1 ∣. INTRODUCTION
Platinum-based chemotherapy is the standard of care treatment for patients with non-resectable, recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC).1 However, the toxicides of platinum must not be ignored. In reality, many patients with non-resectable R/M HNSCC cannot tolerate platinum-based chemotherapy due to their performance status (PS) and/or comorbidities.
Methotrexate (MTX), a folate antimetabolite that inhibits DNA synthesis, repair and cellular replication, has shown a modest response rate (RR) of 10% while RR was 32% for ciplatin/5-FU in R/M HNSCC, although this was in exchange with significantly increased toxicides with dsplatin/5-FU compared to MTX (P = 0.001).2
Cetuximab (CTX) is a chimeric monoclonal antibody directed against the extracellular portion of the epidermal growth factor receptor (EGFR). CTX monotherapy has shown RR of 13% in R/M HNSCC post-platinum-failure.3
Both CTX and MTX have shown single-agent activity in non-resectable R/M HNSCC with favourable toxicity profiles. However, data on combination CTX/MTX are limited. The purpose of this study is to provide outcomes data on combination CTX/MTX in non-resectable R/M HNSCC patients.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Ethical considerations
Approval for data collection and analysis was obtained from the Karmanos Cancer Institute/Wayne State University (Detroit, MI) institutional review board. The requirement for informed consent was waived as all identifying information was removed from the data set prior to analysis.
2.2 ∣. Patients
Retrospectively, data from patients with histology proven unresectable or R/M HNSCC who were treated with CTX/MTX at the Karmanos Cancer Institute between January 2004 and December 2010 were obtained from the pharmacy database.
Demographic characteristics of age, race, gender, PS, number of prior chemotherapy regiments in the R/M HNSCC, number of CTX/MTX cycles and adverse events (AE) were obtained from electronic medical records.
2.3 ∣. Chemotherapy
The regimen consisted of weekly intravenous (IV) MTX 25 mg/m2 plus IV CTX 400 mg/m2 loading dose at week one then 250 mg/m2 weekly maintenance dose. Each cycle consisted of 4 weeks. Concurrent treatment was defined as both agents given together for ≥two cycles.
2.4 ∣. Evaluation of response and toxicity
Pre-treatment and follow-up imaging studies (ie CT and/or MRI) were reviewed by an independent institutional radiologist. Response to treatment was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4. Imaging studies which were performed between 7 and 12 weeks after starting CTX/MTX were reviewed and analysed for response. Progression-free survival (PFS) was defined as time from initiation of treatment until disease progression or death. Overall survival (OS) was defined as time from initiation of treatment until death. Patients who remained alive were censored at the date of last contact. Response rate (RR) was defined as the fraction of patients who achieved a partial response (PR) or complete response (CR).
2.5 ∣. Statistical analysis
The primary endpoint was to estimate the OS. PFS, RR and AE were assessed as secondary endpoints. Descriptive statistics was used to summarise demographic and baseline characteristics among study populations. Continuous variables were summarised with median and range, while counts and percentages were used to summarise categorical variables. The Kaplan-Meier method was used to describe the distribution of the PFS and OS after treatment. Univariable logistic regression analyses were performed to evaluate for treatment responses. Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between four prior chosen predictors (age, number of cycles, prior CTX exposure and number of prior lines) and survival benefit (PFS and OS). The proportional hazard assumption was checked, and no violation was found. All statistical analyses were performed using R (http://www.r-project.org).
3 ∣. RESULTS
A total of 54 patients were included. Median age was 60 years old. Forty (74%) patients were males; 14 (26%) were females. Thirty-one (57%) patients were African American; 22 (41%) were Caucasian. Fourteen (26%) patients had received prior CTX. Twenty-eight (52%) patients had PS 1, and 26 (48%) patients had PS of two or more (Table 1). Table S1 shows the 34 patients who were identified in the pharmacy database for having CTX/MTX ordered, but did not complete two cycles for various reasons. Table S2 summarises the toxicities and cause of death.
TABLE 1.
Patient characteristics
| All (n = 54) | |
|---|---|
| Age at treatment—median (range) | 60 (40, 67.75) |
| Number of cycles—median (range) | 6(2, 8.75) |
| Race—count (%) | |
| AA | 31(57) |
| EA | 22 (41) |
| Other | 1(2) |
| Gender—count (%) | |
| Male | 40 (74) |
| Female | 14 (26) |
| Site—count (%) | |
| Oral cavity: all mouth: lip, molar, gingival ant 2/3 tongue | 14 (26) |
| Oropharyngeal: tonsil, base of tongue, pharynx around oral area | 14 (26) |
| Larynx: supra, infra/sub- and glottic | 21 (39) |
| Hypopharyngeal | 2(4) |
| Unknown primary | 3(6) |
| Received CTX in the past—count (%) | |
| No | 40 (74) |
| Yes | 14 (26) |
| Number of prior lines of palliative chemo—count (%) | |
| None | 22 (41) |
| 1 | 24 (44) |
| ≥2 | 8(15) |
| Performance status—count (%) | |
| 1 | 28 (52) |
| 2 | 25 (46) |
| 3 | 1(2) |
AA, African American; EA, European American.
3.1 ∣. Response to treatment
Twenty-two (41%) patients received CTX/MTX as first-line therapy, while 24 (44%) and 8 (15%) received 1 or ≥2 prior regimens before to receiving CTX/MTX. Prior chemotherapy regimens include taxanes, platinum, 5-flurouracil and gemcitabine. The median number of CTX/MTX cycles received was six (range; 2-8 cycles).
Out of 54 patients who completed two cycles of CTX/MTX, 5 (9.2%) had PR, 34 (63.0%) had stable disease (SD), and 15 (27.8%) had progression of disease (PD). Patients who had a rash toxicity of grade 2 or more were more likely to have disease control to CTX/MTX (OR, 10.22; 95% CI, 1.06-92.81; P = 0.03, Table 2).
TABLE 2.
Univariable logistic regression analyses for RECIST responses
| Univariable | ||
|---|---|---|
| OR (95% CI) | Signif | |
| DCR (=CR + PR + SD) | ||
| Number of prior lines of palliative chemo | ||
| None | ||
| ≥1 | 1.03 (0.16, 8.39) | 0.97 |
| Rash | ||
| Grade ≤ 1 | ||
| Grade ≥ 2 | 10.22 (1.06, 92.81) | 0.03 |
| Hypomagnesemia | ||
| Grade ≤ 1 | ||
| Grade ≥ 2 | 6.67 (0.97, 56.65) | 0.05 |
CI, confidence interval; DCR, disease control rate; OR, odds ratio; Ref., reference; Signif, significance.
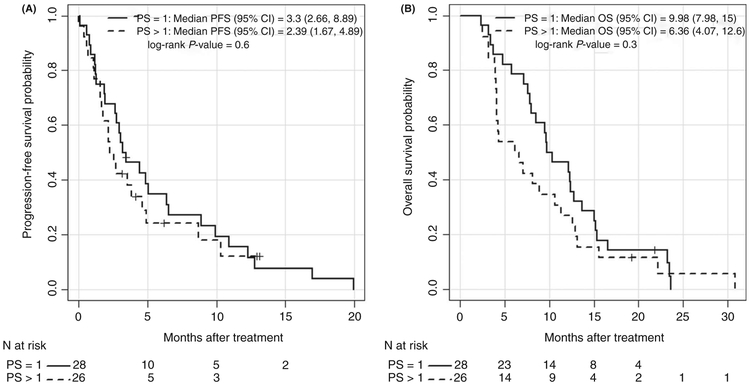
The median PFS was 2.98 (95% CI, 2.16-4.85 months) and the median OS was 8.66 months (95% CI, 7.02-12.26 months) for all patients. The median PFS in the patients with PS of 1 was 3.30 months (95% CI, 2.66-8.89 months) compared with 2.39 months (95% CI, 1.67-4.89 months) in those with PS of 2 or more (PFS HR, 1.19; 95% CI, 0.66-2.13; P = 0.60). The median OS in the patients with PS of 1 was 9.98 months (95% CI, 7.97-15.0 months) compared with 6.36 months (95% CI, 4.07-12.6 months) in those with PS of 2 or more (OS HR, 1.36; 95% CI, 0.78-2.36; P = 0.30). Kaplan-Meier estimates of survival are shown in Figure 1A,B.
FIGURE 1.
A, Kaplan-Meier Progression-free survival (PFS) by PS. B, Kaplan-Meier for Overall Survival (OS) after treatment by PS
The number of cycles received correlated with a smaller risk of death (HR, 0.85; 95% CI, 0.78-0.92; P < 0.001 in a univariable analysis) that was independently predictive even after adjusting for age, prior CTX exposure and number of cycles received in a multivariable Cox regression (HR, 0.84; 95% CI, 0.77-0.91; adjusted P < 0.001, Table 3). At the end of the inclusion period, 50 (94%) patients were deceased, 45 (83%) due to their malignancy, 5 (9%) to non-malignancy-related causes and 2 (4%) due to unknown reasons; 2 (4%) were alive.
TABLE 3.
Univariable and multivariable Cox regression analyses for survival benefit (PFS and OS)
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95% CI) | Signif | HR(95% CI) | Signif | |
| PFS | ||||
| Age at treatment | 0.99 (0.95, 1.02) | 0.44 | 0.98 (0.95, 1.02) | 0.35 |
| Number of cycles | 0.97 (0.90, 1.04) | 0.43 | 0.97 (0.90, 1.04) | 0.34 |
| Received CTX in the past | ||||
| No | Ref. | |||
| Yes | 0.84 (0.43, 1.67) | 0.63 | 0.75 (0.37, 1.52) | 0.43 |
| Number of prior lines of palliative chemo | ||||
| None | Ref. | |||
| ≥1 | 0.98 (0.54, 1.78) | 0.96 | 0.98 (0.54, 1.77) | 0.93 |
| OS | ||||
| Age at treatment | 0.99 (0.96, 1.02) | 0.48 | 0.98 (0.95, 1.02) | 0.34 |
| Number of cycles | 0.85 (0.78, 0.92) | <0.001 | 0.84 (0.77, 0.91) | <0.001 |
| Received CTX in the past | ||||
| No | Ref. | |||
| Yes | 0.90 (0.48, 1.71) | 0.75 | 0.60 (0.30, 1.20) | 0.15 |
| Number of prior lines of palliative chemo | ||||
| None | Ref. | |||
| ≥1 | 1.03 (0.58, 1.83) | 0.91 | 0.91 (0.51, 1.64) | 0.76 |
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; Ref.,reference; Signif, significance.
3.2 ∣. Safety and tolerability
Most patients tolerated the regimen well. Twenty-five (46%) and 28 (52%) patients had neither skin rash nor hypomagnesemia, respectively. Holding treatment was defined as treatment withheld for ≥4 consecutive doses. Treatment was held in only five (9%) patients and that was secondary to toxicities. Only four (7%) and two (4%) patients developed Grade 3 rash and hypomagnesemia, respectively. Twenty-five (46%) had Grade 1-2 rash; 24 (45%) had Grade 1-2 hypomagnesemia (Table S2).
4 ∣. DISCUSSION
The mainstay treatment for R/M HNSCC is systemic chemotherapy. Both MTX and CTX have shown single-agent efficacy in this setting,2,3 but studies of combination CTX/MTX have been limited. Although immune checkpoint inhibition with pembrolizumab and nivolumab has become standard options in platinum-refractory R/M HNSCC, the reported RR is in the range of 13%-16%, PFS of approximately 2 months and OS of approximately 8 months.4,5 Strategies beyond progression on immunotherapy are in dire need.
Judging by the low proportion of patients who developed ≥Grade 3 AE, in addition to the minimal number of patients for whom treatment had to be held, this regimen appears reasonably tolerable.
In our study, we observed a RR of 9.2% with the combination CTX/MTX. The median PFS and OS for all patients in this study were 2.98 (95% CI, 2.16-4.85 months) and 8.66 months (95% CI, 7.02-12.26 months), respectively. Although our study is limited to its retrospective nature, the RR and PFS are similar to previous reports of CTX with/without platinum in the setting of platinum-refractory R/M HNSCC, which has been reported in the range between 10%-13% and 2.2-2.8 months, respectively.6 The OS of 8.66 months exceeds the previously reported numbers of 5.2-6.1 months.6
Our OS data are comparable to the EXTREME trial, although cross-study comparison requires great caution. When compared with platinum-based chemotherapy plus fluorouracil alone, the EXTREME trial showed that the addition of CTX improved OS from 7.4 to 10.1 months. It must be noted that in EXTREME, patients with Karnofsky performance score <70% were excluded,3 whereas in our study, 48% of the patients had a PS of 2 or more. Our findings are also comparable to trials investigating CTX/taxane combination for R/M HNSCC.7,8
Furthermore, efficacy was noted even in the setting of prior exposure to CTX. CTX/MTX can still be a valid option for patients with prior CTX either in the setting of single agent, other combination or with radiation.
This study is limited due to its retrospective nature. Concurrent CTX/MTX was defined as both agents given together for ≥2 cycles. Although this was in an attempt to standardise the regimen to provide the utmost efficacy data when other factors, that is, primary site and lines of treatment varied (Table 1), its potential for causing a selection bias is inevitable. The sample size is relatively small and comes from a single institution. However, this study highlights the safety and efficacy of CTX/MTX for front-line as well as second- and third-line treatment for R/M HNSCC patients. CTX/MTX provides a platinum-free regimen for patients who are unlikely to tolerate platinum, such as those with limited PS which have traditionally been excluded from trials, as well as those with complications from previous platinum. Additionally, given emerging data on the improved efficacy of chemotherapy post-immunotherapy,9,10 this regimen may be of further interest as a potential salvage treatment post-progression on immunotherapy.
5 ∣. CONCLUSION
Our findings highlight the safety and efficacy of CTX/MTX as an option for palliative treatment for R/M HNSCC patients.
Supplementary Material
Keypoints.
Cetuximab (CTX) and methotrexate (MTX) have both shown single-agent activity in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) but data on CTX/MTX combination is limited.
We report safety and efficacy data of 54 R/M HNSCC patients treated with concurrent CTX/MTX.
Median progression-free survival and overall survival were 2.98 months (95% CI, 2.16-4.85 months) and 8.66 months (95% CI, 7.02-12.26 months), respectively.
Grade 3 rash and hypomagnesemia were observed in four (7%) and two (4%) of patients.
CTX/MTX combination could be considered as a palliative treatment option for patients with R/M HNSCC.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and families who contributed data to this retrospective study. All authors have confirmed and declare no conflict of interest to the content of this manuscript.
Footnotes
CONFLICT OF INTEREST
None to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J clin oncol. 1992;10(8):1245–1251. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J clin oncol. 2007;25(16):2171–2177. [DOI] [PubMed] [Google Scholar]
- 4.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum-and cetuximab-refractory head and neck cancer: results from a single-arm, Phase II Study. J Clin Oncol. 2017;35(14):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112(12):2710–2719. [DOI] [PubMed] [Google Scholar]
- 7.Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez Jj. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012;23(4):1016–1022. [DOI] [PubMed] [Google Scholar]
- 8.Jiménez B, Trigo JM, Pajares BI, et al. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49(2):182–185. [DOI] [PubMed] [Google Scholar]
- 9.Sukari A, Nagasaka M, Abdallah N. Responses in patients receiving sequential paclitaxel post progression on PD1 inhibitors. Oral Oncol. 2018;80:100–102. [DOI] [PubMed] [Google Scholar]
- 10.Saleh K, Kochanny S, Khattri A, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with squamous cell carcinoma of the head and neck. J clin oncol. 2018;36(suppl; abstr 6015):6015–6015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.