Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, pulmonary-limited, interstitial lung disease with a poor prognosis. This condition is characterized by different clinical scenarios, ranging from the most typical slow and progressive deterioration of symptoms to a rapid and abrupt decline of lung function. Rapid worsening of clinical course is due to superimposed complications and comorbidities that can develop in IPF patients, with a higher incidence rate compared to the general population. These conditions may require a different management of the patient and a therapy adjustment, and thus it is fundamental to recognize them. High Resolution Computed Tomography (HRCT) is sensitive, but not specific, in detecting these complications, and can evaluate the presence of radiological variations when previous examinations are available; it recognizes ground glass opacities or consolidation that can be related to a large spectrum of comorbidities, such as infection, lung cancer, or acute exacerbation. To reach the final diagnosis, a multidisciplinary discussion is required, particularly when the clinical context is related to imaging findings.
Keywords: IPF, consolidation, ground glass, infection, lung cancer, acute exacerbation, HRCT
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease with a poor prognosis and a median survival rate of 2–3 years after diagnosis [1].
Several complications and comorbidities may occur in patients with IPF, sometimes with a fatal outcome. These conditions include infection, malignancy, pulmonary hypertension, pulmonary embolus, acute exacerbation, drug-induced pneumonitis, and cardiogenic pulmonary oedema [1,2,3].
High Resolution Computed Tomography (HRCT) is fundamental in diagnosing IPF, but also in detecting these complications and comorbidities that can superimpose on the underlying disease.
In this review, we will discuss the imaging findings of the most common parenchymal complications.
2. Imaging
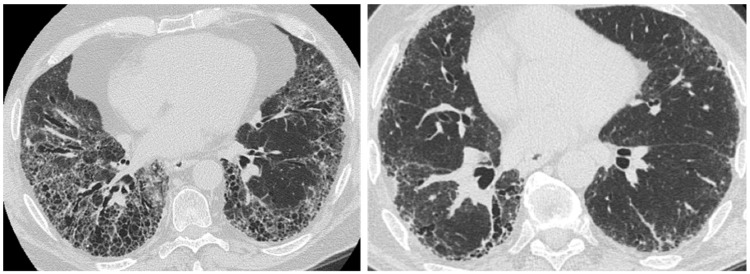
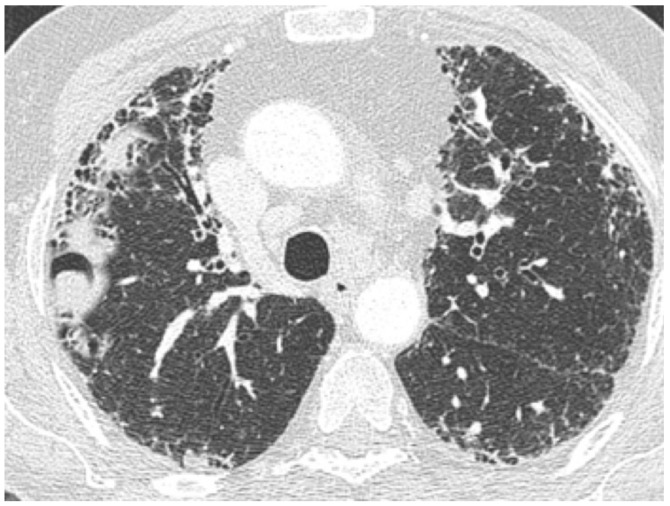
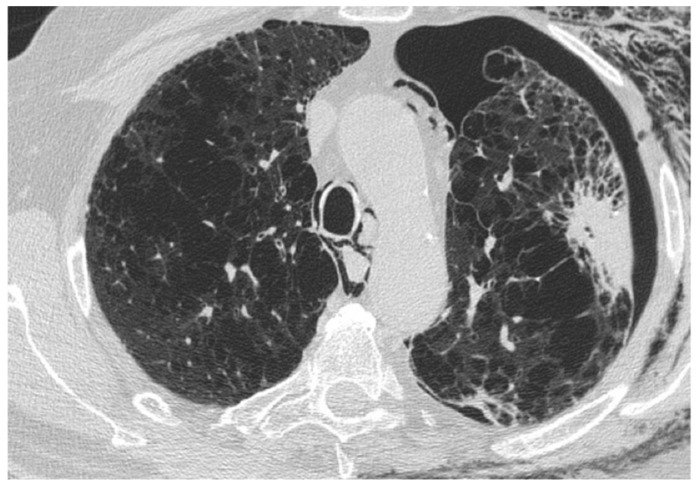
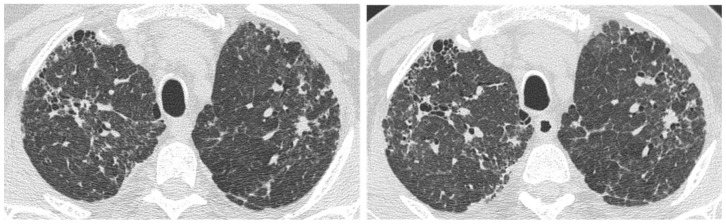
According to the more recent guidelines of American Thoracic Society/European Respiratory Society/ Japanese Respiratory Society/ Latin American Thoracic Association (ATS/ERS/JRS/ALAT) and the Fleischner Society White Paper [4,5], high resolution computed tomography plays a central role in the diagnosis of diffuse interstitial lung diseases, particularly in patients with a clinical suspect of IPF. If a typical (Figure 1) or probable pattern of usual interstitial pneumonia (UIP) (Figure 2) is recognized on HRCT, in association with the appropriate clinical setting, the diagnosis of IPF can be made without surgical biopsy.
Figure 1.
Typical usual interstitial pneumonia (UIP) pattern on High Resolution Computed Tomography (HRCT). Irregular inter- and intralobular septal thickening, with a subpleural, basal predominant. Honeycombing, traction bronchiectasis, and bronchiolectasis are clearly seen in the basal region.
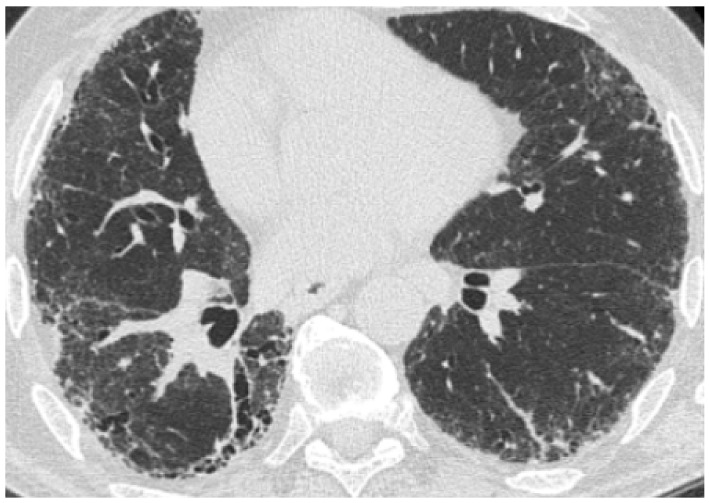
Figure 2.
Probable UIP pattern on HRCT. Irregular inter- and intralobular septal thickening, with a subpleural, basal predominant. Traction bronchiectasis and bronchiolectasis are seen in the fibrotic areas. Honeycombing is not present.
Patients with IPF have a higher risk of comorbidities and lung cancer compared with the general population [1].
HRCT has a role in establishing both acute and chronic parenchymal complications in patients with IPF and, in particular, in detecting areas of parenchymal consolidation or ground glass opacities, which can be an expression of infection, lung cancer, or acute exacerbation [6]. The differential diagnosis among these conditions could be more easily made by the radiologist if clinical data and previous Computed Tomography (CT) examination are available.
2.1. Infection
Patients with IPF may experience pulmonary infections with a higher frequency than patients without IPF. Lung infections are the most frequently occurring comorbidity and are a common cause of hospitalization [7]. It could be difficult to make a differential diagnosis with an acute exacerbation. Some studies suggest that chronic viral infections may have a role in the pathogenesis of IPF, in particular the Epstein–Barr virus and the latent human herpesvirus [8,9,10]. Moreover, the role of bacterial infections during infancy, especially from Staphylococcus and Streptococcus species, is being investigated [11,12,13].
The most frequently observed pathogens are Mycobacterium species and Aspergillus species, along with opportunistic infections such as Pneumocystis jirovecii [2]. The structural changes induced by IPF in the lungs and the impairment of the local host immunity create a favorable environment for mycobacterial colonization. Moreover, concomitant immunosuppressive therapies facilitate the reactivation of tuberculosis [14]. Aspergillosis results from the saprophytic colonization of pre-existing lung cavities.
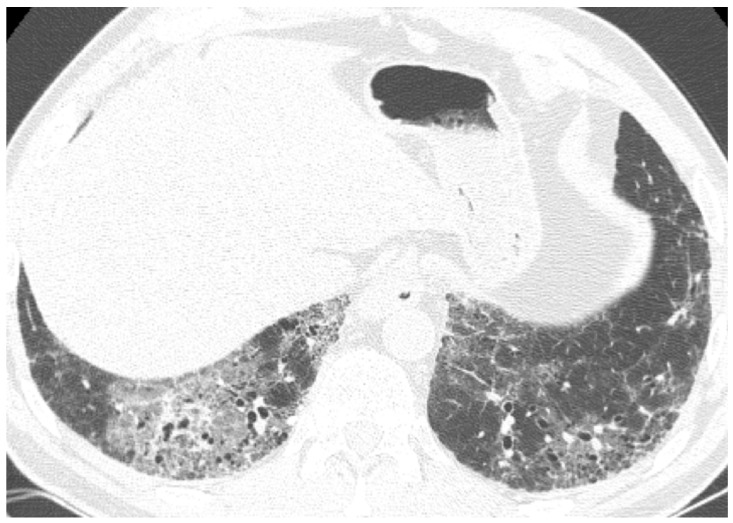
Infections can be occasionally clearly diagnosed, as CT appearance is frequently non-specific, and pre-existing background of parenchymal alterations may mask or alter the typical radiographical pattern of these pathologies. An example can be given by the opportunistic infection of P. jirovecii, which typically manifests on HRCT as bilateral, symmetric ground glass opacity, consolidation, and septal thickening with a perihilar and upper lobe distribution and a subpleural lung sparing. Nevertheless, in IPF P. jirovecii, infection can be undistinguishable from an acute exacerbation of IPF or the CT pattern, showing no change compared to the previous examination, even in a clinical context of infection [2] (Figure 3).
Figure 3.
An example of Pneumocystis jirovecii pneumonia Broncho-Alveolar Lavage-confirmed. HRCT demonstrated patchy areas of ground glass in the upper left lobe superimposed on a fibrotic background.
Tuberculosis has been reported to occur more frequently in IPF patients than the general population [15,16]. A study by Shachor et al. found that the incidence of positive cultures for Mycobacterium tuberculosis in chronic interstitial lung diseases was 4.5 times that of the general population. In a study by Park et al. including 795 IPF patients, pulmonary infections with Mycobacterium tuberculosis and nontuberculous mycobacterium were found in 35 (4.4%) and 16 (2.0%) of patients, respectively. These rates were higher than those in the general population [14]. Patients with IPF are more prone to develop Tuberculosis (TB) reactivation with HRCT, with a peripheral mass-like lesion being shown to exist in more than half of patients, sometimes resembling lung cancer in IPF (Figure 4), but sometimes presenting as subpleural nodules [15]. In 33% of cases, a segmental or lobar coalescent consolidation with or without necrotizing cavitation may be observed, infrequently associated with the typical TB reactivation patterns (patchy multifocal consolidation and centrolobular nodules). It is mandatory to make a correct diagnosis to start an appropriate therapy.
Figure 4.
High Resolution Computed Tomography showing a nodule with spiculated margins, located in the superior left lobe. The final diagnosis after pathological examination on the surgical specimen demonstrated that it was a tuberculoma.
Infections of Aspergillus species in IPF patients may result in the development of aspergilloma, typically developing in pre-existing lung cavities or in a chronic airway invasive aspergillosis. CT can effectively detect early changes, demonstrating the fungal fronds originating from the cavity wall in early stages, with the subsequent detachment and coalescence of the cavity itself resulting in the typical air crescent sign [2,17,18] (Figure 5).
Figure 5.
Figure courtesy of Carlo Florio. In a background of idiopathic pulmonary fibrosis, HRCT showed the typical intracavitary mass with an adjacent “air crescent sign” due to Aspergillus infection.
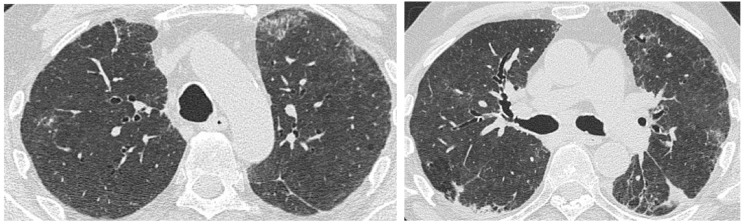
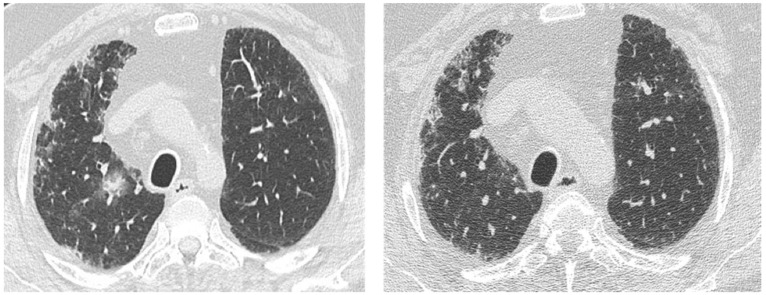
In patients with IPF and bacterial infection, part-solid nodules (Figure 6) or ill-defined nodules (Figure 7) may be seen. The same radiological findings can be present in lung cancer; therefore, in some cases, a correct differential diagnosis is challenging. A Japanese cohort study found that, among patients with IPF that require hospitalization, Gram-negative species are the most common pathogens involved, in particular Haemophilus influenzae [19]. This is in contrast with community-acquired infections, in which Gram-positive bacteria are more commonly isolated.
Figure 6.
HRCT from a patient with idiopathic pulmonary fibrosis, a current smoker. (a) A part-solid nodule located in the upper right lobe, which, according to Fleischner Society, is highly suggestive of lung cancer; (b) 3 month control after corticosteroid and antibiotic therapy showing complete resolution of this opacity.
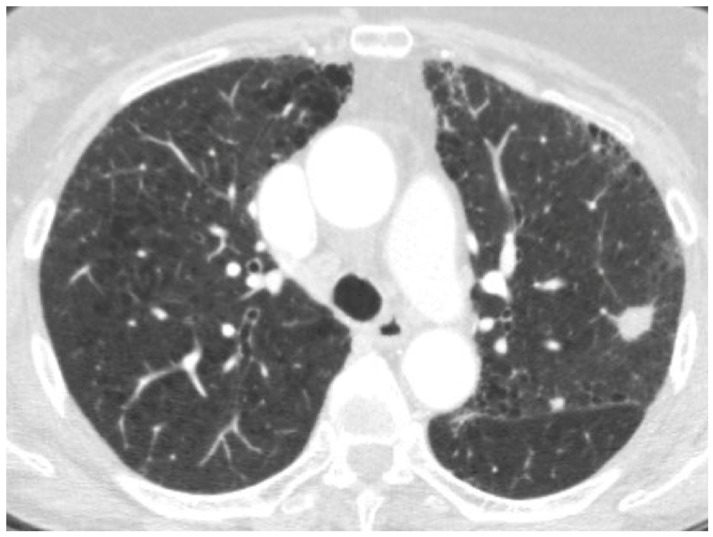
Figure 7.
(a) A case of a patient with idiopathic pulmonary fibrosis with spiculated nodule; (b) the control after corticosteroid and antibiotic therapy showing complete resolution of this opacity.
2.2. Lung Cancer
Patients with IPF have a five-fold increase of developing lung cancer when compared to the general population [20,21], with an incidence rate of 11 cases per 100,000 persons per year. A large meta-analysis estimated a rate of lung cancer prevalence in IPF patients of 13,54% [22]. Some risk factors have been identified, such as age, male sex, and smoking history [22,23].
Both squamous cell carcinoma and adenocarcinoma have been observed, with a higher prevalence of the former [24,25,26]. Moreover, IPF patients experience synchronous tumors with a higher prevalence compared to non-IPF patients [27].
The pathogenesis remains unknown, and repeated episodes of cellular damage caused by inflammatory mediators have been hypothesized. This cellular damage may cause genetic injury to the respiratory epithelium, thus leading to cellular atypia, metaplasia, dysplasia, and finally carcinoma. Considering the already poor survival rate of IPF patients, it is difficult to determine the impact of the diagnosis of lung cancer on patients’ prognosis; moreover, papers reporting survival among IPF patients with lung cancer are limited by small sample sizes [24,28,29]. A study by Ozawa et al. reported a median survival time after lung cancer diagnosis in IPF of 13.1 months [20]. A retrospective analysis of 632 patients with IPF found a 1 year all-cause mortality rate after lung cancer diagnosis of 53.5%, confirming an overall poor survival [30].
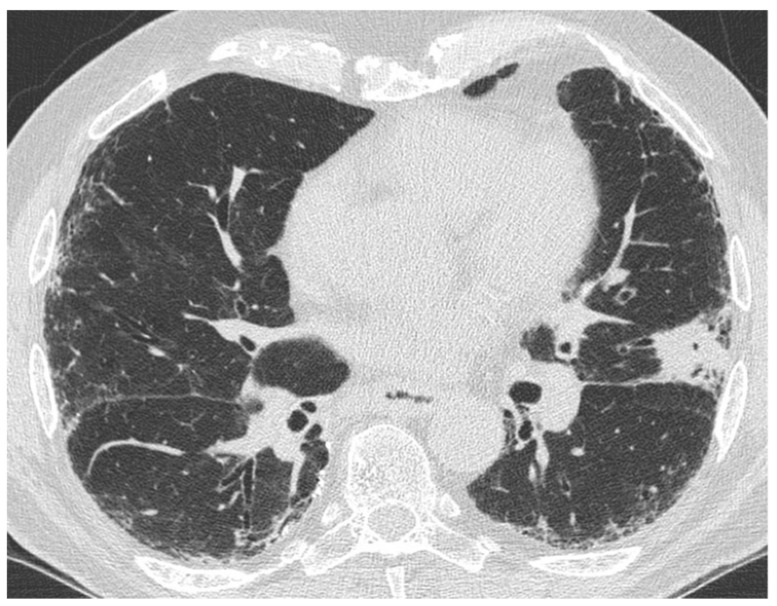
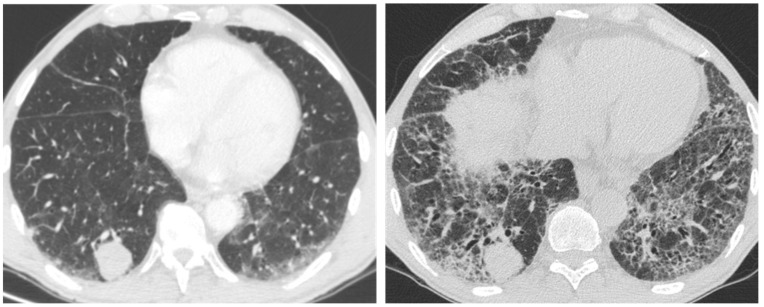
On CT, lung cancer typically manifests as an ill-defined rounded lesion that can mimic an air-space consolidation (Figure 8), or presents as a nodular lesion (Figure 9), making it difficult to distinguish from other entities. In most instances, lung cancer develops in fibrosis areas, typically within or abutted areas of basal and peripheral honeycombing, with a typical prevalence for the lower lobes, compared to the general population where it generally manifests in the upper lobes [31,32].
Figure 8.
HRCT of a 70 year old man showing a consolidation area in the left upper lobe. Pathological examination after biopsy detected an adenocarcinoma.
Figure 9.
HRCT of a 63 year old man coming to the emergency room for an acute chest pain. HRCT showed, in a background of pulmonary idiopathic fibrosis, pneumomediastinum, and pneumothorax, a spiculated mass in the peripheral region of the left upper lobe due to squamous cell carcinoma.
Among invasive adenocarcinomas of the lung, invasive mucinous adenocarcinoma (previously known as mucinous bronchoalveolar carcinoma) may appear on CT as a ground glass opacity, which may have consolidative areas [33]. As shown in Figure 10, this pattern of CT presentation, in the context of a fibrotic lung parenchyma, may mimic an inflammatory area, making a differential diagnosis difficult. Therefore, a malignancy should always be considered as a possible diagnosis in case the lesion does not respond to corticosteroid and antibiotic therapy.
Figure 10.
Figure courtesy of Roberta Polverosi. A well-defined ground glass opacity within a fibrosis area in the right lower lobe, persistent after corticosteroid and antibiotic therapy. The biopsy demonstrated a mucinous bronchioloalveolar carcinoma.
In a CT scan, it could be difficult to distinguish areas of dense fibrosis from lung carcinoma (Figure 11), thus a follow-up is required to evaluate the progression of this consolidation [34].
Figure 11.
(a) HRCT showing a small ill-defined opacity in the upper left lobe of uncertain etiology; (b) the nodule remaining stable at the control HRCT after 3 years.
Analogous pattern on CT may be seen in pulmonary infarction and in TB reactivation. It has been largely demonstrated in the literature that patients with IPF are more prone to suffer from acute thromboembolism, thus developing pulmonary infarction [35,36]. Similar to cancer in IPF, pulmonary infarction is mostly characterized by a peripheral and juxta pleural well-defined opacification without air bronchograms, mostly developing in the lower lobes. The differential diagnosis is made even more difficult by the clinical presentation, characterized by dyspnoea, tachypnea, and hypoxemia that can mimic an acute exacerbation of IPF [37,38].
2.3. Acute Exacerbation
IPF is a progressive and chronic disease with a course that can be unpredictable. Indeed, some patients may manifest a slow and progressive deterioration of lung function, while others may present a rapid progression with a fast decline of the clinical conditions. In some cases, episodes of sudden worsening of symptoms and lung function can occur. These cases are defined as acute exacerbation (AE-IPF).
The revised definition of acute exacerbation of IPF is an acute, clinically significant, respiratory deterioration characterized by evidence of new, widespread alveolar abnormality [39]. It seriously worsens life expectancy, with up to 50% of patients dying during the acute event, and a significant decrease in short-term survival of three to four months after the episode [40,41,42,43,44,45,46]. Moreover, if the patient needs mechanical ventilation, the mortality rate increases to 90% of cases [47].
Although the pathogenesis of acute exacerbations is still unknown, it is thought to share some similarities with acute lung injury (ALI), in which a diffuse alveolar damage (DAD) occurs secondary to an acute event, such as viral infections, gastro oesophageal reflux (GER), surgical lung biopsy or resection, immunosuppressive therapy, bronchoalveolar lavage, or any mechanical procedure (Figure 12).
Figure 12.
HRCT showing a rapid worsening of reticular abnormalities after a Computed Tomography-guided biopsy of a basal rounded mass.
It has also been observed that episodes are more frequent in patients with a physiologically and functionally advanced disease, in combination with a more progressive disease [48], in wintertime [46,49,50], in young people, in patients with coronary artery disease, in cardiogenic pulmonary oedema, and in those with a high body mass index.
The criteria to define AE-IPF are a previous or concurrent diagnosis of IPF, an unexplained worsening or development of dyspnoea within 30 days, and exclusion of alternative causes (left heart failure) [51].
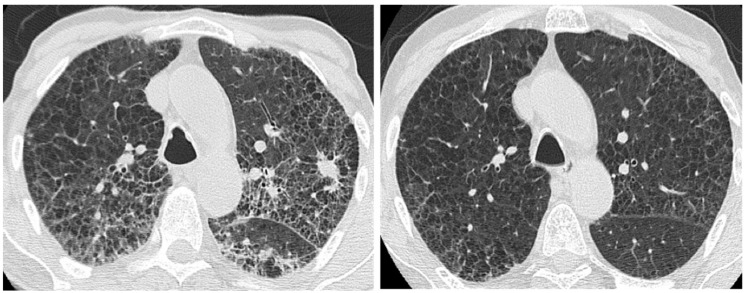
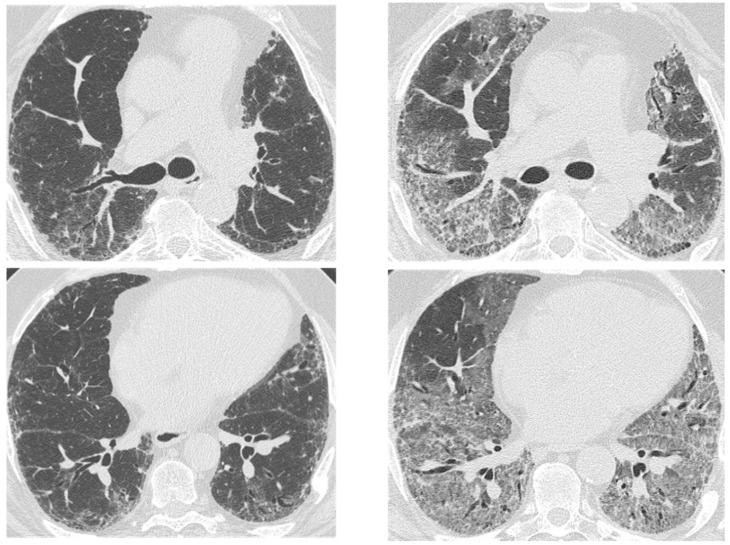
On HRCT, it manifests with new bilateral ground glass abnormality and/or consolidation superimposed on a background reticular or honeycombing (Figure 13) [52]. It has been noticed that the pattern and extent of opacification can be related to the clinical outcome, observing that patients with a diffuse pattern have a higher risk of death compared to those with a peripheral one. If an asymmetrical distribution of the opacification (consolidation or ground glass) is seen on HRCT, this is a predictor of a better outcome in patients with AE-IPF [53].
Figure 13.
HRCT of an IPF patient with acute deterioration of symptoms and lung function, compared to the baseline HRCT. (a,b) A rapid and progressive development of diffuse and symmetric ground glass opacities; (c,d) suggestive of an acute exacerbation. The patient died 3 weeks after.
HRCT is sensitive, but not specific, in recognizing AE-IPF, since other conditions may present a diffuse increase in attenuation of the lung parenchyma in a background of pre-existing fibrosis, and thus these must be taken into account in differential diagnosis. These conditions are pulmonary infections, drug-induced pneumonias, and pulmonary oedema due to heart failure.
In particular, if pleural effusion or profuse septal thickening (an uncommon feature in non-complicated IPF) are associated with the ground glass opacities, heart failure should be highly suspected [2]. Along with pathological conditions, even some technical factors during exam acquisition can determine spurious increased opacification, such as scan acquisition at the end of expiration or intravenous contrast administration [2].
A list of the main parenchymal complications occurring during IPF with their CT scan characteristics is presented in Table 1.
Table 1.
Classification of lung parenchymal complications in patients with idiopathic pulmonary fibrosis (IPF) and HRCT features.
| Acute/Chronic | Complication | HRCT Features | |
|---|---|---|---|
| Acute | Infections | Mycobacterium species | Peripheral mass-like lesion/subpleural nodules/segmental or lobar coalescent consolidation with or without necrotising cavitation. |
| Pneumocystis jirovecii | Ground glass opacity/no change from the baseline in a clinic context of infection. | ||
| Aspergillus | Fungal fronds in a pre-existing cavity in early stages. Subsequent coalescence of the cavity (air crescent sign). | ||
| Acute exacerbation IPF | New bilateral ground glass opacities and/or consolidation on a background of reticular or honeycombing pattern. | ||
| Right heart failure | Profuse septal thickening, ground glass opacities, pleural effusion on a background of reticular or honeycombing pattern. | ||
| Chronic | Lung cancer | Ill-defined rounded lesion, mimicking air space consolidation/nodular lesion developing within peripheral and basal honeycombing areas. Ground glass opacity in fibrosis area (mucinous bronchioloalveolar carcinoma). |
|
If a clinical suspect of AE-IPF is present, to reach the final diagnosis a multidisciplinary discussion is required.
3. Conclusions
HRCT has an increasingly predominant role in the diagnosis of IPF. Moreover HRCT plays a fundamental role in detecting IPF in both acute and chronic parenchymal complications that can seriously worsen the clinical course.
Besides lung cancer, patients with IPF can develop opacities, including inflammatory consolidations, areas of dense fibrosis, infarction due to pulmonary embolism, or acute exacerbation. Clinical presentation plays a crucial role for a correct interpretation of CT findings.
It is important to identify these opacities, particularly through involving an expert thoracic radiologist who can better evaluate the radiological aspects and make a comparison with previous radiological examinations when available.
Author Contributions
Conceptualization, E.B.; methodology, E.B., I.F., C.M.; software, E.B., I.F., C.M.; validation, E.B., I.F., C.M.; formal analysis, E.B., I.F., C.M.; investigation, E.B., I.F., C.M.; resources, E.B.; data curation, E.B.; writing—original draft preparation, E.B.; writing—review and editing, E.B., I.F., C.M., F.S.; visualization, E.B., I.F., C.M.; supervision, M.A.C.; project administration, E.B.; funding acquisition.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Suzuki A., Kondoh Y. The clinical impact of major comorbidities on idiopathic pulmonary fibrosis. Respir. Investig. 2017 doi: 10.1016/j.resinv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd C.R., Walsh S.L.F., Hansell D.M. High-resolution CT of complications of idiopathic fibrotic lung disease. Br. J. Radiol. 2011 doi: 10.1259/bjr/65090500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano-Jiménez E., Hernández González F., Peloche G. Comorbidities and Complications in Idiopathic Pulmonary Fibrosis. Med. Sci. 2018:71. doi: 10.3390/medsci6030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of idiopathic pulmonary fibrosis An Official ATS/ERS/JRS/ALAT Clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 5.Lynch D.A., Sverzellati N., Travis W.D., Brown K.K., Colby T.V., Galvin J.R., Goldin J.G., Hansell D.M., Inoue Y., Johkoh T., et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018 doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 6.Kusmirek J.E., Martin M.D., Kanne J.P. Imaging of Idiopathic Pulmonary Fibrosis. Radiol. Clin. North Am. 2016 doi: 10.1016/j.rcl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Collard H.R., Ward A.J., Lanes S., Cortney Hayflinger D., Rosenberg D.M., Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J. Med. Econ. 2012 doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto K., Hayakawa H., Sato A., Chida K., Nakamura H., Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000 doi: 10.1136/thorax.55.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y.W., Johnson J.E., Browning P.J., Cruz-Gervis R.A., Davis A., Graham B.S., Brigham K.L., Oates Jr J.A., Loyd J.E., Stecenko A.A. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J. Clin. Microbiol. 2003 doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawson W.E., Crossno P.F., Polosukhin V.V., Roldan J., Cheng D.S., Lane K.B., Blackwell T.R., Xu C., Markin C., Ware L.B., et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: Association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2008 doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 11.Richter A.G., Stockley R.A., Harper L., Thickett D.R. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax. 2009 doi: 10.1136/thx.2008.110445. [DOI] [PubMed] [Google Scholar]
- 12.Molyneaux P.L., Cox M.J., Willis-Owen S.A., Mallia P., Russell K.E., Russell A.M., Murphy E., Johnston S.L., Schwartz D.A., Wells A.U., et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am. J. Respir. Crit Care Med. 2014 doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han M.K., Zhou Y., Murray S., Tayob N., Noth I., Lama V.N., Moore B.B., White E.S., Flaherty K.R., Huffnagle G.B., et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: An analysis of the COMET study. Lancet Respir. Med. 2014 doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S.W., Song J.W., Shim T.S., Park M.S., Lee H.L., Uh S.T., Park C.S., Kim D.S. Mycobacterial pulmonary infections in patients with idiopathic pulmonary fibrosis. J. Korean Med. Sci. 2012 doi: 10.3346/jkms.2012.27.8.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung M.J., Goo J.M., Im J.G. Pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. Eur. J. Radiol. 2004 doi: 10.1016/j.ejrad.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Shachor Y., Schindler D., Siegal A., Lieberman D., Mikulski Y., Bruderman I. Increased incidence of pulmonary tuberculosis in chronic interstitial lung disease. Thorax. 1989;44:151–153. doi: 10.1136/thx.44.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraceno J.L., Phelps D.T., Ferro T.J., Futerfas R., Schwartz D.B. Chronic necrotizing pulmonary aspergillosis: Approach to management. Chest. 1997 doi: 10.1378/chest.112.2.541. [DOI] [PubMed] [Google Scholar]
- 18.Roberts C.M., Citron K.M., Strickland B. Intrathoracic aspergilloma: Role of CT in diagnosis and treatment. Radiology. 1987 doi: 10.1148/radiology.165.1.3628758. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki R., Nishiyama O., Sano H., Iwanaga T., Higashimoto Y., Kume H., Tohda Y. Clinical features and outcomes of IPF patients hospitalized for pulmonary infection: A Japanese cohort study. PLoS ONE. 2016 doi: 10.1371/journal.pone.0168164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozawa Y., Suda T., Naito T., Enomoto N., Hashimoto D., Fujisawa T., Nakamura Y., Inui N., Nakamura H., Chida K. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009 doi: 10.1111/j.1440-1843.2009.01547.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoon J.H., Nouraie M., Chen X., Zou R.H., Sellares J., Veraldi K.L., Chiarchiaro J., Lindell K., Wilson D.O., Kaminski N., et al. Characteristics of lung cancer among patients with idiopathic pulmonary fibrosis and interstitial lung disease—Analysis of institutional and population data. Respir. Res. 2018 doi: 10.1186/s12931-018-0899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JafariNezhad A., YektaKooshali M.H. Lung cancer in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. PLoS ONE. 2018 doi: 10.1371/journal.pone.0202360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard R., Venn A., Lewis S., Britton J. Lung cancer and cryptogenic fibrosing alveolitis: A population-based cohort study. Am. J. Respir Crit. Care Med. 2000 doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 24.Aubry M.C., Myers J.L., Douglas W.W., Tazelaar H.D., Stephens T.L.W., Hartman T.E., Deschamps C., Pankratz V.S. Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis. Mayo Clin. Proc. 2002 doi: 10.4065/77.8.763. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G., Nyberg F., Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br. J. Cancer. 2004 doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita H., Tanaka S., Saiki Y., Hara M., Nakata K., Tanimura S., Banba J. Iung cancer associated with usual interstitial pneumonia. Pathol. Int. 1995 doi: 10.1111/j.1440-1827.1995.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 27.Hironaka M., Fukayama M. Pulmonary fibrosis and lung carcinoma: A comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol. Int. 1999 doi: 10.1046/j.1440-1827.1999.00989.x. [DOI] [PubMed] [Google Scholar]
- 28.Kreuter M., Ehlers-Tenenbaum S., Schaaf M., Oltmanns U., Palmowski K., Hoffmann H., Schnabel P.A., Heußel C.P., Puderbach M., Herth F.J., et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc. Diffuse Lung Dis. 2014;31:266–274. [PubMed] [Google Scholar]
- 29.Tomassetti S., Gurioli C., Ryu J.H., Decker P.A., Ravaglia C., Tantalocco P., Buccioli M., Piciucchi S., Sverzellati N., Dubini A., et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015 doi: 10.1378/chest.14-0359. [DOI] [PubMed] [Google Scholar]
- 30.Kato E., Takayanagi N., Takaku Y., Kagiyama N., Kanauchi T., Ishiguro T., Sugita Y. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERS Monogr. 2018 doi: 10.1183/23120541.00111-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H.J., Im J.-G., Ahn J.M., Yeon K.M. Lung Cancer in Patients with Idiopathic Pulmonary Fibrosis: CT Findings. J. Comput. Assist. Tomogr. 1996;20:979–982. doi: 10.1097/00004728-199611000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Sakai S., Ono M., Nishio T., Kawarada Y., Nagashima A., Toyoshima S. Lung Cancer Associated with Diffuse Pulmonary Fibrosis: CT-Pathologic Correlation. J. Thorac. Imagin. 2003 doi: 10.1097/00005382-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Jang H.J., Lee K.S., Kwon O.J., Rhee C.H., Shim Y.M., Han J. Bronchioloalveolar carcinoma: Focal area of ground-glass attenuation at thin-section CT as an early sign. Radiology. 1996 doi: 10.1148/radiology.199.2.8668800. [DOI] [PubMed] [Google Scholar]
- 34.Oh S.Y., Kim M.Y., Kim J.E., Kim S.S., Park T.S., Kim D.S., Choi C.M. Evolving early lung cancers detected during follow-up of idiopathic interstitial pneumonia: Serial CT features. Am. J. Roentgenol. 2015 doi: 10.2214/AJR.14.13587. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard R.B., Smith C., Le Jeune I., Gribbin J., Fogarty A.W. The association between idiopathic pulmonary fibrosis and vascular disease: A population-based study. Am. J. Respir Crit. Care Med. 2008 doi: 10.1164/rccm.200805-725OC. [DOI] [PubMed] [Google Scholar]
- 36.Dalleywater W., Powell H.A., Fogarty A.W., Hubbard R.B., Navaratnam V. Venous thromboembolism in people with idiopathic pulmonary fibrosis: A population-based study. Eur. Respir. J. 2014 doi: 10.1183/09031936.00099614. [DOI] [PubMed] [Google Scholar]
- 37.Panos R.J., Mortenson R.L., Niccoli S.A., King T.E. Clinical deterioration in patients with idiopathic pulmonary fibrosis: Causes and assessment. Am. J. Med. 1990 doi: 10.1016/0002-9343(90)90495-Y. [DOI] [PubMed] [Google Scholar]
- 38.Frazier A.A., Galvin J.R., Franks T.J., Rosado-de-Christenson M.L. Pulmonary Vasculature: Hypertension and Infarction. Radiographics. 2000;20:491–524. doi: 10.1148/radiographics.20.2.g00mc17491. [DOI] [PubMed] [Google Scholar]
- 39.Collard H.R., Ryerson C.J., Corte T.J., Jenkins G., Kondoh Y., Lederer D.J., Lee J.S., Maher T.M., Wells A.U., Antoniou K.M., et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am. J. Respir. Crit. Care Med. 2016 doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 40.Dalpiaz G. Fibrosi polmonare idiopatica. Radiol Med. 2010;115:S130–S138. [Google Scholar]
- 41.Natsuizaka M., Chiba H., Kuronuma K., Otsuka M., Kudo K., Mori M., Bando M., Sugiyama Y., Takahashi H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014 doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 42.Kishaba T., Tamaki H., Shimaoka Y., Fukuyama H., Yamashiro S. Staging of Acute Exacerbation in Patients with Idiopathic Pulmonary Fibrosis. Lung. 2014;192:141–149. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]
- 43.Song J.W., Hong S.B., Lim C.M., Koh Y., Kim D.S. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur. Respir. J. 2011 doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R., Jindal S.K. Acute exacerbation of idiopathic pulmonary fibrosis: A systematic review. Eur. J. Intern. Med. 2008 doi: 10.1016/j.ejim.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Huie T.J., Olson A.L., Cosgrove G.P., Janssen W.J., Lara A.R., Lynch D.A., Groshong S.D., Moss M., Schwarz M.I., Brown K.K., et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology. 2010 doi: 10.1111/j.1440-1843.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 46.Simon-blancal V., Freynet O. Acute Exacerbation of Idiopathic Pulmonary Fibrosis: Outcome and Prognostic Factors. Respiration. 2012;83:28–35. doi: 10.1159/000329891. [DOI] [PubMed] [Google Scholar]
- 47.Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir. Med. 2008 doi: 10.1016/j.rmed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Luppi F., Cerri S., Taddei S., Ferrara G., Cottin V. Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern. Emerg. Med. 2015 doi: 10.1007/s11739-015-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collard H.R., Yow E., Richeldi L., Anstrom K.J., Glazer C. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir. Res. 2013;14:1. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson A.L., Swigris J.J., Raghu G., Brown K.K. Seasonal variation: Mortality from pulmonary fibrosis is greatest in the winter. Chest. 2009;136:16–22. doi: 10.1378/chest.08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryerson C.J., Cottin V., Brown K.K., Collard H.R. Acute exacerbation of idiopathic pulmonary fibrosis: Shifting the paradigm. Eur. Respir. J. 2015;46:512–520. doi: 10.1183/13993003.00419-2015. [DOI] [PubMed] [Google Scholar]
- 52.Silva C.I.S., Müller N.L., Fujimoto K., Kato S., Ichikado K., Taniguchi H., Kondoh Y., Johkoh T., Churg A. Acute Exacerbation of Chronic Interstitial Pneumonia: High-resolution Computed Tomography and Pathologic Findings. J. Thorac. Imagin. 2007;22:221–229. doi: 10.1097/01.rti.0000213588.52343.13. [DOI] [PubMed] [Google Scholar]
- 53.Sokai A., Tanizawa K., Handa T., Kubo T., Hashimoto S., Ikezoe K., Nakatsuka Y., Aihara K., Taguchi Y., Muro S., et al. Asymmetry in acute exacerbation of idiopathic pulmonary fibrosis. ERS Monogr. 2017;3:1–8. doi: 10.1183/23120541.00036-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]