Abstract
The use of opioids in the perioperative period is associated with respiratory depression, impaired gastrointestinal function, post-operative nausea and vomiting (PONV), pruritus, urinary retention, delirium and the potential for developing opioid addiction. Currently the United States is experiencing an epidemic of prescription opioid abuse and deaths from overdose. Many addicts develop their addiction during a routine surgical admission to hospital. More people now die from overdose of synthetic prescription opioids than from heroin and other street drugs. Public education campaigns teaching family members of addicts to reverse opioid induced respiratory depression with naloxone are currently underway. Preventing the development of addiction in the first place during and after the surgical admission however will be more successful at saving lives. Primary prevention of opioid addiction is possible when non-opioid analgesic drugs are used. Employing alternative analgesic drugs in the peri-operative period that have a lower addiction potential and less respiratory depression has therefore become a matter of great national importance. Many powerful non-opioid analgesics are currently available that have more favorable side effect profiles and a lower potential for developing addiction. However, these medications are currently not used as often in routine clinical practice as they should be. Replacing opioids with other analgesics will not only reduce the development of opioid addiction but will also lead to better perioperative outcomes and enhanced patient recovery. This article briefly reviews the opioid alternatives that can significantly reduce or even entirely eliminate the perioperative use of opioids in the majority of surgical procedures.
Keywords: Opioids, Analgesia, Perioperative
Introduction
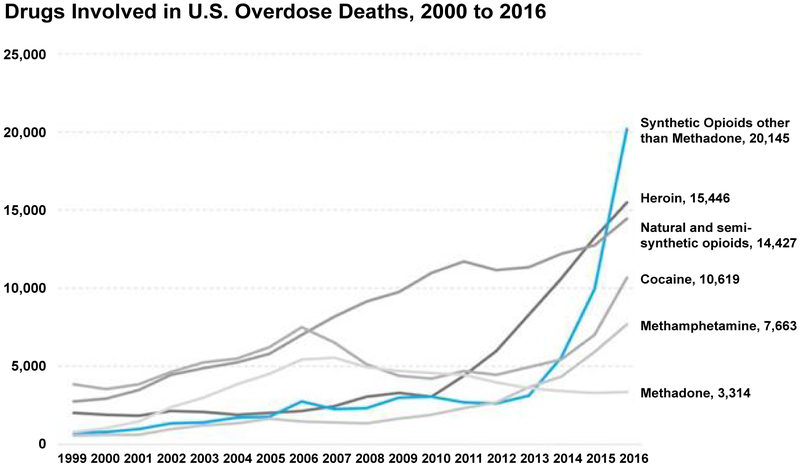
Opioid analgesic drugs have been the most commonly used perioperative pain-relieving medications for a very long time. While they are effective at relieving somatic pain they, unfortunately, do not eliminate neuropathic pain and have a profound potential for developing addiction [1,2]. Opioid addiction is currently an epidemic in the United States and overdose deaths from synthetic opioid drugs have been skyrocketing over the last decade. (Figure 1) Many addicts trace the origin of their opioid addiction back to when they were admitted to the hospital and received opioids as an analgesic modality. There is therefore a large iatrogenic component to the current opioid abuse epidemic. High potency opioids like hydrocodone and oxycodone have a street value that far exceeds that of heroin. [1] Prescription opioids now have become a common cause of overdose deaths. A national strategy needs to be developed to reverse the current epidemic of addiction and overdose deaths. There are currently different approaches to trying to reduce these overdose deaths. Educating addicts, their friends and families about how and when to administer naloxone can be an effective method of secondary prevention [3]. Primary prevention to stop an opioid addiction from developing during the perioperative period in the first place should however be the ultimate goal. In this article we explore the use of non-opioid analgesic drugs to both reduce the risk of developing opioid addiction and the occurrence of opioid related side effects. The use of these alternative drugs in combination as part of a multimodal strategy can lead to enhanced recovery after surgery with a lower potential for developing addiction. Substituting the administration of opioids with alternative analgesics in the perioperative period should be of great priority for health care providers. There should be a frank discussion with patients about the risk of developing an opioid addiction before the operation and counselling should be conducted when the patient’s opioid use appears to be excessive after the operation. Prescription opioid refills will need to be limited and carefully reviewed [4].
Figure 1:
Drugs involved in U.S. Overdose deaths, 2000 to 2016.
Opioid Related Side Effects
The most significant opioid side effect is respiratory depression. This is especially important in patients with obesity, sleep apnea, chronic obstructive pulmonary disease and operations that are associated with a high incidence of post-op respiratory failure [5]. Impaired gastro-intestinal function is a major issue in bowel surgery because postoperative ileus may lead to an anastomotic leak. When opioids are used liberally in major operations opioid induced bowel distension pushes the diaphragm in a cephalad direction and causes atelectasis in both lung bases. This abdominal distension increases the work of breathing and leads to hypoxemia because the collapsed lung bases no longer take part in gas exchange. This often causes postoperative respiratory failure with re-intubation. Abdominal distension is also a common cause for surgical wound dehiscence. Opioid induced gastro-paresis is also a significant contributor to aspiration during anesthesia induction. In the critical care unit (CCU) it leads to inability to absorb nasogastric feeds and malnutrition. The use of opioids as standard critical care sedatives should therefore be eliminated as better agents like dexmedetomidine have become available [6]. Opioid induced PONV is a particularly significant problem following eye surgery, upper gastrointestinal surgery and head and neck and neurosurgery where the Valsalva maneuver associated with the vomiting process may precipitate bleeding or a cerebrospinal fluid leak. Pruritus can be a more significant problem in the recovery room than pain. This is often treated with antihistamines that are only partially effective at alleviating the itch and cause a lot of unwanted sedation. Urinary retention leads to catheterization and urinary tract infections. Postoperative delirium is commonly induced by opioids and can be treated by employing alternative pain management strategies. Opioids depress cell mediated immunity and in in some studies have been found to be associated with an increased tumor recurrence rate after cancer surgery [7,8]. Until further evidence becomes available opioids should be used with caution in cancer surgery.
Alternatives to Opioids in Perioperative Care
Dexmedetomidine
Dexmedetomidine is an alpha-2 agonist with analgesic action that causes far less hypotension than clonidine. Blood pressure transiently rises following dexmedetomidine administration followed by a drop to ten percent below baseline values [9]. Unlike opioids it is not associated with significant respiratory depression, PONV, pruritus, constipation, ileus or delirium [10]. It can reduce intraoperative opioid administration by more than 50% [11]. In laparoscopic surgeries, it has been found to provide adequate analgesia when used as the only analgesic [12]. It has been shown to provide better heart rate control post intubation than fentanyl when used for intravenous induction [13]. It has been used as a PCA drug in combination with opioids and has been found to provide better analgesia with less PONV than opioids alone [14,15]. It should be used in patients with sleep apnea, obesity, gastric bypass surgery and a history of PONV or delirium. It is a powerful bronchodilator and should be used in patients with chronic obstructive airway disease and asthma [16]. It is also an anxiolytic and 20 mcg dexmedetomidine can replace 2 mg midazolam as a pre-operative anxiolytic. It provides enhanced recovery and patient satisfaction after laparotomy [17] and should be considered as part of any enhanced recovery after surgery (ERAS) program. It has a much lower addictive potential than opioids and should therefore be used in preference to opioids as a first line analgesic drug. It has long half-life of two hours and it can be safely administered by intravenous (IV) bolus doses until the desired effect is achieved. Bradycardia is the major dose limiting side effect and this responds readily to atropine [9].
Intravenous acetaminophen
Acetaminophen is a non-opioid analgesic drug with potent antipyretic but very weak anti-inflammatory action [18]. The IV formulation has a more potent analgesic action with faster onset and much higher plasma levels than the oral formulation [19]. There is also less liver toxicity with the IV formulation because IV administration bypasses the first pass metabolism in the liver. There was a 46% reduction in opioid use on day 1 with this drug following hip and knee surgeries with less PONV. Acetaminophen can be toxic to the liver when administered in overdose and the daily IV administration should not exceed 15 mg/kg every 6 hours. The combined daily maximum should not exceed 4 g [20]. Care needs to be taken when using this drug in patients with liver disease and the maximal daily dose in these patients should be limited to 2 g/day [21] (Table 1).
Table 1:
Intravenous and Oral Opioid Replacement Drugs.
| Drug | Dosage |
|---|---|
| IV Dexmedetomidine | 0.25 mcg/kg boluses (0.5-mcg/kg/hr infusion) |
| IV Acetaminophen | 15 mg/kg every 6 hours |
| IV Ketorolac | 30 mg every 6 hours |
| IV Ketamine | 0.25 mg/kg boluses (0.1 mg/kg/hr infusion) |
| IV Lidocaine | 1 mg/kg loading dose (1-2 mg/kg/hr infusion) |
| Oral Gabapentin | 300 mg PO daily |
| Oral Pregabalin | 150 mg PO daily |
| IV Magnesium | 30 mg/kg loading dose (10 mg/kg/hr infusion) |
Intravenous Ketorolac
Ketorolac is a non-steroidal anti-inflammatory drug with significant analgesic action [22]. It can be used to limit opioid side effects and is particularly useful in treating painful uterine cramps which are prostaglandin mediated. It should be avoided in asthmatics because interference with the prostaglandin mechanism can precipitate bronchospasm. There is also the risk of bleeding, renal impairment and gastritis and peptic ulceration when administered over several days. Bleeding was not an issue after breast surgery [23] but after circumcision the ketorolac group had more bleeding [24]. Unlike ketorolac the selective cyclooxygenase 2 inhibitors are not associated with bleeding but have a higher rate of thrombotic events and should be avoided in patients at risk for myocardial infarction or stroke. The opioid sparing effect of ketorolac has been associated with a reduced cancer recurrence rate after breast cancer surgery [25]. Ketorolac also interferes with bone fusion after back surgery and should not be used in these cases [26]. Ketorolac should also best be avoided in the elderly with pre-existing renal impairment.
Ketamine
Ketamine is an NMDA receptor antagonist that has profound analgesic effects at sub-anesthetic doses. Bolus doses should be limited to 0.25 mg/kg to prevent tachycardia and hypertension. Because of its long half-life (2-3 hours) it is reasonable to administer it via IV bolus doses rather than continuing infusion. Bolus doses larger than 0.25 mg/kg should however be avoided especially in patients with coronary artery disease because the resulting tachycardia and hypertension may lead to myocardial ischemia. Ketamine is associated with the wind-down phenomenon [27] and results in reduced pain during the post-operative period. It is also effective at treating neuropathic pain that usually does not respond to treatment with opioids. Recently it has also been noted to have a profound antidepressant effect [28,29]. The most significant side effect is a dysphoric syndrome with hallucinations and out of body experiences at higher doses. This can be suppressed with benzodiazepines but is best avoided by not administering doses greater than 0.5 mg/kg [30,31]. The addictive potential of this drug is, unfortunately, very high and while it can reduce the side effects of opioids it may not be a solution to the current prescription drug abuse epidemic.
Intravenous lidocaine
Lidocaine is an amino-amide local anesthetic that has a profound analgesic effect and has been shown to significantly reduce opioid requirements and side effects. The loading dose is 1-2 mg/kg followed by an infusion of 1-2 mg/kg/hr. [32] The rate should be reduced by 50% every 6 hours. In abdominal operations it reduced ileus, PONV [33] and has been shown to be of similar efficacy to epidural administration of local anesthetic. It may be an effective neuroprotective agent to prevent early post-operative cognitive dysfunction [34]. It is also effective for neuropathic pain. Lidocaine is metabolized in the liver and the administration rate needs to be reduced in low cardiac output states that are associated with poor liver perfusion in order to prevent toxicity. Side effects are perioral paresthesia, metallic taste, tinnitus and seizures [35]. Under anesthesia the only manifestations of toxicity may be bradycardia and wide QRS complexes. Lidocaine toxicity is more likely to manifest when the plasma level reaches 5 mcg/ml [36]. A bolus dose of 1 mg/kg followed by an infusion of 1.5 mg/kg/hr usually leads to a plasma concentration of about 2 mcg/ml [37]. So there is an inbuilt margin of safety when administering this dose. Cardiac arrest is possible with extreme toxicity and given its low therapeutic index it is wise to administer lidocaine by continuous infusion rather than by large intravenous bolus doses. If local anesthetic systemic toxicity is suspected a bolus dose of 1.5 ml/kg intralipid followed by an infusion of 0.25 ml/kg/min should be administered [35].
Gabapentin/Pregabalin
Gabapentinoids are derivatives of the inhibitory neurotransmitter gamma aminobutyric acid (GABA). Both gabapentin 300 mg PO and pregabalin 150 mg PO are effective analgesics that are also useful in neuropathic pain. Their use leads to lower pain scores, reduced opioid consumption and opioid related side effects [38,39]. They can be continued into the post-operative period. Excessive sedation, dizziness and visual disturbances can be a problem with high doses after prolonged periods.
Magnesium
Magnesium (Mg) has analgesic action by regulating calcium flux into the cell and acting as an NMDA receptor antagonist. It also suppresses neuropathic pain. It has been shown to reduce the need for post-operative opioids and improve post-operative pain scores [40]. The loading dose is 30-50 mg/kg and it may be followed up by an intravenous infusion of 10 mg/kg/hr.
Side effects are hypotension and bradycardia that respond readily to standard therapy but are more common when higher doses are administered. When using Mg intra-operatively it is important to reduce the amount of muscle relaxants and anesthetic drugs that are administered to prevent residual neuromuscular block and delayed emergence from anesthesia. Mg potentiates neuromuscular blocking drugs and CNS depressants and these effects need to be taken into consideration. The depth of neuromuscular block should be carefully monitored and adequacy of neuromuscular reversal should be confirmed prior to extubation.
Regional blocks and centroneuraxial blocks
Many procedures can be performed under regional or centroneuraxial anesthesia. The use of catheters for ongoing post-operative infusion can greatly limit or entirely eliminate the use of perioperative opioids. Many perineural regional techniques like paravertebral and pectoral nerve blocks have been used successfully [41]. Local wound infiltration with local anesthetic has become common in lower limb joint replacement surgery because of great efficacy and the absence of motor block [42]. Transversus abdominis plane (TAP) blocks and the erector spinae plane (ESP) block for abdominal and thoracic surgeries lead to better analgesia and reduced opioid use [43,44].
Mixed agonist/antagonist drugs acting on opioid receptors
Mixed agonist/antagonist drugs acting on opioid receptors like dezocine and buprenorphine are generally regarded as less addictive and respiratory depressant than the full agonists but they may also have a lower analgesic ceiling than the full agonists. These drugs do, however, offer significant advantages in the treatment of opioid addicted patients. Dezocine has been shown to alleviate morphine-induced dependence and improve patient experience in both preclinical and clinical studies [45,46].
In summary, the perioperative use of opioids has many detrimental effects on health care outcomes and is currently precipitating an epidemic of prescription opioid addiction and overdose deaths throughout the United States. Many alternative non-opioid analgesic drugs with a lower addiction potential and a better side effect profile are available but are currently underused. At present there is a campaign to educate addicts and their families and friends about naloxone to curb opioid overdose deaths. To get the current prescription opioid abuse epidemic under control however primary prevention by limiting the administration of opioids during hospitalizations will be necessary. Opioid free anesthesia can be achieved during most operations by employing alternative analgesics. The use of these non-opioid analgesic drugs as the alternatives to opioids should be continued after discharge from hospital. Opioid prescriptions and refills will need to be limited and ongoing opioid requests by the patient will need to be assessed carefully. Anesthesia care providers and surgeons need to understand that their perioperative pain management has lasting effects that have the potential to negatively affect their patients for the rest of their lives (Table 2).
Table 2:
Risk and benefits of opioid and non-opioid analgesics.
| Opioid Analgesia | Opioid free/ Opioid Sparing Analgesia | |
|---|---|---|
| Risks | Respiratory depression Need for post-op ventilation with ventilator associated pneumonia Addiction Nausea & Vomiting Gastrointestinal dysfunction, ileus Pruritus Urinary retention |
Bradycardia with dexmedetomidine Hepatic damage with acetaminophen Bleeding, renal impairment and bronchospasm with ketorolac Hallucinations, tachycardia and addiction with ketamine Tinnitus, seizures and cardiac arrest with lidocaine Sedation with gabapentin Hypotension with magnesium |
| Benefits | Analgesia Ready acceptance of poor patient outcomes by peers, due to the conventional nature of the analgesic therapy |
No respiratory depression No addiction except for ketamine Less need for post op ventilation No nausea and vomiting No gastrointestinal dysfunction and ileus No pruritus No urinary retention |
Acknowledgments
Funding Sources
This work was supported by the University of California Davis Health Department of Anesthesiology and Pain Medicine, and NIH grant UL1 TR001860 of the University of California Davis Health.
References
- 1.Mendelson J, Flower K, Pletcher MJ, Galloway GP. Addiction to prescription opioids: characteristics of the emerging opioid epidemic and treatment with buprenorphine. Exp Clin Pharmacol 2008;16(5):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peltz G, Südhof TC. The neurobiology of opioid addiction and the potential for prevention strategies. JAMA 2018;319(20):2071–2. [DOI] [PubMed] [Google Scholar]
- 3.Adans JM. Increasing naloxone awareness and use: the role of healthcare practitioners. JAMA 2018;319(20):2073–4. [DOI] [PubMed] [Google Scholar]
- 4.McEwen S, Prakken S. Reducing the oversupply of prescription opioids. NC Med J 2018;79(3):175–80. [DOI] [PubMed] [Google Scholar]
- 5.Sultana A, Torres D, Schumann R. Indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: Including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract Res Clin Anesthesiol 2017;31(4):547–60. [DOI] [PubMed] [Google Scholar]
- 6.Alfonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 2012;62(1):118–33. [DOI] [PubMed] [Google Scholar]
- 7.Kim R Anesthesia technique and cancer recurrence in oncologic surgery: unravelling the puzzle. Cancer Metastasis Rev 2017;36(1):159–77. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Qu X, Wang Y, Shen H, Liu Q, Du J. Effect of mu agonists on long-term survival and recurrence in nonsmall cell lung cancer patients. Medicine (Baltimore) 2015;94(33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohringer C, Liu H. Is it time for an expanded role of intraoperative dexmedetomidine in contemporary anesthesia practice? Translational Perioperative and Pain Medicine 2018;5(3) 55–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Davy A, Fessler J, Fischler M, LE Guen M. Dexmedetomidine and general anesthesia: a narrative literature review of its major indications for use in adults undergoing non-cardiac surgery. Minerva Anestesiol 2017. December;83(12):1294–1308. [DOI] [PubMed] [Google Scholar]
- 11.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesthesia and Analgesia 2004;98(1):153–58. [DOI] [PubMed] [Google Scholar]
- 12.Jebaraj B, Ramachandran R, Rewari V, Trikha A, Chandralekha, Kumar R, et al. Feasibility of dexmedetomidine as sole analgesic agent during robotic urological surgery: a pilot study. J Anaesthesiol Clin Pharmacol 2017;33(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunalan S, Venkatraman R, Sivarajan G,Sunder P. Comparative evaluation of bolus administration and fentanyl for stress attenuation during laryngoscopy and endotracheal intubation. J Clin Diagn Res 2015;9(9):06–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng K, Liu HY, Wu SR, Cheng H, Ji FH. Effects of combining dexmedetomidine and opioids for postoperative intravenous patient controlled analgesia: a systematic review and meta-analysis. Clin J Pain 2015;31(12):1097–1104. [DOI] [PubMed] [Google Scholar]
- 15.Peng K, Zhang J, Meng XW, Liu HY, Ji FH. Optimization of postoperative intravenous patient controlled analgesia with opioid-dexmedetomidine combinations: an updated meta-analysis with trial sequential analysis of randomized controlled trials. Pain Physician 2017;20(7):569–96. [PubMed] [Google Scholar]
- 16.Groeben H, Mitzner W,Brown RH. Effects of the alpha 2 adreno-receptor agonist dexmedetomidine on bronchoconstriction I dogs. Anesthesiology 2004;100(2):359–63. [DOI] [PubMed] [Google Scholar]
- 17.Xin J, Zhang Y, Zhou L, Liu F, Zhou X, Liu B, et al. Effect of dexmedetomidine infusion for intravenous patient controlled analgesia on the quality of recovery after laparotomy surgery. Oncotarget 2017;8(59):100371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clinical Infectious Diseases 2000;31(5):202–10. [DOI] [PubMed] [Google Scholar]
- 19.O’Neal J The utility of intravenous acetaminophen in the perioperative period. Front Public Health 2013;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeschke H Acetaminophen: dose dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis 2015;33(4):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunchorntavukul C, Reddy K. Acetaminphen-related hepatotoxicity. Clin Liver Dis 2013;17(4):587–607. [DOI] [PubMed] [Google Scholar]
- 22.De Oliveira G, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg 2012;114(2):424–33. [DOI] [PubMed] [Google Scholar]
- 23.Mikhaylov Y, Weinstein B, Schrank TP, Swartz JD, Ulm JP, Armstrong MB, et al. Ketorolac and hematoma incidence in postmastectomy implant-based breast reconstruction. Ann Plast Surg 2018;80(5):472–4. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Remondini T, Dhaliwal N, Frusescu A, Patel P, Cook A, et al. Incidence of bleeding in children undergoing circumcision with ketorolac administration. Can Urol Assoc J 2018;12(1):E6–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival in conservative breast cancer surgery. Br J Anaesth 2014;113(Suppl):i82–87. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Ajiboye RM, Orden MH, Sharma A, Drysch A, Pourtaheri S. The effect of ketorolac on thoracolumbar posterolateral fusion: a systematic review and meta-analysis. Clin Spine Surg 2018;31(2):65–72. [DOI] [PubMed] [Google Scholar]
- 27.Crumb MW, Bryant C, Atkinson TJ. Emerging trends in pain medication management: back to the future: a focus on ketamine. Am J Med 2018;18:30291–2. [DOI] [PubMed] [Google Scholar]
- 28.Tadler SC, Mickey BJ. Emerging evidence for antidepressant actions of anesthetic agents. Curr Opin Anaesthesiol 2018;May [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin 2016;37(7):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers AR, Gancsos MG, Finn ES, Morgan PT, Corlett PR. Ketamine-induced hallucinations. Psuchopathology 2015;48(6):376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aroke EN, Crawford SL, Dungan JR. Pharmacogenetics of ketamine-induced emergence phenomena: a pilot study. Nurs Res 2017;66(2):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estebe JP. Intravenous lidocaine. Best Pract Res Clin Anaesthesiol 2017;31(4):513–21. [DOI] [PubMed] [Google Scholar]
- 33.Weibel S, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K, Eberhart, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 2016;116(6):770–83. [DOI] [PubMed] [Google Scholar]
- 34.Chen K, Wei P, Zheng Q, Zhou J, Li J. Neuroprotective effects of intravenous lidocaine on early postoperative cognitive dysfunction in elderly patients following spine surgery. Med Sci Monit 2015;21:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickerson DM, Apfelbaum JL. Local anesthetic systemic toxicity. Aesthet Surg J 2014;34(7):1111–9. [DOI] [PubMed] [Google Scholar]
- 36.Sucena M, Cachapuz I, Lombardia E. Plasma concentration of lidocaine during bronchospcopy. Rev Port Pneumol 2004;10:287–96. [DOI] [PubMed] [Google Scholar]
- 37.Lauretti GR. Mechanisms of analgesia of intravenous lidocaine. Rev Bras Anestesiol 2008;58(3):280–6. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Liu R, Wang L. A meta-analysis of the preoperative use of gabapentinoids for the treatment of acute postoperative pain following spine surgery. Medicine Baltimore 2017;96(37)e8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochhar A, Chouhan K, Panjiar P, Vajifdar H. Gabapentinoids as part of a multimodal drug regime for pain relief following laparoscopic cholecystectomy: a randomized study. Anesth Essays Res 2017;11(3):676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albrecht E, Kirkham KR, Liu SS, Brull R. Perioperative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anesthesia 2013;68(1):79–90. [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Min S. Postoperative pain management in the postanesthesia care unit: an update. J Pain Res 2017;10:2687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawal N Current issues in postoperative pain management. Eur J Anaesthesiol 2016;33(3):160–71. [DOI] [PubMed] [Google Scholar]
- 43.Amlong CA, Schroeder KM, Andrei AC, Han S, Donnelly MJ. The analgesic efficacy of transversus abdominis plane blocks in ileostomy takedowns: a retrospective analysis. J Clin Anesth. 2012;24(5):373–377. [DOI] [PubMed] [Google Scholar]
- 44.De la Cuadra-Fontaine JC, Altermatt FR. Continuous erector spinae plane (ESP) block: optimizing the analgesia technique. J Cardiothorac Vasc Anesth 2018; March 27[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Wu FX, Babazada H, Gao H, Huang XP, Xi CH, Chen CH, Xi J, Yu WF, Liu R. Dezocine alleviates morphine-induced dependence in rats. Anesthesia and Analgesia 2019;128(6):1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou ZG, Liu R, Tan HL, Ji XY, Yi XL, Song JF. The application of dexmedetomidine combined with dezocine in thoracoscopic radical resection of lung cancer and its effect on awakening quality of patients. Eur Rev Med Pharmacol Sci. 2019. September;23(17):7694–702. [DOI] [PubMed] [Google Scholar]