Abstract
Background
This study aimed to investigate the effects of maresin-1 (MaR1) in a mouse model of caerulein-induced acute pancreatitis (AP).
Material/Methods
Fifty C57BL/6 mice with caerulein-induced AP were divided into the untreated control group (N=10), the untreated AP model group (N=10), the MaR1-treated (low-dose, 0.1 μg) AP model group (N=10), the MaR1-treated (middle-dose, 0.5 μg) AP model group (N=10), and the MaR1-treated (high-dose, 1 μg) AP model group (N=10). Enzyme-linked immunoassay (ELISA) measured serum levels of amylase, lipase, tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 and mRNA was measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Malondialdehyde (MDA), protein carbonyls, superoxide dismutase (SOD), and the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) were measured. Histology of the pancreas included measurement of acinar cell apoptosis using the terminal-deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) assay. Western blot measured Toll-like receptor 4 (TLR4), MyD88, and phospho-NF-κB p65, and apoptosis-associated proteins Bcl-2, Bax, cleaved caspase-3, and cleaved caspase-9.
Results
Following treatment with MaR1, serum levels of amylase, lipase, TNF-α, IL-1β, and IL-6 decreased, MDA and protein carbonyl levels decreased, SOD and the GSH/GSSG ratio increased in a dose-dependent manner. In the MaR1-treated AP mice, inflammation of the pancreas and the expression of inflammatory cytokines, pancreatic acinar cell apoptosis, Bcl-2 expression, and expression of TLR4, MyD88, and p-NF-κB p65 were reduced, but Bax, cleaved caspase-3, and cleaved caspase-9 expression increased.
Conclusions
In a mouse model of caerulein-induced AP, treatment with MaR1 reduced oxidative stress and inflammation and reduced apoptosis.
MeSH Keywords: Apoptosis, Inflammation, Pancreatitis
Background
Acute pancreatitis (AP) is a severe medical condition that can be fatal, as it may lead to multiple organ failure [1]. Worldwide, the incidence of AP is increasing with a reported annual incidence rate of 13–45/100,000 [2]. Approximately 30% of patients with AP develop severe acute pancreatitis (SAP), which presents with an acute abdomen and has high morbidity and mortality [2]. AP is characterized by inflammation, apoptosis of pancreatic acinar cells, and the release of pancreatic enzymes, including amylase and lipase [3,4]. Severe AP is associated with pancreatic necrosis [3,4]. Increased production of reactive oxygen species (ROS) has been shown to be associated with the progression of AP [5]. Oxidative stress has an important role in the pathogenesis of inflammatory disease, including AP [6]. However, the mechanisms involved in the pathogenesis of AP are poorly understood, and effective treatments remain to be developed. Possible developments in the treatment of AP include inhibition of oxidative stress and control of inflammation.
Maresin is a pro-inflammatory degradation product derived from docosahexaenoic acid (DHA) and is synthesized by macrophages, and maresin-1 (MaR1) is the earliest chemical isomer of maresin [7,8]. A recent study showed that MaR1 promoted the resolution of acute inflammation in renal ischemia-reperfusion injury [9]. Studies have shown that MaR1 reduces the inflammatory response and mitochondrial dysfunction induced by sepsis and suppresses acute lung injury induced by lipopolysaccharide in mice [10,11]. A previously published study showed that MaR1 reduced lipopolysaccharide-induced inflammation and apoptosis in human vascular endothelial cells [12]. Also, MaR1 regulates lipid toxicity, apoptosis, and endoplasmic reticulum stress in nonalcoholic fatty liver disease [13]. However, there have been no previous studies on the effect of MaR1 on AP. Therefore, this study aimed to investigate the effects of MaR1 in a mouse model of caerulein-induced AP.
Material and Methods
Experimental animals
Fifty male C57BL/6 mice, 6–8 weeks old and weighing between 22–25 g, were purchased from Shanghai Laboratory Animal Company (SLAC) Limited (Shanghai, China). All animals were housed in temperature-controlled cages with free access to food and water. They were allowed to acclimate to the environment for at least a week before the experiments began. This study was conducted in accordance with the guidelines for the Care and Use of Laboratory Animals and was approved by the Ministry of Science and Technology of China. The study protocols were approved by the Ethics Committee on Animal Experiments of Zhejiang University.
Establishment of the mouse model of caerulein-induced acute pancreatitis (AP)
Fifty C57BL/6 mice with caerulein-induced AP were divided into the untreated control group (N=10), the untreated AP model group (N=10), the MaR1-treated (low-dose, 0.1 μg) AP model group (N=10), the MaR1-treated (middle-dose, 0.5 μg) AP model group (N=10), and the MaR1-treated (high-dose, 1 μg) AP model group (N=10). Mice were injected intraperitoneally with caerulein in saline at a dose of 50 mg/kg (Sigma-Aldrich, St Louis, MO, USA) to establish the model of AP [14,15]. Mice were injected hourly for 6 h, as previously reported [14,15]. The control animals were given intraperitoneal injections of saline vehicle hourly for 6 h. Intraperitoneal injection of MaR1 was given after the last injection of caerulein. After a further 12 hours, the mice were euthanized by dislocation of the neck while anesthetized. Blood and pancreas tissue were harvested for further analysis.
Histopathology
Equal amounts of mouse pancreas tissues were fixed in 4% paraformaldehyde overnight at 4°C and routinely embedded in paraffin wax. Tissue sections at 5 μm were cut onto glass slides, stained with hematoxylin and eosin (H&E), dehydrated, mounted, and coverslipped for light microscopy. The slides (N=8) were examined by light microscopy at a magnification of ×100 by an experienced pathologist who was blinded to the treatment groups.
Measurement of serum levels of amylase and lipase
Blood samples were centrifuged at 3,500 rpm for 10 minutes to obtain the serum. The levels of amylase and lipase in serum were measured by the assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) were measured using ELISA kits, according to manufacturer’s instructions (Shanghai Xitang Biotechnology Co., Ltd., Shanghai, China.
Measurement of markers of oxidative stress in pancreas tissues
The levels of oxidative stress markers in the pancreas were measured, including malondialdehyde (MDA), protein carbonyls, superoxide dismutase (SOD) and the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) according to the manufacturer’s instructions for the kits used (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Terminal-deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) assay
The TUNEL assay was used to examine the apoptotic pancreatic acinar cells in paraffin-embedded pancreatic tissues. Following staining with hematoxylin for 15 min at room temperature, the nuclei of healthy cells were stained blue, while those in apoptotic cells showed brown/yellow staining, which was identified as TUNEL-positive cells. Integrated optical density (IOD) analysis was used to identify the apoptotic cells. Specimens were mounted and examined using routine light microscopy.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from pancreatic tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The purity of total RNA was evaluated by spectrophotometry. Then, cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (K1622) (Fermentas, Thermofisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. RT-qPCR was performed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA). Cycling conditions were 95°C for 10 min followed by 40 cycles of denaturation for 30 sec at 95°C, followed by annealing at 60°C for 30 sec, extension at 72°C for 30 sec. The primers used were:
TNF-α, forward 5′-GGAACACGTCGTGGGATAATG-3′;
TNF-α, reverse: 5′-GGCAGACTTTGGATGCTTCTT-3′;
IL-1β, forward: 5′-GAAATGCCACCTTTTGACAGTG-3′;
IL-1β, reverse: 5′-TGGATGCTCTCATCAGGACAG-3′;
IL-6, forward: 5′-GGTGCCCTGCCAGTATTCTC-3′,
IL-6, reverse: 5′-GGCTCCCAACACAGGATGA-3′;
GAPDH, forward: 5′-TGGATTTGGACGCATTGGTC-3′;
GAPDH, reverse: 5′-TTTGCACTGGTACGTGTTGAT-3′.
GAPDH or U6 was used as an internal control. CTRP3 expression was analyzed using the 2−ΔΔCt method.
Western blot
Pancreas tissues were lysed on ice in RIPA lysis buffer (Beyotime, Shanghai, China). A BCA Protein Quantitation kit was used to measure the concentration of total protein (Beyotime, Shanghai, China). A total of 40 μg of protein was isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% dried skimmed milk powder for 1 h, and incubated with primary antibodies overnight at 4°C. The primary antibodies were obtained from Cell Signaling Technology (Boston, MA, USA) and included anti-Bcl-2 (3498S), anti-Bax (14796S), anti-cleaved caspase-3 (9664S), anti-cleaved caspase-9 (9509T), anti-TLR4 (14358S), anti-MyD88 (4283S), anti-phospho-NF-κB p65 (3033S), anti-NF-κB p65 (8242S), and anti-GAPDH (5174S). Protein expression was normalized to GAPDH levels. The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (ab6721) (Abcam, Cambridge, UK) for 2 h. Enhanced chemiluminescence (ECL) reagent was used for visualization. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyze the results.
Statistical analysis
All experiments were performed in triplicate. Data were analyzed using SPSS version 14.0 software (IBM, Chicago, IL, USA). Data were expressed as the mean ± standard deviation (SD). Statistical comparisons were made using a two-tailed Student’s t-test or one-way analysis of variance (ANOVA). A P-value <0.05 was considered to be statistically significant.
Results
Maresin-1 (MaR1) reduced pancreatic inflammation in the mouse model of caerulein-induced acute pancreatitis (AP)
To determine the effects of MaR1 in the mouse model of AP, routine light microscopy was used to examine the pancreatic tissues. Figure 1 shows pancreatic necrosis and inflammation in the AP mouse model group when compared with the control group. Inflammation and necrosis were reduced following treatment with MaR1, which was most significant in the high-dose MaR1 group. These results indicated that the mouse model of AP was established and that MaR1 treatment reduced the pathological changes in the pancreatic tissue.
Figure 1.
Photomicrographs of the histology of the pancreatic tissues from mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1). Representative photomicrographs from each experimental group at 12 hours after the induction of acute pancreatitis. Hematoxylin and eosin (H&E). Magnification ×100. MaR1 – maresin-1; AP – acute pancreatitis.
MaR1 reduced the levels of serum amylase and lipase in the mouse model of AP
Serum levels of amylase and lipase were used as biochemical markers of AP. The results are shown in Figure 2A, 2B. Serum amylase and lipase levels were significantly increased in the mouse model of AP compared with the control group at 0 h, 6 h, and 12 h after induction of AP. Following treatment with MaR1, the levels of serum amylase and lipase decreased in a concentration-dependent manner at 0 h, 6 h, and 12 h. These findings showed that MaR1 treatment reduced the levels of serum amylase and lipase in the experimental model of AP.
Figure 2.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed reduced levels of serum amylase and lipase. Assay kits measured serum levels of (A) amylase and (B) lipase. *** P<0.001 vs. control; ### P<0.001 vs. AP. MaR1 – maresin-1; AP – acute pancreatitis
MaR1 reduced the expression of markers of oxidative stress in the mouse model of AP
Oxidative stress markers in pancreatic tissue were examined to evaluate the effect of MaR1 on reactive oxygen species (ROS) induced by caerulein. Levels of malondialdehyde (MDA) and protein carbonyls were significantly increased, the levels of superoxide dismutase (SOD) and the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) were significantly decreased in the model group (Figure 3A–3D). Following treatment with MaR1, the levels of MDA and protein carbonyls were reduced, accompanied with an increase in SOD and the GSH/GSSG ratio, and the greatest effect found in the high dose group (Figure 3A–3D). These findings showed that treatment with MaR1 reduced oxidative stress in the mouse model of AP.
Figure 3.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed reduced expression of markers of oxidative stress in the pancreas. The levels of (A) malondialdehyde (MDA), (B) protein carbonyls, (C) superoxide dismutase (SOD), and (D) the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) were measured using kits. *** P<0.001 vs. control; # P<0.05, ## P<0.01, ### P<0.001 vs. AP. MaR1 – maresin-1; AP – acute pancreatitis; MDA – malondialdehyde; SOD – superoxide dismutase; GSH/GSSG – reduced glutathione/oxidized glutathione.
MaR1 reduced the levels of inflammatory cytokines in the mouse model of AP
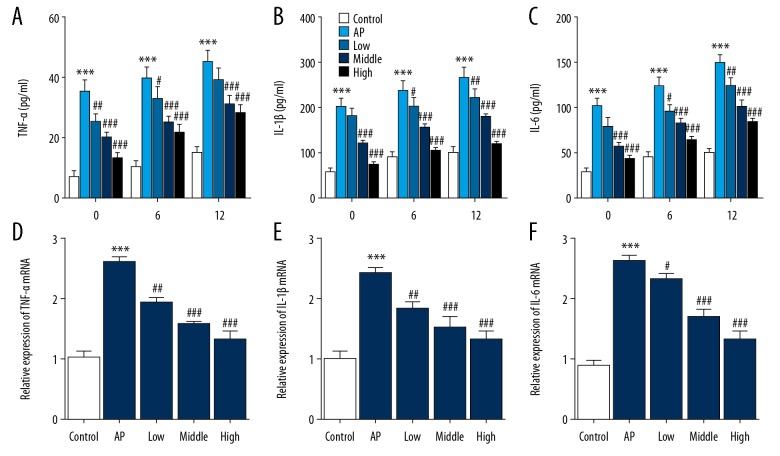
The levels of inflammatory cytokines, levels of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in serum and pancreatic tissues were measured to investigate the effect of MaR1 on inflammatory cytokines. As shown in Figure 4A–4C, the levels of TNF-α, IL-1β, and IL-6 in serum in the model group were significantly increased when compared with the control group at 0 h, 6 h, and 12 h after induction of AP. Following treatment with MaR1, serum levels of TNF-a, IL-1β, and IL-6 were significantly reduced, and the high dose group treated with MaR1 showed the greatest inhibitory effect. The mRNA expression of TNF-α, IL-1β, and IL-6 in pancreatic tissues supported the serum findings (Figures 4D–4F). These results indicated that MaR1 could reduce the expression of inflammatory cytokines in the mouse model of AP.
Figure 4.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed reduced expression of inflammatory cytokines. Serum levels of (A) tumor necrosis factor- α (TNF-α), (B) interleukin-1β (IL-1β), and (C) IL-6 in serum were measured by enzyme-linked immunoassay (ELISA). Expression of (D) TNF-α, (E) IL-1β, and (F) IL-6 in pancreatic tissues were detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). ** P<0.01, *** P<0.001 vs. control; # P<0.05, ## P<0.01 vs. AP. MaR1 – maresin-1; AP – acute pancreatitis.
MaR1 increased apoptosis in pancreatic acinar cells in the mouse model of AP
In this study, the terminal-deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) assay was used to measure the degree of pancreatic acinar cell apoptosis. As shown in Figure 5, animals in the AP model group showed low levels of apoptosis. Following treatment with MaR1, the apoptosis index of pancreatic acinar cells was significantly higher compared with the model group. Also, the expression of apoptosis-associated proteins was measured by Western blot. As shown in Figure 6, the expression of the anti-apoptosis protein, Bcl-2, was downregulated accompanied by upregulation of the expression of the pro-apoptotic proteins Bax, cleaved caspase-3, and cleaved caspase-9 in the mouse model of AP compared with the control. After treatment with MaR1, the expression level of Bcl-2 decreased, while Bax, cleaved caspase-3, and cleaved caspase-9 increased in a concentration-dependent manner. These results showed that MaR1 activated apoptosis in the mouse model of AP.
Figure 5.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed increased apoptosis of pancreatic acinar cells. Photomicrographs of representative images of cell apoptosis in the mouse pancreas measured by the terminal-deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) assay. Magnification ×100. MaR1 – maresin-1; AP – acute pancreatitis; TUNEL – terminal-deoxynucleotidyl transferase-mediated nick end-labeling.
Figure 6.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed increased expression of apoptosis-associated proteins. Western blot shows the expression levels of Bcl-2, Bax, cleaved caspase-3 and cleaved caspase-9. *** P<0.001 vs. control; ## P<0.01, ### P<0.001 vs. AP. MaR1 – maresin-1; AP – acute pancreatitis
MaR1 reduced the expression of proteins in the TLR4/NF-κB signaling pathway in the mouse model of AP
To further investigate the regulatory mechanisms of MaR1 on AP, the expression of proteins in the TLR4/NF-κB signaling pathway were measured by Western blot. As shown in Figure 7, the expression levels of TLR4, MyD88, and p-NF-κB p65 were significantly increased in the AP model group compared with the control group. Decreased expression of TLR4, MyD88, and p-NF-κB p65 was found in a concentration-dependent manner following treatment with MaR1. These results indicated that MaR1 inhibited the TLR4/NF-κB signaling pathway in the mouse model of AP.
Figure 7.
Mice with caerulein-induced acute pancreatitis (AP) treated with maresin-1 (MaR1) showed inhibition of the TLR4/NF-κB signaling pathway. Western blot shows reduced expression levels of TLR4, MyD88, and p-NF-κB p65. *** P<0.001 vs. control; ## P<0.01, ### P<0.001 vs. AP. MaR1 – maresin-1; AP – acute pancreatitis; TLR4 – toll-like receptor 4; p-NF-κB p65 – phospho-NF-κB p65.
Discussion
Acute pancreatitis (AP) is an inflammatory disease that can have a mild to severe course. Because of the limited treatment options, AP continues to be closely associated with high morbidity and mortality [16]. Therefore, the aim of this study was to investigate the effects of maresin-1 (MaR1) in a mouse model of caerulein-induced AP. The findings were that treatment with MaR1 reduced the course of AP by inhibiting oxidative stress and the inflammatory response as well as inducing apoptosis.
Oxidative stress is one of the factors implicated in the progression of AP [17]. Malondialdehyde (MDA) is the final product of lipid peroxidation induced by reactive oxygen species (ROS), which cause pancreatic damage during AP [18]. Protein carbonyls are produced following catalytic oxidation of metal ions [19]. When oxidative stress occurs, excessive production of ROS leads to an imbalance between oxidation and antioxidants in the body, which were reflected in this study by measurement of the levels of superoxide dismutase (SOD), and the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG). In the current study, in the mouse model of AP, MaR1 treatment reduced the levels of MDA and protein carbonyls and increased the levels of SOD and GSH/GSSG, which were findings supported by previous studies [20,21]. These results suggested that MaR1 treatment reduced tissue damage in pancreatitis by suppressing the production of ROS.
Increased activation of inflammatory cytokines contributes to the development of AP [22]. Also, the activation of NF-κB signaling is induced by the increased production of ROS [23]. TNF-α is a dominant regulatory factor in the initiation of AP, and TNF-α is one of the first inflammatory cytokines to increase during AP [24]. Increased accumulation of TNF-α promotes the production of other inflammatory cytokines, including IL-1β and IL-6, which will result in the activation of an inflammatory cascade leading to damage in multiple tissues and organs [25]. The levels of IL-1β and IL-6 are correlated with the severity of AP [26]. Therefore, inhibition of these pro-inflammatory cytokines could reduce the severity of pancreatitis. Previous studies have shown that MaR1 suppressed the inflammatory response and reduced tissue damage in multiple organs [10,11]. For these reasons, the role of MaR1 in caerulein-induced AP was studied. The findings showed that MaR1 reduced pancreatic necrosis and inflammation on histology, and reduced the serum levels of amylase and lipase. Importantly, the expression levels of TNF-α, IL-1β, and IL-6 were significantly reduced. These findings indicate that MaR1 reduced tissue damage in pancreatitis by inhibiting inflammation.
Because cell apoptosis is associated with inflammation, a reduced apoptotic cell index can be an indicator of reduced severity of AP. In the pancreas, inflammation is associated with damaged acinar cells that can undergo apoptosis or necrosis. Apoptotic cells maintain their membrane integrity, but cell necrosis is associated with the release of intracellular contents and triggers an inflammatory cascade [3,27]. Therefore, a switch from necrosis to apoptosis in pancreatic acinar cells may be important in the clinical treatment of AP [3,28]. In the present study, MaR1 treatment in the mouse model of AP significantly increased cell apoptosis in pancreatic tissues, as measured using the terminal-deoxynucleotidyl transferase-mediated nick end-labeling (TUNEL) assay. Also, there was a concentration-dependent reduction in the expression level of Bcl-2 and an increase in Bax, cleaved caspase-3, and cleaved caspase-9.
Toll-like receptors (TLRs), a family of pattern recognition receptors (PRRs), recognize pathogen-associated and damage-associated molecular pattern molecules, and promote the initiation of immune responses [29]. TLR4 has received particular attention because of its role in inflammation [29]. TLR4 interacts with the adaptor molecule, MyD88, and following a series of signal transductions, leads to NF-κB activation and the further synthesis of pro-inflammatory cytokines [30]. The findings from previously published studies have shown that the inhibition of TLR4/NF-κB signaling reduced inflammation and tissue damage in AP [31,32]. The findings of the present study showed that the expression of TLR4, MyD88, and p-NF-κB p65 was significantly downregulated following treatment with MaR1, which consistent with the findings from a previous study [33]. These results confirmed that MaR1 could inhibit the TLR4/NF-κB signaling pathway in a mouse model of AP.
Conclusions
This study aimed to investigate the effects of maresin-1 (MaR1) in a mouse model of caerulein-induced acute pancreatitis (AP). The results showed that treatment of the mouse model with MaR1 reduced pancreatic inflammation, oxidative stress, inflammation, the expression of inflammatory cytokines, and reduced apoptosis of pancreatic acinar cells. These findings support the anti-inflammatory role of MaR1 in this animal model.
Acknowledgments
The authors would like to thank Professors Rui Chen and Dong Ma for their helpful suggestions.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Xia S, Wang J, Kalionis B, et al. Genistein protects against acute pancreatitis via activation of an apoptotic pathway mediated through endoplasmic reticulum stress in rats. Biochem Biophys Res Commun. 2019;509:421–28. doi: 10.1016/j.bbrc.2018.12.108. [DOI] [PubMed] [Google Scholar]
- 2.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–20. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 4.Najenson AC, Courreges AP, Perazzo JC, et al. Atrial natriuretic peptide reduces inflammation and enhances apoptosis in rat acute pancreatitis. Acta Physiol (Oxf) 2018;222(3) doi: 10.1111/apha.12992. [DOI] [PubMed] [Google Scholar]
- 5.Tiruveedi VL, Bale S, Khurana A, Godugu C. Withaferin A, a novel compound of Indian ginseng (Withania somnifera), ameliorates Cerulein-induced acute pancreatitis: Possible role of oxidative stress and inflammation. Phytother Res. 2018;32:2586–96. doi: 10.1002/ptr.6200. [DOI] [PubMed] [Google Scholar]
- 6.Uslukaya O, Turkoglu A, Yazgan UC, et al. The effects of roflumilast on the pancreas and remote organs in a cerulein-induced experimental acute pancreatitis model in rats. Surg Today. 2016;46:1435–42. doi: 10.1007/s00595-016-1329-1. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Zhang H, Luo L, et al. Resolvin D1 improves the resolution of inflammation via activating NF-kappaB p50/p50-mediated cyclooxygenase-2 expression in acute respiratory distress syndrome. J Immunol. 2017 doi: 10.4049/jimmunol.1700315. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu Y, Wu Y, Zhao H, et al. Maresin 1 mitigates renal ischemia/reperfusion injury in mice via inhibition of the TLR4/MAPK/NF-kappaB pathways and activation of the Nrf2 pathway. Drug Des Devel Ther. 2019;13:739–45. doi: 10.2147/DDDT.S188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong J, Wu ZY, Qi H, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol. 2014;171:3539–50. doi: 10.1111/bph.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordgren TM, Heires AJ, Wyatt TA, et al. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res. 2013;14:51. doi: 10.1186/1465-9921-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung TW, Park HS, Choi GH, et al. Maresin 1 attenuates pro-inflammatory reactions and ER stress in HUVECs via PPARalpha-mediated pathway. Mol Cell Biochem. 2018;448:335–47. doi: 10.1007/s11010-018-3392-y. [DOI] [PubMed] [Google Scholar]
- 13.Rius B, Duran-Guell M, Flores-Costa R, et al. The specialized pro-resolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J. 2017;31:5384–98. doi: 10.1096/fj.201700394R. [DOI] [PubMed] [Google Scholar]
- 14.Amiti, Tamizhselvi R, Manickam V. Menadione (vitamin K3) inhibits hydrogen sulfide and substance P via NF-κB pathway in caerulein-induced acute pancreatitis and associated lung injury in mice. Pancreatology. 2019;19:266–73. doi: 10.1016/j.pan.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Velusamy RK, Tamizhselvi R. Protective effect of methylsulfonylmethane in caerulein-induced acute pancreatitis and associated lung injury in mice. J Pharm Pharmacol. 2018;70:1188–99. doi: 10.1111/jphp.12946. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: Management of acute pancreatitis. Can J Surg. 2016;59:128–40. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma R, Yuan F, Wang S, et al. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-kappaB signal pathways in mice. Biomed Pharmacother. 2018;105:599–605. doi: 10.1016/j.biopha.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 18.Kilic Y, Geyikoglu F, Colak S, et al. Carvacrol modulates oxidative stress and decreases cell injury in pancreas of rats with acute pancreatitis. Cytotechnology. 2016;68:1243–56. doi: 10.1007/s10616-015-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafik NM, Abou-Fard GM. Ameliorative effects of curcumin on fibrinogen-like protein-2 gene expression, some oxido-inflammatory and apoptotic markers in a rat model of l-arginine-induced acute pancreatitis. J Biochem Mol Toxicol. 2016;30:302–8. doi: 10.1002/jbt.21794. [DOI] [PubMed] [Google Scholar]
- 20.Tsang SW, Guan YF, Wang J, et al. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci Rep. 2016;6:22859. doi: 10.1038/srep22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco C, Rodriguez AB, Pariente JA. Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in cerulein-induced acute pancreatitis in rats. Hepatobiliary Pancreat Dis Int. 2014;13:442–46. doi: 10.1016/s1499-3872(14)60271-x. [DOI] [PubMed] [Google Scholar]
- 22.Qian Y, Chen Y, Wang L, Tou J. Effects of baicalin on inflammatory reaction, oxidative stress and PKDl and NF-κB protein expressions in rats with severe acute pancreatitis1. Acta Cir Bras. 2018;33:556–64. doi: 10.1590/s0102-865020180070000001. [DOI] [PubMed] [Google Scholar]
- 23.Seo JY, Kim H, Seo JT, Kim KH. Oxidative stress induced cytokine production in isolated rat pancreatic acinar cells: Effects of small-molecule antioxidants. Pharmacology. 2002;64:63–70. doi: 10.1159/000056152. [DOI] [PubMed] [Google Scholar]
- 24.To SQ, Knower KC, Clyne CD. Origins and actions of tumor necrosis factor-alpha in postmenopausal breast cancer. J Interferon Cytokine Res. 2013;33:335–45. doi: 10.1089/jir.2012.0155. [DOI] [PubMed] [Google Scholar]
- 25.Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130–40. doi: 10.1097/shk.0b013e3180487ba1. [DOI] [PubMed] [Google Scholar]
- 26.Chi DZ, Chen J, Huang DP. Influence of interleukin-1beta and interleukin-6 gene polymorphisms on the development of acute pancreatitis. Genet Mol Res. 2015;14:975–80. doi: 10.4238/2015.February.3.5. [DOI] [PubMed] [Google Scholar]
- 27.Xiang H, Wang G, Qu J, et al. Yin-Chen-Hao Tang attenuates severe acute pancreatitis in rat: An experimental verification of in silico network target prediction. Front Pharmacol. 2016;7:378. doi: 10.3389/fphar.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Yang W, Li Y, et al. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. Int Immunopharmacol. 2018;56:18–28. doi: 10.1016/j.intimp.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36:245–71. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- 30.Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J Hepatol. 2016;8:1354–69. doi: 10.4254/wjh.v8.i32.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Junyuan Z, Hui X, Chunlan H, et al. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38MAPK and ERS inhibition. Pancreatology. 2018;18:742–52. doi: 10.1016/j.pan.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Pan LF, Yu L, Wang LM, et al. Augmenter of liver regeneration (ALR) regulates acute pancreatitis via inhibiting HMGB1/TLR4/NF-kappaB signaling pathway. Am J Transl Res. 2018;10:402–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Wu X, Yang L, et al. TLR4-mediated NF-kappaB signaling pathway mediates HMGB1-induced pancreatic injury in mice with severe acute pancreatitis. Int J Mol Med. 2016;37:99–107. doi: 10.3892/ijmm.2015.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]