Abstract
Purpose
To investigate whether tenofovir disoproxil fumarate (TDF) treatment that started from the second trimester had an advantage over TDF treatment that started from the third trimester.
Patients and methods
Twenty 35-year-old pregnant women with hepatitis B virus (HBV) DNA >2×106 IU/mL were prospectively enrolled in this study. All participants were divided into two subgroups: the second trimester group who started TDF treatment at 24–27 weeks and the third trimester group who started TDF treatment at 28–30 weeks. The primary outcome was the change in serum HBV DNA level from baseline to delivery. Each parameter was tested every 4 weeks from TDF initiation to 3 months postpartum.
Results
There were 80 pregnant women in the second trimester group and 49 pregnant women in the third trimester group. The decline in HBV DNA from baseline to delivery was more obvious in the second trimester group (4.8±1.2 log10 IU/mL) than that in the third trimester group (4.3±1.1 log10 IU/mL, p=0.041). The downward shift of haemoglobin (HB) from baseline to delivery was greater in the second trimester group (10.6±10.7 g/L) than in the third trimester group (6.3±12.3 g/L, p=0.041). The decline in HBV DNA from baseline to delivery was linearly related to the start of TDF treatment from the second trimester (β=0.50 and 95% CI: 0.26–0.75, p<0.001). There were no significant differences between the two groups regarding HBV serologic markers and safety indicators.
Conclusion
Starting TDF treatment from the second trimester achieved better viral suppression than starting TDF treatment from the third trimester in highly viraemic pregnant women without increasing additional adverse reactions. HB level needed frequent monitoring during treatment to avoid anaemia.
Registry number
Clinical Trial No. NCT02719808.
Keywords: tenofovir disoproxil fumarate, efficacy, safety, second trimester, third trimester
Introduction
Mother-to-child transmission (MTCT) is a dominant risk factor for developing chronic hepatitis B virus (HBV) infection.1–9 Prevention of perinatal transmission of HBV infection is still a public health concern globally.10–14 Tenofovir disoproxil fumarate (TDF), a nucleotide analogue and potent inhibitor of HBV polymerase,13,15 is recommended as the preferred antiviral treatment for HBV for MTCT prevention. Its high efficiency in viral load reduction, high safety, and low rate of resistant mutations are well appreciated in several major global guidelines.7,11,13,16–20 A previous random control trial (RCT) was conducted to determine the efficacy and safety of TDF therapy in mothers with a high level of HBV DNA by Pan et al The investigators discovered that the rate of MTCT is significantly lower in mothers with TDF therapy than those without antiviral therapy.21 This is supported by several prior systematic reviews and meta-analyses, which demonstrate that TDF therapy in HBV infected mothers in the second or third trimester could block MTCT with high efficacy.4,11,22 In addition, a new prospective single-arm study by Wang et al verified these discoveries of TDF treatment, with a 100% success rate in preventing MTCT in a real-world setting.
Because of the heterogeneous study designs and different therapeutic strategies, efficacy and safety problems of TDF and the exact time for the initiation of therapy have not been well described and studied.23 Furthermore, most of the previous studies started TDF treatment in the third trimester.1,2,5,6,10,11,20,21,24–28 However, data regarding the efficacy and safety in mothers whose treatment commenced in the second trimester (24–27 weeks) are sparse. Whether TDF treatment initiated from the second trimester has advantages over TDF treatment starting from the third trimester in highly viraemic pregnant women is not clear. Therefore, this real-world prospective study aimed to compare the efficacy and safety of TDF treatment starting from the second trimester and third trimester.
Materials And Methods
Patient Selection And Study Setting
In this prospective, single-arm, study patients were recruited from the First Affiliated Hospital of Xi’an Jiaotong University between January 2013 and December 2018. Twenty 35-year-old pregnant women with hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) dual-positive HBV infection were enrolled. The following exclusion criteria were applied: (1) a creatinine (Cr) clearance rate <100 mL/min, alanine aminotransferase (ALT) >5 times the upper limit of normal (ULN), bilirubin >2 mg/dL or evidence of hepatocellular carcinoma, renal dysfunction, or hepatic dysfunction; (2) co-infection with HIV, hepatitis C virus, or hepatitis D virus; (3) a HBV treatment history within 6 months; (4) an abortion history or clinical manifestation of an inevitable abortion; (5) congenital foetal deformity; (6) haemoglobin (HB) <8 g/100 mL, neutrophils <1000/mm3, or albumin <2.5 g/100 mL; (7) special medicine treatment required during the pregnancy; (8) the biological father of the infant has chronic HBV infection; (9) serum HBV DNA titre<2×106 (6.3 log10) IU/mL; (10) did not receive TDF treatment. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University and registered in Clinical Trial (registration number: NCT02719808). All patients signed the written consent form and all protocols conformed to the standards of the Declaration of Helsinki.
Study Procedures And Data Collections
All participants were started on an oral dose of 300 mg/day of TDF (GSK, China) from the second trimester or third trimester until 1 month postpartum. Antiviral treatment was in accordance with the guidelines and regulations for the prevention of vertical transmission of HBV in pregnancy (Chinese Medical Association-Society of Obstetrics and Gynecology, 2013). Mothers were followed up every 4 weeks from the initiation of TDF to 3 months postpartum by patient visits to the outpatient clinic. Mothers’ HBV DNA, HBV serologic markers, aspartate aminotransferase (AST), ALT, serum albumin (ALB), HB, blood urea nitrogen (BUN), and Cr were tested before TDF treatment and continued every visit.
Laboratory Evaluation
The primary outcome was the change in serum HBV DNA levels of mothers from baseline (before treatment) to delivery. The HBV DNA was tested with the PCR-fluorescence probe method (Daan GENE, China). The secondary efficacy outcomes for mothers were HBsAg, HBeAg, hepatitis B e antibody (HBeAb), and hepatitis B core antibody (HBcAb) levels and secondary safety outcomes for mothers were AST, ALT, ALB, HB, BUN, and Cr. A severe ALT flare was defined as a level above 5–10 times the ULN and a serious ALT flare was defined as a level more than 10 times the ULN.21
Statistical Analysis
Study populations were divided into two subgroups: patients who started TDF treatment from the second trimester (24–27 weeks) and patients who started TDF treatment in the third trimester (28–30 weeks). Baseline characteristics and laboratory results are presented as percentages for categorical variables, means ± standard deviation for normally distributed continuous variables, and medians [interquartile ranges (Q3–Q1)] for non-normally distributed continuous data. HBV DNA level was transformed to a logarithmic scale (log10 IU/mL). The changes in each indicator from baseline to delivery and from delivery to 3 months postpartum were compared between the two groups. Pearson χ2 test, modified by Fisher’s exact test, was used to compare the two groups for categorical variables. The t-test and Mann–Whitney U-test for nonparametric analysis were used to compare two groups for continuous variables. A mixed model with repeated measures analysis was used to evaluate the effect of trimester on the change in serum HBV DNA level and other serum parameters during the follow-up period. Age and family history of HBV were adjusted in the model. p1 was calculated to test whether the effect of the group (second trimester vs third trimester) is significant in the mixed linear model. p2 was calculated to test whether the effect of follow-up time is significant in the mixed linear model. Multiple linear regression analysis was used to analyse the relationship between HBV DNA decline (baseline-delivery) and baseline factors, including age, family history of HBV, initiated trimester, HBV DNA, HBsAg, HBeAg, ALT, HB, caesarean section, education, domicile, number of pregnancies before this delivery, and number of deliveries before this delivery. The regression coefficient and 95% confidence interval (CI) were depicted. p<0.05 was considered significant. All data analyses were conducted with R 3.5.1 and SPSS 24.0 (SPSS, IL, USA).
Results
Study Population
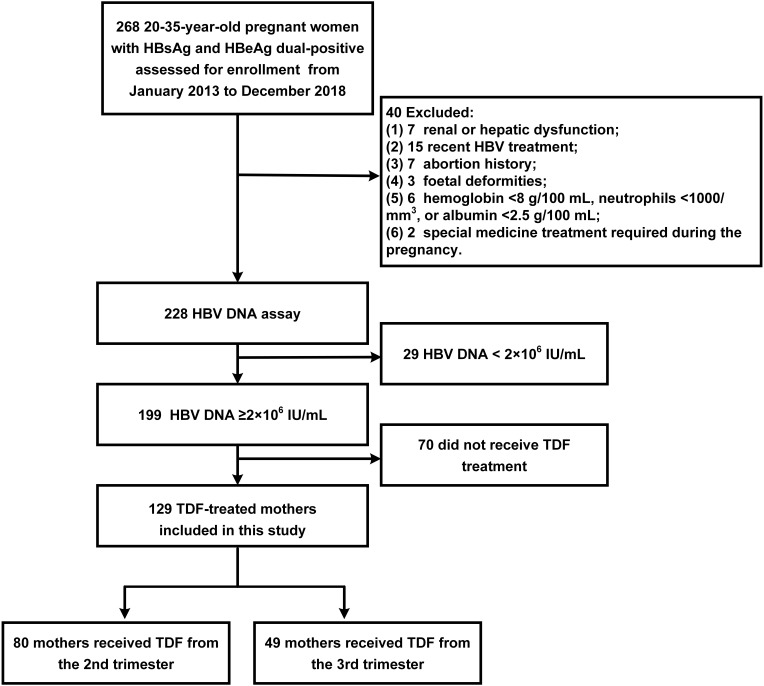
Among 268 pregnant women that were screened, 129 were included in the final study. Patients who had renal or hepatic dysfunction (n=7), recent HBV treatment (n=15), abortion history (n=7), foetal deformities (n=3), HB<8 g/100 mL, neutrophils <1000/mm3, albumin <2.5 g/100 mL (n=6), special medicine treatment (n=2), HBV DNA<2×106 IU/mL (n=29), or did not receive TDF treatment (n=70) were excluded. Among the remaining 129 mothers, there were 80 mothers who received TDF treatment from the second trimester and 49 mothers who received TDF treatment from the third trimester. There were no lost follow-ups from baseline to the final visit 12 weeks post-partum. The screening process of the study population is shown in Figure 1. Maternal and infant baseline characteristics are presented in Table 1. The mothers’ mean ages at enrolment were 29.1±3.6 years and 29.4±4.3 years in the second trimester and third trimester groups, respectively. There were 20 mothers (25%) in the second trimester group and 6 mothers (12.2%) in the third trimester group who had a family history of HBV. HBV DNA loads at baseline were 7.7±1.0 log10 IU/mL and 7.8±1.0 log10 IU/mL in the second trimester and the third trimester groups, respectively. In infants, the mean weights in the second trimester and the third trimester groups were 3.3±0.3 kg and 3.2±0.4 kg, respectively. The lengths in the two groups were 48.7±2.1 cm and 48.6±1.8 cm, respectively. The HBV infection rates in infants were 0% in both groups. None of the basic characteristics showed significant differences between the two groups (all p>0.05), except the number of delivery before this delivery.
Figure 1.
The screening process of study population.
Abbreviations: HBsAg, hepatitis B surface antigen; HBeAg, hepatitis Be antigen; HBV, hepatitis B virus; DNA, deoxyribonucleic acid; TDF, tenofovir disoproxil fumarate.
Table 1.
Maternal And Infant Characteristics At Baseline
| Variables | TDF Treatment From The 2nd Trimester n=80 | TDF Treatment From The 3rd Trimester n=49 | p |
|---|---|---|---|
| Mothers | |||
| Age (y),mean±sd | 29.1±3.6 | 29.4±4.3 | 0.704 |
| Family history of hepatitis B, n(%) | 20 (25) | 6 (12.2) | 0.079 |
| Domicile, n(%) | 0.271 | ||
| Rural | 58 (72.5) | 31(63.3) | |
| City | 22 (27.5) | 18(36.7) | |
| Education, n(%) | 0.118 | ||
| Below college | 55 (68.8) | 27 (55.1) | |
| College and above | 25 (31.3) | 22 (44.9) | |
| Number of pregnancy before this delivery, n(%) | 0.496 | ||
| One | 36 (45) | 27 (55.1) | |
| Two | 23 (45) | 15 (30.6) | |
| Three | 15 (18.8) | 5 (10.2) | |
| Four | 4 (5) | 2 (4.1) | |
| Five | 2 (2.5) | 0 (0) | |
| Number of delivery before this delivery, n(%) | 0.002 | ||
| None | 56 (70) | 45 (91.8) | |
| One | 23 (28.8) | 3 (6.1) | |
| Two | 1 (1.3) | 1 (2) | |
| Type of this delivery, n(%) | 0.785 | ||
| Vaginal | 31 (49.2) | 20 (46.5) | |
| Caesarean section | 32 (50.8) | 23 (53.5) | |
| Laboratory evaluation, mean±sd | |||
| HBV DNA-log10 (IU/mL) | 7.7±1.0 | 7.8±1.0 | 0.509 |
| HBsAg ×103(IU/mL) | 35.6±33.9 | 41.1±30.9 | 0.272 |
| HBeAg ×103(s/co) | 1.2±0.6 | 1.4±1.0 | 0.531 |
| HbeAb (s/co) | 43.4±19.9 | 47.2±20 | 0.29 |
| HBcAb (s/co) | 8.3±3.4 | 8.5±2.6 | 0.087 |
| AST (IU/L) | 25.8±13 | 26.7±12 | 0.654 |
| ALT (IU/L) | 26.2±18.5 | 28.2±13.5 | 0.185 |
| HB (g/L) | 117.6±8.3 | 118.7±8.9 | 0.644 |
| ALB (g/L) | 38.0±2.4 | 38±2.5 | 0.889 |
| BUN (mmol/L) | 3.1±0.7 | 3.1±0.7 | 0.924 |
| Cr (umol/L) | 40.6±4.8 | 40.2±6 | 0.802 |
| Infants | |||
| Gestational age (wks),mean±sd | 38.8±1.6 | 39.1±1.6 | 0.524 |
| Weight (kg),mean±sd | 3.3±0.3 | 3.2±0.4 | 0.816 |
| Length (cm),mean±sd | 48.7±2.1 | 48.6±1.8 | 0.587 |
| Head circumference (cm),mean±sd | 32.7±1.0 | 32.8±1.0 | 0.94 |
| APGAR score (1 min),mean±sd | 9.9±0.3 | 9.9±0.4 | 0.678 |
| HBV infection at 7 months after birth, n(%) | 0(0) | 0(0) | NA |
Abbreviations: TDF, tenofovir disoproxil fumarate; HBV-DNA, hepatitis B virus deoxyribonucleic acid; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis Be antigen; HBeAb, hepatitis Be antibody; HBcAb, hepatitis B core antibody; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HB, hemoglobin; ALB, albumin; BUN, blood urea nitrogen; Cr, creatinine; NA, not available.
Maternal Efficacy And Safety Evaluation
Results regarding the efficacy and safety parameters in two changes (baseline-delivery and delivery 3 months postpartum) are listed in Table 2. The decline in HBV DNA from baseline to delivery was greater in the second trimester group (4.8±1.2 log10 IU/mL) than that in the third trimester group (4.3±1.1 log10 IU/mL, p=0.041). HBcAb level increased after delivery (−0.9±1.8 s/co) in the second trimester group, whereas it decreased (0.1±1.9 s/co, p=0.002) from delivery to 3 months postpartum in the third trimester group. The shift downward in HB from baseline to delivery was greater in patients who started TDF treatment from the second trimester (10.6±10.7 g/L) than that in patients who started from the third trimester (6.3±12.3 g/L, p=0.041). Other changes in efficacy and safety indicators were not significant.
Table 2.
Changes Of Effective And Safety Parameters Of Pregnant Women Who Initiated TDF Therapy From The 2nd Trimester And The 3rd Trimester
| Variables | TDF Treatment From The 2nd Trimester n=80 | TDF Treatment From The 3rd Trimester n=49 | p |
|---|---|---|---|
| HBV DNA-log10 (IU/mL) | |||
| Baseline-delivery | 4.8±1.2 | 4.3±1.1 | 0.041 |
| Delivery-3 months postpartum | −4.5±1.4 | −4.6±0.9 | 0.622 |
| HBsAg ×103(IU/mL) | |||
| Baseline-delivery | 16.7±39.8 | 20.8±232.2 | 0.649 |
| Delivery-3 months postpartum | −33.9±37.2 | −43.5±48.8 | 0.726 |
| HBeAg ×103(s/co) | |||
| Baseline-delivery | 0.2±0.2 | 0.2±0.3 | 0.987 |
| Delivery-3 months postpartum | −0.3±0.2 | −0.1±0.2 | 0.280 |
| HbeAb (s/co) | |||
| Baseline-delivery | 4.8±16.9 | 4.5±16.8 | 0.926 |
| Delivery-3 months postpartum | −0.5±13.2 | −2.3±14.4 | 0.465 |
| HBcAb (s/co) | |||
| Baseline-delivery | 0.5±3.2 | −0.2±2.2 | 0.165 |
| Delivery-3 months postpartum | −0.9±1.8 | 0.1±1.9 | 0.002 |
| AST (IU/L) | |||
| Baseline-delivery | −1.2±15.3 | −1.0±12.9 | 0.925 |
| Delivery-3 months postpartum | −12.8±40.5 | −14.0±30.3 | 0.933 |
| ALT (IU/L) | |||
| Baseline-delivery | 0.02±21.9 | −2.0±31.4 | 0.674 |
| Delivery-3 months postpartum | −30.9±58.3 | −32.1±50.7 | 0.956 |
| HB (g/L) | |||
| Baseline-delivery | 10.6±10.7 | 6.3±12.3 | 0.041 |
| Delivery-3 months postpartum | −15.9±13.9 | −8.4±15.1 | 0.179 |
| ALB (g/L) | |||
| Baseline-delivery | 1±5.5 | 0.9±4.7 | 0.971 |
| Delivery-3 months postpartum | −6.0±4.0 | −7.4±4.4 | 0.462 |
| BUN (mmol/L) | |||
| Baseline-delivery | −0.3±0.3 | −0.3±0.6 | 0.933 |
| Delivery-3 months postpartum | −1.0±1.5 | −0.7±0.7 | 0.665 |
| Cr (umol/L) | |||
| Baseline-delivery | −6.9±9.1 | −10.2±8.0 | 0.499 |
| Delivery-3 months postpartum | 1±2.3 | 2.4±6.2 | 0.149 |
Abbreviations: TDF, tenofovir disoproxil fumarate; HBV-DNA, hepatitis B virus deoxyribonucleic acid; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis Be antigen; HBeAb, hepatitis Be antibody; HBcAb, hepatitis B core antibody; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HB, hemoglobin; ALB, albumin; BUN, blood urea nitrogen; Cr, creatinine.
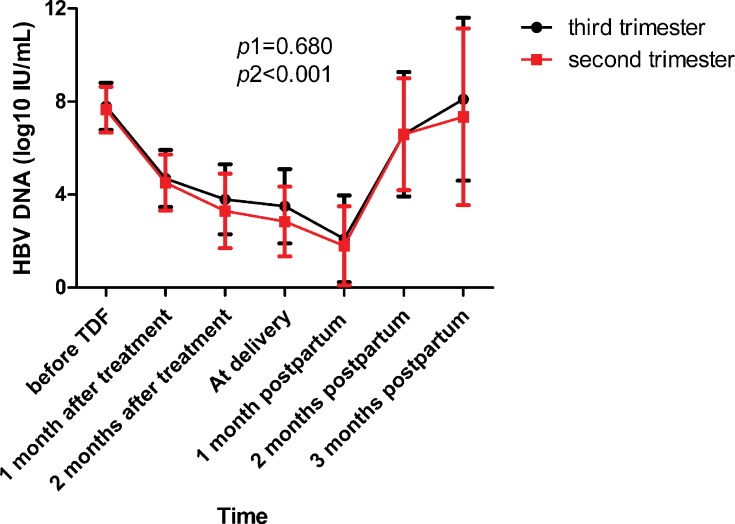
All mothers followed TDF treatment from enrolment to 1 month postpartum without early withdrawal. The alteration process in HBV DNA from baseline before TDF treatment to 3 months postpartum between the two groups is shown in Figure 2. The two groups of patients had a similar variation trend during the whole follow-up period (p1=0.680). All mothers’ DNA levels were lower than 200,000 IU/mL (5.30 log10 IU/mL) at delivery. HBV DNA declined to the lowest point at 1 month postpartum and increased after TDF cessation in both groups (p2<0.001).
Figure 2.
The dynamic changes in the level of HBV DNA.
Notes: p1 was calculated to test whether the effect of the group (sencond trimester vs third trimester) is significant in the mixed linear model. p2 was calculated to test whether the effect of follow-up time is significant in the mixed linear model.
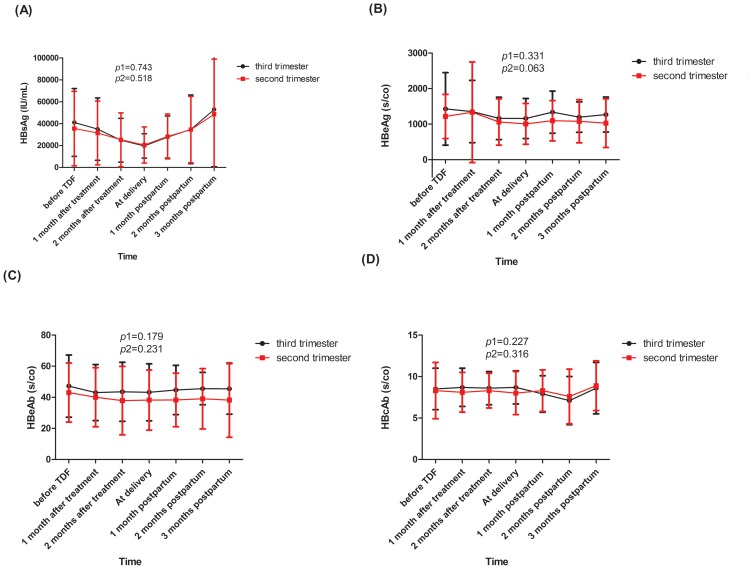
The dynamic alterations of HBsAg, HBeAg, HBeAb, and HBcAb were followed from baseline until 3 months postpartum and their variation trends in the two groups are shown in Figure 3. There was no significant difference between the patients in the second trimester and the third trimester group (all p1>0.05) and the two groups had a similar change in HBV serologic markers over time (all p2>0.05).
Figure 3.
The dynamic changes in the level of HBsAg (A), HBeAg (B), HBeAb (C) and HBcAb (D).
Notes: p1 was calculated to test whether the effect of the group (sencond trimester vs third trimester) is significant in the mixed linear model. p2 was calculated to test whether the effect of follow-up time is significant in the mixed linear model.
Abbreviations: HBsAg, hepatitis B surface antigen; HBeAg, hepatitis Be antigen; HBeAb, hepatitis Be antibody; HBcAb, hepatitis B core antibody.
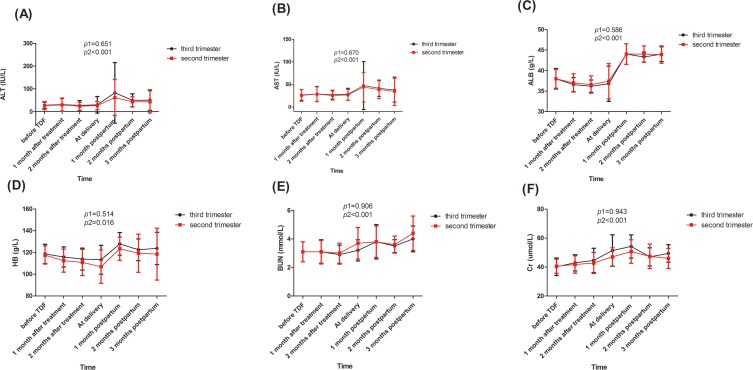
Dynamic changes in safety parameters for mothers are shown in Figure 4. All of these safety markers showed significant time-varying effects during the follow-up period in the two groups (all p2<0.05). However, there was no significant difference between the two groups (all p1>0.05).
Figure 4.
The dynamic changes of safety indicators: ALT (A), AST (B), ALB (C), HB (D), BUN (E), Cr (F).
Notes: p1 was calculated to test whether the effect of the group (sencond trimester vs third trimester) is significant in the mixed linear model. p2 was calculated to test whether the effect of follow-up time is significant in the mixed linear model.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; HB, haemoglobin; BUN, blood urea nitrogen; Cr, creatinine.
Adverse events between the second trimester and third trimester groups are listed in Table 3. There were 20 mothers (25%) in the second trimester group and 14 mothers (28.6%) in the third trimester group who had foetal distress, which had the highest incidence rate among maternal complications. There were 5 mothers (6.2%) in the second trimester group and 3 mothers (6.1%) in the third trimester group who had an ALT flare. The mean peak ALT values in patients who had an ALT flare were 313.6±119.0 IU/L in the second trimester group and 378±112.6 IU/L in the third trimester group. There were no significant differences in adverse events between the two groups.
Table 3.
Maternal Adverse Events Of Pregnant Women Who Initiated TDF Therapy From The 2nd Trimester And The 3rd Trimester
| Events | TDF Treatment From The 2nd Trimester n=80 | TDF Treatment From The 2nd Trimester n=49 | p |
|---|---|---|---|
| Maternal complications, n(%) | |||
| Intrahepatic cholestasis of pregnancy | 2 (2.5) | 3 (6.1) | 0.300 |
| Pregnancy-induced hypertension | 5 (6.3%) | 1 (2%) | 0.270 |
| Fetal distress | 20 (25) | 14 (28.6) | 0.654 |
| Pregnancy-induced diabetes mellitus | 1 (1.3) | 0 (0) | 1 |
| FGR | 1 (1.3) | 0 (0) | 1 |
| Macrosomia | 0 (0) | 1 (2) | 0.199 |
| Preterm delivery | 1 (1.3) | 1 (2) | 0.724 |
| Breech delivery | 4 (5) | 3 (6.1) | 0.784 |
| Placental abruption | 4 (5) | 0 (0) | 0.112 |
| Cord around the infant’s neck | 19 (23.8) | 9 (18.4) | 0.472 |
| Postpartum hemorrhage | 0 (0) | 0 (0) | NA |
| Laboratory abnormality | |||
| Grade 1 or 2, n(%) | |||
| ALT level 1.1–5 ULN | 38 (47.5) | 22 (44.9) | 0.774 |
| Anemia | 16 (20.0) | 7 (14.3) | 0.331 |
| Grade 3 or 4, n(%) | |||
| Increase of Cr≥0.5 mg/dl from baseline | 0 (0) | 0 (0) | NA |
| Severe ALT flare | 3 (3.8) | 1 (2) | 0.586 |
| Serious ALT flare | 2 (2.5) | 2 (4.1) | 0.606 |
| ALT flare, n(%) | |||
| At any time point during the follow-up period | 5 (6.2) | 3 (6.1) | 0.991 |
| Baseline-delivery | 1 (1.3) | 0 (0) | 1 |
| Postpartum period | 4 (5) | 3 (6.1) | 0.784 |
| Peak ALT value (IU/L),mean±sd | 313.6±119.0 | 378.0±112.6 | 0.479 |
Abbreviations: TDF, tenofovir disoproxil fumarate; FGR, fetal growth restriction; ALT, alanine aminotransferase; ULN, upper limit of normal; Cr, creatinine; NA, not available.
Related Factors Of HBV DNA Decline
Correlation analysis of HBV DNA decline are listed in Table 4. The decline in HBV DNA from baseline to delivery had a positive correlation with starting TDF treatment from the second trimester (β=0.50 and 95% CI: 0.26–0.75, p<0.001) and HBV DNA level (log10 IU/mL) before treatment (β=0.92 and 95% CI: 0.78–1.07, p<0.001).
Table 4.
Factors Associated With The Decline In HBV DNA From TDF Initiation To Delivery By Multiple Linear Regression Analysis
| Variables | β | 95% CI | p |
|---|---|---|---|
| Age (y) | 0.02 | −0.01 to 0.05 | 0.215 |
| Family history of hepatitis B | −0.13 | −0.42 to 0.16 | 0.375 |
| Second trimester vs third trimester | 0.5 | 0.26 to 0.75 | <0.001 |
| HBV DNA-log10 (IU/mL) | 0.92 | 0.78 to 1.07 | <0.001 |
| HBsAg (IU/mL) | −0.0004 | −0.004 to 0.004 | 0.809 |
| HBeAg (s/co) | −0.02 | −0.19 to 0.15 | 0.777 |
| ALT (IU/L) | −0.01 | −0.02 to 0.001 | 0.076 |
| HB (g/L) | 0.008 | −0.01 to 0.02 | 0.241 |
| Caesarean section | −0.21 | −0.44 to 0.03 | 0.081 |
| Education (College and above vs below college) | −0.07 | −0.34 to 0.21 | 0.628 |
| Domicile (City vs Rural) | −0.02 | −0.30 to 0.25 | 0.870 |
| Number of pregnancy before this delivery | −0.08 | −0.21 to 0.04 | 0.186 |
| Number of delivery before this delivery | 0.05 | −0.23 to 0.35 | 0.713 |
Abbreviations: β, regression coefficient; CI, confidence interval; HBV-DNA, hepatitis B virus deoxyribonucleic acid; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis Be antigen; ALT, alanine aminotransferase; HB, hemoglobin.
Discussion
This real-world prospective cohort study compared the efficacy and safety of TDF treatment starting from the second and third trimester. The first main result was that the reduction in viral load was greater in TDF treatment from the second trimester than that in TDF treatment from the third trimester in highly viraemic pregnant women according to the multiple linear regression analysis. Secondly, patients in the second trimester and third trimester groups successfully decreased HBV MTCT. The infection rates in infants were 0% in both groups. The two groups had a similar variation trend in HBV DNA and other HBV serologic markers from baseline to 3 months postpartum. Lastly, no evident deleterious events were observed in both groups; however, the decline in HB was more obvious during pregnancy when TDF treatment was started in the second trimester than that in the third trimester.
A recent meta-analysis demonstrated that lamivudine and telbivudine have better antiviral effects when administered from the second trimester than that from the third trimester.4 Few studies compare the differences in efficacy and safety of TDF treatment from different initiated trimesters. Only one study observed that starting TDF therapy from 24 weeks showed a more rapid viral decline than from 28 weeks of gestation (p<0.001) in HBV-infected mothers resistant to lamivudine.29 However, this study did not investigate whether TDF treatment from the second trimester could achieve a better effect in pregnant women without a drug-resistant history. In our study, we provided a more comprehensive evaluation regarding efficacy and safety issues by comparing TDF treatment from the second and third trimesters in highly viraemic pregnant women (HBV DNA >2×106 IU/mL) without previous antiviral treatment.
Before this, Pan, in an RCT, observed that the reduction in HBV DNA from baseline to delivery was 3.56 (3.02–4.32) log10 IU/mL in patients who received TDF treatment from 30–32 weeks of gestation until postpartum week 4.21 The pregnant women from Chen’s study started TDF from gestational weeks 30–32 until 1 month postpartum.20 They discovered that the reduction in HBV DNA (baseline-delivery) was 3.89±0.87 log10 IU/mL.20 Similarly, in a trial by Greenup, the mean reduction in HBV DNA was 3.64±0.9 log10 IU/mL (baseline-delivery) in pregnant women who started TDF treatment at 32 weeks of gestation and discontinued at 12 weeks postpartum.24 In our study, the reductions of HBV DNA (baseline-delivery) were 4.8±1.2 log10 IU/mL in patients who started the TDF treatment from the second trimester (24–27 weeks) and 4.3±1.1 log10 IU/mL in the third trimester group (28–30 weeks). None of the infants in our study were infected with HBV. The better antiviral effect we achieved can be attributed to the earlier starting time we used than that by other investigators. Although the best duration of antiviral treatment and initiation time are unknown, our study suggests that it is better to start TDF treatment from 24 weeks and no later than 27 weeks of gestation until 1 month postpartum, which could achieve comparatively effective results, as indicated by HBV DNA. Regarding safety issues, TDF treatment from the second trimester did not increase additional risk, unlike TDF treatment in the third trimester. However, we discovered that TDF led to a decline in HB concentration, especially in the second trimester group, and HB level returned to normal after delivery. Another important discovery from our study was that HBV DNA level before treatment was positively correlated with the decline degree of HBV DNA. One conceivable explanation is that TDF is a nucleotide reverse transcriptase inhibitor that prevents the virus from replicating and stops the swift growth of the virus. Therefore, patients with a higher viral load are more sensitive to TDF treatment.
There are inevitable limitations in our study. Firstly, because of the limited sample size in our study, some differences may not have been observed between the two groups, such as HBsAg and HBeAg levels between the two groups and mean DNA level during the follow-up period between the two groups. However, we discovered that the reduction in HBV DNA was more rapid with TDF treatment from the second trimester than from the third trimester. Secondly, we did not stratify patients based on HBV DNA or HBsAg level for an earlier initiation because of the relatively small number of patients in the groups. Thirdly, there was a short follow-up duration in the study. Finally, adverse events, especially the long-term deleterious effects in infants when mothers started TDF from the second trimester, need to be observed in a future study.
Conclusion
This study showed that starting TDF treatment from the second trimester (24–27 weeks) may achieve better viral suppression than TDF treatment from the third trimester in highly viraemic pregnant women without increasing additional adverse reactions. HB level may decline during treatment and needs frequent monitoring during the TDF treatment period, especially in patients who received TDF from the second trimester. Our discoveries warrant additional research with an enlarged sample, particularly in RCTs, to assess the optimal initiation time of TDF treatment.
Acknowledgments
This study was funded by the National Natural Science Fund, China (81771615), Shaanxi Science and Technology Coordination Innovation Project Plan, China (2016KTCL03-01), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (XJTU1AF-CRF-2018-008) and Shaanxi Key Science and Technology Innovation Team Project (2019TD-031).
Data Availability
No further data will be shared and we will continue this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378(10):911–923. doi: 10.1056/NEJMoa1708131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cressey TR, Harrison L, Achalapong J, et al. Tenofovir exposure during pregnancy and postpartum in women receiving tenofovir disoproxil fumarate for the prevention of mother-to-child transmission of hepatitis B virus. Antimicrob Agents Chemother. 2018;62(12). doi: 10.1128/AAC.01686-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29:133–139. [DOI] [PubMed] [Google Scholar]
- 4.Song J, Yang F, Wang S, et al. Efficacy and safety of antiviral treatment on blocking the mother-to-child transmission of hepatitis B virus: A meta-analysis. J Viral Hepat. 2019;26(3):397–406. [DOI] [PubMed] [Google Scholar]
- 5.Jourdain G, Ngo-Giang-Huong N, Cressey TR, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infect Dis. 2016;16:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RS, McMahon BJ, Lok ASF, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology. 2016;63(1):319–333. [DOI] [PubMed] [Google Scholar]
- 7.Dionne-Odom J, Tita AT, Silverman NS; Society for Maternal-Fetal M. #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214(1):6–14. [DOI] [PubMed] [Google Scholar]
- 8.Beasley RP. Hepatitis-B virus - the major etiology of hepatocellular-carcinoma. Cancer. 1988;61(10):1942–1956. [DOI] [PubMed] [Google Scholar]
- 9.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Bian Q, Zhu Y, et al. Real-world study of tenofovir disoproxil fumarate to prevent hepatitis B transmission in mothers with high viral load. Aliment Pharmacol Ther. 2019;49(2):211–217. doi: 10.1111/apt.15064 [DOI] [PubMed] [Google Scholar]
- 11.Hyun MH, Lee YS, Kim JH, et al. Systematic review with meta-analysis: the efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus. Aliment Pharmacol Ther. 2017;45(12):1493–1505. doi: 10.1111/apt.14068 [DOI] [PubMed] [Google Scholar]
- 12.Cui F, Woodring J, Chan P, Xu F. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China. Int J Epidemiol. 2018;47(5):1529–1537. doi: 10.1093/ije/dyy077 [DOI] [PubMed] [Google Scholar]
- 13.Papatheodoridis G, Buti M, Cornberg M, et al. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–185. [DOI] [PubMed] [Google Scholar]
- 14.Rapicetta M. New perspectives and models for hepatitis B vaccines and immunization. Vaccine. 2008;27(25):3271–3275. doi: 10.1016/j.vaccine.2009.01.063 [DOI] [PubMed] [Google Scholar]
- 15.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–2455. doi: 10.1056/NEJMoa0802878 [DOI] [PubMed] [Google Scholar]
- 16.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 19.Korean Association for the Study of the L. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22(1):18–75. doi: 10.3350/cmh.2016.22.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HL, Lee CN, Chang CH, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62(2):375–386. doi: 10.1002/hep.27837 [DOI] [PubMed] [Google Scholar]
- 21.Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324–2334. doi: 10.1056/NEJMoa1508660 [DOI] [PubMed] [Google Scholar]
- 22.Brown RS Jr., McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology. 2016;63(1):319–333. doi: 10.1002/hep.28302 [DOI] [PubMed] [Google Scholar]
- 23.Fan R, Yin XR, Hou JL. Prevention of peripartum hepatitis B transmission. N Engl J Med. 2016;375(15):1496–1498. doi: 10.1056/NEJMc1609991 [DOI] [PubMed] [Google Scholar]
- 24.Greenup AJ, Tan PK, Nguyen V, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol. 2014;61(3):502–507. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014. doi: 10.1002/hep.27034 [DOI] [PubMed] [Google Scholar]
- 26.Yi W, Pan CQ, Li MH, et al. The characteristics and predictors of postpartum hepatitis flares in women with chronic hepatitis B. Am J Gastroenterol. 2018;113(5):686–693. doi: 10.1038/s41395-018-0010-2 [DOI] [PubMed] [Google Scholar]
- 27.Kochaksaraei GS, Castillo E, Osman M, et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat. 2016;23(1):15–22. doi: 10.1111/jvh.2015.23.issue-1 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen V, Tan PK, Greenup AJ, et al. Anti-viral therapy for prevention of perinatal HBV transmission: extending therapy beyond birth does not protect against post-partum flare. Aliment Pharmacol Ther. 2014;39(10):1225–1234. doi: 10.1111/apt.12726 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Liu J, Qi C, et al. Efficacy of tenofovir disoproxil fumarate to prevent vertical transmission in mothers with lamivudine-resistant HBV. Antivir Ther. 2015;20(7):681–687. doi: 10.3851/IMP2981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No further data will be shared and we will continue this research.