Abstract
The free-living amoeba Naegleria fowleri was identified as the etiological agent of primary amoebic meningoencephalitis that caused the deaths of two children in Peoria, Arizona, in autumn of 2002. It was suspected that the source of N. fowleri was the domestic water supply, which originates from ground water sources. In this study, ground water from the greater Phoenix Metropolitan area was tested for the presence of N. fowleri using a nested polymerase chain reaction approach. Phylogenetic analyses of 16S rRNA sequences of bacterial populations in the ground water were performed to examine the potential link between the presence of N. fowleri and bacterial groups inhabiting water wells. The results showed the presence of N. fowleri in five out of six wells sampled and in 26.6% of all ground water samples tested. Phylogenetic analyses showed that β- and γ-proteobacteria were the dominant bacterial populations present in the ground water. Bacterial community analyses revealed a very diverse community structure in ground water samples testing positive for N. fowleri.
The waterborne pathogen Naegleria fowleri has long been associated with the fatal disease of primary amoebic meningoencephalitis. Healthy children and young adults with a history of swimming in freshwater lakes or ponds are usually among those who acquire the infection, which is fatal if not identified early (Carter, 1968; Carter, 1970; Carter, 1972; Marciano-Cabral, 1988). Naegleria fowleri trophozoites infect humans by entering the nasal cavity, attach to the nasal mucosa, and enter the central nervous system. In the USA, 121 people died by infection from N. fowleri between the years of 1937 to 2007, including six deaths in 2007 (Centers for Disease Control and Prevention, 2007).
Previous studies have shown the presence of N. fowleri in soil, surface water, treated water, thermal effluents, environmental waters with elevated temperatures (including hot springs), and water distribution systems (MacLean et al., 2004; Marciano-Cabral, 1988). Recently, N. fowleri detected in the residual water from household pipes and sinks was identified as the causative agent in the primary amoebic meningoencephalitis infections that resulted in the death of two children in Arizona (Marciano-Cabral et al., 2003). In the latter case, the suspected vector of infection was the potable water supply from a warm water aquifer in the area. Moreover, it is assumed that N. fowleri (ranging in size from 7–20 μm) could not be present in well water due to the low organic content of the water and the barriers provided by well casing, which typically screens materials larger than 5 μm.
Although detection of N. fowleri has traditionally required cultivation methods, most species of Naegleria are morphologically indistinguishable, requiring molecular methods to further identify species of interest. Specifically, the Mp2Cl5 gene has recently been used to discriminate between N. fowleri and closely related species (Reveiller et al., 2002; Blair et al., 2008). In this study, the Mp2Cl5–polymerase chain reaction (PCR) assay was used to detect the presence of N. fowleri in well water samples from the greater Phoenix Metropolitan area. A recent study (Blair et al., 2008) detected heterotrophic bacteria and N. fowleri in ground water samples, suggesting that these bacteria may serve as a potential food source for the amoebae. Thus, phylogenetic analyses of 16S rRNA gene ground water clones were also performed to determine the potential correlation between bacterial community composition and the presence of N. fowleri. The overall objectives of this study were to identify the presence of N. fowleri in warm ground water aquifer samples and to associate its presence with specific microbial communities in the water samples. The information from this study will lead to a better understanding of the ecology of N. fowleri in warm ground water environments.
Materials and Methods
Environmental Wells Sampled
Well samples were collected and processed from six public water supply wells (labeled as TON1, VAL1, SAF3, PX244, SC85, and SC86) from the greater Phoenix Metropolitan area from winter 2004 through autumn 2005. Representative sites were selected based on temperature and pump type. Sites were chosen based on variable temperatures (29–48.1°C) to investigate if N. fowleri presence was a potentially temperature-dependent phenomenon. Pump type (submersible or oil-lubed turbine) was also considered because the oil lubricant was thought to serve a potential food-source for bacteria, which in turn could serve as a food source for N. fowleri. In addition, well water samples from the Mason Drinking Water Treatment Plant in Cincinnati, Ohio, were collected and used for detection limit experiments and as environmental negative controls, as described below.
Greater Phoenix Metropolitan Area Ground Water Sampling and Filtration
The following parameters were measured for selected water samples in situ: water temperature, pH, dissolved oxygen, and conductivity measurements. Conductivity, pH, and temperature were measured using an Oakton Portable pH/Con 10 Series Meter (Oakton Instruments, Vernon Hills, IL). Dissolved oxygen was measured using an Orion Model 830 Dissolved Oxygen Meter (Thermo Fisher Scientific, Inc., Waltham, MA).
Heterotrophic bacteria were measured for selected water samples collected from the greater Phoenix Metropolitan area and the Mason Drinking Water Treatment Plant in Cincinnati, Ohio. Water samples were collected and transported back to the lab and analyzed for heterotrophic bacteria according to standard method 9215B (Clesceri et al., 1998).
Wells from the greater Phoenix Metropolitan area were sampled during winter (December), late summer (August), and early autumn (September) to determine if seasonality had an impact on the detection of N. fowleri in the environment. In the field, each well water sample was passed through two serially arranged filters (pore size, 0.5 and 1.0 μm, respectively) (Micro-Wynd II D-PPPY cartridge filter; Cuno Inc., Meriden, CT). The volume of water filtered for each well ranged from 236.6 to 4402.1 L. Filters were transported to the laboratory in iced coolers and stored at –80°C until further processing.
Mason Ground Water Sampling, Spiking, and Filtration
Four 100-L control well water samples were collected from the Mason Drinking Water Treatment Plant in Cincinnati, Ohio, and transported to the laboratory immediately for processing. These water samples were considered negative environmental controls because N. fowleri levels were below the limit of detection for the assay used in this study. One of the four 100-L samples was directly filtered using two serially arranged filters (pore size, 0.5 μm) (Micro-Wynd II D-PPPZ cartridge filter), while three remaining 100-L control samples were first spiked with 103, 104, and 105 N. fowleri cells and then filtered as mentioned above. Pure culture N. fowleri (ATCC 30894) was grown in Cline medium (Cline et al., 1983) at 37°C. Naegleria fowleri cell counts were performed using a hemacytometer injected with 10 μL of well-mixed N. fowleri culture at stationary growth phase. Cell counts were performed by counting all 25 sections of the hemacytometer via a light microscope.
DNA Extraction
A portion of each filter (approximately 1.61 cm2) was removed aseptically using a sterile razor blade and placed in a 2-mL tube containing bead beating solution from a MoBio Soil DNA Extraction Kit (Mo Bio Laboratories, Inc., Carlsbad, CA). Genomic DNA extractions were performed as suggested by the manufacturer’s instructions. The DNA extracts from each filter were used as a template in PCR assays to determine the presence or absence of N. fowleri in each sample. DNA was also extracted from 1 mL of N. fowleri (ATCC 30894) pure culture cell suspension using the same DNA extraction method.
Molecular Methods
Naegleria fowleri DNA extracts were serially diluted in sterile water (1:1, 1:10, 1:100, and 1:1000). Aliquots were then used to determine the detection limits of PCR assays targeting N. fowleri (Table 1). Primers and genes targeted in this study are listed in Table 1. Detection of N. fowleri was performed using a nested-PCR assay as described previously (Reveiller et al., 2002). DNA extracts from N. fowleri cultures and each filter were used as a template in the first PCR assays, which targeted the Mp2Cl5 gene unique to the Naegleria genus. Polymerase chain reaction products from the first PCR reaction were used as a DNA template for the second PCR assay, which targeted a conserved region within the amplified Mp2Cl5 gene unique to N. fowleri. All PCR assays were performed in triplicate. Polymerase chain reaction products were visualized using a 2% ethidium bromide–stained agarose gel.
Table 1.
Description of polymerase chain reaction assays used in this study.
| PCR† assay name | Target microorganism | Target gene | Forward primer name | Forward primer sequence (5′–3′) | Reverse primer name | Reverse primer sequence (5′–3′) | Reference |
|---|---|---|---|---|---|---|---|
| Naegleria assay | general Naegleria | Mp2Cl5 | Mp2Cl5.for | TCTAGAGATCCAACCAATGG | Mp2Cl5.rev | ATTCTATTCACTCCACAATCC | Reveiller et al., 2002 |
| N. fowleri assay | N. fowleri | Mp2Cl5 | Mp2Cl5.for-in | GTACATTGTTTTTATTAATTTCC | Mp2Cl5.rev-in | GTCTTTGTGAAAACATCACC | Reveiller et al., 2002 |
| Universal 16S assay | universal DNA | 16S rDNA | S-D-Bact-0008-a-S-20 | AGAGTTTGATCCTGGCTCAG | S-D-Bact-0926-a-S-20 | CCGTCAATTCCTTTRAGTTT | Liu et al., 1997 |
| Sequencing assay | n/a | 16S rDNA gene | S-D-Bact-0338-a-S-18 | GCTGCC TCCCGT AGGAGT | n/a | n/a | Stackebrandt et al., 1991 |
PCR, polymerase chain reaction.
To identify predominant bacterial populations associated with well water samples, DNA extracts from selected samples were used to develop 16S rRNA gene clone libraries. Clone libraries were developed using primers 8F and 926R and the protocol described by Liu et al. (1997). Polymerase chain reaction products were cloned into chemically competent Escherichia coli cells using the TOPO TA Cloning Kit (TOPO10 Electrocomp Cells; Invitrogen Corp., Carlsbad, CA) following the manufacturer’s instructions. M13 primers were used in PCR assays to screen for transformants containing correct size product inserts. Polymerase chain reaction products (n = 209) were submitted to Cincinnati Children’s Hospital Medical Center DNA Sequencing Facility for partial sequencing using primer 338F (Stackebrandt and Goodfellow, 1991). Nucleic acid sequences were edited using BioEdit v7.0 (Hall, 1999) and aligned using ARB software before constructing phylogenetic trees (Ludwig et al., 2004). Aligned sequences of the environmental samples and their close relatives were used to construct phylogenetic trees using inferred from 705 sequence positions using the neighbor-joining algorithm with the Kimura correction. Additionally, 16S rRNA gene sequences were assigned to phylogenetically consistent high-order bacterial taxonomy using the Ribosome Database Project classifier tool to describe the bacterial community structure of four different well samples (Wang et al., 2007).
Data Analysis
To determine potential parameters driving N. fowleri presence, physical and chemical parameters for each different well tested were correlated to N. fowleri PCR-positive frequency using linear regression. A Student’s t test was performed to determine if there were significant differences between physical and chemical parameters for PCR-positive and PCR-negative samples.
Results and Discussion
Naegleria fowleri Detection and Environmental Parameters
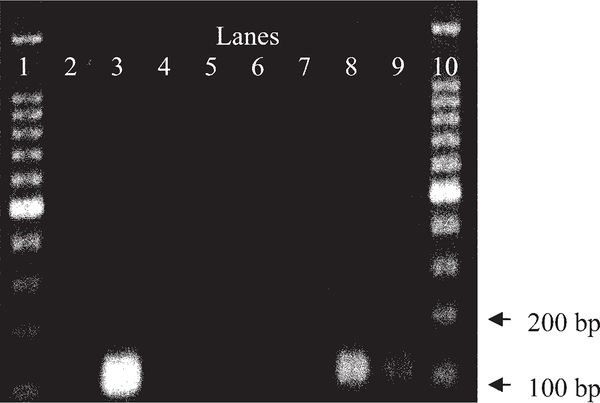
The detection limit of the nested N. fowleri PCR assay was determined using genomic DNA and spiked ground water samples. Experiments with spiked ground water samples showed that the assay could detect 10 cells per mL (Fig. 1). No PCR signals were detected in the negative control Mason ground water samples. When the N. fowleri nested PCR assay was applied to ground water samples collected in Phoenix, approximately 27% of all samples tested positive for N. fowleri (Table 2; Fig. 2). Five out of the six wells sampled tested positive during this study, although all samples that tested positive for N. fowleri were taken from wells during the late summer and early autumn. Although seasonal patterns have been previously observed for N. fowleri (Dorsch et al., 1983; Griffen, 1972; Griffen, 1983; Marciano-Cabral et al., 2003), our results indicate that there was no correlation between PCR-positive detection of N. fowleri and water temperature (Table 3). Naegleria fowleri was detected in water samples with temperatures ranging from 29 to 47°C, suggesting the thermo-tolerance of this microorganism. None of the physical or chemical parameters tested for in this study showed a correlation to PCR detection of N. fowleri (Table 4).
Fig. 1.
Gel image (2% agarose gel stained with ethidium bromide showing the sensitivity of Naegleria fowleri nested PCR assay in Mason ground water spiked with increasing concentrations of N. fowleri. Lane 1: 100-bp DNA ladder; lane 2: negative control with sterile water; lane 3: positive control with N. fowleri genomic DNA; lane 4: Mason ground water filter sample with 0.1 N. fowleri cells per mL; lane 5: Mason ground water duplicate filter sample with 0.1 N. fowleri cells per mL; lane 6: Mason ground water filter sample with 1.0 N. fowleri cells per mL; lane 7: Mason ground water duplicate filter sample with 1.0 N. fowleri cells per mL; lane 8: Mason ground water filter sample with 10.0 N. fowleri cells per mL; lane 9: Mason ground water duplicate filter sample with 10.0 N. fowleri cells per mL; lane 10: DNA ladder.
Table 2.
Polymerase chain reaction results for environmental samples from the greater Phoenix Metropolitan area.
| Amplification results for samples collected in summer, autumn, and winter for Naegleria fowleri (number positive/samples tested) |
|||
|---|---|---|---|
| Well name | Late summer | Autumn | Winter |
| TON1 | – | 3/6 | 0/2 |
| VAL1 | – | 4/13 | – |
| SAF3 | – | 2/5 | 0/2 |
| PX244 | 0/2 | 0/3 | 0/2 |
| SC85 | 2/2 | 0/3 | – |
| SC86 | 1/2 | 0/3 | – |
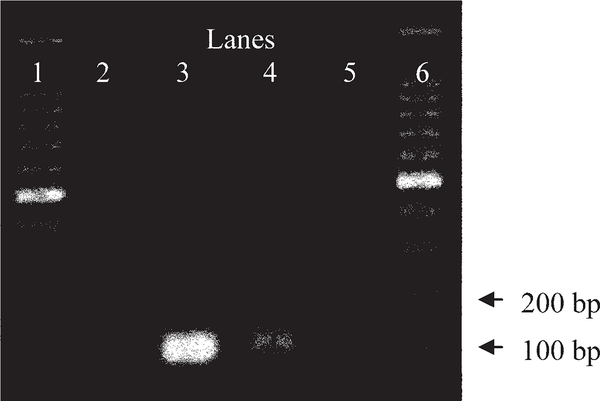
Fig. 2.
Gel image (2% agarose gel stained with ethidium bromide) showing the intensity of Naegleria fowleri polymerase chain reaction assay amplification products on environmental samples. Lane 1: 100-bp DNA ladder; lane 2: negative control with sterile molecular grade water; lane 3: positive control with N. fowleri genomic DNA; lane 4: TON1 well site sampled during early fall from the greater Phoenix Metropolitan area; lane 5: TON1 well site sampled during early fall from the greater Phoenix Metropolitan area; lane 6: DNA ladder.
Table 3.
Student’s t test of physical and chemical parameters for samples testing positive and negative for Naegleria fowleri.
| Temperature | Conductivity | pH | HPC† | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
| —°C— | μS | S μm−1 | —cfu per 100 mL— | ||||
| 47 | 43.9 | 968 | 840 | 8.4 | 8.6 | 1 | 2 |
| 48.1 | 44.2 | 921 | 896 | 8.3 | 8.6 | 2 | 3 |
| 35.9 | 47.9 | 352 | 910 | 8.6 | 8.6 | 8 | 11 |
| 35.6 | 46.8 | 343 | 912 | 8.6 | 8.6 | 1 | 1 |
| 31.2 | 35.4 | 365 | 2310 | 8.4 | 8.1 | 7 | 3 |
| 31.4 | 35.1 | 470 | 2310 | 8.2 | 8 | 15 | 2 |
| 29.2 | 34.7 | 624 | 2280 | 7.7 | 8.3 | 21 | 3400 |
| 34.5 | 2230 | 8.3 | 3200 | ||||
| 30.5 | 326 | 8.5 | 50 | ||||
| 30.5 | 321 | 8.5 | 23 | ||||
| 34.7 | 340 | 8.8 | 1 | ||||
| 34.5 | 340 | 8.8 | 1 | ||||
| 34.1 | 444 | 8.1 | 2 | ||||
| 34.3 | 437 | 8.1 | 13 | ||||
| 34.5 | 381 | 8.3 | 1 | ||||
| 34.8 | 378 | 8.3 | 1 | ||||
| 37.9 | 447 | 8 | 1 | ||||
| 37.7 | 457 | 8.2 | 1 | ||||
| 31.2 | 478 | 8.2 | 83 | ||||
| 31.1 | 487 | 8.4 | 32 | ||||
| 29.2 | 611 | 7.8 | 10 | ||||
| 29.1 | 616 | 7.6 | 52 | ||||
| 29 | 617 | 7.7 | 19 | ||||
| p = 0.652 | p = 0.149 | p = 0.794 | p = 0.152 | ||||
cfu, colony-forming units; HPC, heterotrophic plate count.
Table 4.
Correlation of physical and chemical parameters to polymerase chain reaction–positive detection of Naegleria fowleri.
| Parameter | Average | Correlation coefficient |
|---|---|---|
| pH | 8.2 | 0.1698 |
| Conductivity, μS | 547.2 | 0.119 |
| Temperature, °C | 35.1 | 0.0464 |
| HPC,† cfu per 100 mL | 288.6 | 0.0265 |
| DO, mg L−1 | 5.4 | 0.0512 |
cfu, colony-forming units; DO, dissolved oxygen; HPC, heterotrophic plate count.
A potentially important factor in the ecology of N. fowleri was that the occurrence of N. fowleri in the sampled locations could relate to the use of oil-lubed turbine pumps because lubricant released from the pumps could influence the growth of bacterial populations in the ground water, which consequently could serve as a food source for N. fowleri (Singh and Dutta, 1984; Marciano-Cabral et al., 2003). However, in this study, N. fowleri was not restricted to a specific pump type (Table 5), and therefore no connection could be made between the presence of N. fowleri and potential impact of oil on the ground water bacterial populations. No pattern was evident between the presence of N. fowleri and pH and conductivity. This is not surprising because pH does not appear to be important factor controlling the ecology of Naegleria species (Kyle and Noblet, 1985). Similarly, heterotrophic counts, which ranged from <100 to 800 cfu per 100 mL, did not appear to correlate with the presence of N. fowleri.
Table 5.
Summary of environmental samples analyzed for bacterial populations.
| Sample name | HPC† | Water temperature | Dissolved oxygen | pH | Conductivity | Filter size | Pump type | PCR results for Naegleria fowleri |
|---|---|---|---|---|---|---|---|---|
| CFU per 100 mL | °C | mg L−1 | S μm−1 | μm | ||||
| TON1A‡ | 200 | 43.9 | 4.1 | 8.6 | 840 | 1.0 | submersible | negative |
| TON1B | 300 | 44.2 | 4.2 | 8.6 | 896 | 0.5 | submersible | negative |
| TON1C | <100 | 47.0 | not measured | 8.4 | 968 | 1.0 | submersible | positive |
| SC85A | 700 | 31.2 | not measured | 8.4 | 365 | 1.0 | oil lube turbine | positive |
HPC, heterotrophic plate count; PCR, polymerase chain reaction.
TON1, VAL1, SAF3, PX244, SC85, and SC86 are well names. Letters A, B, and C listed after well names designate specific samples from the wells.
Bacterial Population Ecology of Selected Water Samples
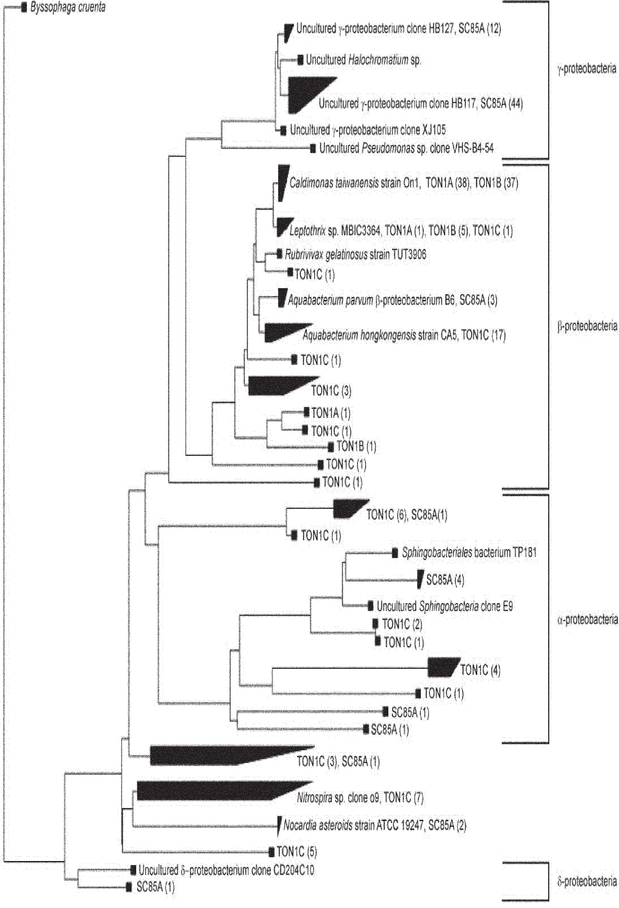
To determine whether there was a link between the presence of N. fowleri and predominant bacterial populations in the well water, 16S rRNA gene clone libraries were developed for selected well samples that tested positive (n = 126 sequences) or negative (n = 83 sequences) for N. fowleri (Table 5). Three of the samples were from one well, two of which were negative for the presence of N. fowleri (TON1A and TON1B). The other two samples (TON1C and SC85A) tested positive for N. fowleri. A total of 209 clones were analyzed and used in the development of a phylogenetic tree (Fig. 3). Most clones were closely related to β-proteobacteria, specifically to Caldimonas (n = 75), Aquabacterium (n = 17), and Leptothrix (n = 7). Members of these genera are commonly found in aquatic habitats. For example, C. taiwanensis was recently isolated from a hot spring with recorded growth temperatures between 35 and 60°C (Chen et al., 2005), and Aquabacterium species were originally detected and isolated from drinking water in Germany (Kalmbach et al., 1999). Sequences closely related to γ-proteobacteria were also abundant, with a total of 56 clones, most of which were closely related to uncultured Pseudomonas species. Proteobacteria of the α- and δ-subclasses were not as abundant. Sequences related to Nitrospira (n = 7) and Nocardia (n = 2) were also retrieved from the water samples.
Fig. 3.
Phylogenetic tree of 16S rRNA gene sequences derived from well samples SC85A, TON1A, TON1B, and TON1C and bacterial members from the α, β, γ, and δ proteobacteria, as well as Nitrospira and Nocardia genera as points of reference. Samples TON1A (n = 40 sequences) and TON1B (n = 43 sequences) tested negative for N. fowleri, whereas SC85A (n = 70 sequences) and TON1C (n = 56 sequences) tested positive for N. fowleri. The phylogenetic tree was constructed in ARB using the neighbor joining algorithm with a Kimura correction (Ludwig et al., 2004). Numbers in parentheses indicate the number of sequences derived from a given well sample.
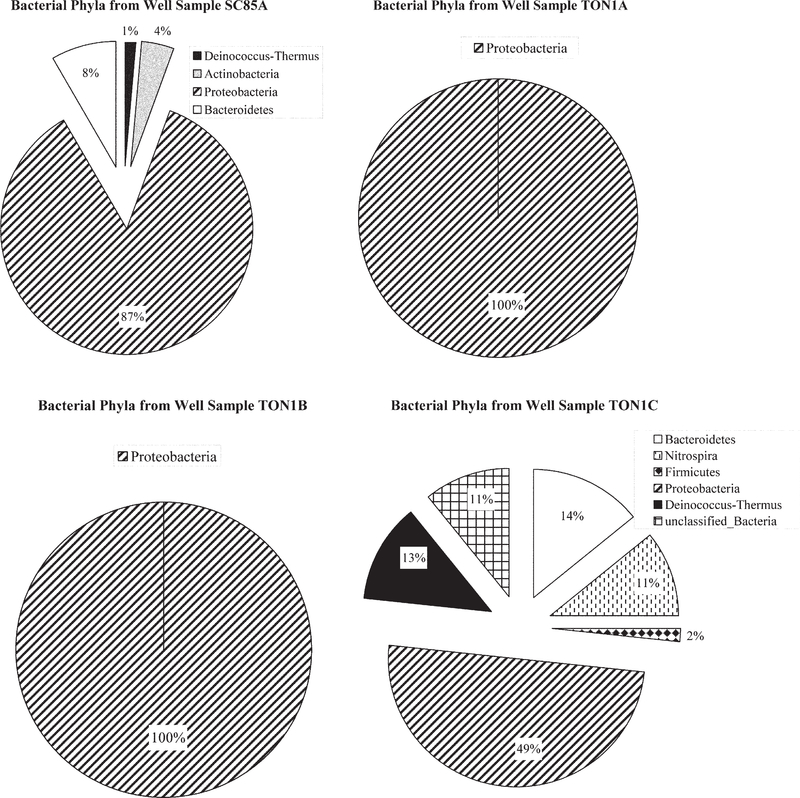
Phylogenetic classification indicated that sequences retrieved from well samples TON1A and TON1B, which were negative for N. fowleri, consisted only of β-proteobacteria. Clones derived from two samples testing positive for N. fowleri (SC85A and TON1C) comprised a much more diverse community structure, including members from at least four different bacterial phyla (Fig. 4). Although further sequencing efforts are needed to saturate bacterial diversity within these samples, these data suggest a potential association between high bacterial diversity and the presence of N. fowleri in well water. Several studies have examined the feeding habits and growth rates of free-living amoeba, including Naegleria species (Brown et al., 1983; Danso and Alexander, 1975; Danso et al., 1975; Singh and Dutta, 1984). Most of these studies have been performed in laboratory settings with specific bacteria, such as E. coli, Klebsiella species, Enterobacter cloacae, and Rhizobium meliloti. In contrast, little is known about the feeding preference of free-living amoeba in environmental settings. Free-living amoebae, including N. fowleri, have been isolated from waters containing high levels of filamentous cyanobacteria (Kyle and Noblet, 1985). In such cases, cyanobacteria were assumed to serve as a food source. Naegleria species have also been isolated from waters with high coliform levels, but no clear patterns have been established because typically the levels of enteric bacteria in surface waters do not support amoebal survival (Brown et al., 1983). A small number of bacterial sequences from samples SC85A and TON1C (wells that tested positive for N. fowleri) were members of the Bacteroidetes and Firmicutes phyla; previous research has shown that these enteric bacterial groups positively correlated with the occurrence of N. fowleri (Brown et al., 1983).
Fig. 4.
Bacterial phyla represented in well samples SC85A, TON1A, TON1B, and TON1C using the Ribosomal Database Project Bayesian classifier (Wang et al., 2007). SC85A and TON1C tested positive for Naegleria fowleri, and TON1A and TON1B tested negative for N. fowleri by polymerase chain reaction.
Summary
In this study, β- and γ-proteobacteria were present in samples that were both positive and negative to N. fowleri. Samples that were negative for N. fowleri were primarily associated with clones within the Caldimonas and Leptothrix clades. These genera are known to oxidize iron and manganese (Takeda et al., 2002; van Veen et al., 1978). These metals have been reported to positively influence the levels of free-living amoeba in environmental waters (Duma, 1981; Kyle and Noblet, 1985), although the mechanism of action is unknown. According to drinking water utilities who participated in this study, iron and manganese concentrations in the region where the samples were collected are generally low, and therefore the presence of metal oxidizers might influence the availability of metals for amoeba. Although additional evidence is needed to understand the importance of metal-oxidizing bacteria in the survival of amoeba, it should be noted that amoeba prefer gram-negative, nonpigmented bacteria (Singh and Dutta, 1984), and pigments are known to possess iron chelating activity.
In conclusion, this study has confirmed the presence of N. fowleri in warm ground water aquifers using a nested PCR assay. Although physical and chemical parameters did not correlate with PCR detection of N. fowleri, phylogenetic and Bayesian community structure analyses revealed a more rich community structure associated with wells positive for N. fowleri. Future studies should address the ecology and in situ feeding habits of N. fowleri, including the role metal availability has in the survival of this pathogenic amoeba.
Acknowledgments
The authors thank Greater Cincinnati Water Works and Dr. Jorge Santodomingo. Funding was provided by the US EPA (PR-OH-03-00572) to DBO.
Abbreviations
- PCR
polymerase chain reaction
Contributor Information
Ian Laseke, Dep. of Civil and Environmental Engineering, Univ. of Cincinnati, Cincinnati, OH.
Jill Korte, U.S. Environmental Protection Agency Region 9, Drinking Water Section, San Francisco, CA.
Regina Lamendella, Dep. of Civil and Environmental Engineering, Univ. of Cincinnati, Cincinnati, OH.
Edna S. Kaneshiro, Dep. of Biological Sciences, Univ. of Cincinnati, Cincinnati, OH
Francine Marciano-Cabral, Dep. of Microbiology and Immunology, Medical College of Virginia/Virginia Commonwealth Univ., Richmond, VA..
Daniel B. Oerther, Dep. of Civil and Environmental Engineering, Univ. of Cincinnati, Cincinnati, OH; Dep. of Biological Sciences, Univ. of Cincinnati, Cincinnati, OH.
References
- Blair B, Sarkar P, Bright KR, Marciano-Cabral F, and Gerba CP. 2008. Naegleria fowleri in well water [letter]. Emerg. Infect. Dis 2009 February 13 Available at http://www.cdc.gov/EID/content/14/9/1499.htm (verified 3 Sept. 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Cursons RT, Keys EA, Marks M, and Miles M. 1983. The occurrence and distribution of pathogenic free-living amoebae in thermal areas of the North Island of New Zealand. N. Z. J. Mar. Freshwater Res 17:59–69. [Google Scholar]
- Carter RF 1968. Primary amoebic meningoencephalitis: Clinical, pathological and epidemiological features of six fatal cases. J. Pathol. Bacteriol 96:1–25. [DOI] [PubMed] [Google Scholar]
- Carter RF 1970. Description of a Naegleria species isolated from two cases of primary amoebic meningoencephalitis and of the experimental pathological changes induced by it. J. Pathol 100:217–244. [DOI] [PubMed] [Google Scholar]
- Carter RF 1972. Primary amoebic meningo-encephalitis: An appraisal of present knowledge. Trans. R. Soc. Trop. Med. Hyg 66:193–213. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Primary amebic meningoencephalitis—Arizona, Florida, and Texas, 2007. MMWR 57:573–577. [PubMed] [Google Scholar]
- Chen WM, Chang JS, Chiu CH, Chang SC, Chen WC, and Jiang CM. 2005. Caldimonas taiwanensis sp. nov., a amylase producing bacterium isolated from a hot spring. Syst. Appl. Microbiol 28:415–420. [DOI] [PubMed] [Google Scholar]
- Clesceri LS, Greenberg AE, and Eaton AD. 1998. Standard methods for the examination of water and wastewater (20th ed.). American Public Health Association, Washington, DC. [Google Scholar]
- Cline M, Marciano-Cabral F, and Bradley SG. 1983. Comparison of Naegleria fowleri and Naegleria gruberi cultivated in the same nutrient medium. J. Eukaryot. Microbiol 30:387–391. [DOI] [PubMed] [Google Scholar]
- Danso SKA, and Alexander M. 1975. Regulation of predation by prey-density: The protozoan-Rhizobium relationship. Appl. Environ. Microbiol 29:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso SKA, Keys SO, and Alexander M. 1975. Protozoa and the decline of Rhizobium populations added to soil. Can. J. Microbiol 21:884–895. [DOI] [PubMed] [Google Scholar]
- Dorsch MM, Cameron AS, and Robinson BS. 1983. The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans. R. Soc. Trop. Med. Hyg 77:372–377. [DOI] [PubMed] [Google Scholar]
- Duma RJ 1981. Study of pathogenic free-living amoebas in freshwater lakes in Virginia Environmental Health Effects Research Series. EPA no. 600/1–800-037. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Griffen JL 1972. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science 178:869–870. [DOI] [PubMed] [Google Scholar]
- Griffen JL 1983. The pathogenic amoeboflagellate Naegleria fowleri: Environmental isolations competitors, ecologic interactions, and the flagellate-empty habitat hypothesis. J. Eukaryot. Microbiol 30:403–409. [DOI] [PubMed] [Google Scholar]
- Hall TA 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser 41:95–98. [Google Scholar]
- Kalmbach S, Manz W, Wecke J, and Szewzky U. 1999. Aquabacterium gen. nov., with description of Aquabacterium citratiphilum sp. nov., Aquabacterium parvum sp. nov. and Aquabacterium commune sp. nov., three in situ dominant bacterial species from the Berlin drinking water system. Int. J. Syst. Bacteriol 49:769–777. [DOI] [PubMed] [Google Scholar]
- Kyle DE, and Noblet GP. 1985. Vertical distribution of potentially pathogenic free-living amoebae in freshwater lakes. J. Eukaryot. Microbiol 32:99–105. [DOI] [PubMed] [Google Scholar]
- Liu WT, Marsh TL, Cheng H, and Forney LJ. 1997. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol 63:4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Bretteske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüßmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, and Schleifer K-H. 2004. ARB: A software environment for sequence data. Nucleic Acids Res. 32:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean RC, Richardson RL, Lepardo R, and Marciano-Cabral F. 2004. The identification of Naegleria fowleri from water and soil samples by nested PCR. Parasitol. Res 93:211–217. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F 1988. Biology of Naegleria spp. Microbiol. Rev 52:114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F, Maclean R, Mensah A, and Lapat-Polasko L. 2003. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl. Environ. Microbiol 69:5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveiller FL, Cabanes P, and Marciano-Cabral F. 2002. Development of a nested PCR assay to detect the pathogenic free-living amoeba Naegleria fowleri. Parasitol. Res 88:443–450. [DOI] [PubMed] [Google Scholar]
- Singh BN, and Dutta GD. 1984. Small free-living aerobic amoebae: Soil as a suitable habitat, isolation, culture, classification, pathogenicity, epidemiology and chemotherapy. Indian J. Parasitol 8:1–23. [Google Scholar]
- Stackebrandt E, and Goodfellow M (ed.) 1991. Nucleic acid techniques in bacterial systematics. Wiley, Chichester, UK. [Google Scholar]
- Takeda M, Kamagata Y, Ghiorse WC, Hanada S, and Koizumi J. 2002. Caldimonas manganoxidans gen. nov., sp. nov., a poly(3-hydroxybutyrate)-degrading, manganese-oxidizing thermophile. Int. J. Syst. Evol. Microbiol 52:895–900. [DOI] [PubMed] [Google Scholar]
- van Veen WL, Mulder EG, and Deinema MH. 1978. The Sphaerotilus-Leptothrix group of bacteria. Microbiol. Rev 42:329–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, and Cole JR. 2007. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol 73:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]