 Trans,trans,trans-[Pt(N3)2(OH)2(4-picoline)2] is potently photocytotoxic (λirr = 420 nm) towards cancer cell lines whilst being minimally toxic in the absence of irradiation.
Trans,trans,trans-[Pt(N3)2(OH)2(4-picoline)2] is potently photocytotoxic (λirr = 420 nm) towards cancer cell lines whilst being minimally toxic in the absence of irradiation.
Abstract
A series of trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes of the form trans,trans,trans-[Pt(N3)2(OH)2(L1)(L2)] where L = pyridine, 2-picoline, 3-picoline, 4-picoline, thiazole and 1-methylimidazole have been synthesised and characterised, and their photochemical and photobiological activity evaluated. Notably, complexes 19 (L1 = py, L2 = 3-pic) and 26 (L1 = L2 = 4-pic) were potently phototoxic following irradiation with visible light (420 nm), with IC50 values of 4.0 μM and 2.1 μM respectively (A2780 cancer cell line), demonstrating greater potency than the previously reported complex 1 (L1 = L2 = py; 6.7 μM); whilst also being minimally toxic in the absence of irradiation. Complexes with mixed N-(heterocyclic)imine ligands 19 and 20 (L1 = py, L2 = 4-pic) were particularly photocytotoxic towards cisplatin resistant (A2780cis) cell lines. Complex 18 (L1 = py, L2 = 2-pic) was comparatively less photocytotoxic (IC50 value 14.5 μM) than the other complexes, despite demonstrating the greatest absorbance at the irradiation wavelength and the fastest half-life for loss of the N3 → Pt LMCT transition upon irradiation (λirr = 463 nm) in aqueous solution. Complex 29 (X1 = X2 = thiazole) although potently phototoxic (2.4 μM), was also toxic towards cells in the absence of irradiation.
Introduction
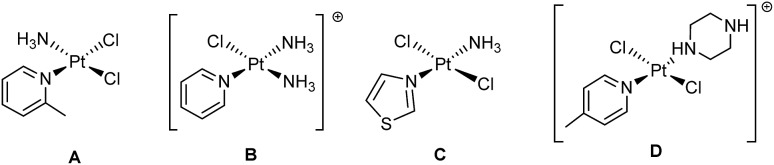
For cancers treated with chemotherapy, approximately half of patients currently receive a platinum(ii) drug, typically in combination with other therapies.1,2 The widely-used platinum(ii) complexes cisplatin,3 carboplatin4 and oxaliplatin5,6 all include ammine or aliphatic amine ligands, and exhibit potent activity against a number of different cancers. Development of resistance to treatment is a serious problem for platinum-based drugs, and a number of strategies have been explored to combat this, including the use of alternative ligand classes and geometries.7 In addition to the use of aliphatic amines, Pt(ii) complexes incorporating N-heterocyclic amines also show promise: picoplatin (AMD 473, A) which incorporates 2-picoline has been studied in clinical trials for the treatment of small cell lung cancer in 2007 (Fig. 1).8
Fig. 1. Pt(ii) compounds (A) picoplatin, (B) pyriplatin, (C) trans-[PtCl2(NH3)(thiazole)] and (D) trans-[PtCl2(4-pic)(piperazine·H)]+ which exhibit promising anti-cancer properties.
Picoplatin was developed to tackle the problem of platinum drug deactivation by S-donor molecules, such as glutathione, which can lead to the development of resistance. The steric hindrance caused by the methyl substituent on the pyridyl ring in close proximity to the Pt centre is thought to reduce the rates of side-reactions which can diminish drug potency.9 The pyridyl-complex pyriplatin (B) is highly cytotoxic towards a number of tumour cell lines; forming monofunctional Pt-DNA adducts which are proposed to evade DNA repair and inhibit transcription. The positive charge on the complex makes cellular uptake through organic cation transporters more likely than through passive diffusion or copper transporters – uptake mechanisms which are implicated for cisplatin – giving it the potential to be effective against cisplatin-resistant lines.10 Studies in zebrafish suggest that monofunctional complexes such as pyriplatin and phenanthriplatin may also generate less severe auditory side-effects (ototoxicity), compared with cisplatin.11 In addition to the ligands themselves, the use of aromatic pyridine-like ligands or substituted amines in a trans rather than a cis geometry in a Pt complex can give rise to cytotoxic activity in cisplatin-resistant cell lines.12,13
Trans complexes such as trans-[PtCl2(NH3)(thiazole)] (Fig. 1C) which include non-pyridyl heterocyclic amines such thiazole also demonstrate a good combination of cytotoxicity, aqueous solubility and in vivo activity,14 and trans-[PtCl2(4-pic)(piperazine·H)]+ (Fig. 1D) which incorporates picolyl and piperazine ligands is also highly cytotoxic towards cancer cells, with a mechanism of action distinct from cisplatin.15
Another strategy in the development of more selective anti-cancer drugs is the use of PtIV prodrugs;16 oxidation of square-planar PtII complexes to octahedral PtIV complexes incorporates two additional ligands into the axial positions. These low-spin 5d6 PtIV complexes are kinetically more inert than their PtII precursors, minimising unwanted side-reactions in advance of reduction in cellulo.17–20
PtIV azido complexes are a promising class of stimuli-responsive prodrugs which may be non-toxic in the dark but exhibit potently photocytoxic effects when irradiated with visible light.21 Complexes with amine ligands in a trans geometry can be more photocytotoxic than their corresponding cis isomers,22 and replacing aliphatic amines (e.g. NH3) with pyridyl ligands can enhance the photocytotoxic activity of a PtIV diazido complex when irradiated with longer – and therefore more clinically relevant – wavelengths of light; trans,trans,trans-[Pt(py)(NH3)(N3)2(OH)2] and trans,trans,trans-[Pt(py)2(N3)2(OH)2]23 exhibit IC50 values of 25.4 μM and 6.7 μM, respectively, (A2780 ovarian cancer cell line) when irradiated with visible light (420 nm) irradiation, whilst being non-toxic in the absence of irradiation. Use of other N-heterocyclic amine ligands like thiazole in e.g. trans,trans,trans-[Pt(N3)2(OH)2(methylamine)(thiazole)] can also result in potently photocytotoxic complexes.24
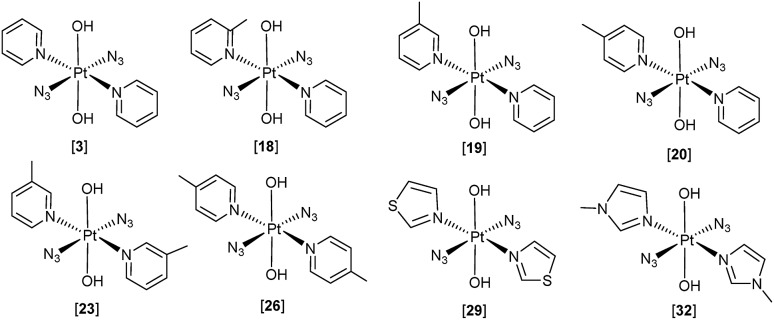
These structure–activity relationships suggested that PtIV azido complexes with N-heterocyclic amine ligands in a trans geometry require further investigation as potential photactivatable anti-cancer complexes. Here we present the synthesis, characterisation, photochemical and photobiological evaluation of a series of novel trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes (Fig. 2).
Fig. 2. PtIV diazido complexes reported previously (3), and studied here: trans,trans,trans-[Pt(N3)2(OH)2(2-picoline)(pyridine)] (18), trans,trans,trans-[Pt(N3)2(OH)2(3-picoline)(pyridine)] (19), trans,trans,trans-[Pt(N3)2(OH)2(4-picoline)(pyridine)] (20), trans,trans,trans-[Pt(N3)2(OH)2(3-picoline)2] (23), trans,trans,trans-[Pt(N3)2(OH)2(4-picoline)2] (26), trans,trans,trans-[Pt(N3)2(OH)2(thiazole)2] (29), trans,trans,trans-[Pt(N3)2(OH)2(1-methylimidazole)2] (32).
Results
Synthesis and characterisation
The precursor PtII complexes 1 and 2,238–11, 21, 24, 27 and 30 were synthesised using established methods (see ESI†).25 Complexes 3,238–11, 21, 24,26 and complexes 27 and 30 (ref. 27) have been reported previously. The novel PtIV-diazido complexes 18, 19, 20, 23, 26, 29 and 32 were synthesised from their PtII-diazido precursors via H2O2 oxidation, and characterised by 1H, 13C, 195Pt-NMR spectroscopy, ESI-MS and UV-vis spectroscopy (ESI).
X-ray diffraction
N.B. Pt-azido atom labelling: Pt–Nα–Nβ–Nγ
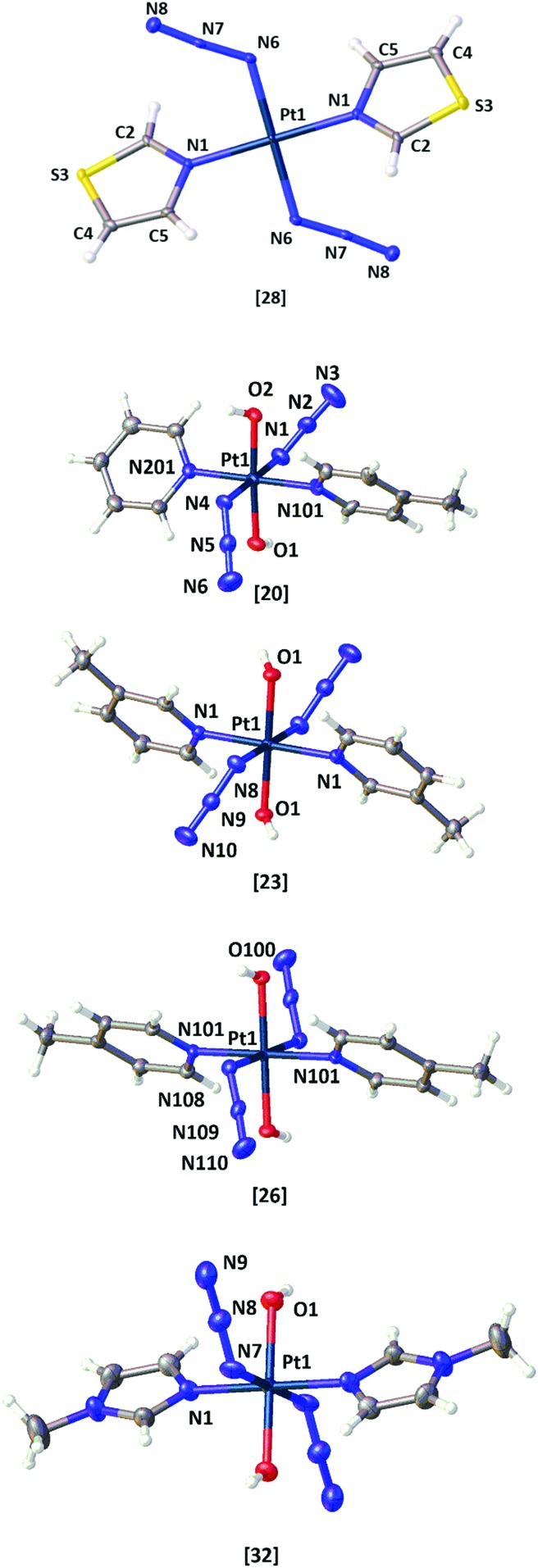
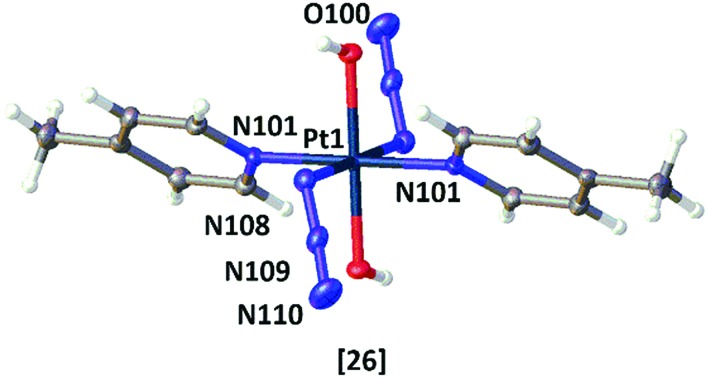
Single crystals suitable for X-ray diffraction studies were obtained for the novel PtII complex trans-[Pt(N3)2(tz)2] 28 (where tz = thiazole), and confirmed that the thiazole ligand is N- rather than S-coordinated to the PtII centre (Fig. 3 and Tables S1–S3†). X-ray crystallographic structures were also obtained for four of the novel PtIV azido picoline and imidazole complexes: 20 (py, 4-pic); 23 (3-pic, 3-pic); 26 (4-pic, 4-pic) and 32 (mim, mim), Fig. 3; crystals suitable for X-ray diffraction were grown as detailed in the ESI.† Crystallographic data for 20, 26, 23 and 32 are summarized in Tables S4–S8.† The crystal structures of the PtIV complexes all have an octahedral geometry with an [N4O2] coordination sphere around the metal centre. The structures of complexes 23 (3-pic, 3-pic), 26 (4-pic, 4-pic) and 32 (mim, mim) which each contain two identical amine ligands are highly symmetrical, with an O–Pt–O bond angle of 180.0(0)°. In contrast, the structure of complex 20 (py, 4-pic) is slightly distorted, with an O–Pt–O angle of 176.5(2)°. The two symmetrical picolyl complexes 23 and 26 have similar bond lengths and angles; with lengths of Pt–Nα (2.048(2) Å; 2.046(2) Å), Nα–Nβ (1.217(3) Å; 1.225(4) Å) and Nβ–Nγ (1.147(3) Å; 1.152(4) Å) and angles Nα–Nβ–Nγ (174.5(2)°; 175.6(3)°) and Pt–Nα–Nβ (115.56(16)°; 115.3(2)°) respectively. Complex 20 (py, 4-pic) showed similar Pt–Nα bond lengths to 23 and 26 (2.021(8) Å/2.038(7) Å) whereas in complex 32 (mim, mim) this bond was slightly elongated to 2.051(5) Å. In both complex 32 and complex 20 the Pt–NαNβ angle was less acute (118.8(4)°; 118.3(6)°/117.2(6)° respectively) when compared with the symmetric bis picolyl complexes.
Fig. 3. X-ray crystallographic structures of: trans-[Pt(N3)2(tz)2] 28; trans,trans,trans-[Pt(N3)2(OH)2(py)(4-pic)] 20; trans,trans,trans-[Pt(N3)2(OH)2(3-pic)2] 23; trans,trans,trans-[Pt(N3)2(OH)2(4-pic)2] 26; trans,trans,trans-[Pt(N3)2(OH)2(mim)2] 32. Thermal ellipsoids are displayed at 50% probability; figures were generated using Olex2.28.
Solution chemistry
Solubility
The trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes showed considerable variation in their maximum aqueous solubilities (Table 1), and were stable in D2O in the dark at ambient temperature (20–25 °C) for >2 weeks, as determined by 1H-NMR spectroscopy.
Table 1. Maximum aqueous solubility of the PtIV diazido complexes at 20 °C.
| Complex | Solubility/mM |
| 3 | 34 |
| 18 | 20 |
| 19 | 20 |
| 20 | 20 |
| 23 | 3 |
| 26 | 4 |
| 29 | 1 |
| 32 | 82 |
ESI-MS
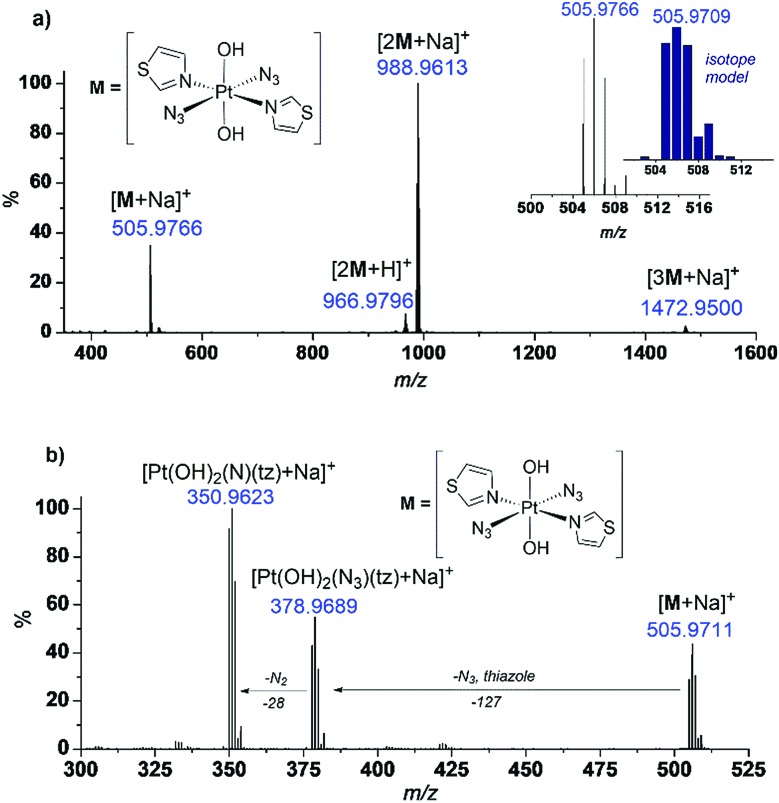
As commonly observed for PtIV diazido complexes, the complexes were detected by ESI-MS as a series of sodiated adducts, as exemplified by the thiazole complex trans,trans,trans-[Pt(OH)2(N3)2(tz)2] (29) (Fig. 4a). Speciation typically varies between H+ and Na+ adducts, depending on the mass spectrometer.
Fig. 4. (a) ESI-MS of complex trans,trans,trans-[Pt(OH)2(N3)2(tz)2] (29) in H2O; (b) MS/MS of species [29 + Na]+ (505.9711 m/z) revealing loss of neutral fragments N3˙, N2 and the thiazole ligand.
MSMS studies
Collision-induced dissociation can provide insight into the stability of a metal complex and the lability of the various ligands attached to the metal centre. Selective MSMS fragmentation of the sodiated complex [29 + Na]+ (Fig. 4b) gave similar fragmentation products to those which we previously reported for the dipyridyl complex, trans,trans,trans-[Pt(OH)2(N3)2(py)2] (3);23 in both cases fragmentation products were detected, e.g. for complex 29: [Pt(OH)2(N3)(tz) + Na]+ (378.97 m/z) following loss of the aromatic ligand and N3˙, and [Pt(OH)2(N)(tz) + Na]+ (350.96 m/z) following further loss of N2. The necessity of the products to be charged in order to be detected by ESI-MS does mean that these species represent only the positively-charged subset of the fragmentation products which are likely to be formed.
NMR spectroscopy
The complexes were characterised by 1H, 13C and 195Pt NMR spectroscopy. 195Pt NMR resonances for the novel PtIV complexes were all observed within the range 954–987 ppm (D2O), apart from complex 18 (py, 2-pic), for which the resonance was significantly more deshielded, being observed at 1132 ppm.
UV-vis absorption spectra
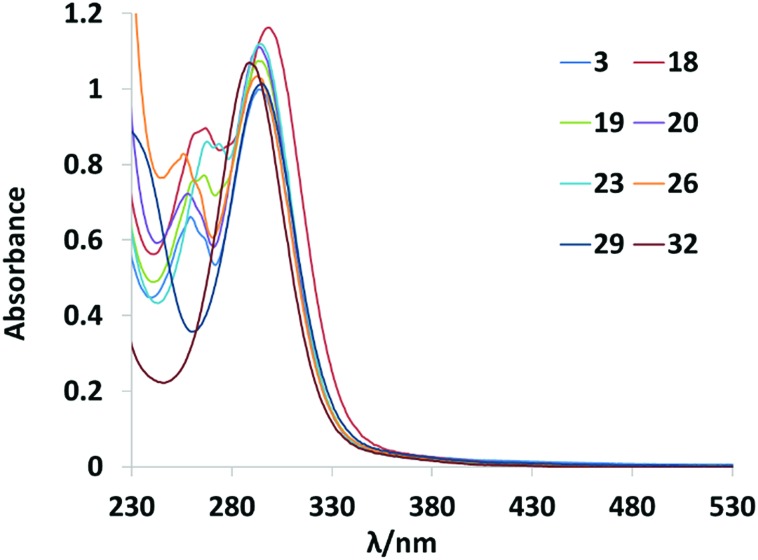
The UV-vis spectra of the PtIV diazido complexes in H2O are overlaid in Fig. 5. The absorption maxima (λmax) corresponding to the LMCT N3 → Pt transition for the complexes lies in the range 290–297 nm. Complex 18 (py, 2-pic) has the longest wavelength absorption maximum (297 nm), whereas 32 (mim, mim) has the shortest (290 nm). Complexes incorporating pyridyl derivatives have two main absorption bands in the UV region, whereas those incorporating thiazole or 1-methylimidazole have only one main absorbance band.
Fig. 5. UV-vis spectra of eight Pt(iv)-diazido complexes (60 μM, H2O).
TD-DFT
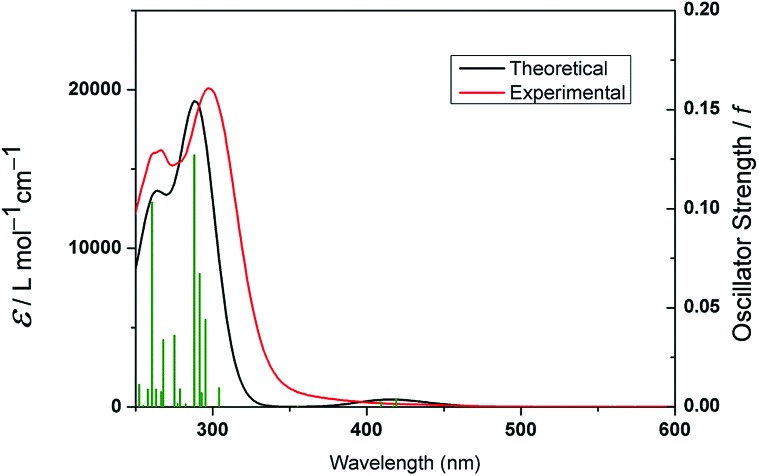
Because complex 18 (py, 2-pic) had the longest λmax, DFT (restricted and unrestricted), TDDFT calculations were carried out to characterize the singlet and triplet excited states. The theoretical UV-vis spectrum was simulated by the calculation of 32 singlet states using water as a solvent. The theoretical and experimental UV-vis spectra show reasonable agreement, with the absorption maximum underestimated by 9 nm (experimental: 297 nm, 20 091 M–1 cm–1, theoretical 288 nm, 19 282 M–1 cm–1) and the shoulder with only 1 nm difference (theoretical: 267 nm, 13 520 M–1 cm–1; experimental: 268 nm, 16 061 M–1 cm–1) (Fig. 6). As was previously reported for complex 3, these transitions can be assigned to dissociative 1LMCT (N3 → Pt) and mixed 1LMCT/1IL (OH → Pt, N; IL = interligand) transitions. The singlet excited states as well as the percentage contribution are presented in Tables S9, S10 and Fig. S1.†
Fig. 6. Calculated (red) and experimental (black) absorption spectra of 18. The excited states are shown as vertical green bars with heights equal to the extinction coefficients. The theoretical spectrum was obtained using GAUSSUM 2.2.29.
Photochemistry
Solution photochemistry
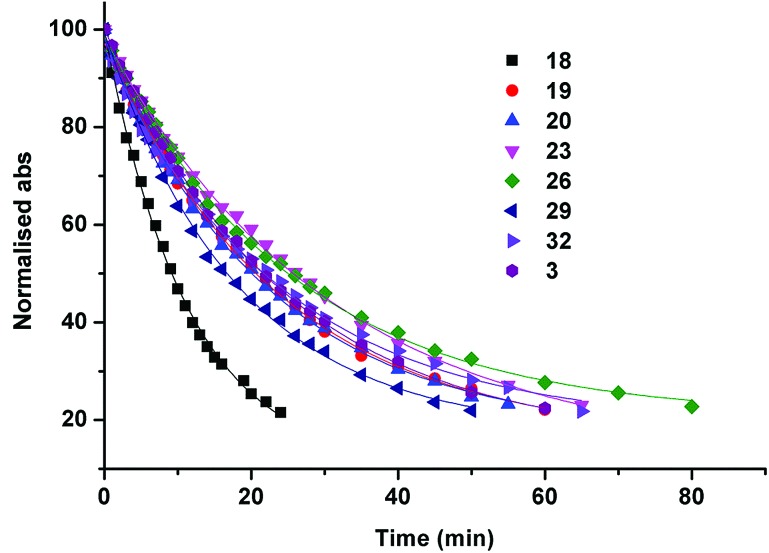
Solutions of the PtIV diazido complexes (60 μM, H2O) were irradiated with blue light (463 nm, 57 mW cm–2). The decrease in intensity of the λmax N3 → Pt band was monitored over time for each complex by UV-vis spectroscopy until ca. 22% of the original intensity remained (Fig. S2†). The data were fitted to y = y0 + AeRox, with r2 > 0.98 (Fig. 7). The fastest half-life of photodecomposition was observed for complex 18 (py, 2-pic); 2.5-fold faster than for the slowest for complexes 23 (3-pic, 3-pic) and 26 (4-pic, 4-pic) (Table S11†). The photosensitivity of complexes 18, 19 and 20 was also investigated with longer-wavelength green light (λ = 517 nm, 30 mW cm–2); despite minimal visible absorption at this wavelength the complexes clearly demonstrated photoinduced loss of the N3 → Pt band, although the process was significantly slower (>6 hours) to achieve a conversion comparable to that observed with 463 nm irradiation (Fig. S3†).
Fig. 7. Rate of decrease of the normalised absorbance (290–297 nm) for the Pt(iv) diazido complexes corresponding to the loss of the N3 → Pt LMCT band to an extent of ca. 22% of the original intensity, when irradiated with blue light (463 nm, 57 mW cm–2).
Irradiation of complexes 19, 20, 23, 26 and 32 (2 mM in PBS/D2O pH* 7.4, 1 mM for 29, due to low solubility) with 420 nm light (1 h, 24.7 mW cm–2) in the presence of 5′-GMP (2 mol eq.) resulted in formation of 5′-GMP platinum adducts (determined by 1H NMR spectroscopy). Photoejection of the N-heterocyclic ligand was not observed for any complex. The percentage of bound 5′-GMP was quantified by integration of the diagnostic 5′-GMP sugar C1 protons in the 1H NMR spectral region 5.50–6.00 ppm (Table S12†). The highest conversion to new 5′-GMP adducts was seen for 29 (tz, tz) (80%), and the lowest for 32 (mim, mim) (48%) with the remaining compounds showing intermediate conversions. Complex 18 showed moderate 5′-GMP binding (52%).
Complex 18, as the most rapidly photoreduced complex in the absence of 5′-GMP (Fig. 7), was irradiated in the presence of 5′-GMP (9 mM in PBS/D2O pH* 7.4, 2 mol eq. 5′-GMP) with 420 nm light (45 min, 7 mW cm–1) and was more extensively investigated by 1H, 195Pt NMR spectroscopy and LC-MS. The intensity of the 195Pt NMR spectroscopic signal corresponding to the PtIV starting material (1132 ppm) rapidly decreased (Fig. S4A†), and a new resonance corresponding to [PtII(N3)(py)(2-pic)(5′-GMP)] appeared at –2195 ppm. A smaller signal (–2110 ppm) was also detected upfield of the main resonance in the PtII region (Fig. S4B†). After two weeks in the dark, the signal at –2110 ppm had disappeared, and a new broad resonance corresponding to the bis 5′-GMP adduct [PtII(py)(2-pic)(5′-GMP)2] appeared at –2280 ppm (Fig. S4C†). The samples were analysed by LC-MS shortly after irradiation, and again after two weeks, confirming the proposed speciation and the relative increase in concentration of the bis adduct (Fig. S5†). This is in contrast to our observations for 3, which rapidly formed two approximately equal intensity signals corresponding to the mono (–2212 ppm) and bis (–2288 ppm) substituted 5′-GMP adducts.23
Phototoxicity
The novel trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes PtIV complexes were evaluated against human ovarian (A2780), cisplatin-resistant ovarian (A2780cis) and oesophageal (OE19) cancer cell lines, and the previously reported compounds (FM165 and 3) were also evaluated for comparison. Phototoxicity (IC50 values) and phototoxicity indices (PI) are shown in Table 2. Complexes 19, 20, 23, 26, and 32 were non-toxic in the absence of irradiation (defined as IC50 > 200 μM). Complex 18 (py, 2-pic) exhibited moderate dark toxicity in both of the cell lines in which it was evaluated (A2780 and A2780cis), and complex 29 (tz, tz) exhibited some dark toxicity in the A2780 cell line, but no toxicity in either of the A2780cis or OE19 lines.
Table 2. Phototoxicity of the PtIV complexes trans,trans,trans-[Pt(N3)2(OH)2(L1)(L2)] following irradiation with blue light (420 nm, 5 J cm–2, TL03, filtered <400 nm).Values are in μM.
| Complex | L1 | L2 | A2780 |
A2780cis |
OE19 |
||||||
| IC50 | 95% CI | a PI | IC50 | 95% CI | PI | IC50 | 95% CI | PI | |||
| 18 | py | 2-pic | 14.5 | 12.7–16.7 | 7.3 d | 15.5 | 11.2–21.5 | 5.3 d | ND | ND | ND |
| 19 | py | 3-pic | 4.0 | 3.0–5.4 | >51.5 | 3.3 | 1.9–5.9 | >71.0 | 5.5 | 3.2–9.4 | >38 |
| 20 | py | 4-pic | 5.4 | 4.3–6.8 | >38 | 4.6 | 3.8–5.6 | >45 | 12.3 | 7.9–19.1 | >17 |
| 23 | 3-pic | 3-pic | 7.2 | 6.0–8.7 | >27.8 | 10.4 | 8.7–12.2 | >19.3 | ND | ND | ND |
| 26 | 4-pic | 4-pic | 2.1 | 1.6–2.7 | >95 | 4.1 | 3.3–5.1 | >48.8 | 8.2 | 5.5–12.0 | >24 |
| 29 | Thiazole | Thiazole | 2.4 | 2.2–2.7 | 48 d | 2.9 | 1.6–5.2 | >71.0 | 7.6 | 5.4–10.7 | >27 |
| 32 | mim | mim | 56.3 | 40.2–78.9 | >3.7 | 164.2 | Wide | >1.3 | ND | ND | ND |
| FM165 c | py | NH3 | 25.4 | 22.2–29.1 | >9.6 | b ND | ND | ND | ND | ND | ND |
| 3 | py | py | 6.7 | 3.6–13.7 | >45 | ND | ND | ND | 8.4 | ND | >45 |
aPI = phototoxic index (ratio of cytotoxicity following irradiation vs. in the dark). Explicit cytotoxicity values in the dark will be added to the ESI if they become available.
bND = not determined.
cFM165 = trans,trans,trans-[Pt(N3)2(OH)2(NH3)(py)].
d= cytotoxic in the absence of irradiation. mim = 1-methylimidazole.
Aside from complex 18 (py, 2-pic); the py/n-pic family of complexes (19, 20, 23, 26) all exhibited potent phototoxicity with minimal toxicity in the dark. Compared with 3 (py, py) as a benchmark,2326 (4-pic, 4-pic) demonstrated greater phototoxicity in the lines tested, being 3-fold more potent towards the A2780 line (2.1 μM vs. 6.7 μM) with similar phototoxicity (8.2 μM (26) vs. 8.4 μM (3)) in the OE19 line. In the lines tested, complex 23 (3-pic, 3-pic) was less potent than 26 (4-pic, 4-pic), and within error, was similar to complex 3.
The mixed ligand complexes 19 (py, 3-pic) and 20 (py, 4-pic) showed slightly greater phototoxicity than 3 (py, py) in the A2780 ovarian line (4.0 μM and 5.4 μM respectively compared to 6.7 μM (3)), but encouragingly, demonstrated greater photototoxicity in the cisplatin resistant line A2780cis (3.3 μM (19) and 4.6 μM (20)) than in the A2780 line. We previously determined that – with UVA irradiation – 3 was significantly less phototoxic in A2780cis (14.5 μM) than in A2780 (1.4 μM). Furthermore, 19 (py, 3-pic) showed the most potent phototoxicity (5.5 μM) towards OE19 cells of all the compounds tested.
Complex 29 (tz, tz) demonstrated potent phototoxicity (2.9 μM in A2780cis cells; 7.6 μM in OE19) compared with our previously reported thiazole complex trans,trans,trans-[Pt(N3)2(OH)2(NH2CH3)(thiazole)] (6.4 μM in A2780cis; 19.3 μM in OE19)24 with blue light (420 nm) irradiation, without any dark toxicity towards these cell lines. Complex 32 (mim, mim) demonstrated good aqueous solubility, but relatively low phototoxicity in the A2780 and A2780cis lines; due to this modest activity it was not evaluated against the OE19 cell line.
Despite potent photocytotoxicity under blue light irradiation (420 nm), complex 20 (py, 4-pic) showed no phototoxicity following irradiation with green light (515 nm) in either A2780 or OE19 cell lines.
Discussion
Synthesis and photochemistry
Seven novel trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes of form the trans,trans,trans-[Pt(N3)2(OH)2(L1)(L2)], where L1 and L2 = pyridine, 2, 3, 4-picoline, thiazole or 1-methylimidazole (18, 19, 20, 23, 26, 29 and 32) have been synthesised from their PtII-diazido precursors via H2O2 oxidation, and characterised by 1H, 13C, 195Pt-NMR spectroscopy, ESI-MS and UV-vis spectroscopy.
The single crystal X-ray crystal structures were obtained for one PtII diazido precursor (28) and four of the novel Pt(IV)-diazido complexes (20, 23, 26, 32). Although slight distortions from octahedral geometry were observed in the complexes bearing two different N-heterocyclic amine ligands in trans position, the key bond lengths and angles within the structures are relatively similar to the previously reported trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complex 3. Although an X-ray crystal structure was not been obtained for the PtIV azido bis-thiazole derivative, it is likely that N-coordination of the thiazole is retained in 29 following oxidation.
The PtIV azido compounds demonstrated significant variation in aqueous solubility over the range 1–82 mM depending on the N-heterocyclic amine ligands; complexes which were mixed-ligand isomers (py, n-pic) 18, 19, 20 exhibited similar aqueous solubility (20 mM) as did the bis (n-pic, n-pic) complexes (3–4 mM) and inclusion of two thiazole ligands significantly decreased the aqueous solubility (1 mM). These data indicate that the position of n-pic substitution has a negligible effect on the solubility.
UV-vis spectroscopy revealed that of all the complexes reported, 18 (2-pic, 2-pic) has the longest λmax in the UV-vis absorption spectrum. Consistent with this, irradiation (463 nm) revealed 18 to be the most rapidly photoreduced complex, and DFT and TDDFT calculations showed higher intensity transitions for 18 in the 410–470 nm region compared to the previously reported complex 3 (py, py). Irradiation (420 nm) of the novel PtIV complexes in the presence of 5′-GMP resulted in the rapid formation of both mono and bis 5′-GMP Pt adducts, with the exception of the sterically crowded complex 18 which initially formed only the mono adduct [Pt(N3)(2-pic)(py)(5′-GMP)], whilst a smaller amount of bis-substituted adduct [Pt(2-pic)(py)(5′-GMP)2] formed significantly more slowly. This photochemical speciation was significantly different from that observed for 3 (py, py) which we previously demonstrated forms both mono and bis 5′-GMP adducts immediately.23 The reduction potential of cis,trans,cis-[PtIVCl2(OH)2(NH3)(L)] has been reported to increase by 56 mV by replacing L = pyridine with 2-picoline: attributed to the steric clash between the N-ligand and the platinum, destabilising the Pt–O bond and facilitating reduction to Pt(ii).30 We reasoned that such destabilization may be beneficial for the photochemical reduction of Pt(iv)–diazido complexes if sufficient stability in the dark could be retained.
Attempts to obtain crystals of 18 suitable for diffraction were unsuccessful. Nevertheless, the DFT-optimized geometry of 18 (Table S9†) resembled the asymmetries in the structure of 20, albeit with slightly overestimated bond distances, particularly for Pt–O bonds.
Phototoxicity
The choice of N-heterocyclic amine ligands plays a crucially important role in the photochemical and photobiological properties of trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes; variation in the position of a single methyl group within the series of n-pic complexes is sufficient to destabilise a complex (18: py, 2-pic) such that it becomes cytotoxic in the absence of irradiation towards both A2780 and A2780cis ovarian cancer cell lines. Variations in cytotoxicity between picoline analogues has precedent; for trans-[PtIIX2(3-pic)2] and trans-[PtIIX2(4-pic)2] (X = Cl–, OAc–) the 4-picoline complexes were more cytotoxic than 3-pic derivatives (Pam 212 and Pam 212-ras cell lines).31 In general, changing the position of a substituent on pyridine ligands has a significant effect on the cytotoxic properties; evaluation of trans-[PtCl2(3-acetylpyridine)2] and trans-[PtCl2(4-acetylpyridine)2] in a range of cell lines showed that the 4-acetylpyridine complex was more cytotoxic.32
Phototoxicity studies (λirr 420 nm) of the (py)(n-pic) series of complexes in different cell lines showed a general trend from most phototoxic to least phototoxic: 26 (4-pic, 4-pic) > 19 (py, 3-pic) > 20 (py, 4-pic) > 3 (py, py) > 23 (3-pic, 3-pic) > 18 (py, 2-pic). Despite already being cytotoxic in the absence of irradiation and the most rapidly photoreduced in solution, 18 (py, 2-pic) was the least phototoxic of this series; being nearly 7-fold less phototoxic than 26 (4-pic, 4-pic; A2780). Although the inclusion of the sterically demanding 2-picoline ligand results in a shift of LMCT band towards longer wavelengths and also in rapid photodecomposition of 18 (λirr 420 nm), this does not appear to translate to greater phototoxicity in cellulo. This may be due to excessive crowding close to the platinum centre and the slow formation of bifunctional adducts for 18 when compared with other complexes, as indicated by 5′-GMP binding studies. Picoplatin (Fig. 1) – which also contains 2-picoline – also forms DNA crosslinks and binds plasma proteins more slowly than cisplatin. However, picoplatin is likely to form different DNA lesions compared to 18 due to its cis geometry, and the NH3 of picoplatin is also less sterically demanding than the pyridine of 18.
Encouragingly, little or no cross-resistance was observed for the mixed ligand complexes 19 (py, 3-pic) and 20 (py, 4-pic) in the cisplatin-resistant ovarian cancer cell line (A2780cis) than towards the ovarian (A2780) line. In contrast, the bis ligand complexes: 23 (3-pic, 3-pic) and 26 (4-pic, 4-pic) and 32 (mim, mim) and the previously reported 3 (py, py – with UVA light)23 all demonstrated relatively lower phototoxicity against the cisplatin resistant line (A2780cis) than the A2780 line. This indicates that the use of these mixed ligand systems may be effective at circumventing cisplatin-resistance; however, investigation of a wider panel of cell lines – particularly those which are more closely representative of patient-derived ovarian tumours than A2780 cells33 will clarify if this efficacy is more widely observed.
Although all the complexes were slightly less potent towards the oesophageal line than the ovarian cancer cell lines, the phototoxicity of 19 (py, 3-pic; 5.5 μM) towards the OE19 oesophageal cancer cell line is notable. Oesophageal cancer is one of the most deadly cancers worldwide because of its extremely aggressive nature and poor survival rate. Ranking sixth among all cancers in mortality, it is increasing in incidence in Western populations.34 It is also a superficial cancer which may benefit from photochemotherapy with light of shorter wavelengths (e.g. blue rather than green) to prevent damage to underlying healthy tissue, since light penetration is wavelength-dependent.35
Our previously reported thiazole complex (tz, methylamine) showed no dark cytotoxicity (IC50 > 232.9 μM) and good phototoxicity under 420 nm irradiation (IC50: 28.2 (A2780); (A2780cis) 6.4; (OE19) 19.3 μM).24 Although the bis thiazole complex 29 (tz, tz) reported here showed at least 2-fold greater phototoxicity in all three cell lines, 29 was also slightly cytotoxic in the absence of irradiation towards A2780 cells only (IC50 115.2 μM; phototoxic index = 48). PtIV diazido complexes which are cytotoxic towards some cell lines in the absence of irradiation (e.g.18, 29) could potentially be derivatised and developed as redox-activatable PtIV prodrugs (e.g. satraplatin, which has been evaluated in Phase III trials for prostate cancer),2 particularly if they show suitable pharmacological characteristics (e.g. good solubility). However, the complexes would need to be potently (IC50 < 10 μM) cytotoxic which may be achievable through judicious axial ligand choice. Predicting the biological rates of reduction of PtIV complexes (e.g. by cytochrome c, metallothioneins, glutathione, ascorbate) is not straightforward;36 reduction potentials measured in solution do not necessarily correlate with rates of reduction by reducing agents.37 Reduction can occur through either inner or outer sphere mechanisms, depending on the bridging capabilities of ligands.38 If there are other ligands present which can bridge to reducing agents to facilitate reduction (e.g. Cl, OH) then complexes with axial halide (Cl/I) groups are generally reduced most rapidly, followed by bis COOR complexes, then mixed OH/COOR, with OH/OH complexes reduced most slowly.39 However, trends in reduction rates and cytotoxicity are highly complex-dependent: mixed OH/COOR compounds incorporating cisplatin and ethacrynic acid show more facile reduction (which translates to greater in vivo activity) than the corresponding bis COOR complexes, despite the presence of Cl ligands.40 In its current form, complex 29 is not sufficiently cytotoxic in the absence of irradiation to be developed as a redox-active PtIV prodrug and since it exhibited modest aqueous solubility (1 mM). Any (axial) modifications made to improve cytotoxicity would also need to improve solubility.
Replacing the pyridyl, picolyl or thiazole ligands with methylimidazole significantly decreased the phototoxicity of the complex (32). Investigation of the lipophilicity and cellular accumulation of this complex is anticipated to shed more light on the likely reason(s) for the lower phototoxicity.
Experimental
For materials, methods and procedures see ESI.†
Conclusions
We have reported the synthesis and characterisation of seven novel trans-di-(N-heterocyclic)imine dihydroxido diazido PtIV complexes which have been characterised by 1H, 13C, 195Pt-NMR spectroscopy, ESI-MS and UV-vis spectroscopy. Photochemical and photobiological experiments show that complexes 19 (py, 3-pic), 20 (py, 4-pic), 23 (3-pic, 3-pic), 26 (4-pic, 4-pic), and 32 (mim, mim) are non-toxic in the absence of irradiation in the cell lines investigated. The picolyl complexes 19, 20 and 26 were more potently phototoxic in all cell lines than our previously reported complex 3 (py, py) when irradiated with blue light. Based on their good aqueous solubility (20 mM), dark stability and potent phototoxicity – particularly towards cisplatin resistant cell lines – complexes 19 (py, 3-pic) and 20 (py, 4-pic) in particular warrant further investigation as photoactivatable PtIV prodrugs. Complex 19 also shows good activity towards the oesophageal cancer line. Investigation of the cellular accumulation of these PtIV azido complexes is anticipated to shed further light on the observed biological activity;41 these experiments are currently underway.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
NF is supported by the Wellcome Trust (201406/Z/16/Z); Cancer Research UK (CR-UK) grant number C5255/A18085, through the Cancer Research UK Oxford Centre; and the John Fell Fund. NF thanks Profs. Stephen Faulkner and Andrew Weller for advice and support. The Oxford Diffraction Gemini XRD system was obtained through the Science City Advanced Materials project: Creating and Characterising Next Generation Advanced Materials, with support from Advantage West Midlands (AWM) and part funded by the European Regional Development Fund (ERDF).We also thank the ERC (grant no. 247450), MRC (grant no. G0701062) and EPSRC (grant no. EP/G006792) for their support for this work.
Footnotes
†Electronic supplementary information (ESI) available: Materials and methods, synthesis and characterisation data, X-ray crystallographic tables, biological data. CCDC deposits 1904755 (28), 1904751 (20), 1904753 (26), 1904752 (23), 1904754 (32) contain the crystallographic data. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02644d
References
- Kelland L. Nat. Rev. Cancer. 2007;7:573. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Wheate N. J., Walker S., Craig G. E., Oun R. Dalton Trans. 2010;39:8113. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- Breglio A. M., Rusheen A. E., Shide E. D., Fernandez K. A., Spielbauer K. K., McLachlin K. M., Hall M. D., Amable L., Cunningham L. L. Nat. Commun. 2017;8:1. doi: 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer R. J., Ater J., Allen J., Phillips P., Geyer R., Nicholson H. S., Jakacki R., Kurczynski E., Needle M., Finlay J., Reaman G., Boyett J. M. J. Neurosurg. 1997;86:747. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- Bruno P. M., Liu Y., Park G. Y., Murai J., Koch C. E., Eisen T. J., Pritchard J. R., Pommier Y., Lippard S. J., Hemann M. T. Nat. Med. 2017;23:461. doi: 10.1038/nm.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. B., Alqazzaz A., Hwang J. R., Qi X., Keegan A. D., Kim A. J., Winkles J. A., Woodworth G. F. J. Neuro-Oncol. 2018:497. doi: 10.1007/s11060-018-2979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D. J., Wang J., Zhou J. Y., Wu G. S. Biochem. Biophys. Res. Commun. 2010;394:600. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravera M., Gabano E., Zanellato I., Bonarrigo I., Escribano E., Moreno V., Font-Bardia M., Calvet T., Osella D. Dalton Trans. 2012;41:3313. doi: 10.1039/c2dt11874b. [DOI] [PubMed] [Google Scholar]
- Holford J., Sharp S. Y., Murrer B. A., Abrams M., Kelland L. R. Br. J. Cancer. 1998;77:366. doi: 10.1038/bjc.1998.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M., Lippard S. J., Cvitkovic E., Raymond E., Bieche I., Serova M., Faivre S., Broggini M., Emami S., Lovejoy K. S., Erba E., Gespach C. Mol. Cancer Ther. 2011;10:1709. doi: 10.1158/1535-7163.MCT-11-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. D., Hruska H. L., Ruggles H. K., Williams K. M., Smith M. E. PLoS One. 2018;13:1. doi: 10.1371/journal.pone.0192505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natile G., Coluccia M. Coord. Chem. Rev. 2001;216–217:383. [Google Scholar]
- Aris S. M., Farrell N. P. Eur. J. Inorg. Chem. 2009:1293. doi: 10.1002/ejic.200801118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dange Y., Gupta M. S., Gewirtz D. A., Khan Q. A., Pommier Y., Povirk L. F., Kohlhagen G., DeMasters G., Farrell N. Biochem. Pharmacol. 2004;68:857. doi: 10.1016/j.bcp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Najajreh Y., Perez J. M., Navarro-Ranninger C., Gibson D. J. Med. Chem. 2002;45:5189. doi: 10.1021/jm0201969. [DOI] [PubMed] [Google Scholar]
- Ravera M., Gabano E., McGlinchey M. J., Osella D. Inorg. Chim. Acta. 2019;492:32. [Google Scholar]
- Gibson D. J. Inorg. Biochem. 2019;191:77–84. doi: 10.1016/j.jinorgbio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Yap S. Q., Chin C. F., Hong Thng A. H., Pang Y. Y., Ho H. K., Ang W. H. ChemMedChem. 2017;12:300. doi: 10.1002/cmdc.201600577. [DOI] [PubMed] [Google Scholar]
- Ravera M., Gabano E., Zanellato I., Gallina A., Perin E., Arrais A., Cantamessa S., Osella D. Dalton Trans. 2017;46:1559. doi: 10.1039/c6dt03749f. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z., Bonnitcha P., Wexselblatt E., Klein A. V., Najajreh Y., Gibson D., Hambley T. W. Chem.–Eur. J. 2013;19:1672. doi: 10.1002/chem.201203159. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang X., Jin S., Muhammad N., Guo Z. Chem. Rev. 2019;119:1138. doi: 10.1021/acs.chemrev.8b00209. [DOI] [PubMed] [Google Scholar]
- Farrer N. J., Woods J. A., Munk V. P., Mackay F. S., Sadler P. J. Chem. Res. Toxicol. 2010;23:413. doi: 10.1021/tx900372p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer N. J., Woods J. A., Salassa L., Zhao Y., Robinson K. S., Clarkson G., Mackay F. S., Sadler P. J. Angew. Chem., Int. Ed. Engl. 2010;49:8905. doi: 10.1002/anie.201003399. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Woods J. A., Farrer N. J., Robinson K. S., Pracharova J., Kasparkova J., Novakova O., Li H., Salassa L., Pizarro A. M., Clarkson G. J., Song L., Brabec V., Sadler P. J. Chem.–Eur. J. 2013;19:9578. doi: 10.1002/chem.201300374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman G. B., Thompson R. J. Inorg. Synth. 1963;7:249. [Google Scholar]
- Tessier C., Rochon F. D. Inorg. Chim. Acta. 1999;295:25. [Google Scholar]
- Farrell N., Kelland L., Roberts J., Van Beusichem M. Cancer Res. 1992;52:5065. [PubMed] [Google Scholar]
- Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H. J. Appl. Crystallogr. 2009;42:339. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle N. M., Tenderholt A. L., Langner K. M. J. Comput. Chem. 2008;29:839. doi: 10.1002/jcc.20823. [DOI] [PubMed] [Google Scholar]
- Battle A. R., Choi R., Hibbs D. E., Hambley T. W. Inorg. Chem. 2006;45:111. doi: 10.1021/ic060273g. [DOI] [PubMed] [Google Scholar]
- Quiroga A. G., Pérez J. M., Alonso C., Navarro-Ranninger C., Farrell N. J. Med. Chem. 2006;49:224. doi: 10.1021/jm050804v. [DOI] [PubMed] [Google Scholar]
- Rakić G. M., Grgurić-Sipka S., Kaluderović G. N., Gómez-Ruiz S., Bjelogrlić S. K., Radulović S. S., Tesić Z. L. Eur. J. Med. Chem. 2009;44:1921. doi: 10.1016/j.ejmech.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Ince T. A., Sousa A. D., Jones M. A., Harrell J. C., Agoston E. S., Krohn M., Selfors L. M., Liu W., Chen K., Yong M., Buchwald P., Wang B., Hale K. S., Cohick E., Sergent P., Witt A., Kozhekbaeva Z., Gao S., Agoston A. T., Merritt M. A., Foster R., Rueda B. R., Crum C. P., Brugge J. S., Mills G. B. Nat. Commun. 2015;6:1. doi: 10.1038/ncomms8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. World J. Gastroenterol. 2013;19:5598. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A., Conde R., Fontolliet C., Wagnieres G., Van den Bergh H., Monnier P. Gastrointestinal Endoscopy. 2003;57:897. doi: 10.1016/s0016-5107(03)70027-3. [DOI] [PubMed] [Google Scholar]
- Wexselblatt E., Gibson D. J. Inorg. Biochem. 2012;117:220. doi: 10.1016/j.jinorgbio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z., Wexselblatt E., Hambley T. W., Gibson D. Chem. Commun. 2012;48:847. doi: 10.1039/c1cc16647f. [DOI] [PubMed] [Google Scholar]
- Lemma K., Berglund J., Farrell N. P., Elding L. I. J. Biol. Inorg Chem. 2000;5:300. doi: 10.1007/pl00010658. [DOI] [PubMed] [Google Scholar]
- Kenny R. G., Chuah S. W., Crawford A., Marmion C. J. Eur. J. Inorg. Chem. 2017;2017:1596. [Google Scholar]
- Lee K. G. Z., Babak M. V., Weiss A., Dyson P. J., Nowak-Sliwinska P., Montagner D., Ang W. H. ChemMedChem. 2018;13:1210. doi: 10.1002/cmdc.201800105. [DOI] [PubMed] [Google Scholar]
- Pizarro A. M., McQuitty R. J., Mackay F. S., Zhao Y., Woods J. A., Sadler P. J. ChemMedChem. 2014;9:1169. doi: 10.1002/cmdc.201402066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.