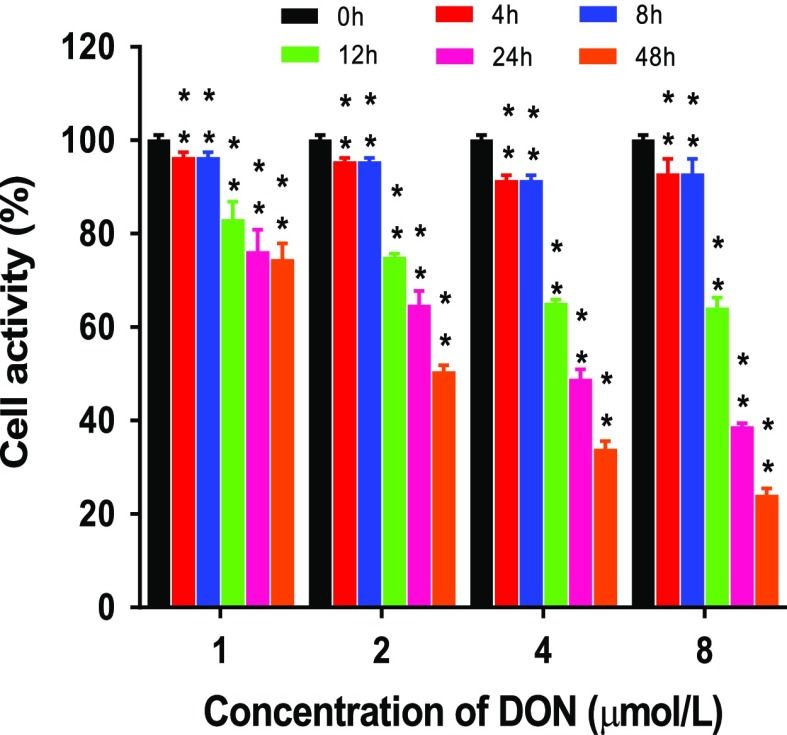
The effect of DON on cell activity was measured via MTS assay. As can be seen from Figure 2, the activity of cells decreased apparently with both the increase of DON concentration (1–8 μM) and the prolongation of DON exposure time (4–48 h), which is consistent with the trend obtained from the study of Tang12 by treating IPEC-J2 cells with DON of different concentrations (1, 2 μg/mL) for different durations (0–4 d). From the perspective of the cell survival rate, it can be seen from the experiment that DON attenuated the activity of IPEC-J2 cells and produced cytotoxicity to the cells even at low dosage. Given that when treated with DON for 12 h, the IC20 value of DON was around 1 μM (0.279 μg/mL), herein, 1 μM DON was employed to conduct follow-up experiments.
Figure 2.
Cell viability (%) of IPEC-J2 cells treated with DON of various concentrations. IPEC-J2 cells were treated with DON of various concentrations (1, 2, 4, 8 μM) for 4, 8, 12, 24, and 48 h.
Effect of Autophagy Inhibitor 3-MA on DON Cytotoxicity
Figure 3 manifests the effect of 3-MA on the cytotoxicity induced by DON to IPEC-J2 cells on the aspects of cell activity and morphology. In the control group, the IPEC-J2 cells were firmly connected and plump, possessing well-proportioned size and shape and filling the entire field of view (Figure 3A). Compared with the control group, after 12 h exposure to DON, IPEC-J2 cells showed a decrease in cell density and the cell morphology was stretched. In addition, the convex structure gradually decreased, and the cell membrane became fuzzy (Figure 3B). The results revealed that DON caused damage to IPEC-J2 cells. After 12 h, cells became more homogeneous in the 3-MA group compared to the DON group (Figure 3C). There may be considerable that cell damage caused by DON might be associated with DON-induced autophagy. In contrast with the control group, exposure to DON for 12 h resulted in a remarkable decrease in the activity of IPEC-J2 cells, contrary to the result of co-treatment with 3-MA and DON when compared with the DON group (Figure 3D), suggesting that the cytotoxicity of DON may be related to DON-induced autophagy.
Figure 3.
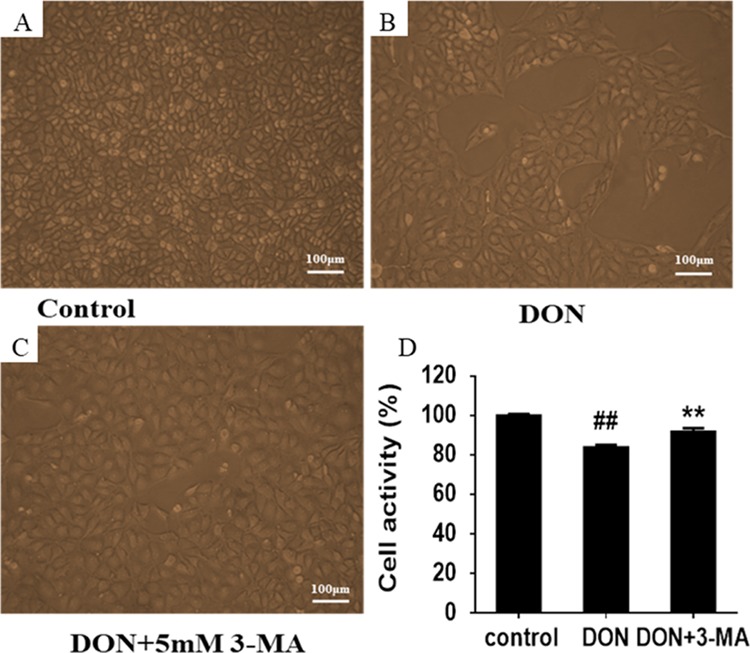
Effects of 3-MA and DON on the morphology and activity of IPEC-J2 cells. (A) Morphology of in control group. IPEC-J2 cells were cultured with or without DON (1 μM) and 3-MA (5 mM) for 12 h. (B) Morphology of IPEC-J2 cells in DON group. (C) Morphology of IPEC-J2 cells in 3-MA group. (D) Effects of 3-MA on DON-induced activity of IPEC-J2 cells. ## means P ≤ 0.01 vs the healthy control group; ** means P ≤ 0.01 vs DON group.
Effect of 3-MA on DON-Induced Autophagy
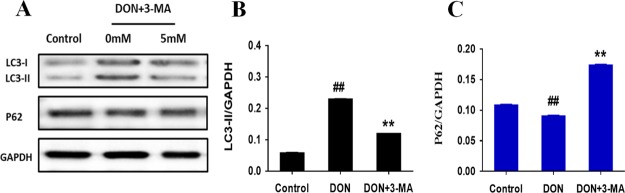
The autophagy inhibitor (3-MA) was also used to treat DON-injured IPEC-J2 cells to corroborate that DON exposure induces apoptosis and autophagy. Subsequently, the effect of 3-MA on the expressions of autophagy-related proteins was detected by the Western blot to verify whether DON-induced autophagy could be intervened after treating IPEC-J2 cells with 5 mM 3-MA for 12 h. The results are shown in Figure 4. Compared with the control group, exposure to DON for 12 h significantly upregulated the expression of autophagic protein LC3-II as well as downregulated the expression of protein p62, indicating that DON-induced autophagy, as reported by Han.25 The differences between the 3-MA-treated culture and positive controls were that 3-MA significantly upregulated the expression of protein p62 and downregulated the expression of LC3-II, indicating that 3-MA notably reversed the autophagy induction of DON. The above results show that DON-induced autophagy, which was blocked by 3-MA.
Figure 4.
Expression levels of autophagy-related proteins and the ratios of autophagic markers (p62 and LC3-II) to GPADH with immunoblotting analysis in IPEC-J2 cells treated with 3-MA and DON. IPEC-J2 cells were cultured with or without DON (1 μM) and 3-MA (5 mM) for 12 h. (A) p62/SQSTM1 and LC3 detected by Western blot in IPEC-J2 cells. (B) Ratio of LC3-II to GAPDH measured by densitometric analysis. (C) Ratio of p62 to GAPDH measured by densitometric analysis. ## means P ≤ 0.01 vs the healthy control group; ** means P ≤ 0.01 vs DON group.
Effect of 3-MA on DON-Induced Apoptosis
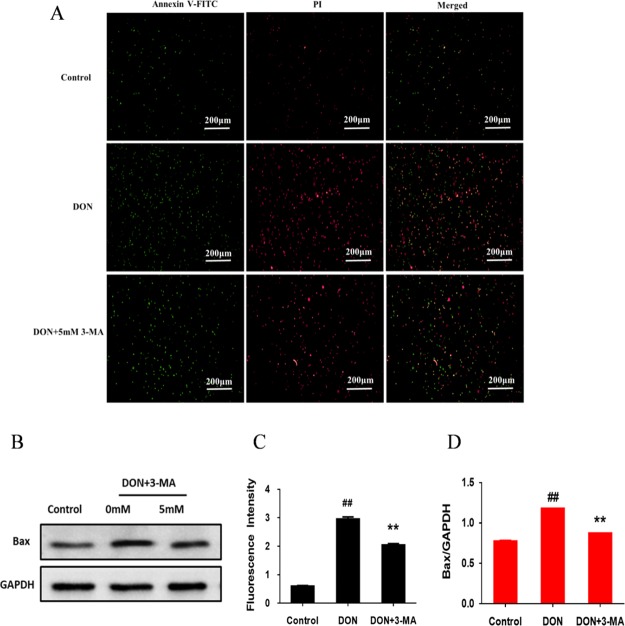
With the aim of investigating the relationship between DON-induced apoptosis and autophagy, we further investigated the effect of 3-MA on apoptosis and the expression of apoptosis-related protein. IPEC-J2 cells were double-stained with Annexin V-FITC and PI for detection of early apoptotic and late apoptotic/dead cells, respectively, and quantitative analysis in terms of fluorescence intensity (positively correlated with the degree of apoptosis) was carried out by applying ImageJ software. As is depicted in Figure 5C, compared with the negative control, the fluorescence intensity was significantly increased for 12 h DON exposure, suggesting the apoptosis of IPEC-J2 cells because of the induction of DON. On the contrary, compared with the DON group, co-treating IPEC-J2 cells with 3-MA and DON for 12 h presented an opposite result, illustrating that DON may induce apoptosis by activating autophagy. The results of the effect of 3-MA on the expression of apoptosis-related protein Bax with Bax/GAPDH representing the degree of apoptosis (positively correlated with the content of Bax) were presented in Figure 5D. In comparison with the control group, 12 h DON exposure significantly upregulated the expression of apoptotic protein Bax, which indicated that DON induced apoptosis. The addition of 3-MA significantly downregulated the expression of apoptotic protein Bax as compared with the DON group, suggesting that 3-MA significantly prevented apoptosis induced by DON. These results indicated that DON-induced autophagy promotes apoptosis, and DON may induce apoptosis by triggering excessive autophagy. In conclusion, DON-induced autophagy and apoptosis are responsible for the decline in the quality of IPEC-J2 cells.
Figure 5.
Effect of 3-MA on DON-induced apoptosis. IPEC-J2 cells were cultured with or without DON (1 μM) and 3-MA (5 mM) for 12 h. (A) Detection of apoptotic and dead cells by fluorescent microscope (4× magnification); (B) GAPDH and Bax detected by Western blot in IPEC-J2 cells. (C) Fluorescence intensity of Annexin V-FITC/PI double-stained IPEC-J2 cells; (D) the ratio of Bax to GAPDH measured by densitometry analysis. ## means P ≤ 0.01 vs control group; ** means P ≤ 0.01 vs DON group.
Effects of PI3K Pathway Inhibitor and Activator on the Morphology and Activity of DON-Injured Cells
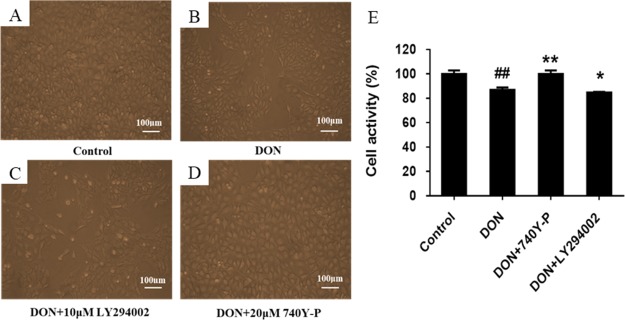
It has been documented that the PI3K-AKT-mTOR signaling pathway is one of the classical pathways for negatively regulating autophagy. To further investigate the mechanism of DON-induced autophagy, DON and the PI3K-AKT-mTOR pathway activator or inhibitor were selected to co-treat IPEC-J2 cells for 12 h and then the effects on cell activity and morphology were observed to preliminarily verify the mechanism that DON induced autophagy. The results are shown in Figure 6. In the control group, the IPEC-J2 cells were firmly connected and plump, having well-proportioned size and shape and filling the entire field of view (Figure 6A). After exposure to DON for 12 h, by comparison with the control group, IPEC-J2 cells showed a reduction in cell density and the cell morphology was stretched with the convex structure decreased, and the cell membrane became fuzzy (Figure 6B). The results showed that DON caused damage to IPEC-J2 cells. After co-treatment with PI3K inhibitor LY294002 and DON for 12 h, compared with the DON group, branching appeared around the cell and the cell deformity was more acute. What is worse, the gap between cells became larger and the intercellular connection and original morphology were lost. After co-treatment with PI3K activator 740Y-P and DON for 12 h, in comparison to the DON group, the cell density became larger and the cell morphology was restored to a certain extent. Meanwhile, compared with the DON group, cell activity was significantly decreased (P < 0.05) after treatment with LY294002 and DON for 12 h, which was opposite to the result of co-treatment with 740Y-P and DON. The results suggest that DON may regulate the PI3K-AKT-mTOR pathway, and the cytotoxicity of DON may be related to DON-induced autophagy and the PI3K-AKT-mTOR pathway.
Figure 6.
Effects of PI3K pathway inhibitor LY294002 and activator 740Y-P on the morphology and activity of DON-injured cells. IPEC-J2 cells were cultured with or without DON (1 μM) and PI3K pathway activator 740Y-P (20 μM)/inhibitor LY294002 (10 μM) for 12 h. (A) Morphology of IPEC-J2 cells in control group. (B) Morphology of IPEC-J2 cells in DON group. (C) Morphology of IPEC-J2 cells in LY294002 group. (D) Morphology of IPEC-J2 cells in 740Y-P group. (E) Effects of 740Y-P and LY294002 on DON-induced activity of IPEC-J2 cells. ## means P ≤ 0.01 vs the healthy control group; ** means P ≤ 0.01 vs DON group; * means P ≤ 0.05 vs DON group.
Effects of PI3K Pathway Inhibitor and Activator on DON-Induced Apoptosis
IPEC-J2 cells were double-stained with Annexin V-FITC and PI for detection of early apoptotic and late apoptotic/dead cells, respectively, and ImageJ software was used to perform quantitative analysis in terms of fluorescence intensity to evaluate the effects of PI3K pathway inhibitor LY294002 and activator 740Y-P on DON-induced apoptosis, with the aim of exploring the effect of the PI3K-AKT-mTOR pathway on DON-induced apoptosis. Figure 7B shows the sum of fluorescence intensity of early apoptotic and late apoptotic/dead cells measured by a fluorescence microscope. As can be seen from Figure 7B, compared with the control group, 12 h treatment with DON made the fluorescence intensity significantly increased, indicating that DON induced apoptosis of IPEC-J2 cells. After co-treating IPEC-J2 cells with PI3K inhibitor LY294002 and DON for 12 h, the fluorescence intensity was significantly enhanced when contrasted with the DON group, contrary to the results of co-treatment with DON and PI3K activator 740Y-P. The results indicate that DON may induce apoptosis by inhibiting the PI3K-AKT-mTOR signaling pathway.
Figure 7.
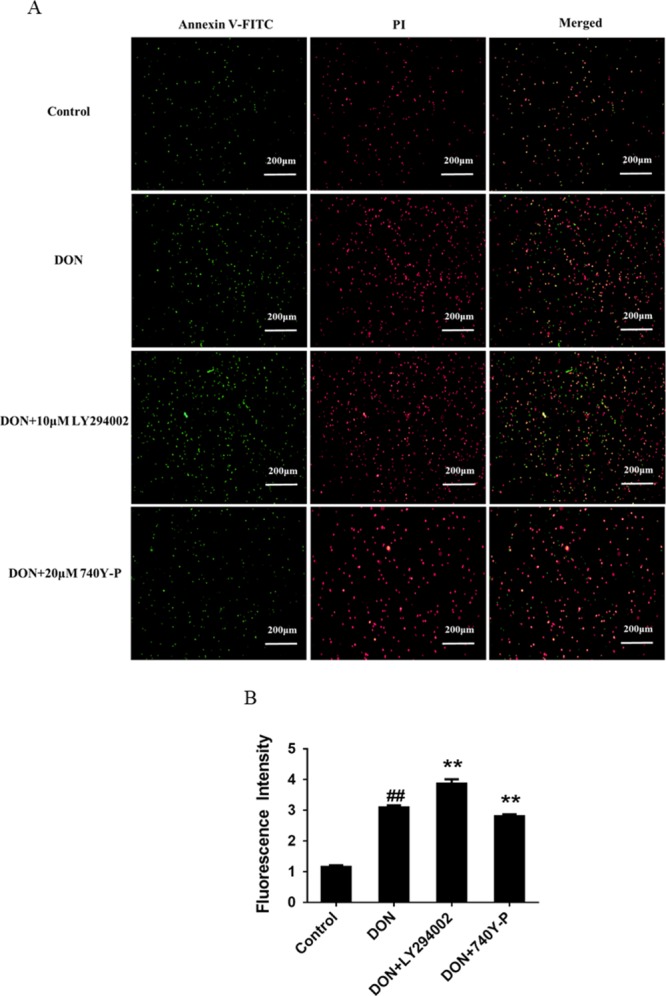
Effects of PI3K pathway inhibitor LY294002 and activator 740Y-P on DON-induced apoptosis. IPEC-J2 cells were cultured with or without DON (1 μM) and PI3K pathway activator 740Y-P (20 μM)/inhibitor LY294002 (10 μM) for 12 h. (A) Detection of apoptotic and dead cells by a fluorescent microscope (4× magnification). (B) Fluorescence intensity of Annexin V-FITC/PI double-stained IPEC-J2 cells. ## means P ≤ 0.01 vs control group; ** means P ≤ 0.01 vs DON group.
PI3K-AKT-mTOR Pathway is Involved in DON-Induced Autophagy
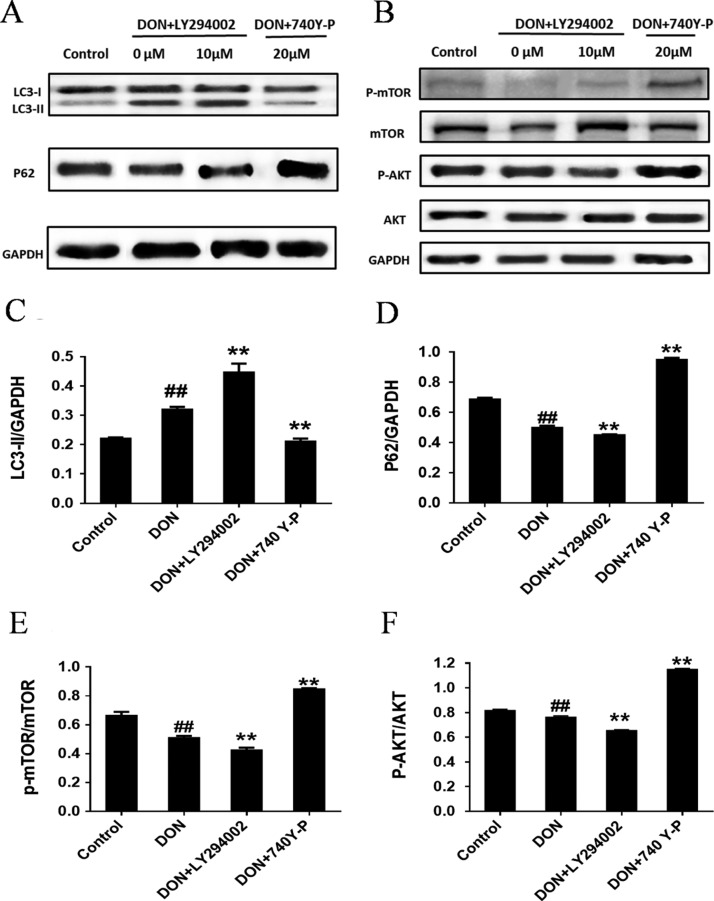
The expression results of autophagy-related and the PI3K-AKT-mTOR pathway-related proteins (p-mTOR, mTOR, p-AKT and AKT proteins) with treatment of IPEC-J2 cells by PI3K-AKT-mTOR pathway inhibitor LY294002 and activator 740Y-P are shown in Figure 8. After 12 h treatment of IPEC-J2 with DON, compared with untreated cells, the autophagy-related protein LC3-II was significantly increased, besides, p62 protein and both p-mTOR/mTOR and p-AKT/AKT significantly decreased. After co-treating IPEC-J2 cells with DON and LY294002 for 12 h, compared with the DON group, the autophagy-related protein LC3-II was upregulated, p62 protein expression was significantly downregulated, and both p-mTOR/mTOR and p-AKT/AKT were downregulated, the results of which were opposite to that of co-treatment with 740Y-P and DON. These results indicate that DON induces autophagy by inhibiting the PI3K-AKT pathway, that is, the PI3K-AKT-mTOR pathway is involved in DON-induced autophagy.
Figure 8.
Effects of PI3K-AKT-mTOR pathway inhibitor LY294002 and activator 740Y-P on autophagy and PI3K-AKT-mTOR pathway-associated protein expression levels detected by immunoblotting analysis. IPEC-J2 cells were cultured with or without DON (1 μM) and PI3K/AKT pathway activator 740Y-P (20 μM)/inhibitor LY294002 (10 μM) for 12 h. (A,B) p62/SQSTM1 and LC3 as well as mTOR, p-mTOR, AKT and p-AKT proteins detected by Western blot in IPEC-J2 cells. (C) Ratio of LC3-II to GAPDH measured by densitometry analysis. (D) Ratio of p62 to GAPDH measured by densitometry analysis. (E) Ratio of p-mTOR to mTOR measured by densitometry analysis. (F) Ratio of p-AKT to AKT measured by densitometry analysis. ## means P ≤ 0.01 vs the healthy control group; ** means P ≤ 0.01 vs DON group.
Discussion
DON is the most prevalent mycotoxin, whose contamination to food and feed is a severe worldwide problem. The contamination level of DON in cereals reaches to about 1.2–19 mg/kg.33−35 The gastrointestinal tract is the first barrier against external pollutants and pathogens, playing a pivotal role in local and systemic immune responses.4,5 Many in vivo and in vitro research studies have demonstrated that DON affects IEC function by egulating cell proliferation and activity, as well as jeopardizing intestinal barrier function.7,10,11 As a result, for human and animal, the ingestion of DON-contaminated cereals would incur impacts of different degrees on the health of the body. Therefore, IPEC-J2 cells were selected as cell models to further study on toxicological effects of DON. In this study, IPEC-J2 cells were treated with DON of various concentrations for different durations and it was found that DON reduced cell activity in a time- and dose-dependent manner. Considering that when treated with DON for 12 h, the cell activity was significantly inhibited and the IC20 value of DON was about 1 μM (0.279 μg/mL), therefore, 1 μM DON was used to conduct subsequent experiments.
The levels of LC3-II and p62 were positively36 and negatively correlated37 with the degree of autophagy, respectively. Generally, there are two indicators, namely, LC3-II/internal reference (GAPDH) and LC3-II/LC3-I values, for semiquantitatively reflecting the degree of autophagy by Western blot. At present, the epitopes detected by the LC3 antibody are located at the N-terminal of LC3. However, because the binding of the PE group may cause a conformational change in the N-terminal region, the LC3 antibody has a stronger affinity for LC3-II than LC3-I and is prone to false positive. Therefore, this experiment utilized LC3-II/GAPDH to represent the degree of autophagy. Bax is a pro-apoptotic protein which is one of the typical proteins of apoptosis. Accordingly, we chose LC3-II and p62 proteins as markers of autophagy and Bax protein as a marker of apoptosis.
Autophagy, called as “self-eating”, is a conservative way of cell self-degradation, which is a process of degradation and reuse of damaged organelles and macromolecules through lysosomes. Apoptosis, called as “self-killing”, is an autonomous process of cell death in order to maintain homeostasis.38 Autophagy and apoptosis are key factors regulating cell death, and the relationship between them is complex and even conflicting.39 On the one hand, autophagy acts as a protective mechanism against cell apoptosis and alleviates cell death, on the other hand, autophagy acts as a damage mechanism to accelerate cell apoptosis and promote cell death.40 To investigate the relationship between apoptosis and autophagy induced by DON, we determined the cell viability, apoptosis, and autophagy after treating IPEC-J2 cells with 3-MA and DON for 12 h. 3-MA were used for blocking the autophagy induced by DON. It turned out that DON upregulated the expression of autophagy-related protein LC3-II, downregulated p62 protein expression, and upregulated Bax protein expression, indicating that DON induced autophagy and apoptosis. 3-MA, autophagy inhibitor, significantly downregulated the expressions of both autophagic protein LC3-II and apoptotic protein Bax and upregulated the expression of p62 protein in DON-injured IPEC-J2 cells, indicating that 3-MA could suppress the apoptosis and autophagy and alleviate the cell damage induced by DON. These results demonstrated that autophagy induced by DON was essential to apoptotic cell damage, and inhibition of autophagy could weaken DON-induced apoptosis in IPEC-J2 cells. DON-induced autophagy and apoptosis are the reasons for the decline in the quality of IPEC-J2 cells. These results are consistent with the notion that Han et al. treated porcine oocytes with DON and found that DON induced autophagy/apoptosis in porcine oocytes.25
However, there is little information to understand how the DON-induced autophagy and apoptosis at the cellular level. Previous research studies have shown that autophagy and apoptosis were related to oxidative stress induced by DON.41−43 DON-dependent ROS production has been confirmed in vitro test, and the effect of DON (25–250 ng/mL) on the generation of NO is negligible.44 ROS could induce autophagy and apoptosis by regulating PI3K-AKT,20 p38 MAPK,21 JNK,22 and AMPK.23,24 Zhang et al.5 confirmed the association of JAK/STAT, p38 MAPK, and PI3K/AKT pathways with enteric toxicity induced by DON. Therefore, we speculated that the cytotoxic mechanism exhibited by DON is highly correlated with the fact that DON induced autophagy by regulating the PI3K-AKT-mTOR pathway, thereby inducing apoptosis.
To verify this hypothesis, we first studied the effects of DON on autophagy and apoptosis pathways and the relationship between them in IPEC-J2 cells. Subsequently, the regulation of DON on the PI3K-AKT-mTOR signaling was confirmed. Finally, the link between autophagy and the PI3K-AKT-mTOR signaling pathway was investigated. The results showed that the 3-MA and PI3K activator 740Y-P significantly increased the activity of DON-injured cells, effectively restored cell morphology, maintained normal cell morphology, and inhibited DON-induced apoptosis (Annexin V-FITC/PI double staining), while PI3K inhibitors showed the opposite trend. These results verify that DON may suppress the PI3K-AKT-mTOR signaling pathway and that apoptosis as well as autophagy can be triggered owing to the inhibition of PI3K-AKT-mTOR pathway. It was found that cell activity was significantly decreased by DON and then the co-treatment with DON and 740Y-P for 12 h significantly increased the cell activity, whereas the cell activity was significantly decreased by co-treating IPEC-J2 cells with LY294002 and DON. After 12-h treatment of IPEC-J2 cells with DON, p-mTOR/mTOR and p-AKT/AKT were significantly decreased. After IPEC-J2 cells were co-treated with LY294002 and DON for 12 h, the expression of autophagy-related protein LC3-II was notably upregulated, while the expression of p62 protein, p-mTOR/mTOR and p-AKT/AKT were significantly downregulated, which, however, took on an opposite trend in contrast with the results of co-treatment with 740YP and DON. The above results further demonstrate that DON induces autophagy by inhibiting the PI3K-AKT-mTOR signaling pathway.
In conclusion, our study elucidated that DON exposure decreased cell viability and induced apoptosis with a concomitant increase of autophagy, which indicated that DON-induced autophagy contributed to apoptosis in IPEC-J2 cells. Furthermore, the following experiments illuminated that DON could induce autophagy and apoptosis by inhibiting the PI3K-AKT-mTOR signaling pathway. Altogether, our results indicate that DON induces cytotoxicity and apoptosis through the activation of autophagy by negatively regulating the PI3K-AKT-mTOR signaling pathway. These findings may provide a new recognition of the molecular mechanism of DON cytotoxicity and provide a new strategy to prevent and treat DON-induced cytotoxicity.
This study was supported by the Natural Science Foundation of Hubei Province (No. 2018CFC821), the National Natural Science Foundation of China (No. 31302139), and Innovation Foundation of Hubei Key Laboratory for Processing and Transformation of Agricultural Products (No. 2019HBSQGDKFB01).
The authors declare no competing financial interest.
Notes
§ Co-first author: Wenyan Guo.
References
- Park J.; Chang H.; Kim D.; Chung S.; Lee C. Long-Term Occurrence of Deoxynivalenol in Feed and Feed Raw Materials with a Special Focus on South Korea. Toxins 2018, 10, 127–139. 10.3390/toxins10030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Castillo M.-Á.; Montes R.; Navarro A.; Segarra R.; Cuesta G.; Hernández E. Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 2008, 21, 423–427. 10.1016/j.jfca.2008.03.009. [DOI] [Google Scholar]
- Pierron A.; Mimoun S.; Murate L. S.; Loiseau N.; Lippi Y.; Bracarense A.-P. F. L.; Liaubet L.; Schatzmayr G.; Berthiller F.; Moll W.-D. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. 10.1007/s00204-015-1592-8. [DOI] [PubMed] [Google Scholar]
- Oswald Z.-Q.; Wang S.-B.; Wang R.-G.; Zhang W.; Wang P.-L.; Su X.-O. Phosphoproteome Analysis Reveals the Molecular Mechanisms Underlying Deoxynivalenol-Induced Intestinal Toxicity in IPEC-J2 Cells. Toxins 2016, 8, 270–288. 10.3390/toxins8100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygelen V.; De Vos M.; Willemen S.; Fransen E.; Casteleyn C.; Van Cruchten S.; Van Ginneken C. Age-related differences in mucosal barrier function and morphology of the small intestine in low and normal birth weight piglets1. J. Anim. Sci. 2014, 92, 3398–3406. 10.2527/jas.2014-7742. [DOI] [PubMed] [Google Scholar]
- Pinton P.; Tsybulskyy D.; Lucioli J.; Laffitte J.; Callu P.; Lyazhri F.; Grosjean F.; Bracarense A. P.; Kolf-Clauw M.; Oswald I. P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. 10.1093/toxsci/kfs239. [DOI] [PubMed] [Google Scholar]
- Boeira L. S.; Bryce J. H.; Stewart G. G.; Flannigan B. The effect of combinations of Fusarium mycotoxins (deoxynivalenol, zearalenone and fumonisin B1) on growth of brewing yeasts. J. Appl. Microbiol. 2000, 88, 388–403. 10.1046/j.1365-2672.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Guslandi M. Role of Probiotics in Crohn’s Disease and in Pouchitis. J. Clin. Gastroenterol. 2015, 49, S46–S49. 10.1097/mcg.0000000000000351. [DOI] [PubMed] [Google Scholar]
- Diesing A.-K.; Nossol C.; Panther P.; Walk N.; Post A.; Kluess J.; Kreutzmann P.; Dänicke S.; Rothkötter H.-J.; Kahlert S. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicol. Lett. 2011, 200, 8–18. 10.1016/j.toxlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Pinton P.; Graziani F.; Pujol A.; Nicoletti C.; Paris O.; Ernouf P.; Di Pasquale E.; Perrier J.; Oswald I. P.; Maresca M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015, 59, 1076–1087. 10.1002/mnfr.201500005. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Li J.; Li F.; Hu C.-A. A.; Liao P.; Tan K.; Tan B.; Xiong X.; Liu G.; Li T. Autophagy protects intestinal epithelial cells against deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free Radical Biol. Med. 2015, 89, 944–951. 10.1016/j.freeradbiomed.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Pierron A.; Mimoun S.; Murate L. S.; Loiseau N.; Lippi Y.; Bracarense A. P. F. L.; Schatzmayr G.; Jian W. H.; Zhou T.; Moll W. D. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105. 10.1038/srep29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva E. O.; Gerez J. R.; Drape T. d. C.; Bracarense A. P. F. R. L. Phytic acid decreases deoxynivalenol and fumonisin B1-induced changes on swine jejunal explants. Toxicol. Rep. 2014, 1, 284–292. 10.1016/j.toxrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.; Gu X.; Tong Y.; Wang X.; Wu J.; Chang C. Protective effects of mannan/β-glucans from yeast cell wall on the deoxyniyalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Int. J. Biol. Macromol. 2019, 135, 619–629. 10.1016/j.ijbiomac.2019.05.180. [DOI] [PubMed] [Google Scholar]
- Jing K.; Lim K. Why is autophagy important in human diseases?. Exp. Mol. Med. 2012, 44, 69–72. 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras-Sandoval D.; Pérez-Rojas J. M.; Hernández-Damián J.; Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Lin W.; Xu G. Autophagy: A Role in the Apoptosis, Survival, Inflammation, and Development of the Retina. Ophthalmic Res. 2018, 61, 65–72. 10.1159/000487486. [DOI] [PubMed] [Google Scholar]
- Wang K.; Chu D.; Wu J.; Zhao M.; Li B.; Du W.; Du J.; Guo R. Cinobufagin induced cell apoptosis and protective autophagy through the ROS/MAPK signaling pathway. Life Sci. 2019, 116642. 10.1016/j.lfs.2019.116642. [DOI] [PubMed] [Google Scholar]
- Mi Y.; Xiao C.; Du Q.; Wu W.; Qi G.; Liu X. Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radical Biol. Med. 2016, 90, 230–242. 10.1016/j.freeradbiomed.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Sui X.; Kong N.; Ye L.; Han W.; Zhou J.; Zhang Q.; He C.; Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Li Y.; Luo Q.; Yuan L.; Miao C.; Mu X.; Xiao W.; Li J.; Sun T.; Ma E. JNK-dependent Atg4 upregulation mediates asperphenamate derivative BBP-induced autophagy in MCF-7 cells. Toxicol. Appl. Pharmacol. 2012, 263, 21–31. 10.1016/j.taap.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Deeb D.; Gao X.; Jiang H.; Janic B.; Arbab A. S.; Rojanasakul Y.; Dulchavsky S. A.; Gautam S. C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 2010, 79, 350–360. 10.1016/j.bcp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J.-M.; Seo M.-J.; Baek J.-Y.; Chu H.; Han S. H.; Min T. S.; Cho C.-s.; Yun C.-H. Alpha-eleostearic acid induces autophagy-dependent cell death through targeting AKT/mTOR and ERK1/2 signal together with the generation of reactive oxygen species. Biochem. Biophys. Res. Commun. 2010, 391, 903–908. 10.1016/j.bbrc.2009.11.161. [DOI] [PubMed] [Google Scholar]
- Han J.; Wang Q.-C.; Zhu C.-C.; Liu J.; Zhang Y.; Cui X.-S.; Kim N.-H.; Sun S.-C. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol. 2016, 300, 70–76. 10.1016/j.taap.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Portal-Núñez S.; Esbrit P.; Alcaraz M. J.; Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem. Pharmacol. 2016, 108, 1–10. 10.1016/j.bcp.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Aoki M.; Blazek E.; Vogt P. K. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 136–141. 10.1073/pnas.98.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A.; Ogier-Denis E.; Blommaart E. F. C.; Meijer A. J.; Codogno P. Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 2000, 275, 992–998. 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Bin B.-H.; Bhin J.; Yang S. H.; Choi D.-H.; Park K.; Shin D. W.; Lee A.-Y.; Hwang D.; Cho E.-G.; Lee T. R. Hyperosmotic stress reduces melanin production by altering melanosome formation. PLoS One 2014, 9, e105965 10.1371/journal.pone.0105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira S.-M.; Stauffer F.; Schnell C.; García-Echeverría C. PI3K inhibitors for cancer treatment: where do we stand?. Biochem. Soc. Trans. 2009, 37, 265–272. 10.1042/bst0370265. [DOI] [PubMed] [Google Scholar]
- Kondo Y.; Kanzawa T.; Sawaya R.; Kondo S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Li Y.; Li C. Autophagy plays a positive role in zinc-induced apoptosis in intestinal porcine epithelial cells. Toxicol. in Vitro 2017, 44, 392–402. 10.1016/j.tiv.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Tralamazza S. M.; Bemvenuti R. H.; Zorzete P.; de Souza Garcia F.; Corrêa B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. 10.1016/j.foodchem.2015.09.063. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lu Y.; Wang L.; Chang F.; Yang L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. 10.1016/j.foodchem.2015.11.047. [DOI] [PubMed] [Google Scholar]
- Abouzied M. M.; Azcona J. I.; Braselton W. E.; Pestka J. J. Immunochemical assessment of mycotoxins in 1989 grain foods: evidence for deoxynivalenol (vomitoxin) contamination. Appl Environ Microbiol 1991, 57, 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N.; Yoshimori T.; Levine B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Wang H.; Zhu J.; Xu J.; Ding K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. 10.1016/j.bbrc.2014.05.101. [DOI] [PubMed] [Google Scholar]
- Wang W.; Luo S.-M.; Ma J.-Y.; Shen W.; Yin S. Cytotoxicity and DNA Damage Caused from Diazinon Exposure by Inhibiting the PI3K-AKT Pathway in Porcine Ovarian Granulosa Cells. J. Agric. Food Chem. 2019, 67, 19–31. 10.1021/acs.jafc.8b05194. [DOI] [PubMed] [Google Scholar]
- Sun M.; Wang S.; Jiang L.; Bai Y.; Sun X.; Li J.; Wang B.; Yao X.; Liu X.; Li Q.; Geng C.; Zhang C.; Yang G. Patulin Induces Autophagy-Dependent Apoptosis through Lysosomal-Mitochondrial Axis and Impaired Mitophagy in HepG2 Cells. J. Agric. Food Chem. 2018, 66, 12376–12384. 10.1021/acs.jafc.8b03922. [DOI] [PubMed] [Google Scholar]
- Li S.; Jiang Z.; Chai W.; Xu Y.; Wang Y. Autophagy activation alleviates nonylphenol-induced apoptosis in cultured cortical neurons. Neurochem. Int. 2019, 122, 73–84. 10.1016/j.neuint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Yu M.; Chen L.; Peng Z.; Wang D.; Song Y.; Wang H.; Yao P.; Yan H.; Nüssler A.; Liu L. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins 2017, 9, 188–207. 10.3390/toxins9060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.-H.; Wang X.; Yang W.; Nüssler A. K.; Xiong L.-Y.; Kuča K.; Dohnal V.; Zhang X.-J.; Yuan Z.-H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch. Toxicol. 2014, 88, 1309–1326. 10.1007/s00204-014-1280-0. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Miao Y.; Juan F.; Wei B.; Di W.; Liping H.; Ping Y.; Nüssler A. K.; Hong Y.; Liegang L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. 10.1016/j.fct.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Ji G. E.; Park S. Y.; Wong S. S.; Pestka J. J. Modulation of nitric oxide, hydrogen peroxide and cytokine production in a clonal macrophage model by the trichothecene vomitoxin (deoxynivalenol). Toxicology 1998, 125, 203–214. 10.1016/s0300-483x(97)00178-9. [DOI] [PubMed] [Google Scholar]