Abstract
Giant cell tumors of bone are relatively rare in the axial skeleton, accounting for approximately 6.7% of all cases. Due to their anatomical complexity, difficult access and proximity to vital neurovascular structures, management of these tumors poses a huge challenge on the treating surgeon. Several data series reported on axial GCTB involve short series of limited cases with varied methods used in their local control due to which, proper guidelines are unavailable for the management of such difficult cases. Though the present data support the use of denosumab for effective management of these lesions but there is varied consensus on dosage and duration of treatment. This review article summarizes the basic features and treatment modalities related to axial GCTB stressing on multidisciplinary approach to achieve optimum outcomes.
Keywords: Giant cell tumor, Axial, Management, Curettage, Angioembolization, Denosumab
1. Introduction
GCTB (Giant cell tumor of Bone) is benign locally aggressive tumor arising at ends of long bone commonly around the knee in epiphyseal location. These usually occurs in mature skeleton with peak age of occurrence in 3rd decade. Function preserving intralesional curettage with adequate disease clearance and appropriate reconstruction has been the main stay of treatment wherever possible. The most appropriate and widely accepted mode of local therapy in extremity GCTB is surgery, which can be in the form of intralesional curettage when adequate disease clearance is possible and function of the limb is retained. Cases with multiple recurrence or with multiple planar soft tissue component or with joint involvement requires resection for adequate disease control. Various modalities have been employed for reconstruction of these bony defects to minimize surgical morbidity and retain function.
Axial location of occurrence poses a great challenge in diagnosis and management of GCTB. Spine and sacrum have been reported to be approximately involved in about 6–7% of cases.1 Sacral GCTB account for 2–8% cases of all GCTB, which is more common than GCTB of pelvis.2,3 Among the spinal skeleton affected by GCTB, the mobile spine comprises 54.8% of cases and sacrum is affected in 45.2%.4 Slight female predominance is noted, just as in extremity GCTB.1,5,6 Compared to extremity GCTB, spine GCTB has been reported to have high recurrence rates, with published reports up to 41.7%.7 Pulmonary metastasis in axial GCTB has been reported to be ranging from 0 to 13.7%.8,9
Due to complex anatomy and proximity to vital neurovascular structure, the adequate disease clearance either by intra-lesional extended curettage or resection is always a challenge and hence associated with high rate of local recurrence. The surgical procedures are also associated with high morbidity like neurovascular deficit and injury to visceral organs hence there is more stress on devising less morbid treatment strategies which can balance good local control with minimal treatment related morbidities. The present-day management of GCTB od axial spine is multi-disciplinary involving an integrated approach from musculoskeletal oncologist, intervention radiologist, medical and radiation oncologists.
1.1. Clinical presentation
Pain is the most common symptom, which is secondary to gross osseous destruction, which is localized and mechanical in type. It tends to increase with disease growth leading to extensive bony destruction and instability of spine. If left untreated, patients can present with radicular symptoms, loss of power in extremities, cauda equina syndrome, bowel and bladder disturbance, perineal hypoesthesia as well as erectile dysfunction.8,10 Symptoms may vary according to the location of disease. Neurological symptoms appear much earlier with posterior column disease and vertebral disease. Sacral lesions tend to stay dormant and are detected much later.
1.2. Evaluation
A systematic approach involving hematological, radiological and histopathological investigation is used to confirm the diagnosis. In addition to routine blood investigation, alkaline phosphatase is done to rule out any systemic disorder like Brown tumor. Local staging of the disease is done by radiographs in two perpendicular planes. Magnetic resonance imaging (MRI) is the investigation of choice to assess the local extent and relationship of diseases to adjacent vital structures. Sometimes a Computed tomography (CT) scan is very helpful in assessing the extent of bone destruction. This may help to great in deciding appropriate surgical procedure (intralesional curettage or en-block resection). Most of these lesions are visualized as an expansile lytic lesion lacking sclerotic rim.9 On Magnetic Resonance Imaging (MRI),these lesions usually have low to intermediate signal as seen on T1 and low to similar signal changes to spinal cord in T2 images in 63–96% cases.11 Cystic changes and areas of hemorrhage can also be seen in the form of high signal intensity on both T1 and T2.12 Fluid-fluid levels are also appreciable in large GCTB with secondary aneurysmal cystic changes. Such lesions can be differentiated from primary ABCs, which appear as complete cystic lesions with multiple fluid-fluid levels without evidence of any soft tissue component. Clinico-pathological diagnosis must be confirmed with histopathological evaluation in these lesions. Various other neoplastic entities like ABC, osteoblastoma, Ewing’s sarcoma and chordoma can be the differentials in axial location. A CT guided core needle biopsy is preferred, which has the advantage of being image guided and can secure representative tissues from difficult location and minimizing the risk of injury to vital structures. The approach must be planned in consultation with the treating surgical team. Most of the biopsy procedures are done via posterolateral or transpedicular approach for spine lesions, and via midline posterior approach for sacral lesions.13
2. Management
2.1. Principles
The management of axial GCTB requires an integrated multidisciplinary approach. All cases must be discussed in multidisciplinary clinic involving all treatment stakeholders. Careful radiological evaluation must be done to decide the extent of disease which is essential to decide the correct approach required to manage these lesions. If treated adequately, surgical options provide best chance of local disease control but are plagued with high risk of intraoperative and post-operative complications like high operative blood loss, transfusion related complications, prolonged ICU stay and injury to surrounding vital structure like spinal cord, major vessels and visceral organs like lung, kidney and GI tract, depending on location. Being an inherent benign disease, the morbidity associated are life long and require intense rehabilitation for a prolonged period. Over the years, the emphasis has been shifting from more morbid and aggressive surgical approach to a multimodality conservative approach. Advent of specialized minimally invasive techniques like selective angioembolization and availability of anti-osteoclastic drugs like bisphosphonates and recently denosumab, has changed the way we manage these lesions.
2.2. Surgical management
As in extremity GCTBs, the two standard surgical options for managing an axial GCTB are – Intra-lesional curettage or En-bloc resection. Pre-operative angioembolization is essential as it not only offers marked reduction in intraoperative blood loss (decreasing need of blood transfusion and preventing related complications) but also helps in better disease clearance by improving intraoperative visibility (Fig. 1).
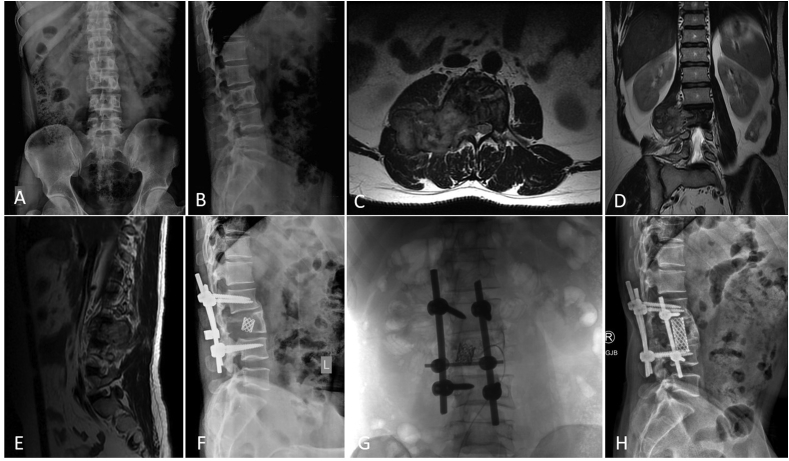
Fig. 1.
shows the imaging of a 38-year old male diagnosed with L3 Vertebral GCTB (A,B – Plain Radiograph of Lumbosacral spine Anteroposterior and Lateral views showing lytic lesion in L3 vertebral body; C,D,E − MRI Spine axial, coronal and sagittal views with GCTB of L3 and large soft tissue component compressing the cord) treated with 2 cycles of angioembolization followed by Decompression Laminectomy of L3 vertebra + radial screw + cage fixation (F) followed by another angioembolization to control the recurrence (G) and a static and well ossified lesion on 4-year follow up imaging (H – Plain Radiograph Lateral view).
Rationale: The standard treatment for any GCTB is complete surgical removal. En-bloc resection is the treatment of choice in spinal GCTB, provided the result of surgery does not lead to worsening of neurologic symptoms. In a mobile spine, total spondylectomy has better relapse-free survival in comparison to subtotal spondylectomy, even when total spondylectomy is done with piece-meal resection.14 Resection in sacral GCTB can be undertaken when lower segments are involved and when it does not necessitate removal of S2 segment. Spinopelvic stabilization may be needed when there is instability,15 manifesting in the form of persistent pain and neurologic symptoms. Cases not responding to serial angioembolization need curettage, though the procedure results in massive blood loss, wound complications16 and high risk of local recurrence.17,18
Challenges: Surgery at axial sites is limited by the complexity of anatomy, higher risk of uncontrolled bleeding, inability to achieve negative margin without significant morbidity such as neurologic deficits and loss of bowel and bladder function, and high chance of local recurrence in attempted curettage cases.19 Axial GCTB often invades spinal canal, causing compression of cord (roots below L1), which is why complete curettage is hardly possible and adjuvants like phenol, cement and liquid nitrogen, cannot be used in such cases.
2.3. Conservative management
Selective Arterial Embolization (SAE): SAE is an effective minimally invasive, hence a less morbid procedure in the management of axial GCTBs. Assessment of tumor vascularity is done by angiography prior to the procedure, which also helps in identification of vessels of adequate caliber to facilitate angioembolization. It helps to reduce pain, decrease tumor size, and promote healing by ossification, as visible on radiography. Various materials have been used for embolization, such as absorbable gelatin or polyvinyl alcohol particles for peripheral occlusion, and stainless-steel coils for central occlusion. Superabsorbent polymer microspheres have also been used.20 Successful angioembolization is confirmed with a post-procedure angiography. The reported complications include common peroneal palsy, muscle weakness, mild numbness, and temporary loss of bowel and bladder control, which is greatly acceptable compared to neurologic deficits which are observed post definitive radiotherapy or surgery. Occlusion of one vessel leads to development of further collaterals, as seen on follow-up angiography. Hence, this procedure needs to be repeated at frequent intervals until no significant vascularity is noted. Review of articles on SAE has shown varied frequency of angioembolizations, ranging from once every 3 weeks to once every 8 weeks and thus, a consistent interval is not known yet.10 Angiography and subsequent SAE is also stopped if patient has no new symptoms or progression of disease on follow-up imaging. A short series shows 9 cases of sacral GCTBs solely treated with SAE with excellent progression-free disease control in 7 out of 9 cases and the authors of this series recommend use of SAE as the primary modality of treatment in unresectable GCTB of sacrum.21 A review article by He et al., including 9 articles with a total of 44 cases, shows favorable results on local control and overall survival when SAE was used as the primary modality for managing axial GCTB.10 The local control rates at 2yrs, 5yrs and 10yrs have been reported to be 93.2% (41/44), 90.9% (40/44), and 81.8% (36/44) in the same series.
Zoledronic acid and SAE: Bisphosphonates have a proven role as an adjuvant therapy in the management of GCTB. It aids in reducing local recurrence for cases treated with intralesional curettage.22,23 The most commonly used agent is Zoledronic acid amongst all bisphosphonates. Although limited data is available investigating the efficacy of combining Zoledronic acid with SAE, the benefits of this combination in large and unresectable GCTB have been reflected in a case series by Balke et al.24
Denosumab: Denosumab is a fully human monoclonal antibody blocking the receptor activator of NF-kappa B ligand (RANKL) signaling pathway, which plays a role in the pathogenesis of GCTB. It prevents interaction between RANK and RANKL resulting in osteoclast mediated bone destruction. Denosumab therapy is beneficial in managing large axial GCTBs or GCTBs present at challenging anatomical locations by downstaging tumors which were deemed inoperable or required morbid surgery. It also helps in creating a sclerotic rim around the lesions, thereby providing an adequate mechanical scaffold for intralesional curettage.
In an open label, parallel group, phase II study concerning safety and dosage of Denosumab in adults and efficiency mature adolescents (15), though no treatment related deaths were reported, a variety of adversities noted in the form of hypophosphatemia, back pain, pain in extremities in 18% cases, 5% had hypocalcemia, 1% cases had osteonecrosis of jaw (3 cases in total of which 2 had recent tooth extraction) and 2 of them needed further surgical intervention of tooth/jaw. Up to 84% patients experienced improvement in clinical symptoms in the form of decrease in pain and improvement in function (16). Histologic response was noted in form of depletion of giant cells >90% compared to baseline histology. Objective response was seen in 74% cases (defined as partial/complete response) on MRI/CT/FDG-PET by various criteria and mean time to objective response was 3.1 months. There is cessation of lysis in bone, new bone formation as seen on plain radiograph, plain CT and decrease in metabolic activity on response-evaluation PET.
Review of 54 cases of unresectable GCTB for treated with monthly denosumab after initial loading dose, with a median time on Denosumab for 54 months revealed risk of ONJ in 9% cases (median ONJ free survival of 92% at 5-yrs), hypophosphatemia and atypical femoral fracture of 4% in the overall series treated with denosumab. Follow-up data in the same series showed disease progression in 40% cases after discontinuation of Denosumab at median duration of 8 months after discontinuation of therapy (7–15months range).25
Denosumab can be combined with SAE. This approach can be used as a per treatment or neoadjuvant setting to reduce the risk of surgery. These two modalities can synergistically promote sclerosis and result in significant pain relief and relieving neurological symptoms.26 (Fig. 2).
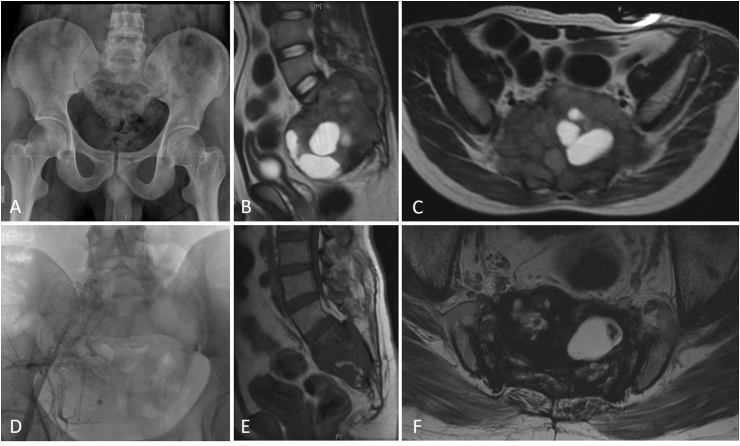
Fig. 2.
shows images of a 32-year male with a large sacral GCT (A – Plain Radiograph Pelvis Anteroposterior view; B,C – MRI Pelvis Sagittal and Axial views showing the sacral bony lesion with a large soft tissue component), treated with Denosumab and Angioembolization (D) with response and controlled disease after 5 years of follow up (E,F – MRI Pelvis Sagittal and Axial views showing significant reduction in soft tissue component).
Controversies – Denosumab is a recent drug and is still in investigation. There are multiple trials going on looking at mid to long term side effects in addition to exploring the options of dose modification and altering the duration of treatment.25 There are few reports of de-novo conversion of GCTB to sarcoma after the use of Denosumab.27 Though extremely concerning, this needs to be further evaluated and current literature neither accept or refute such challenge.28
Radiotherapy – In the current era of availability of angioembolization and Denosumab, radiotherapy (RT) has been among the last modalities to be used for the fear of sarcomatous transformation.29 The present day indications for the use of radiotherapy include multiple recurrent GCTB after surgical or denosumab failure (Fig. 3). It may also be helpful in relieving pain in palliative patients. In a systemic review of use of radiotherapy as a sole option of treatment in 42 cases of unresectable GCTB by Yifei et al.,4 all cases had responded to RT (100% response) but 9 patients (21.4%) had local failure at mean of 11.3 months, which was defined as local progression of disease at last follow-up. The mean radiotherapy dosage used was 45Gy (21–65). The review suggested that use of megavoltage was safe in unresectable GCTB, compared to earlier reported local failure and risk of sarcomatous transformation with orthovoltage. All local failures noted were within first 2 years post treatment and no failure noted beyond 2 yrs. Neurologic complications reported post RT were pain, residual spasticity and parasympathetic dysfunction. The most feared complication in cases that were treated with definitive RT is sarcomatous transformation, which has been reported to be from 0 to 11% with mean latency period of 9yrs.30 There was significant difference in local control in surgery followed by radiotherapy versus radiotherapy alone.17 Post radiation Recurrences and sarcomatous transformation are difficult to manage and have guarded prognosis.
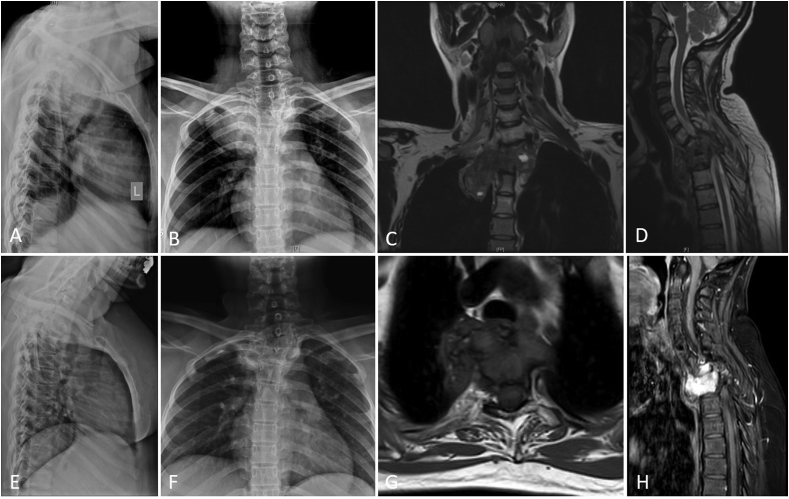
Fig. 3.
shows images of a 32-year female with D1-2 Vertebral GCT (A,B – Plain Radiograph Cervicodorsal spine Lateral and Anteroposterior views; C,D – MRI Spine Coronal and Sagittal views showing D1-2 vertebral lesion with intrathoracic extension) managed with Denosumab and definitive RT (50.4Gy/28#), given in view of poor response to the former with evident response (E,F – Plain Radiograph Lateral and Anteroposterior views; G,H – MRI Spine Axial and Sagittal views post Denosumab and RT).
Use of heavy particle therapy like proton beam/carbon ion therapy has not yet been really explored and there is very limited literature available except for anecdotal case reports.31 These therapies may be utilized in limited indication in place of routine Radiotherapy techniques in order to minimize side effect and achieve long term local disease control.
3. Conclusion
GCTB occurring in the axial skeleton pose a great challenge in terms of diagnosis and management. A multimodality management is required for optimum outcomes. Focus is shifting from more morbid surgical treatment to a more conservative multidisciplinary approach to decrease long term morbidity. A meticulous evaluation and precise decision making are required to plan the line of management – Surgical versus Conservative. Angioembolization used as a pre-operatively or as a definitive procedure in unresectable cases, or in combination with Denosumab, which plays a role in limiting the extensiveness of the tumor. Introduction of denosumab has accelerated the progress in further refining the management though optimum dosage and duration of treatment is still debatable. Radiotherapy is being reserved for very few multi treatment refractory cases due to the possibility of sarcomatous transformation of the lesion. Role of newer heavy particle therapy may change the management of these lesions in future.
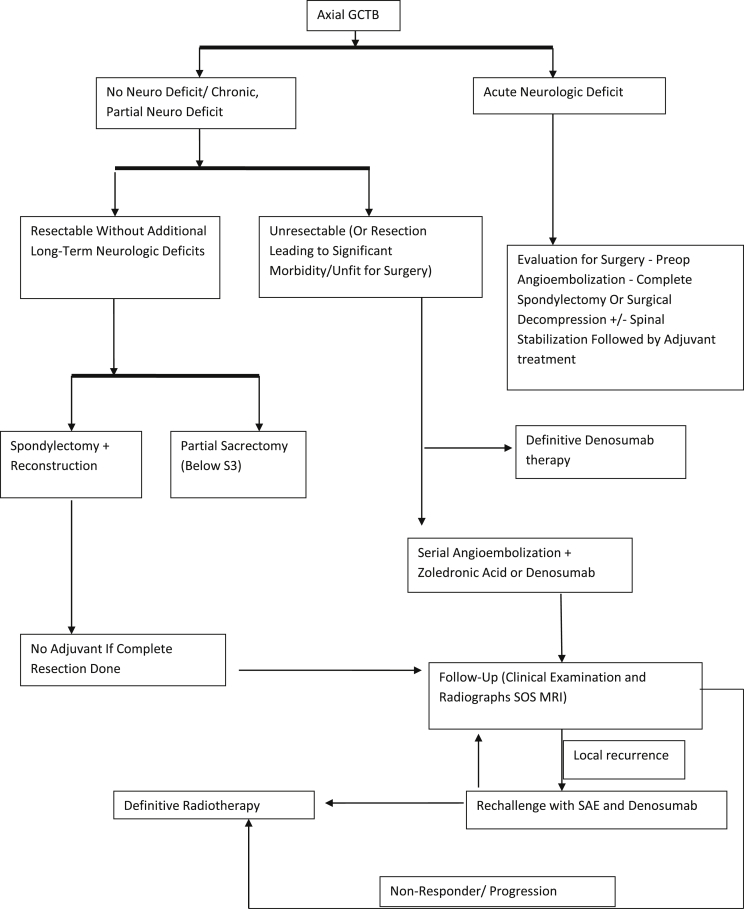
Even though further studies are necessary to systematize the management of these perplexing tumors, research material available at present has enabled us to define a current standard of practice for axial GCTBs (Fig. 4).
Fig. 4.
Algorithm – management of axial GCTB.
Author contribution
Navaneeth Kamath: Patient evaluation, data collection, data analysis and manuscript writing.
Jasmine Agarwal: Patient evaluation, data collection, data analysis and manuscript writing and editing.
Ashish Gulia: Conceptualization, Supervision, patient evaluation, data collection, data analysis, manuscript editing, formal analysis
Contributor Information
Navaneeth Kamath, Email: navaneethkmckamath3@gmail.com.
Jasmine Agarwal, Email: jasmineagarwal2810@gmail.com.
Ashish Gulia, Email: aashishgulia@gmail.com.
References
- 1.Balke M., Henrichs M.P., Gosheger G. Giant cell tumors of the axial skeleton. Sarcoma. 2012;2012:410973. doi: 10.1155/2012/410973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thangaraj R., Grimer R.J., Carter S.R., Stirling A.J., Spilsbury J., Spooner D. Giant cell tumour of the sacrum: a suggested algorithm for treatment. Eur Spine J. 2010;19(7):1189–1194. doi: 10.1007/s00586-009-1270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Heijden L., van de Sande M.A.J., van der Geest I.C.M. Giant cell tumors of the sacrum--a nationwide study on midterm results in 26 patients after intralesional excision. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(9):1949–1962. doi: 10.1007/s00586-014-3263-5. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y., Xu W., Yin H. Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. Eur spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(8):1754–1760. doi: 10.1007/s00586-015-3834-0. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco C.H., Murray J.A. vol 20. 1989. Giant cell tumors; pp. 395–405. (Orthopedic Clinics of North America). [PubMed] [Google Scholar]
- 6.Larsson S.E., Lorentzon R., Boquist L. Giant-cell tumor of bone. A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg Am. 1975;57(2):167–173. [PubMed] [Google Scholar]
- 7.Bhojraj S.Y., Nene A., Mohite S., Varma R. Giant cell tumor of the spine: a review of 9 surgical interventions in 6 cases. Indian J Orthop. 2007;41(2):146–150. doi: 10.4103/0019-5413.32047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin C., McCarthy E.F. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J. 2010;30:69–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber S., Ollivier L., Leclere J. Imaging of sacral tumours. Skelet Radiol. 2008;37(4):277–289. doi: 10.1007/s00256-007-0413-4. [DOI] [PubMed] [Google Scholar]
- 10.He S., Xu W., Sun Z. Selective arterial embolization for the treatment of sacral and pelvic giant cell tumor: a systematic review. Orthop Surg. 2017;9(2):139–144. doi: 10.1111/os.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester D.M., Donald Resnick M.D., Gen Niwayama M.D. 3 Volumes. W. B. Saunders; Philadelphia: 1995. pp. 3785–3806. (Diagnosis of Bone and Joint Disorders). Arthritis Rheum. 1995;25(4):480. [Google Scholar]
- 12.Kwon J.W., Chung H.W., Cho E.Y. MRI findings of giant cell tumors of the spine. Am J Roentgenol. 2007;189(1):246–250. doi: 10.2214/AJR.06.1472. [DOI] [PubMed] [Google Scholar]
- 13.Puri A., Shingade V.U., Agarwal M.G. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skelet Radiol. 2006;35(3):138–143. doi: 10.1007/s00256-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 14.Xu W., Li X., Huang W. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol. 2013;20(3):804–810. doi: 10.1245/s10434-012-2707-6. [DOI] [PubMed] [Google Scholar]
- 15.Doita M., Harada T., Iguchi T. Total sacrectomy and reconstruction for sacral tumors. Spine (Phila Pa 1976) 2003;28(15):E296–E301. doi: 10.1097/01.BRS.0000083230.12704.E3. [DOI] [PubMed] [Google Scholar]
- 16.Beadel G.P., McLaughlin C.E., Aljassir F. Iliosacral resection for primary bone tumors: is pelvic reconstruction necessary? Clin Orthop Relat Res. 2005;438:22–29. doi: 10.1097/01.blo.0000180046.97466.bc. [DOI] [PubMed] [Google Scholar]
- 17.Leggon R.E., Zlotecki R., Reith J., Scarborough M.T. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196–207. doi: 10.1097/01.blo.0000128643.38390.07. [DOI] [PubMed] [Google Scholar]
- 18.Turcotte R.E., Sim F.H., Unni K.K. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993;291:215–221. [PubMed] [Google Scholar]
- 19.Shen C.C., Li H., Shi Z.L., Tao H.M., Yang Z.M. Current treatment of sacral giant cell tumour of bone: a review. J Int Med Res. 2012;40(2):415–425. doi: 10.1177/147323001204000203. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi K., Osuga K., Hori S. Transarterial embolization (TAE) of sacral giant cell Tumor (GCT) using spherical permanent embolic material superabsorbant polymer microsphere (SAP-MS) SpringerPlus. 2013;2:666. doi: 10.1186/2193-1801-2-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosalkar H., Jones K., King J., Lackman R. Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results. Spine (Phila Pa 1976) 2007;32:1107–1115. doi: 10.1097/01.brs.0000261558.94247.8d. [DOI] [PubMed] [Google Scholar]
- 22.Shi M., Chen L., Wang Y., Wang W., Zhang Y., Yan S. Effect of bisphosphonates on local recurrence of giant cell tumor of bone: a meta-analysis. Cancer Manag Res. 2019;11:669–680. doi: 10.2147/CMAR.S187316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu Z.S., Sen R., Dhiman A., Sharma P., Siwach R., Rana P. Effect of intravenous zoledronic acid on histopathology and recurrence after extended curettage in giant cell tumors of bone: a comparative prospective study. Indian J Orthop. 2018;52(1):45–50. doi: 10.4103/ortho.IJOrtho_216_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balke M., Campanacci L., Gebert C. Bisphosphonate treatment of aggressive primary, recurrent and metastatic Giant Cell Tumour of Bone. BMC Canc. 2010;10:462. doi: 10.1186/1471-2407-10-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmerini E., Chawla N.S., Ferrari S. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Ji T., Yang Y., Wang Y., Sun K., Guo W. Combining of serial embolization and denosumab for large sacropelvic giant cell tumor: case report of 3 cases. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000007799. e7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broehm C.J., Garbrecht E.L., Wood J., Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015;2015:767198. doi: 10.1155/2015/767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alaqaili S., Abduljabbar A M., Altaho A Jaffr, Khan A A., Alherabi J A. Malignant sarcomatous transformation of benign giant cell tumor of bone after treatment with denosumab therapy: a literature review of reported cases. Cureus. 2018;10 doi: 10.7759/cureus.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micke O., Bruns F., Eich H.T. Radiation therapy for giant cell tumors of bone: long-term results of a multicenter study in Germany. Int J Radiat Oncol. 2005;63:S108. [Google Scholar]
- 30.Thomas D.M., Skubitz K.M. Giant cell tumour of bone. Curr Opin Oncol. 2009;21(4):338–344. doi: 10.1097/CCO.0b013e32832c951d. [DOI] [PubMed] [Google Scholar]
- 31.Huh A., Villelli N., Martinez D. Denosumab treatment for a residual giant cell tumor of the clivus: a case report and review of the literature. World Neurosurg. 2018;118:98–101. doi: 10.1016/j.wneu.2018.06.242. [DOI] [PubMed] [Google Scholar]