Abstract
Introduction:
Anaplastic thyroid cancer (ATC) is rare but fatal thyroid cancer responsible for majority of thyroid cancer related mortality. ATC may originate de novo or from preexisting differentiated thyroid cancer. Complex interaction between different gene mutation has been suggested to be the main causative factor for origin of ATC in both pathways. Mostly affected pathways are MAP kinase and PI3CA kinase. Hence, we decided to study the frequent alterations in both the pathways in ATC patients.
Methodology:
Clinico-pathological data of 34 ATC patients were collected retrospectively and Formalin Fixed Paraffin Embedded (FFPE) blocks were taken out for genetic analysis. DNA and RANA were isolated from FFPE tissues. BRAF V600E mutations were screened by RFLP PCR method and confirmed by sequencing. RAS, PI3CA and p53 mutations were checked by sequencing. RET/PTC translocations were screened by Real Time PCR.
Results:
A total of 34 patients were studied: Mean age 58.6+ 11.6 years with F:M- 1.8:1, 60% had history of goiter. Most common presenting symptom was rapidly growing thyroid mass followed by dyspnea, dysphasia and hoarseness of voice. Extent of disease was local, locoregional and metastatic in 32%, 35% and 33% respectively. 57.6% were euthyroid, 20.5 % were hyperthyroid while functional status were not available in 11.7%. FNAC was suggestive of ATC only in 52.9% cases. 15 (44%) were operated. BRAF V600E mutations were observed in 10/34 (29.4%). Interestingly, all three ATC patients with DTC components had previous history of goiter with rapid increase in size and BRAF V600E mutation, while BRAF was positive only in 7/31 (22.5%) of patients with no DTC component. Mean survival of 3.5 months in BRAF positive cases in comparison to 5.5 months in BRAF negative ATC. RAS mutations were found to be positive in 5.8%, and none had RET-PTC/PI3CA mutations. P53 mutation was positive in 7 patients. 3 patients presented with history of rapid increase in size of previous goiter while rest 4 patients presented with rapidly increasing thyroid swelling of 1 to 3 months. At presentation 2 patients has disease localized to thyroid, 4 has loco-regional disease and one patient presented with metastasis. 5 out of these 7 patients were operated (Total thyroidectomy:3, thyroidectomy with neck dissection:2). Mean survival was 4 months (1-6 months).
Conclusion:
BRAF V600E was the commonest mutation followed by p53 of the 5 genes tested and BRAF was more common in patients with previous history of longstanding goiter or differentiated thyroid cancer. This provides an indirect evidence of neoplastic transformation of PTC to ATC.
Keywords: Anaplastic, genetic alteration, thyroid
INTRODUCTION
Thyroid cancer is the most prevalent endocrine malignancy accounting for 1% of cancers worldwide. More than 95% of thyroid cancer are well differentiated tumors that respond to surgery followed by radioactive iodine (RAI) therapy and thyroid hormone suppression. Anaplastic thyroid cancer constitutes <2% of thyroid cancers but carries almost 100% mortality.[1,2]
ATCs originate from follicular cells and may either derive de novo or from pre-existing well differentiated thyroid cancers (WDTCs) such as papillary thyroid carcinomas (PTCs), follicular thyroid carcinomas (FTCs) or even from poorly-differentiated thyroid carcinomas (PDTCs) by accumulation of various genetic alterations.[3,4,5,6,7,8] Numerous studies have explored ATC mutational landscape and have increased our understanding of the molecular pathogenesis of thyroid cancer. Among the genetic alterations involved in thyroid tumourigenesis, BRAF mutations, RAS mutations and RET rearrangements are important in differentiated thyroid carcinomas.
The beneficial effect of BRAF inhibition in ATC with activating BRAF mutations has been recently reported.[9,10] Other genetic alterations include gain of function mutations in the PIK3CA gene, mutations in the CTNNB1, loss of function alterations of tumor suppression genes such as PTEN, and mutation or inactivation of P53 gene. Hence the knowledge of the tumor mutation status is needed for optimizing and tailoring the treatment with kinase inhibitors as well as in understanding the heterogeneity and failure of existing treatment approaches.[9,11] We sought to study the genetic alterations in ATC with the aim of finding out whether there is any difference in mutations in those ATC which are thought to develop from a pre-existing DTC as compared to those develop de-novo and to look for any clinco-pathological correlation with a particular mutation/inactivation.
METHODS
Sample Collection
Study was conducted at department of endocrine surgery, SGPGI. Data of all patients diagnosed with anaplastic thyroid carcinoma from 1990 to 2018 were collected and 34 patients with a complete clinicopathological record and adequate tissue samples for genetic analysis were selected for the study. The cases with incomplete records and with poor tissue samples were excluded from the study. Formalin Fixed Paraffin Embedded (FFPE) blocks were taken out for genetic analysis. The study was approved by Institutional Ethics committee. Institute ethics committee: JAN 2017. Ethics number 2017-3-IMP-95.
Methods of gene analysis
DNA isolation
The tumor areas were confirmed and marked from the slides stained by Heamatoxylin and eosin stain (H and E stain). Eight sections of 10 micron from each FFPE tissue blocks were subjected to DNA extraction using QIAamp FFPE tissue kit (Qiagen, Germany). The quality and quantity of the DNA was measured by using the Nano Drop 2000c (Thermo Fisher Scientific, US).
RNA isolation
Total RNA was isolated from 34 FFPE ATC tissues by using Recover All Total Nucleic Acid isolation kit for FFPE (Thermo Fisher Scientific, US). Quantity and quality were measured by Nano Drop 2000c (Thermo Fisher Scientific, US and stored in -80°C). Yield and quality of RNA was not affected by the procedure and storage. Further cDNA was synthesized by using Revert Aid First strand cDNA synthesis kit (Thermo Fisher Scientific, US).
Point mutation analysis
The presence of BRAF V600E and K601E, NRAS codon 61, HRAS codon 61, and KRAS codons 12 and 13 point mutations was analyzed using two different techniques.
PCR-RFLP and sequencing for BRAF
The most common BRAF V600E mutation reported in thyroid carcinomas is confined to exon 15. We therefore amplified BRAF exon 15 by polymerase chain reaction (PCR) using the following primers: forward '5GCTTGCTCTGATAGGAAAATGAG3'; reverse '5GATACTCAGCAGCATCTCAGG3'. The denatured PCR products were electrophoresed [Figure 1a] and digestion of the 237-base pair (bp) PCR fragment with restriction endonuclease TspRI showed 3 major bands of 117 bp, 87 bp, and 33 bp for the wild type allele [Figure 1b]. The T1799A mutation abolished the restriction sites, which resulted in a prominent band of 237 bp from the mutant allele and residual bands from the normal allele.[12] Randomly selected three BRAF positive samples were sequenced [Figure 1c] using Applied Biosystems 3500 genetic analyzer and reconfirmed the presence of BRAF mutations.
Figure 1.
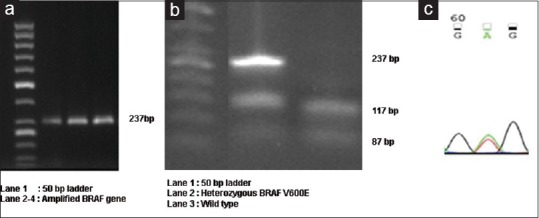
(a) BRAF gene amplicon of 237 bp (b) Representative figure showing wild type and heterozygous BRAF mutation (c) Presence of BRAF V600E confirmed by sequencing
RET/PTC rearrangements: RET/PTC1, RET/PTC3, rearrangements was detected from RNA by RTPCR with primers designed to flank the respective fusion point. Quality of RNA in each sample was assessed by amplification of the -GAPDH gene. Primers and Taqman probe for RET/PTC1 were used as described previously in detail.[12]
RAS and PI3CA mutation detection
Point mutations in codons 12/13 of the H-RAS, K-RAS and codon 61 of N-RAS genes were analyzed by Sanger sequencing method [Figure 2]. DNA isolated from the FFPE tissue was amplified for KRAS, HRAS and NRAS using the following PCR primers: codon 12/13 KRAS-A (5′-GGCCTGCTGAAAATGACTGA-3′) and D (5′-TAGCTGTATCGTCAAGGCAC-3′), codon 12/13 of HRAS 5′-TGAGGAGCGATGACGGAA-3′ and 5′-GCGCTAGGCTCACCTCTAT-3′, codon 61 NRAS: 5′-CCTGTTTGTTGGACATACTG-3 and 5′-CCTGTAGAGGTTAATATC CG-3′.
Figure 2.
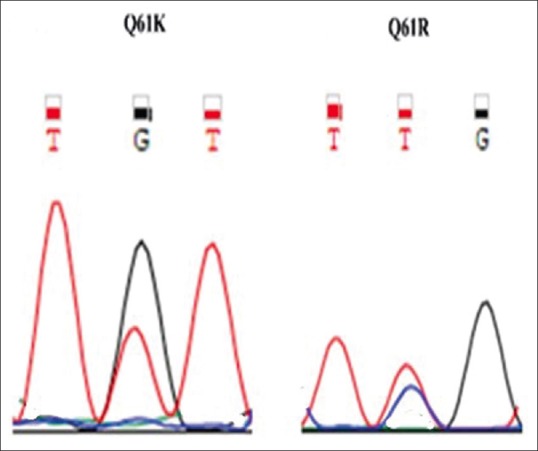
RAS mutations Q61K and Q61R mutation
Using specific primers (exon 9 forward, 5′-ATCATCTGTGAATCCAGA-3′; exon 9 reverse, 5′-TTAGCACTTACCTGTGAC-3′; exon 20 forward, 5′-TGACATTTGAGCAAAGACC-3′; and exon 20 reverse, 5′-GTGTGGAATCCAGAGTGA-3′), the portions of exon 9 and 20 of PI3K gene were amplified. Further, these portions were sequenced using ABI capillary sequencer.
P53 IHC
The sections were cut at 4μ thickness on polylysine coated slides, deparaffinised and rehydrated in graded alcohols. Antigen retrieval was done in EDTA buffer at pH 8.9 followed by endogenous peroxidase blocking with 3% H2O2. Sections with primary monoclonal antibody for P53 (dilution - 1:200, source P 53 polyclonal antibody from Cell Signaling Technology, United States) were incubated for 1 hour at room temperature. Secondary antibody (UltraVision Quanto Detection System, Thermo Fisher) was incubated for 30 minutes at room temperature. Diaminobenzidine was used as chromogen and followed by counterstaining with Mayer's hematoxylin and mounting in DPX. Nuclear expression was considered as positive staining.
RESULTS
A total of 34 patients were studied. Mean age was 58.6 ± 11.6 years. Female:male ratio was 1.8:1 [Table 1]. 60% had history of previous goiter.
Table 1.
Age and sex distribution of patients (n=34)
| Parameters | |
|---|---|
| Age (years) | 58.6 (36-80) |
| Sex (F:M) | 1.8:1 |
| H/o goitre-n (%) | 15 (44.1) |
| Thyroid function | |
| Euthyroid- n (%) | 23 (67.6) |
| Hyperthyroid- n (%) | 7 (20.5) |
| Not available- n (%) | 4 (11.7) |
Most common presenting symptom was rapidly growing thyroid mass followed by dyspnea, dysphasia and hoarseness of voice. The extent of disease was local, locoregional and metastatic in 32, 35 and 33% respectively [Tables 2, 3 and 4]. 57.6% were euthyroid, 20.5% were hyperthyroid while functional status was not available in 4 patients. FNAC was suggestive of ATC only in 52.9% cases [Table 5]. 15 (44%) patients were operated [Table 6].
Table 2.
Presenting complaints (n=34)
| Symptoms | Frequency | Percent |
|---|---|---|
| Neck mass | 29 | 85.3 |
| Dyspnoea | 1 | 2.9 |
| Hoarseness of voice | 2 | 5.9 |
| Pain | 1 | 2.9 |
| Recurrent goiter | 1 | 2.9 |
Table 3.
Overall symptoms (n=34)
| Symptoms | No. | Percentage |
|---|---|---|
| Dyspnea | 17 | 50 |
| Dysphagia | 12 | 35.2 |
| Hoarseness of voice | 12 | 35.2 |
| Cervical pain | 5 | 14.7 |
| Weight loss | 5 | 14.7 |
| VC palsy | 6 | 17.6 |
Table 4.
Extent of disease (n=34)
| Parameters | n (%) |
|---|---|
| Tumour size | 8.0 (4.0-15) |
| Cervical lymphadenopathy | 18/34 (52.9%) |
| Extent of disease (%) | |
| Local | 32.4 |
| Locoregional | 35.3 |
| Metastatic | 265 |
| Sites of metastasis (%) | |
| Lung | 66.7 |
| Bone | 22.2 |
| Liver | 11.1 |
Table 5.
Fine needle aspiration cytology (n=34)
| FNAC finding | n (%) |
|---|---|
| Anaplastic thyroid cancer | 18 (52.9) |
| Follicular neoplasm | 1 (2.9) |
| Inadequate | 1 (2.9) |
| Medullary thyroid cancer | 4 (11.8) |
| N/A | 4 (11.8) |
| Poorly differentiated cancer | 1 (2.9) |
| Positive for malignancy | 3 (8.8) |
| Papillary thytoid cancer | 1 (2.9) |
| Squamous cell carcinoma | 1 (2.9) |
Table 6.
Surgical procedure (n=14)
| Surgical Procedure | n (%) |
|---|---|
| Debulking | 2 (5.9) |
| Near Total Thyroidectomy | 1 (2.9) |
| Total Thyroidectomy | 7 (20.6) |
| Total Thyroidectomy + CCLND | 2 (5.8) |
| Total Thyroidectomy + MRND | 3 (8.8) |
BRAF V600E mutations were observed in 10/34 (29.4%). Interestingly, all three ATC patients with DTC components had previous history of goiter with rapid increase in size and BRAF V600E mutation, while BRAF was positive only in 7/31 (22.5%) of patients with no DTC component. Mean survival of 3.5 months in BRAF positive cases in comparison to 5.5 months in BRAF negative ATC. RAS mutations were found to be positive in 5.8%, and none had RET-PTC/PI3CA mutations. P53 mutation was positive in 7 patients. 3 patients presented with history of rapid increase in size of previous goiter while rest 4 patients presented with rapidly increasing thyroid swelling of 1 to 3 months [Table 7]. At presentation 2 patients has disease localized to thyroid, 4 has loco-regional disease and one patient presented with metastasis. 5 out of these 7 patients were operated (Total thyroidectomy: 3, thyroidectomy with neck dissection: 2). Mean survival was 4 months (1-6 months). There were no concomitant mutations present however, immunohistochemistry of P53 was positive in 3 BRAF mutated cases (3/7) and one RAS positive patient (1/7) [Figure 3].
Table 7.
Genetic profile (n=34)
| Genes | No of positive cases | Percentage |
|---|---|---|
| BRAF | 10 | 29.4% |
| RAS | 4 | 11.76% |
| PI3CA | 0 | 0 |
| RET/PTC | 0 | 0 |
| p53 | 7 | 20.5 |
Figure 3.
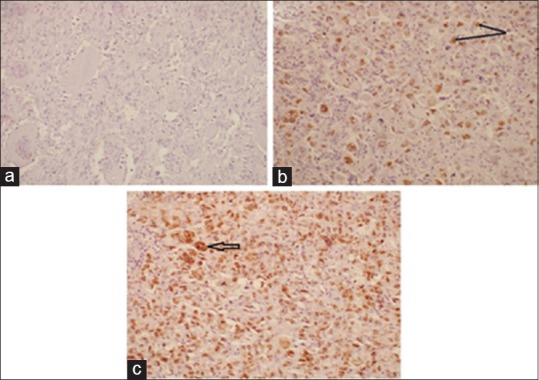
(a) Negative p53 immunostaining in poorly differentiated carcinoma of thyroid (Magnification - 20X, IHC). (b) Strong nuclear expression (arrow) of p53 in epithelial cells of anaplastic carcinoma. The interspersed lymphomonuclear infiltrate is negative for p53. (Magnification - 20X, IHC) (c) Stong nuclear expression of p53 in spindled and epithelial cells along with tumor giant cells (arrow) in anaplastic carcinoma (Magnification - 20X, IHC)
DISCUSSION
Anaplastic thyroid cancer has poor survival despite aggressive conventional therapy. Ina previous publication from the authors[13] mean survival in a series of 100 patients was only 3 months. It is still therefore a “viper in the thyroid cancer pit.”[14] Thus there is a need to look beyond the conventional forms of treatment such as molecular therapy. Genetic alterations are highly prevalent in anaplastic thyroid cancer in comparison to differentiated thyroid cancer. Zhi Liu[15] et al. has shown that overall, 46 of 48 ATC (95.8%) harboured at least one genetic alteration either in MAP kinase pathway or PIK3/Akt pathway. In our study overall 21 of 34 (67%) patients tested positive for at least one mutation. BRAF V600E mutations were observed in 10/34 (29.4%). It was the most common mutation in our study.
BRAF mutation has been documented to be the most common mutation in papillary thyroid cancer.[16,17] The high prevalence of BRAFV600E mutation in ATC supports the hypothesis that many ATCs actually represent a progressive malignant degeneration of BRAF-mutated, well-differentiated thyroid carcinomas.[18] This gene is a pivotal component of the MAPK pathway and reduces the activity of p21kip1 in thyroid tumors, stimulating the cell cycle machinery. Although clinical studies of BRAF inhibitors in advanced non RAI-responsive differentiated thyroid carcinomas have shown encouraging results with frequent early responses, in a relevant fraction of patients this effect was of limited duration, with frequent relapse or no response. Interestingly, all three ATC patients with DTC components had previous history of goiter with rapid increase in size and BRAF V600E mutation, which again support the hypothesis of origin of ATC from progressive degeneration of differentiated thyroid cancer.
The high prevalence of BRAFV600E mutation in ATC supports the hypothesis that many ATCs actually represent a progressive malignant degeneration of BRAF-mutated, well-differentiated thyroid carcinomas.[18] In our study, BRAF mutations were found in 29.4% of cases. All mutations were p.V600E and were mutually exclusive to (K-H-N)-RAS mutation. Despite a well-known correlation between the presence of a BRAF mutation and tumor aggressiveness in PTCs,[19,20] BRAF mutations are less frequent in ATCs (as in our cohort) than in PTCs (prevalence of 45-50%).[21,22]
Since BRAF mutations do not appear to occur during tumor dedifferentiation but are relatively early and frequent events in PTC carcinogenesis,[23] all ATCs may not be derived from PTCs. Therefore BRAF mutations seem to most prevalent mutation in PDTC.[24] BRAF-mutant cells have been reported to be MEK dependent and selectively more sensitive to MEK inhibition than either RAS-mutant or both BRAF and RAS wild-type cells. As MEK is a key downstream signaling mediator of the MAP-kinase pathway, the use of co-inhibitors has been proposed in anticancer therapies.[25]
An obstacle to the efficacy of treatments based on the inhibition of BRAF V600E is the presence of activating mutations of RAS. This proto-oncogene is located upstream RAF in the MAPK cascade. Activating mutations of this protein reactivate the MAPK pathway, making BRAFV600E inhibition inefficient.[18] The high prevalence of RAS activating mutations in ATC (60%) makes the inhibition of the MAPK pathway by kinase inhibitors unsuccessful. Moreover, papillary thyroid carcinoma and ATC exhibit concomitant BRAFV600E and RAS mutations, although a rare occurrence.[19,20] In light of these considerations, the pharmacological inhibition of the MAPK pathway looks less promising than the inhibition of the PI3K/Akt/mTOR pathway.
P53 was second most common mutation in our study found in 7 of 34 (20.5%) patients. This mutation has been suggested to be associated with de novo origin of anaplastic thyroid cancer. In contrast to our study in which BRAF was the most common mutation, few other studies suggest that p53 is the most common mutation in anaplastic thyroid cancer.[21] The prevalence rate in the literature ranges from 48%[24] to up to 70-80%.[26,27] It has been suggested that wild type p53 gene helps in expression of sodium iodide symporter I thyroid follicular cells allowing radioiodine therapy. Inactivating mutation in p53 leads to loss of sodium iodide symporter and radio-iodine avidity, thereby resulting in poor prognosis[28] In our study P53 mutation was positive in 7 patients. 3 of 7 patients presented with history of rapid increase in size of previous goiter while rest 4 patients presented with rapidly increasing thyroid swelling of 1 to 3 months. At presentation, 2 patients has disease localized to thyroid, 4 had loco-regional disease and one patient presented with metastases. Mean survival of these 7 patients was 4 months.
Incidence of RAS mutation is relatively low in anaplastic thyroid cancer. These mutations are frequent in a variety of thyroid tumors from benign follicular adenomas (20-25%) to aggressive ATCs (20-30%) indicating a role in early tumorigenesis.[29] It was found to be positive in 5.8% of our cases. Libero Santarpia et al. in 2008[30] studied 36 samples of ATCs and found RAS mutation in 6% of cases which is close to that of our study. On the other hand, RAS mutations are the commonest molecular alteration in poorly differentiated carcinoma.[31] RAS mutation has not been shown to be associated with overall prognosis in ATC.
The prevalence of PIK3/AKT pathway alteration in our series was much lower than that reported in the literature for ATCs (15-25%) and close to that found for follicular carcinomas.[18] It is suggested that PIK3CA alterations often cooperate with other Oncogenic events.[32]
Although RET/PTC rearrangement is a common mutation in papillary thyroid cancer, it is not common in anaplastic thyroid cancer. We analyzed all 3 types of RET/PTC rearrangement in 34 samples. None of the samples tested positive for RET/PTC rearrangement. A single RET mutation has been identified in a series of 22 ATCs,[33] associated with TP53, CTNNB1 and RASAL1 mutations Roderick M. et al.[34] analyzed RET/PTC rearrangement in 8 ATC tumor samples and did not find any sample positive.
CONCLUSION
BRAF mutation is responsible for majority of ATC originating from or in combination of differentiated thyroid cancer. This provide and indirect evidence of origin of ATC from preexisting DTC. BRAF positive ATC has poor survival than those of BRAF negative cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, et al. Immediate causes of death in thyroid carcinoma: Clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–9. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 3.Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321–30. doi: 10.1002/1097-0142(19900715)66:2<321::aid-cncr2820660221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid Off J Am Thyroid Assoc. 2012;22:1104–39. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 5.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: A 50-year experience at a single institution. Surgery. 2001;130:1028–34. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 6.Lam KY, Lo CY, Chan KW, Wan KY. Insular and anaplastic carcinoma of the thyroid: A 45- year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg. 2000;231:329–38. doi: 10.1097/00000658-200003000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornett WR, Sharma AK, Day TA, Richardson MS, Hoda RS, van Heerden JA, et al. Anaplastic thyroid carcinoma: An overview. Curr Oncol Rep. 2007;9:152–8. doi: 10.1007/s11912-007-0014-3. [DOI] [PubMed] [Google Scholar]
- 8.DeLellis RA. Pathology and Genetics of Tumours of Endocrine Organs (World Health Organization Classification of Tumours) Lyon: IARC Press; 2004. [Google Scholar]
- 9.Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368:684–5. doi: 10.1056/NEJMc1215697. [DOI] [PubMed] [Google Scholar]
- 10.Marten KA, Gudena VK. Use of vemurafenib in anaplastic thyroid carcinoma: A case report. Cancer Biol Ther. 2015;16:1430–3. doi: 10.1080/15384047.2015.1071734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra A, Di Crescenzo V, Garzi A, Cinelli M, Carlomagno C, Tonacchera M, et al. Genetic mutations in the treatment of anaplastic thyroid cancer. BMC Surg. 2013;13:44. doi: 10.1186/1471-2482-13-S2-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19:1351–61. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan R, Agarwal A, Lal P, Kumari N, Jain M, Chand G, et al. Clinico-pathological profile of anaplastic thyroid carcinoma in an endemic goiter area. Indian J Endocrinol Metab. 2018;22:793–7. doi: 10.4103/ijem.IJEM_264_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seshadri KG. Anaplastic cancer of the thyroid: The viper in the pit. Indian J Endocrinol Metab. 2019;23:1–2. doi: 10.4103/ijem.IJEM_91_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–16. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 16.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–7. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 17.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 18.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 20.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 22.George N, Agarwal A, Kumari N, Agarwal S, Krisnani N, Gupta SK. Mutational profile of papillary thyroid carcinoma in an endemic goiter region of North India. Indian J Endocrinol Metab. 2018;22:505–10. doi: 10.4103/ijem.IJEM_441_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH. BRAF mutations in anaplastic thyroid carcinoma: Implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–63. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 24.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: A clinicopathologic study of 58 patients. Cancer. 2006;106:1286–95. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 25.Catalanotti F, Solit DB, Pulitzer MP, Berger MF, Scott SN, Iyriboz T, et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013;19:2257–64. doi: 10.1158/1078-0432.CCR-12-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–84. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti MA. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest. 1993;91:1753–60. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Li D, Chen Z, Yang J, Ma Y, Cai H, et al. Wild-Type P53 induces sodium/iodide symporter expression allowing radioiodide therapy in anaplastic thyroid cancer. Cell Physiol Biochem. 2017;43:905–14. doi: 10.1159/000481640. [DOI] [PubMed] [Google Scholar]
- 29.Wiseman SM, Griffith OL, Gown A, Walker B, Jones SJM. Immunophenotyping of thyroid tumors identifies molecular markers altered during transformation of differentiated into anaplastic carcinoma. Am J Surg. 2011;201:580–6. doi: 10.1016/j.amjsurg.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93:278–84. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 31.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: The turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–64. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 32.Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–8. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 33.Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24:2318–29. doi: 10.1093/hmg/ddu749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–8. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]


