Abstract
Context:
There is no consensus about the inflection point for 25 hydroxy vitamin D below which the intact PTH level increases.
Objective:
Determine the relationship/inflection point between 25 hydroxy vitamin D and parathormone levels.
Materials and Methods:
We performed a population-based analysis on a nonobese cohort (n = 405).
Results:
Prevalence of vitamin D deficiency was 58.76% (n = 228). Vitamin D insufficiency was found in 34.56% (n = 140). An inverse relationship between 25 hydroxy vitamin D (25(OH)D) and intact PTH exist, but strength of such relationship is weak (r = −0.16, P = 0.018). With respect to the 25(OH)D cut-off of 16 ng/mL by IOM (EAR linked), proportion of persons with high intact PTH was higher in the group with lower 25(OH)D compared with higher 25(OH)D group (P = 0.005) and it was similar for RDA linked cut-off of 20 ng/mL also (P = 0.017). LOWESS method revealed two inflection points at which PTH levels change. A less conspicuous inflection point was found at 32 ng/mL (95% CI, 27–36), which reasonably corroborates with the current cutoff of definition of vitamin D sufficiency, and the second, steeper inflection point was found at 16.5 ng/ml (95% CI, 14.9–18.8) which corroborates with the IOM supported EAR linked value of 25(OH)D level in general population and possible definition of vitamin D deficiency.
Conclusions:
There are possibly two inflection points at which PTH levels change in relation to 25(OH)D levels
Keywords: Inflection point, vitamin D deficiency, vitamin D insufficiency
INTRODUCTION
In recent times, there has been several publications related to vitamin D deficiency from various parts of the globe. The major bone of contention has been around the critical level of 25 hydroxy vitamin D (25(OH)D) for definition of vitamin D deficiency/sufficiency. Some researchers have suggested use of population-based normative values to define cutoffs, whereas others have relied on biological/physiological impact of deficiency including skeletal and extra-skeletal effects occurring when 25(OH)D levels are below a certain limit. Other investigators have tried to define insufficiency states on the basis of possible inflection point at which serum PTH starts to rise with falling levels of 25(OH)D (secondary hyperparathyroidism). Although controversial, there is some consensus that a level >30 ng/mL should be regarded as vitamin D sufficiency and a level <20 ng/mL should be regarded as vitamin D deficiency.[1] The level between 20 and 30 ng/mL is customarily taken as the state of insufficiency,[2] which is likely to corroborate with PTH elevation without any major clinical manifestation. But studies performed in different populations have suggested differences in the inflection point. Thus, the estimates of the inflection point from various study varies from 15 ng/mL (Thomas et al.[3]) to 44 ng/mL.[4] There are other studies which have failed to demonstrate such co-relationships. These differences possibly reflect differences in genetic, ethnic, environmental, and other factors in different populations. Again, Mason et al.[5] while explaining the global pandemic of vitamin D deficiency clarified that the level of 25(OH)D linked to EAR (estimated average requirement) of 400 IU/day has its mean value at 16 ng/mL and the RDA (recommended daily allowance) of 600 IU/day has its mean corresponding 25(OH)D value of 20 ng/ml. However, these cutoffs did not take account of the possible extra skeletal manifestations of vitamin D deficiency. It is on this premise that we ventured to look at the relationship between 25(OH)D levels and PTH in an Indian rural population and determine any inflection point, which might help clinicians in deciding possible clinically relevant cutoffs of vitamin D deficiency/insufficiency.
MATERIALS AND METHODS
A population-based observational study was undertaken to compare the metabolic health (anthropological and biochemical) between Tribal and non-tribal population in underdeveloped rural areas in the District of Birbhum (23.6687° N, 87.6828° E), West Bengal, India. The results presented herein are part of the data collected of the aforementioned study.
The population had adequate exposure to sunlight and was normocalcemic, normophosphatemic, euthyroid with normal renal and liver function test during winter.
Clearance from the Institutional Ethics committee of Institute of Post Graduate Medical Education and research was obtained. Approval from the ethics committee was obtained and the date of the approval is 26.08.2014.
About 405 individuals (adult males and adult non-pregnant females) including all ethnicity/caste were included in the study to make an appropriate representation of rural West Bengal.
Individuals were included in the study only if they agreed to give informed written consent and did not have any definite documented chronic infective or inflammatory illness. All patients had history of adequate exposure to sun and no history of pharmacological vitamin D supplementation. It may be noted that in India there is no recommendations of fortification of food items (including milk and bread).
We collected blood samples for biochemical tests in fasting state. Anthropometric data including height, weight, waist circumference, and blood pressure were measured using standard methods and body mass index (BMI) was calculated. History of addiction, including tobacco and alcohol, was documented.
Blood samples drawn were allowed to clot and serum was separated by centrifuging on site and samples were immediately sent for biochemical examination at an NABL (National Accreditation Board for Testing and Calibration Laboratories) accredited laboratory of West Bengal (appropriately transported on dry ice). Calcium, phosphorus, intact PTH, 25(OH)D, creatinine, albumin, and alkaline phosphatase levels were estimated. Additionally, SGPT, SGOT, free T4, TSH, anti-TPO antibody apart from other metabolic parameters, e.g., FBS, HbA1c, fasting serum insulin, creatinine, lipid profile, uric acid, free T4, TSH, anti-TPO antibody were also collected, and data for these tests was analyzed and presented elsewhere. Specifically, 25(OH)D and intact PTH was done by chemiluminescence method (Immulite 1000) and calcium, phosphate and alkaline phosphatase was done by spectrophotometry. The reference range for intact PTH was 14–72 pg/mL. The reference ranges for calcium, phosphate, and alkaline phosphate were 8.4–10.4 mg%, 2.0–4.5 mg%, and 20–140 IU/L, respectively.
All analyses were conducted using SAS 9.4 (SAS Institute). Continuous data are presented as mean ± standard deviation (SD) (if normally distributed) or median (interquartile range) (if skewed), and categorical variables are presented as proportions. Comparison of two groups was done by Mann–Whitney U-test and comparison of multiple groups was done by Kruskal-Wallis test. Comparison of proportion was done by Fisher's exact test. Correlation between parameters was done by Spearman's correlation test. LOWESS method (locally weighted scatter plot smoothing) was used to generate a smoothed curve to see the graphical relationship between iPTH and 25(OH)D.
RESULTS
About 405 individuals were included in the study of whom 205 persons (50.62%) were from tribal population and 200 persons (49.38%) were from nontribal population. Of those 405 persons, 232 were female (57.28%) and 173 were male (42.72%) (range was 18–68 years). The mean (±SD) age was 38.53 ± 11.74 years. The population was nonobese with mean (±SD) weight 53.89 (±11.89) kg, BMI 22.32 (±4.54) kg/m2, and waist-hip ratio 0.87 (±0.10).
Table 1 summarizes the results of parameters which were normally distributed. The mean serum calcium level was 9.5 ± 0.66 mg/dL. The mean serum phosphorus level was 3.3 ± 0.57 mg/dL.
Table 1.
Baseline serum calcium and phosphorus (normally distributed)
| Variables | Mean±SD |
|---|---|
| S. Calcium (mg/dL) | 9.52±0.66 |
| S. Phosphorus (mg/dL) | 3.32±0.57 |
Table 2 summarizes the results of parameters which were not normally distributed. The median value of 25(OH)D level was 18.8 ng/mL (IQR: 13.7–23.2) and that of intact PTH was 52.3 pg/mL (IQR: 35.6–76.2).
Table 2.
Baseline parameters of data which were not normally distributed
| Variables |
n=405 |
|
|---|---|---|
| Median | IQ Range | |
| ALT (U/L) | 41 | 32-54 |
| AST (U/L) | 29 | 23-37 |
| iPTH (pg/mL) | 52.3 | 35.6-76.2 |
| ALP (U/L) | 100 | 84-121 |
| Vitamin D (ng/mL) | 18.87 | 13.7-23.3 |
| Albumin (g/L) | 3.9 | 3.6-4.2 |
We also stratified the 25(OH)D levels in four groups at an increment interval of 10 ng/mL to examine the relationship between intact PTH and 25(OH)D levels. Using the current definition, the number and proportion of persons with vitamin D sufficiency (>30 ng/mL) was 9.1% (37/405), vitamin D insufficiency (≥20–30 ng/mL) was 34.56% (140/405), vitamin D deficiency (<20 ng/mL) was 56.29%, (228/405), and severe vitamin D deficiency (<10 ng/mL) was 9.62% (39/405). The corresponding median (with IQR) of intact PTH level was 42.3 pg/mL (IQR 33.6–75.0), 48 pg/mL (IQR 34.1–63.9), 54.7 pg/mL (IQR 36.9–81.5), and 61.5 pg/mL (IQR 37.5–87.9), respectively, for vitamin D level >30, ≥20– 30, 10–20, and <10 ng/mL.
We also analyzed the level of Intact PTH in our population with respect to the EAR (estimated average requirement) and RDA (recommended daily allowance) related cutoff of vitamin D as proposed by Institute of Medicine (IOM) [Tables 3 and 4]. EAR for vitamin D that actually reflects the median of the distribution of dietary requirements, in fact, suggest the most likely requirement for the population. This EAR linked mean 25(OH)D level is set at 16 ng/mL. On the other hand, RDA reflects the estimated requirement for people at the highest end of the distribution. This RDA linked mean 25(OH)D level is set at 20 ng/mL. These cutoffs are based on the established effect vitamin D on skeletal health only. Though the assumption of the EAR and RDA assume minimal to no sun exposure, our population was homogeneous with respect to the adequate sun exposure. It is known that obesity and overweight are associated with lower circulating concentrations of 25(OH)D. But the population in which the study was done had a normal mean BMI of 22.3 kg/m2 (±4.54). In our study subjects having 25(OH)D ≤16 ng/mL (equivalent to IOM EAR of 400 IU vitamin D daily intake), 46.4% (64/138) had elevated intact PTH as compared with 31.5% (84/287) for those having 25(OH)D >16 ng/mL (P = 0.005 as computed by Fisher's exact test). Again, in patients having 25(OH)D ≤20 ng/mL (equivalent to IOM RDA of 600–800 IU vitamin D daily intake), 41.7% (95/228) had elevated iPTH compared with 29.9% (53/177) for those having 25(OH)D > 20 ng/mL (P = 0.017 as computed by Fisher's exact test). Here also, the statistical significance was stronger when the EAR-based cutoff was used.
Table 3.
Vitamin D and iPTH distribution based on EAR of Vitamin D (IOM)
| Vitamin D (EAR based) | Intact PTH |
Total | P | |
|---|---|---|---|---|
| Normal | Elevated | |||
| ≤16 ng/ml | 74 (53.6%) | 64 (46.4%) | 138 | 0.005 |
| >16 ng/ml | 183 (68.5%) | 84 (31.5%) | 267 | |
| 257 63.5%) | 148 (36.5%) | 405 | ||
Table 4.
Vitamin D and iPTH distribution based on RDA of Vitamin D (IOM)
| Vitamin D (RDA based) | Intact PTH |
Total | P | |
|---|---|---|---|---|
| Normal | Elevated | |||
| ≤20 ng/mL | 133 (58.3%) | 95 (41.7%) | 228 | 0.017 |
| >20 ng/mL | 124 (70.1%) | 53 (29.9%) | 177 | |
| 257 (63.5%) | 148 (36.5%) | 405 | ||
To explore any biochemical changes with decreasing 25(OH)D level, we compared serum alkaline phosphatase (U/L) and serum phosphorus level in different 25(OH)D groups as <10, 10 to <20, 20 to <30, and >30 ng/mL. The levels of serum alkaline phosphatase among the groups were not different and were 109 ± 40.3, 109 ± 43.2, 103 ± 29, and 104 ± 29.2 U/L respectively (P = 0.641 by KruskalWallis test). More specifically, post hoc analysis comparing two groups as <10 and >30 ng/mL, the difference did not reach a statistical significance (P = 0.37 by Mann–Whitney U-test).
The levels of serum phosphate among the the groups were not different and were 3.3 ± 0.55, 3.3 ± 0.59, 3.3 ± 0.55, and 3.4 ± 0.54 mg/dL respectively (P = 0.76 by KruskalWallis test). Post hoc analysis comparing two groups as <10 and >30 ng/mL, this difference also did not reach a statistical significance (P = 0.681).
To evaluate the relationship between 25(OH)D and PTH at, we first performed Spearman correlation analysis, both unadjusted and after adjustment for current BMI.
On Spearman correlation analysis, serum 25(OH)D and PTH were inversely correlated (r = −0.16, P = 0.018).
These inverse correlations were unchanged upon adjustment for BMI (r = −0.17, P = 0.0009). The adjusted analyses were performed as Spearman partial correlation test.
Because the scatter plots revealed a complex relationship, we used the (locally weighted scatter plot smoothing) LOWESS method to fit the data and generate a smoothed curve. Without prior assumption of the shape of a relationship, a smoothed curve can be constructed by fitting successive regression functions in the local neighborhood. The radium of neighborhood is determined by a smoothing parameter, the fraction of the data that is used around each point. An optimal smoothing parameter is obtained by minimizing the corrected Akaike Information Criterion because it considers both the goodness of fit and model complexity [Figure 1].
Figure 1.
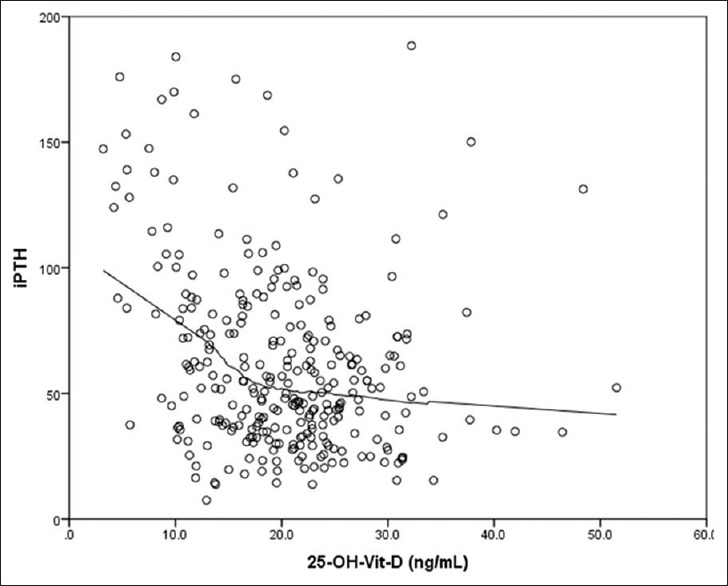
Relationship between intact PTH and vitamin D
The LOWESS fit visually suggested that there might be a threshold level of 25(OH)D around which the relationship between 25(OH)D and PTH would differ. Specifically, there appeared to be a threshold level of 25(OH)D 1) below which PTH started to rise steeply and 2) above which it would somewhat plateau.
To determine the best model to fit the relationship between 25(OH)D and PTH and to obtain an optimal 25(OH)D threshold based on this model, we used segmented regression analyses consisting of three segments that connect in a smooth fashion. With this methodology, the entire data are split into three parts around a given 25(OH)D level, such that a different regression model can be fit for each segment of the data.
We found a curvilinear relationship between 25(OH)D and PTH up to the threshold at which PTH is suppressed. The analysis revealed that the optimal curvilinear model was actually quadratic in shape, with a 25(OH)D threshold of 16.5 ng/mL (95% CI, 14.9–18.8) below which there is a steep rise in the iPTH.
We had also found a curvilinear relationship between 25(OH)D and PTH up to the threshold above which PTH is suppressed. The analysis revealed that the optimal curvilinear model was actually quadratic in shape, with a 25(OH)D threshold of 32 ng/mL (95% CI, 27–36) after which PTH plateaus.
The starting estimates for x were visually provided by the smoothed curves from LOWESS fit, and the other parameters were provided by fitting their own model (linear or curvilinear part) to the whole data.
The model can be written as
E[PTH25(OH)D] = d{a + b *25(OH)D + c*{25(OH)D}2}, 25(OH)D < x and 25(OH)D ≥ x and
Where x is the threshold, a3, b3, and c3 are unknown parameters and d = a + b*x + c*x2
Proc NLIN in SAS was run, which uses a nonlinear least-squares estimation method to estimate these unknown parameters in each model in an iterative process. The process requires a starting estimate for each parameter, until the estimate converges to a certain value. The final estimate minimizes mean squared errors (MSEs). The starting estimates for x were visually provided by the smoothed curves from LOWESS fit, and the other parameters were provided by fitting the curvilinear model to the whole data. We also used MSE to fit the said model and subsequently chose the best model with the smallest MSE. The estimated x in the best model is considered as the optimal 25(OH)D thresholds to achieve maximal suppression of PTH.
The reference used is from the documentation of SAS 9.2 proc NLIN segmented model, i.e., https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_nlin_sect033.htm.
DISCUSSION
The inverse relation between falling serum 25(OH)D concentration and rising parathyroid hormone level is well known and has in past been used by researchers to define vitamin D insufficiency rather than vitamin D deficiency, which leads to a spectrum of bone mineral problems. With progressively declining serum 25(OH)D levels, parathyroid glands are maximally stimulated by negative feedback to produce supra-physiological parathyroid hormone leading to secondary hyperparathyroidism.[6]
Vitamin D deficiency is defined by most experts as a 25(OH)D level of <20 ng/mL.[1,6] Studies by several researchers have suggested that there is an inverse correlation between 25(OH)D with parathyroid hormone until 25(OH)D reaches 30–40 ng/mL, at which point parathyroid hormone levels begin to plateau/nadir especially those who are on calcium sufficient diet.[6] This is the justification for setting the lower normal range for 25(OH)D at around 30 ng/mL.
Though several published studies suggests that levels of parathyroid hormone rise when levels of 25(OH)D fall <30 ng/mL, this assumption is controversial.[2] The relationship of PTH and 25(OH)D is not curvilinear, and data indicate that there is discordance between studies about PTH levels when 25(OH)D levels are between 20 and 30 ng/mL.[2] In fact, no absolute threshold level of serum 25(OH)D is evident at which the serum level of PTH levels starts rising.[2]
However, study by Thomas et al. showed stratifying the level of vitamin D concentrations in increments of 5 ng/mL showed that the slope of the relation between serum 25(OH)D and parathyroid hormone concentrations was not significantly different (P = 0.13) from zero for serum 25(OH)D level >15 ng/ml.[3] Thus, parathyroid hormone concentrations have been found to increase when serum 25(OH)D concentrations fall <15 ng/mL, suggesting that the inflection point, if any, is likely to be around 15 ng/mL. The PTH level in that study was found to be 36 ± 25 ng/mL for those with vitamin D level >15 ng/mL. It was 48 ± 43 and 66 ± 69 ng/mL for vitamin D level between 8 and 15 and <8 ng/mL, respectively (P < 0.001).
Hollick et al. evaluated 1,536 community-dwelling women to find out relationship between serum 25(OH)D and PTH in those receiving chronic therapy for osteoporosis (≥1 year) found 206 (16.5%) to have secondary hyperparathyroidism.[7] There was a significant inverse correlation between serum PTH and 25(OH)D; r = −0.283, P < 0.001. This correlation was also performed for whole study population and a significant and consistent correlation between serum PTH and 25(OH)D was found (r = −0.290, P < 0.001). The authors suggested that the strength of the relation is probably underestimated for the nonlinear association between the two variables. When the same was analyzed using a quadratic model, PTH level began to rise with 25(OH)D concentrations <29.8 ng/mL. At 25(OH)D concentrations >29.8 ng/mL, PTH level appeared to reach a plateau of 27.2 pg/mL. The prevalence of secondary hyperparathyroidism was significantly higher among subjects with lower serum 25(OH)D concentrations (P < 0.001 for trend test).
Steingrimsdottir et al. from a study of 944 healthy persons found that after adjusting for relevant factors, serum PTH was lowest in the group with a 25(OH)D level of >18 ng/mL but highest in the group with a serum 25(OH)D level of <10 ng/mL.[8]
Data from the study by Chapuy et al.[4,9] from a relatively large population of about 440 person from five cities across France showed a novel finding after splitting the subjects into two groups, those with 25(OH)D values above and below 32 ng/mL. For those with 25(OH)D levels >32 ng/mL, there was no relationship between the two variables, whereas <32 ng/mL, there is a highly significant, inverse relationship. Thus, the estimates of the inflection point from various study varies from 15[3] to 44 ng/mL.[4]
Recently, Kroll et al. characterized the temporal relationship between 25(OH)D and intact PTH during multiple seasons in US to find relationship between 25(OH)D and iPTH by analyzing the population weekly mean concentrations of 25(OH)D and iPTH from 3.8 million laboratory results.[10] In this mega-retrospective analysis, they found that 25(OH)D and iPTH levels vary in an inverse sinusoidal pattern throughout the year and 25(OH)D remains higher in summer and lower in winter months. Despite this huge database, they did not mention any inflection point of 25(OH)D above which iPTH level rises, which actually may define biochemical vitamin D deficiency.
In our study, 405 persons from rural West Bengal, India, we found that a sizeable proportion of persons are having vitamin D deficiency 58.76% (n = 228) with level <20 ng/mL. This is in keeping with findings of other studies world over. Again the number of persons with severe vitamin D deficiency with level <10 ng/mL was also significant at 9.62% (n = 39). However, none of them had any clinical/skeletal manifestations of deficiency. Using the data from whole population studied, we found that an inverse relationship between vitamin D and intact PTH does exist but strength of such inverse relationship is not strong enough, though it remains statistically significant. Using cutoffs proposed by IOM, we could also establish that the number of persons having high intact PTH was also statistically significantly higher in the cohort having lower vitamin D level compared with those with higher vitamin D level.
These inverse correlations upon adjustment for BMI was (r = −0.17, P = 0.0009) with the adjusted analyses performed as Spearman partial correlation test. This is somehow similar to the finding by Hollick et al.[7] (r = −0.283, P < 0.001). As suggested by the author in their study population, the strength of the relation is probably underestimated for the nonlinear association between the two variables holds true in our study population also.
Most importantly, by nonparametric LOWESS method, we found there are two inflection points at which changes in PTH level occurs. The first and less conspicuous inflection point was found to be around 32 ng/mL (95% CI, 27–36) at which point the PTH level starts to gradually rise and this corroborates with the current cutoff of definition of vitamin D sufficiency and the second and steeper inflection point was found to be around 16.5 ng/mL (95% CI, 14.9–18.8), which corroborates with the IOM supported EAR linked value of vitamin D level in general population and possibly reflects levels below which vitamin D deficiency occurs.
The less steep inflection point of vitamin D below which intact PTH level rose around 32 ng/mL is also in consonance with the study done by Chapuy et al.[9] Again the prominent inflection point of vitamin D below which intact PTH level rose sharply is in strong agreement with the study of Thomas et al.,[3] which proposes that inflection point if any is likely to be around 15 ng/mL. Our data suggest that there is a weak continuous inverse relation between intact PTH and vitamin D. However, there are definite inflection points where this relationship is strong and significant which corresponds to currently defined cut points of vitamin D insufficiency and vitamin D deficiency, respectively.
Reappraisal of the findings of previous investigators in the light of our study results would suggest that there is no controversy and that the various researchers had looked at this relevant relationship in parts and not in totality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the grant provided by West Bengal Department of Science and Technology, India, for conducting the study.
REFERENCES
- 1.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. ; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ. Vitamin D deficiency. N Engl J Med. 2011;364:248–54. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 3.Thomas KK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–9. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency - Is there really a pandemic? N Engl J Med. 2016;375:1817–20. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 8.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 9.Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ. EPIDOS Study Group. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. J Clin Endocrinol Metab. 1996;81:1129–33. doi: 10.1210/jcem.81.3.8772587. [DOI] [PubMed] [Google Scholar]
- 10.Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One. 2015;10:e0118108. doi: 10.1371/journal.pone.0118108. [DOI] [PMC free article] [PubMed] [Google Scholar]


