Abstract
Background
Pregnant women and newborns are at risk for vitamin D deficiency (VDD). Also, poor health outcomes for pregnant women with VDD are reported in the published literature.
Objective
The aim of this systematic review was to estimate the prevalence of hypovitaminosis D and the associated risk factors for hypovitaminosis D in Middle Eastern pregnant women and their newborns.
Results
The prevalence of circulating 25-hydroxyvitamin D (25(OH)D) 50 nmol/L as a marker of vitamin D status in pregnant women and their newborns was between 24.5-98% and 22-100%, respectively. The prevalence of 25(OH) D 25 nmol/L in pregnant women and their newborns was over a wide range between 16.7-80% and 22-82%, respectively. Predictors for low maternal and neonatal 25(OH)D concentrations included decreased vitamin D synthesis due to reduced exposure to sunlight and decreased nutritional intake of vitamin D. A predictor of low neonatal 25(OH)D concentrations included maternal vitamin D status and the correlation between vitamin D concentrations in maternal and cord blood.
Conclusion
The high prevalence of VDD in the pregnant women of the Middle East underscores the necessity of implementing national prevention and intervention strategies. A clear policy for clinicians and healthcare workers is needed for screening and maintaining sufficient vitamin D status during pregnancy.
Keywords: Vitamin D, Pregnancy, Newborns, Cord blood, Middle East.
1. Introduction
Vitamin D deficiency (VDD) is a global public health problem in all age groups (1). There are four recent meta-analyses showing that pregnant women and newborns are at a risk for VDD (2-5). A number of studies have reported that pregnancy alone increases the risk of VDD (6). Results of two recent meta-analyses showed that there was a significant relationship between maternal VDD and adverse maternal, fetal, and postnatal outcomes (7, 8).
Poor health outcomes for pregnant women with VDD have been reported and include: gestational diabetes mellitus (7-11), preeclampsia (7-9, 12-14), intrahepatic cholestasis of pregnancy (15), preterm labor (16, 17), cesarean section delivery (18-20), periodontal disease (21, 22), and human immunodeficiency virus (HIV) progression (23). Poor health outcomes for newborns as a consequence of maternal VDD have also been reported, including increased risk of low birth weight mainly due to prematurity (7), small-for-gestational-age (7-9, 20, 24-27), body composition and cardiovascular disease risk factors in the offspring (28, 29), abnormal skeletal development (30-35), abnormal immune development (24, 26, 27, 30, 36-38), affected respiratory health including wheezing and asthma (24, 27, 30, 36, 39, 40), type 1 diabetes (26, 27, 30, 34-36), and abnormal neurocognitive development in childhood (41). VDD rickets occurs most commonly during early infancy and is prevalent in infants of mothers who have poor vitamin D stores (42).
The countries of the Middle East - Bahrain, Cyprus, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, and Yemen - have a high incidence of VDD and rickets
(43-44). Although the Middle East has a hot, sunny and arid climate; and is located within the latitudes from 12N-42 N; both of which allow for vitamin D synthesis from ultraviolet B (UVB) rays for most months of the year and for more than 8 hr/day (45), there exists significant VDD. Therefore it is important to determine the mother's vitamin D status and associated factors during pregnancy in order to prevent neonatal VDD and related complications (46-48).
A number of recent reviews have included the rapidly growing body of literature on vitamin D during pregnancy. However, because few studies conducted in the Middle East and North Africa were population-based, extrapolation to the vitamin D nutritional status of pregnant women and their neonates in that region is limited (44). In the past five yr, only one review focused on maternal and newborn vitamin D status, and in this review, just 13% of 95 studies were from countries of the Eastern Mediterranean which includes the countries of the Middle East and Afghanistan, Djibouti, Libya, Morocco, Pakistan, Somalia, Sudan, Tunisia, and not Bahrain, Israel, Occupied Palestinian territories, and Greece (49).
To address this lack of a review representing the Middle East specifically, we undertook a systematic review of the literature on hypovitaminosis D and risk factors in pregnant women and their newborns in the countries of the Middle East.
2. Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines.
Search strategy
This study is a systematic review of the published literature about vitamin D status in pregnant women and their newborns in the Middle East. It was conducted by exploring international electronic databases PubMed and Scopus with the following MeSH and Entry Terms: (“Infants, Newborn” OR “Newborn Infant” OR “Newborn Infants” OR “Newborns” OR “Newborn” OR “Neonate” OR “Neonates” OR “Women, Pregnant” OR “Pregnant Woman” OR “Woman, Pregnant” OR “Blood, Fetal” OR “Bloods, Fetal” OR “Fetal Bloods” OR “Cord Blood” OR “Blood, Cord” OR “Bloods, Cord” OR “Cord Bloods “OR” Umbilical Cord Blood” OR “Blood, Umbilical Cord” OR “Bloods, Umbilical Cord” OR “Cord Blood, Umbilical” OR “Cord Bloods, Umbilical” OR “Umbilical Cord Bloods”) AND (“Vitamin D” OR “Deficiency, Vitamin D” OR “Deficiencies, Vitamin D” OR “Vitamin D Deficiencies”) AND (“Middle East” OR “Near East” OR “Arab Countries” OR “Palestine” OR “Bahrain” OR “Iran” OR “Iraq” OR “Israel” OR “Jordan” OR “Kuwait” OR “Lebanon” OR “Egypt” OR “Cyprus” OR “Oman” OR “Qatar” OR “Saudi Arabia” OR “Syria” OR “Turkey” OR “United Arab Emirates” OR “Yemen”).
The period of publication was from 2000 to 2017. Publications were searched and reference lists were hand-searched. The confirmed sources were examined using a data extraction form. Based on the protocol, we reviewed all cross-sectional studies, prospective studies, and case-control studies, which had been conducted on the status of vitamin D in pregnant women and their newborns in the Middle East in the desired time limit.
The process of study selection
Study selection was performed in three phases: In the first phase, titles were scanned according to the selection criteria. The accepted titles were entered into the abstract review phase to identify studies for eligibility. In the next phase, abstracts were reviewed; the study was excluded if the reviewer found that the study met one or more exclusion criterion/criteria. The last phase was performed to determine if the full texts should be included for data extraction. In total, 1,857 articles were investigated, of which, 1,722 papers were excluded due to the lack of consistency with the goals of the study; as for example, studies on the general population (teenage, children, men, or breastfeeding women), and studies that provided no new empirical data (reviews, editorial letters, and brief items). Moreover, 12 papers were ruled out due to lack of access to the original article; for instance, because of the type of language (Turkish or Arabic language). Finally, 51 papers were included in this study (Figure 1).
Data extraction, data elements, and quality assessment
Each study was evaluated using a data extraction form. We developed a data extraction form to collect key indicators of each study, including study design, definition of VDD and vitamin D insufficiency, as well as assays used and characteristics of the study population. We extracted data on these indicators as reported in the articles. We collected data from the included studies and organized the results in a table format. The study outcomes are presented in the Results section.
We assessed the study quality by using data reported on representativeness and validity. A study was considered representative if: (1) this feature of the study was explicitly addressed in the corresponding full-text article, or (2) any statement made by the authors suggested that the actual sample reflected the target population. Validity was evaluated using information about the (25(OH)D) measure (e.g., participation of the laboratory in the International Vitamin D Quality Assessment Scheme, DEQAS) (50).
At any point, any disagreement between reviewers was resolved by means of meeting and discussion among all authors to establish a consensus.
Figure 1.
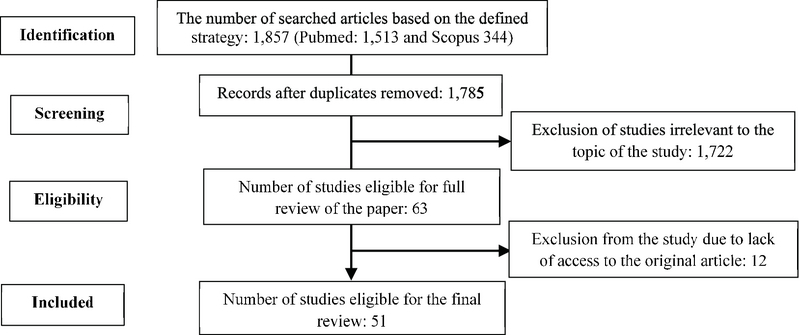
Identification of studies.
3. Results
Table I shows the studies conducted to estimate VDD for pregnant women and newborns in Iran, Turkey, and Arabic countries. Estimates are reported using various assay methodology for the measurement of circulating 25-hydroxyvitamin D (25(OH)D) concentration, which serves as the indicator of vitamin D status. This metabolite (25(OH)D) is difficult to measure, with large variations between methods and among laboratories even when the same methods are used (51). This review revealed that the prevalence of (25(OH)D) concentration 50 nmol/L in pregnant women and
their newborns was between 24.5-98% and 22-100%, respectively. The prevalence of (25(OH)D) concentration 25 nmol/L in pregnant women and their newborns was over a wide range between 16.7-80% and 22-82%, respectively (Table II).
A number of factors can limit the cutaneous production of vitamin D and dietary sources of vitamin D (52), especially in pregnant women (53). As shown in Tables II and III, predictors for low maternal and neonatal (25(OH)D) concentrations included decreased vitamin D synthesis, likely due to reduced exposure to sunlight and decreased nutritional intake of vitamin D.
Table 1.
Methods for laboratory testing of Vitamin D status (25(OH)D concentration) in reviewed studies
|
| ||
| Assay | Studies | Total |
| Enzyme-binding immunoassay | Aly et al., 2013 (47); Al-Shaikh et al., 2016 (54); Al-Faris, 2016 (55); Alfaleh et al., 2014 (56); Aljebory, 2013 (57); Al Kalbani et al., 2011 (58); Khalessi et al., 2015 (59); Ainy et al., 2006 (60); Asemi et al., 2010 (61); Zahediasl and Eyni., 2004 (62); Pehlivan et al., 2002 (63) | 11 |
| Radioligand-binding assay | Bassir et al., 2001 (64) | 1 |
| Radioimmunoassay | Salek et al., 2008 (65); Maghbooli et al., 2008 (66); Maghbooli et al., 2007 (67); Narchi et al., 2010 (68); GÜR et al., 2014b (69); Bener et al., 2013 (70); Molla et al., 2005 (71); Güven et al., 2012 (72); (Kelishadi et al., 2013) (73) | 9 |
| Enzyme-linked immunosorbent assay | Soheilykhah et al., 2010 (11); Kazemi et al., 2009 (74); Asadi et al., 2015 (75) Rostami et al., 2015 (76); Rahbar et al., 2015 (77); Akhlaghi et al., 2015 (78); Abbasian et al., 2016 (79); Khosravi and Entekhabi, 2016 (80); Mirbolouk et al., 2016 (81); Gur et al., 2014 (82); Al-Ajlan et al., 2015 (83); Aydogmus et al., 2015 (84); Gür et al., 2014a (85); Halicioglu et al., 2012 (86) | 13 |
| Electrochemiluminescence | Alp et al., 2016 (6); Hatami et al., 2014 (87); Jafarzadeh et al., 2015 (88); Shor et al., 2015 (89); Al Emadi and Hammoudeh, 2013 (90); Zuhur et al., 2013 (91); Yildiz et al., 2012 (92); Parlak et al., 2015 (93) | 8 |
| High-performance liquid chromatography | Güven et al., 2012 (72); Gunduz et al., 2016 (94); Ustuner et al., 2011 (95); Klcaslan et al., 2017 (96); Ylmaz et al., 2017 (97); Khuri-Bulos et al., 2014 (98); Narchi et al., 2011 (99); Ergür et al., 2009 (100) | 8 |
| Liquid chromatography-tandem mass spectrometry | Ates et al., 2016 (101) | 1 |
| 51 | ||
Table 2.
Prevalence of hypovitaminosis D in pregnant women and their newborns in the Middle East
|
| |||||||||||
| Author, year, ref no | Year | Country-city | Latitude N | Seasons | Trimester of pregnancy | N | Maternal age, Years (X SD) | 25(OH)D ng/mL or (nmol/L) | Type of study and predictors | ||
| Mean SD | Vitamin D deficiency (VDD) | Vitamin D insufficiency (VID) | |||||||||
| Author, year, ref no | Year | Country-city | Latitude N | Seasons | Trimester of pregnancy | N | Maternal age, Years (X SD) | 25(OH)D ng/mL or (nmol/L) | Type of study and predictors | ||
| Mean SD | Vitamin D deficiency (VDD) | Vitamin D insufficiency (VID) | |||||||||
| (Al-Shaikh et al., 2016) (54) | 2014 | Saudi Arabia-Riyadh | 24.7 | Winter & Spring | Third | 1000 | 29.03 5.7 | Mothers: 30.5 19.6 nmol/L | 20 ng/mL (50 nmol/L) mothers: 86.4 % | Cross-sectional study | |
| (Al-Faris, 2016) (55) | 2010 | Saudi Arabia-Riyadh | 24.7 | Spring | First | 160 | - Median: mothers: 49.9 nmol/L | 20 ng/mL (50 nmol/L) mothers: 50% | Cross-sectional study: age group, educational level, sun exposure frequency, and daytime and daily practice of exercise were significantly associated with vitamin D status | ||
| (Shor et al., 2015) (89) | 2011 | Israel-Jerusalem | 31.7 | Spring | Third | 208 | 28.3 5.8 | Mothers: 15.0 51.7 ng/mL | 20 ng/mL (50 nmol/L) mothers: 89.9% newborns: 88.5% | 20-30 ng/mL (50-75nmol/L) mothers: 5.8% newborns: 5.8% | Cross-sectional study: a high correlation was found between maternal and fetal vitamin D serum concentrations (r= 0.85, p 0.001) |
| (Al-Ajlan et al., 2015) (83) | - Saudi Arabia-Riyadh | 24.7 | - First | 515 | 28.7 6.1 | Mothers: 19.1 15.1 nmol/L | 10 ng/mL (25 nmol/L) mothers: 68% | 10-20 ng/mL (25-50 nmol/L) mothers: 26.2% | Cross-sectional study | ||
| (AlFaleh et al., 2014) (56) | 2013 | Saudi Arabia-Riyadh | 24.7 | Winter | Third | 200 | - - | 10ng/mL (25 nmol/L) newborns: 59.5% | 20 ng/mL (50 nmol/L) newborns: 28% | Cross-sectional study | |
| (Khuri-Bulos et al., 2014) (98) | 2010-2011 | Jordan-Amman | 31 | All | Third | 3,731 | Median: 27 | Newborns: 8.6 nmol/L | 20 ng/mL (50 nmol/L) newborns: 94.1% | Prospective study: lower gestational age, maternal smoke exposure, birth during winter months were associated with lower infant vitamin D levels | |
| (ALjebory, 2013) (57) | 2012 | Iraq-Baghdad | 33.3 | Spring & Summer & Autumn | Third | 50 | - Mothers: 40.6 nmol/L newborns: 44.9 nmol/L | 20 ng/mL (50 nmol/L) mothers: 40% newborns: 22% | 20-30 ng/mL (50-75 nmol/L) mothers: 38% newborns: 66% | Cross-sectional study: positive correlation between 25(OH)D concentration in maternal and cord blood (r= 0.762, p= 0.0001). Sunlight exposure | |
| (Bener et al., 2013) (70) | 2011 | Qatar-Doha | 25.28 | All | Third | 1,873 | 20 ng/mL (50 nmol/L) mothers: 48.4% | Prospective study | |||
| (Aly et al., 2013) (47) | 2011 | Saudi Arabia-Al Khafji | 28.42 | - Third | 92 | 33 6.2 | Mothers: 46.6 16.5 nmol/L newborns: 40.1 18 nmol/L | 12ng/mL (30 nmol/L) mothers:14.2% | 12-20 ng/mL (30-50 nmol/L) mothers: 50% | Cross-sectional study: maternal serum 25(OH)D strongly correlated with cord blood 25(OH)D (r= 0.89, p= 0.01). Decreasing trends across the categories of 25(OH)D were found for lower social class, those living in rural areas with a history of inadequate sun exposure, and ultiparous women | |
| (Al Emadi and Hammoudeh, 2013) (90) | 2007-2010 | Qatar-Doha | 25.28 | All | First | 97 | Range (22-37) | Mothers: 17.2 ng/mL | - - | Prospective study | |
| (Narchi et al., 2011) (99) | 2007 | United Arab Emirates-Al-`Ain | 24 | Autumn | Third | 27 | - Mothers: 35.5 nmol/L newborns: 44.7 nmol/L | 10ng/mL (25 nmol/L) mothers: 30% newborns: 22% | 10-20 ng/mL (25-50 nmol/L) mothers: 48% newborns: 22% | Prospective study | |
| (Al Kalbani et al., 2011) (58) | 2010 | Oman-Muscat | 23.58 | Spring & Summer | First & second | 103 | 10 ng/mL (25 nmol/L) mothers: 34% | 25-30 ng/mL (50-75 nmol/L) mothers: 65% | Prospective study | ||
| (Narchi et al., 2010) (68) | 2007 | United Arab Emirates-Al-Ain | 24 | Autumn | All | 75 | Median 27 | Mothers: antenatal visit: 17.3 10.5 ng/mL after delivery: 14.4 9.8 ng/mL | 10ng/mL (25 nmol/L) mothers: antenatal visit: 26% after delivery: 32% | 10-20 ng/mL (25-50 nmol/L) Mothers: Antenatal visit: 42% After delivery: 45% | Prospective study |
| (Molla et al., 2005) (71) | 2005 | Kuwait-Kuwait city | 29 | - Third | 214 | 27.5 4.2 | Mothers: 14.6 10.7 ng/mL newborns: 8.2 6.7 ng/mL | 10ng/mL (25 nmol/L) mothers: 40% newborns: 60% | Cross-sectional study: the vitamin D status of the mothers and neonates were highly correlated (r= 0.790, p 0.001) | ||
| (Kilicaslan et a., 2017) (96) | 2014 | Turkey-Konya | 37.9 | Winter | Third | 100 | 26.75 5.30 | Mothers: 11.4 6.2 ng/mL newborns: 8 5.0 ng/mL | 10ng/mL (25 nmol/L) mothers: 53% newborns: 70% | 10-30 ng/mL (25-75 nmol/L) mothers: 47% newborns: 30% | Cross-sectional study: 25(OH)D concentrations were found to be higher in the women who had received vitamin D. In the cord blood, 86.4% of VDD was attributed to the VDD in the mother (R= 0.864) |
| (Yilmaz et al., 2017) (97) | 2014-2015 | Turkey-Samsun | 41.3 | All | Third | 750 | - Newborns: 11.4 10.2 ng/mL | 20 ng/mL (50 nmol/L) newborns: 87% | 20-30 ng/mL (50-75 nmol/L) newborns: 9% | Cross-sectional study | |
| (Ates et al., 2016) (101) | 2012-2014 | Turkey-Istanbul | 41 | All | First | 229 | 29.49 4.879 | Mothers: 13 9.4 ng/mL | 19ng/mL (47.5 nmol/L) mothers: 82.1%, | 20-29 ng/mL (50-72.5 nmol/L) mothers:13.5% | Prospective study: a high prevalence of VDD was related to dress code, use of multivitamins and season at sampling |
| (Alp et al., 2016) (6) | 2012-2013 | Turkey-Erzurum | 39.4 | All | Third | 81 | 29.9 3.4 | Mothers: 7.1 6.5 ng/mL newborns 7.0 6.6 ng/mL | 5-15 ng/mL (12.5-37.5 nmol/L) mothers: 34.5% newborns: 30.9% | - Prospective study: strong positive correlation between maternal serum and umbilical cord 25(OH)D concentrations (r= 0.624; p 0.001). Dressing style, lack of sunlight in the house | |
| (Gunduz et al., 2016) (94) | 2013 | Turkey-Ankara | 40 | All | Third | 92 | 30.4 4.6 | Mothers: 22.9 16.2 ng/mL | 20ng/mL (50 nmol/L) mothers: 55.4% | 20-32 ng/mL (50-80 nmol/L) mothers: 22.6% | Cross-sectional study |
| (Parlak et al., 2015) (93) | 2012-2013 | Turkey-LSES cities | 37.35 | All | Third | 97 | 27.1 4.5 | mothers: 5.0 3.3 ng/mL newborns: 4.3 2.4 ng/mL | 20ng/mL (50 nmol/L) mothers: 98% newborns: 100% | Prospective study/ strong positive correlation between maternal serum and umbilical cord 25(OH)D levels (r= 0.735, p 0.05). Covered dressing style, not receiving any vitamin D supplementation, and primigravida women | |
| (Aydogmus et al., 2015) (84) | 2013-2014 | Turkey-Izmir | 38.4 | All | Third | 152 | - - | 20 ng/mL (50 nmol/L) mothers: 44.6% | - Prospective study | ||
| (Gür et al., 2014a) (85) | 2012 | Turkey-İzmir and Erzurum- | 38.4, 39.9 | Summer & Autumn | Second | İzmir: 387 erzurum: 245 | Izmir: 28.4 4.5 erzurum: 29.1 5.1 | - 20 ng/mL (50 nmol/L) mothers: izmir: 34.5% erzurum: 75.5% | - Cross-sectional study: clothing style, fish consumption, seaside holiday duration, and 1,200 IU/day vitamin D replacement had an effect on 25(OH)D concentrations in pregnant subjects in İzmir, whereas only holiday duration and 1,200 IU/day vitamin D replacement affected 25(OH)D concentrations in Erzurum | ||
| (Gür et al., 2014) (82) | 2012 | Turkey-Izmir | 38.4 | Summer & Autumn | Second | 208 | 28.5 | Mothers: 22.4 11.2 ng/mL | 20 ng/mL (50 nmol/L) mothers: 51.5% | Prospective study | |
| (GÜR et al., 2014b) (69) | 2009-2010 | Turkey-Ankara | 39.9 | Autumn & Winter | Second | 99 | - - | 20 ng/mL (50 nmol/L) mothers: 62.6% newborns: 58.6% | 21-29 ng/mL (52.5-72.5 nmol/L) mothers: 18.2% newborns: 15.2% | Cross-sectional study | |
| (Zuhur et al., 2013) (91) | 2010-2011 | Turkey-Istanbul | 41 | All | Second | 402 | 30.85 5.6 | Mothers: 33 16.2 nmol/L | 10 ng/mL (25 nmol/L) mothers: 35.6% | 10-19.9 ng/mL (25-49.9 nmol/L) mothers: 48.8% | Cross-sectional study |
| (Güven et al., 2012) (72) | 2008 | Turkey-Ankara | 40 | Winter | Third | 101 | - - | Severe 12 ng/mL (30 nmol/L) newborns: 32% | - Cross-sectional study | ||
| (Yildiz et al., 2012) (92) | - Turkey-Izmir | 38.4 | Winter | Third | 250 | - Mothers: 11.5 5.9 ng/mL newborns: 10.9 5.9 ng/mL | - - | Cross-sectional study: 25(OH)D concentrations of mothers and umbilical cord blood samples were found to be correlated (r= 0.548, p 0.01) | |||
| (Ustuner et al., 2011a) (95) | 2008-2009 | Turkey-Ankara | 40 | Aurumn & Winter | Third | 79 | 26.4 5.5 | Mothers: 12.0 7.2 ng/mL | Severe 10 ng/mL (25 nmol/L) mothers: 45.6% | 10-32 ng/mL (25-80 nmol/L) mothers: 51.9% | Cross-sectional study: in patients who used multivitamin supplements, 25(OH)D concentrations were significantly higher (p 0.046) |
| (Halicioglu et al., 2012) (86) | 2008 | Turkey-Izmir | 38.4 | Spring | Third | 258 | 27.2 4.9 | Mothers: 11.5 5.4 ng/mL newborns: 11.5 6.8 ng/mL | 10 ng/mL (25 nmol/L) mothers: 50.4% 20 ng/mL (50 nmol/L) mothers: 90.3% | Cross-sectional study: uncovered dressing style, sufficient consumption of dairy products, and multivitamin use during gestation | |
| (Ergür et al., 2009) (100) | 2003-2005 | Turkey-Ankara | 40 | All | Third | 70 | 29.7 4.3 | 25ng/mL (62.5 nmol/L) mothers: 81.4% newborns: 97.2% | Case-control study | ||
| (Pehlivan et al., 2002) (63) | 2000 | Turkey-Kocaeli | 40.8 | Spring | Third | 78 | 26.1 5.1 | Mothers: 17.5 10.3 nmol/L | 10ng/mL (25 nmol/L) mothers: 79.5% | 10-16 ng/mL (25-40 nmol/L) mothers:15.3% | Cross-sectional study: the risk factors associated with low maternal 25(OH)D concentrations were low educational level, insufficient intake of vitamin D from the diet, and “covered” dressing habits |
| (Abbasian et al., 2016) (79) | 2012-2013 | Iran-Shahrood | 36.4 | Winter & Spring | Third | 284 | 26.6 5.3 | 8 ng/mL (20 nmol/L) mothers: 1.1% newborns: 2.5% | 8-12 ng/mL (20-30 nmol/L) mothers: 60.2% newborns: 48.9% | Cross-sectional study: weak correlation between maternal serum and cord blood 25(OH)D concentrations (r= 0.12, p=0.053) | |
| (Mirbolouk et al., 2016) (81) | 2013-2014 | Iran-Rasht | 37.3 | All | All | 176 | 27.5 4.6 | Mothers:15.6 9.8 ng/mL | 20 ng/mL (50 nmol/L) mothers: 69% | 20-30 ng/mL (50-75 nmol/L) mothers: 21% | Cross-sectional study: there was a positive correlation between 25(OH)D concentrations and vitamin D consumption as a supplementary one before pregnancy (r = 2.473, p= 0.001) |
| (Khosravi and Entekhabi, 2016) (80) | 2015 | Iran-Tehran | 35.7 | - Third | 49 | Mothers: 26.1 8.4 ng/mL newborns: 17.2 10.4 ng/mL | 20 ng/mL (50 nmol/L) mothers: 24.5% newborns: 71.4% | 20-30 ng/mL (50-75 nmol/L) mothers: 46.9% newborns: 12.2% | Cross-sectional study: serum 25OHD concentration of the mothers and their neonates were significantly correlated (r = 0.446, p 0.001) | ||
| (Khosravi and Entekhabi, 2016) (80) | 2015 | Iran-Tehran | 35.7 | - Third | 49 | Mothers: 26.1 8.4 ng/mL newborns: 17.2 10.4 ng/mL | 20 ng/mL (50 nmol/L) mothers: 24.5% newborns: 71.4% | 20-30 ng/mL (50-75 nmol/L) mothers: 46.9% newborns: 12.2% | Cross-sectional study: serum 25OHD concentration of the mothers and their neonates were significantly correlated (r = 0.446, p 0.001) | ||
| (Rostami et al., 2015) (76) | 2014 | Iran-Masjed Soleimam | 32 | Summer | First | 1,581 | 28.8 5.5 | Mothers: 13.1 6.4 ng/mL | 20 ng/mL (50 nmol/L) mothers: 84.4% | Cross-sectional study: mean serum 25(OH)D concentrations was significantly associated with the duration of sun exposure, use of sunscreens, type of hijab, and type of dwelling (p 0.0001) | |
| (Akhlaghi et al., 2015) (78) | 2013-2014 | Iran-Mashhad | 36.26 | All | Third | 190 | 27.6 4.3 | Mothers: 27.3 4.0 ng/mL newborns: 14.9 8 ng/mL | 12 ng/mL(30 nmol/L) mothers: 33.2% | 12-20 ng/mL (30-50 nmol/L) mothers: 52.1% | Cross-sectional study: insufficient sunlight exposure and maternal skin type were its main risk factor. Birth during winter months was associated with lower infant vitamin D levels |
| (Rahbar et al., 2015) (77) | 2014 | Iran-Semnan | 35.2 | - First | 180 | Mothers: 25.9 18.0 ng/mL | 10 ng/mL (25 nmol/L) mothers: 16.7% | 11-32 ng/mL (27.5-80 nmol/L) mothers: 54.4% | Cross-sectional study | ||
| (Asadi et al., 2015) (75) | 2012-2014 | Iran-Tehran | 35.7 | All | Third | 186 | 28.5 6 6.0 | Median mothers: 11.7 0.12 ng/mL | 20 ng/mL (50 nmol/L) mothers: 74.4% | 20-30 ng/mL (50-75 nmol/L) mothers: 23.3% | Cross-sectional study |
| (Khalessi et al., 2015) (59) | 2011-2012 | Iran-Tehran | 35.7 | All | Third | 102 | 26.2 10.0 | Mothers: 31.5 nmol/L | 10 ng/mL (25 nmol/L) mothers: 48% | 10-20 ng/mL (25-50 nmol/L) mothers: 27.5% | Cross-sectional study |
| (Jafarzadeh et al., 2015) (88) | - Iran-Shahr-e Kord | 32.3 | - First & second | First: 155 second: 64 | 27.4 5.3 | Mothers: first: 25.9 45.6 ng/mL second: 24.1 39.5 ng/mL | - - | Cross-sectional study | |||
| (Hatami et al., 2014) (87) | 2012 | Iran-Bushire | 28.9 | Spring & Summer | Third | 100 | 27.6 6.1 | Mothers: 13.5 10.78 ng/mL | 20 ng/mL (50 nmol/L) mothers: 76% | 20-30 ng/mL (50-75 nmol/L) mothers: 14% | Cross-sectional study |
| (Kelishadi et al., 2013) (73) | 2013 | Iran-Isfahan | 32.65 | - Third | 100 | - Median mother: 15.1 ng/mL newborns: 15.7ng/mL | - - | Cross-sectional study: the air quality had an inverse and independent association with 25(OH)D concentrations of mothers and their neonates. | |||
| (Soheilykhah et al.., 2010) (11) | 2007-2009 | Iran-Yazd | 31.9 | All | Second | 204 | 27.4 5.1 | 20 ng/mL (50 nmol/L) mothers: 78.4% | 20-30 ng/mL (50-75 nmol/L) mothers: 10.3% | Case-control study | |
| (Asemi et al., 2010) (61) | 2008-2009 | Iran-Kashan | 32.98 | All | Second & Third | 147 | - - | 10 ng/mL (25 nmol/L) mothers: 35.2% | 11-32 ng/mL (27.5-80 nmol/L) mothers: 60.6% | Cross-sectional study | |
| (Kazemi et al., 2009) (74) | 2005 | Iran-Zanjan | 36.6 | Winter & Summer | Third | 67 | 28.5 5 | Mothers: 19.4 3.9 nmol/L newborns: 16.7 2.9 nmol/L | 10 ng/mL (25 nmol/L) mothers: 71% newborns: 67% | Cross-sectional study: A positive correlation was found between maternal and cord blood 25(OH)D concentration (r 0.55, p 0.001). | |
| (Salek et al., 2008) (65) | 2005 | Iran-Isfahan | 32.65 | Summer | Third | 88 | 25.5 5.3 | Mothers: 52.2 35.6 ng/mL newborns: 27.4 11.4 ng/mL | 35 ng/mL (87.5 nmol/L) mothers: 26.1% 26ng/mL (65 nmol/L) newborns: 53.4% | - Cross-sectional study | |
| (Maghbooli et al., 2008) (66) | 2005 | Iran-Tehran | 35.7 | - Second | 741 | Mothers: 9.2 7.3 ng/mL | 10 ng/mL (25 nmol/L) mothers: 70.6% | 10-13.9 ng/mL (25-34.9 nmol/L) Mothers: 15.9% | Cross-sectional study | ||
| (Maghbooli et al., 2007) (67) | 2002 | Iran-Tehran | 35.7 | Winter | Third | 552 | - Mothers: 27.8 21.71 nmol/L newborns: 18.1 11.6 nmol/L | 14 ng/mL (35 nmol/L) mothers: 66.8% newborns: 93.3% | Cross-sectional study: A significant correlation between maternal and cord blood serum 25(OH)D concentrations. | ||
| (Ainy et al., 2006) (60) | 2002-2003 | Tehran-Iran | 35.7 | All | All | 48 | 26.2 5.0 | - 20 ng/mL (50 nmol/L) mothers: first trimester: 60% second trimester: 48% Third trimester: 47% | Prospective study | ||
| (Zahediasl and Eyni, 2004) (62) | 2002 | Iran-Tehran | 35.7 | - First | 52 | 25.9 5.0 | - 10 ng/mL (25 nmol/L) mothers: 59.6% | - Cross-sectional study | |||
| (Bassir et al., 2001) (64) | 2001 | Iran-Tehran | 35.7 | - Third | 50 | - Mothers: 12.8 26 nmol/L newborns: 4.94 9.4 nmol/L | 10 ng/mL (25 nmol/L) mothers: 80% newborns: 82% | - Prospective study: A significant and positive correlation between maternal and cord serum 25-OHD concentrations (r= 0.88; p 0.0001). | |||
Table 3.
Risk factors associated with low maternal and neonatal 25(OH)D concentrations in the Middle East
|
| |||
| Maternal | Decreased vitamin D synthesis due to reduced exposure to sunlight | Lifestyle that decreases the time spent outdoors and use of sunscreens | |
| Personal factors | Cultural practices when clothing covers more of the body surface | ||
| Air pollution | |||
| Environmental factors | Season | ||
| Decreased dietary sources of vitamin D | Dietary habits | ||
| Vitamin D replacement | |||
| Newborns | <Correlation between vitamin D status in maternal and cord blood as measured by total circulating 25(OH)D concentration | ||
4. Discussion
In this section, vitamin D status in mothers and their newborns and predictors for low maternal and neonatal (25(OH)D) concentrations in the Middle East will be discussed in detail.
Vitamin D status in mothers and their newborns
A US-based study from California reported extensive vitamin D insufficiency and deficiency during pregnancy that particularly affected mothers
and their newborns (53) Our review revealed the prevalence of (25(OH)D) concentrations 50 nmol/L in pregnant women and their newborns was over a wide range, but at minimum, more than a third-to-half of the women and their newborns had frank VDD, and more than 75% had either deficiency or insufficiency. Our findings of VDD in mothers and their newborns in the Middle East are consistent with the previous systematic review that had shown a high prevalence of hypovitaminosis D among pregnant women (9, 49, 102, 103). Results of this study are similar to other studies in the United States and Europe. In more recent reports, 18% of pregnant women in the UK (103), 42% in northern India (104), 61% in New Zealand (105), and 60-84% of pregnant non-Western women in the Netherlands (106) were reported to have serum (25(OH)D) concentrations 25 nmol/L. Results of this study are consistent with the previous reports and indicate that there is a widespread problem of VDD that persists in mothers and their neonates in the Middle East. The rates of deficiency vary, reflecting the definitions of VDD and insufficiency used. In the studies cited, estimates were based on the different categorization of (25(OH)D) concentrations such as 10, 20, 25, and 35 nmol/L. There is no universally agreed upon definition of VDD and insufficiency (107).
According to the United States' Institute of Medicine, serum 25-hydroxyvitamin D (25(OH)D) concentrations 50 nmol/L (20 ng/mL) are considered as low vitamin D status in adults and children (108). Based on clinical laboratory classifications, vitamin D insufficiency is defined as 50 to 80 nmol/L ( 20 to 32 ng/mL), and sufficiency as 80 nmol/L ( 32 ng/mL) (109-110). Zeghoud and colleagues proposed (25(OH)D) concentrations 30 nmol/L (12 ng/mL) as the cutoff for diagnosing hypovitaminosis D in the newborn (111). It is generally accepted that concentrations of 30 ng/mL are ideal, and concentrations 20 ng/mL are considered deficient (112). The Institute of Medicine in the United States has indicated that serum (25(OH)D) concentrations 50 nmol/L (or 20 ng/mL) are adequate for pregnant women (108); however, Holick (2009) and Dawson-Hughes and colleagues have defined optimal concentrations as 75 nmol/L or 30 ng/mL (113). Hollis and Wagner in their randomized controlled trial of vitamin D supplementation in 350 pregnant women (110) found that the conversion of (25(OH)D) to (1,25(OH)D) is optimized at a (25(OH)D) concentration of at least 100 nmol/L (40 ng/mL), the only time in the lifecycle that (25(OH)D) and (1,25(OH)D) are so highly correlated. This is also found in the neonate at birth, but beyond the neonatal period this relationship no longer exists (114).
Factors influencing vitamin D status in newborns including the correlation between 25(OH)D concentrations in maternal and cord blood
Newborns receive their vitamin D via the placenta throughout pregnancy in the form of 25(OH)D (46). Eight studies in this review have shown a strong positive correlation between maternal and fetal circulating 25(OH)D concentrations (6, 47, 57, 67, 71, 73, 89, 93). The results of this review are consistent with other systematic reviews of studies reporting serum (25(OH)D) concentration in maternal and newborn populations in the Americas, Europe, Eastern Mediterranean region, South-East Asia, Western Pacific, and African countries (49). Most of the investigators have reported that there is a positive and strong correlation between maternal and cord blood (25(OH)D) concentrations (33, 110, 115-117), and this is supported by recent studies from many countries, including India (103), the United Kingdom (118), Greece (119), and the US (110, 117, 120). Not surprisingly, these studies further demonstrated a high prevalence of VDD in mother-infant pairs at birth.
Factors influencing Vitamin D status in mothers
Decreased vitamin D synthesis due to personal and environmental factors
In this review, personal factors associated with decreased vitamin D synthesis included lifestyle (6, 47, 55, 76, 85), and cultural conditions (6, 76, 85, 86, 93, 101) due to greater coverage of the body with clothing that resulted in lower duration as well as less surface area exposed to the sun (121, 122), less participation in outdoor activities (123), and the use of sunscreen, which blocks UVB radiation, and thus vitamin D synthesis in the skin (124). Similar results were observed in studies in the Middle East and countries with Muslim communities (125-126). Women residing in the Middle East and Southeast Asia tend to spend very little time in the sunlight due to cultural and social reasons (127). Studies in Nigeria and Gambia have shown that VDD in newborns at birth is rare when mothers' exposure to sunlight is unrestricted (128). Decreased time spent outdoors may be related to increasing urbanization and increasing time spent indoors at work. Shade reduces the amount of solar radiation by 60% and windowpane glass blocks UVB radiation (123).
Countries of the Persian Gulf region—Bahrain, Iran, Iraq, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates—have become increasingly modernized, resulting in lifestyle transformation based on technology, sedentary activity, and lack of sunlight. These factors have led to a higher prevalence of VDD (129). Besides urbanization, there are multiple factors that limit sunlight exposure such as the hot environment/weather (130), social norms, and religious habits (129-131). Women avoid going outdoors due to more urbanized lifestyles and aesthetic reasons; for example, the culture tends to favor a fair skin that is not suntanned. Therefore, even in the privacy of their own homes, women from such cultural background tend to avoid sun exposure (130). This is in marked contrast to the more Western cultures where tanning and darkening of the skin pigment among white/Caucasian women are considered to enhance their beauty. Cultural norms thus play a role in the time spent outdoors and in the sun, and both directly affect vitamin D status.
As mentioned earlier, sun exposure is also greatly affected by clothing styles that cover most of the body, which leads to reduced vitamin D production in the skin (129). Thus, limited sun exposure in the Middle East appears to be mostly due to cultural practices, clothing styles and limited outdoor activity (132). Other factors such as cloud cover of the sun (133), atmospheric pollution (121, 133, 134), geographic latitude (46), and season (122) are environmental factors influencing the amount of UVB radiation reaching earth (127). In this study, factors associated with vitamin D status included air pollution (98, 73) and seasons (98, 101). Our data indicate that exposure to ambient urban air pollution during pregnancy can significantly contribute to VDD in newborns at the time of delivery. A cohort study in France reported an inverse correlation between air pollution and (25(OH)D) concentrations in cord blood (135). The association between air pollution and low vitamin D status has also been reported among children (133, 136) and women (137, 138). Historically, this was the basis of rickets in children living in industrialized Great Britain where air pollution prevented UVB from reaching beyond the coal and pollutant layer (139). Air pollution decreases vitamin D synthesis in the skin in two ways: Pollution reduces the amount of UVB radiation reaching the skin (121) and also the amount of time that people are outdoor (140).
In a study of newborns in Amman, Jordan, Khuri-Bulos et al., reported that birth during winter months was associated with lower infant 25(OH)D concentrations (98). In a US cohort of 100 infants who were born at latitude 32°N, both the groups of African-American and Caucasian infants exhibited a seasonal variation in vitamin D status (46). An alternative explanation can be that when pregnancy was in winter, these infants accrued lower stores of vitamin D in utero (141). Other studies have shown that the prevalence of VDD during the winter months was higher than the summer months (119, 142-143). Vitamin D status is typically low in the winter months due to variation in cutaneous photosynthesis of pre-vitamin D at higher latitudes above 30° (144). In addition, in the winter the mothers use warmer clothing and the less body surface exposure reduces the cutaneous synthesis of vitamin D (46).
Decreased dietary sources of vitamin D due to dietary habits and vitamin D replacement
The results of this review study showed that another predictor for low 25(OH)D concentrations included decreased dietary sources of vitamin D due to dietary habits and lack of vitamin D replacement (85, 86, 93, 101). Dawodu and colleagues (2015), in the Global Exploration of Human Milk study, concluded that the prevalence of VDD in diverse populations appears to depend on sunlight exposure behaviors and vitamin D supplementation use (145). This is in support of the findings in this study. Vitamin D is a fat-soluble vitamin, which is found
naturally only in a few foods, such as fish-liver oils, fatty fish, egg yolks, liver, and mushrooms (146, 147). Among the dietary sources, the only ones that seem to affect 25(OH)D synthesis are fish (85) and mushrooms (147) if consumed in sufficient quantity. Promoting fish and mushroom consumption in pregnant women may be reasonable with respect to 25(OH)D concentration (85); however, with the former, there is concern about heavy metal exposure and the transmission of this to the developing fetus (148).
The currently recommended dose of vitamin D during pregnancy differs in different parts of the world. In the United States, the daily recommended dose by the Institute of Medicine is 400-600 IU/day (149), while the Endocrine Society suggests a daily dose of 2000 IU/day. In the UK, the recommendation is 400 IU/day (150), and in Turkey, it is 200 IU/day (86). There are other studies that suggest that the requirement for optimal vitamin D status during pregnancy is 4,000 IU/day (110). Based on this sytematic review that shows widespread deficiency throughout the Middle East in pregnant women, higher intake is likely necessary for vitamin D supplementation during pregnancy for women living in the Middle East (74) and those with an increased body mass index (151). Different studies suggest that the daily vitamin D intake during pregnancy should be 600 IU (152), 1,000 IU (112, 115, 153), 2,000 IU (107, 154), and 4,000 IU (109-110, 155-157), and that increased supplementation would help to eliminate vitamin D insufficiency without apparent toxicity in pregnant women and their infants. There is ample evidence that a daily dose of 4,000 IU/day vitamin D will safely achieve vitamin D sufficiency in more than 95% of women and the 100 nmol/L circulating concentration of 25(OH)D that is necessary for optimal conversion of 25(OH)D to 1,25(OH)D (158-159).
5. Conclusions
The Middle East, in most of its geographic regions, has sufficient sunshine for the adequate photocutaneous synthesis of vitamin D; however, most of the Middle Eastern women cannot benefit from this source because of cultural practices, such as clothing and veiling among Muslim women, and spending most time indoors. The results of this study raise the concern that newborns in the Middle East are entering the world with a vitamin D deficiency that begins in utero, with documented consequences on later health such as low birth weight, small-for-gestational age (7, 9), abnormal skeletal development, type 1 diabetes (35), abnormal immune development (37), wheezing, asthma (40), abnormal neurocognitive development (41), and the concern for epigenetic factors that can adversely affect health later in life (160).
The high prevalence of hypovitaminosis D in the Middle East underscores the necessity of implementing national preventive strategies. These strategies for the prevention of VDD in women and their newborns at birth begin with vitamin D sufficiency in women during pregnancy. The preventive strategies need to encompass public awareness and also guidelines for health care personnel concerning screening and supporting vitamin sufficiency (161). In most countries, there is no routine screening for VDD nor for insufficiency during pregnancy. A 2013 study and 2009 review have recommended that women with one or more risk factors for low serum 25(OH)D should be screened at the beginning of gestation and in mid-pregnancy and early in the antenatal period, especially for those with risk factors (47, 116). Such screening programs already have been implemented in centers in the US with a positive effect (162).
Based on this current view, more than 75% of Middle Eastern women are either frankly vitamin D deficient or insufficient, which directly reflects their reduced exposure to sunlight and decreased dietary sources of vitamin D, thus making them significantly more likely to have lower 25(OH)D concentrations. These findings lend support to a screening of pregnant women and their newborns for VDD in all Middle Eastern women. If a mother has VDD or insufficiency and sunlight exposure is limited, an alternative for the pregnant woman is vitamin D supplementation during pregnancy to ensure vitamin D sufficiency for both herself and for her newborn (33, 163). Daily doses of 4,000 units/day are recommended for achieving vitamin D sufficiency throughout pregnancy (110, 164). Because vitamin D status is inversely associated with obesity parameters, women with higher BMIs will need higher doses of vitamin D supplementation (165-167). Dietary intake alone is not sufficient to maintain normal vitamin D status because a considerable amount of vitamin D is acquired by cutaneous synthesis when exposed to sun (121, 137). Therefore, public health awareness about the predictors of low maternal and neonatal 25(OH)D concentrations in the Middle East and also the need to encourage vitamin D sufficiency by modest sunshine exposure and adequate maternal vitamin D intake during pregnancy are needed.
Strengths and Weaknesses of the Study
A strength of our study was the use of a comprehensive search strategy with broad search terms. A potential weakness is that we restricted the review to studies published in the English language in two international electronic databases (Scopus, PubMed), so relevant studies may have been missed.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Palacios C., Gonzalez L. Is vitamin D deficiency a major global public health problem? The Journal of Steroid Biochemistry and Molecular Biology. 2014;144(Part A):138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagenau T., Vest R., Gissel T. N., Poulsen C. S., Erlandsen M., Mosekilde L., Vestergaard P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporosis International. 2009;20(1):133–140. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- 3.Mithal A., Wahl D. A., Bonjour J. P., Burckhardt P., Dawson-Hughes B., Eisman J. A., El-Hajj Fuleihan G., Josse R. G., Lips P., Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis International. 2009;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 4.Arabi A., El Rassi R., El-Hajj Fuleihan G. Hypovitaminosis D in developing countries—prevalence, risk factors and outcomes. Nature Reviews Endocrinology. 2010;6(10):550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 5.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D. A., Pierroz D. D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. British Journal of Nutrition. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 6.Alp H., Tekgündüz K. Ş., Akkar M. K. Maternal and cord blood vitamin D status in high-altitude pregnancy. The Journal of Maternal-Fetal & Neonatal Medicine. 2015;29(4):571–575. doi: 10.3109/14767058.2015.1011119. [DOI] [PubMed] [Google Scholar]
- 7.Aghajafari F., Nagulesapillai T., Ronksley P. E., Tough S. C., O'Beirne M., Rabi D. M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346(7902) doi: 10.1136/bmj.f1169.f1169 [DOI] [PubMed] [Google Scholar]
- 8.Wei S.-Q., Qi H.-P., Luo Z.-C., Fraser W. D. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;26(9):889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 9.Nassar N., Halligan G. H., Roberts C. L., Morris J. M., Ashton A. W. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. American Journal of Obstetrics & Gynecology. 2011;205(3):208.e1–208.e7. doi: 10.1016/j.ajog.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 10.Lau S. L., Gunton J. E., Athayde N. P., Byth K., Wah Cheung N. Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Medical Journal of Australia. 2011;194(7):334–337. doi: 10.5694/j.1326-5377.2011.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 11.Soheilykhah S., Mojibian M., Rashidi M., Rahimi-Saghand S., Jafari F. Maternal vitamin D status in gestational diabetes mellitus. Nutrition in Clinical Practice. 2010;25(5):524–527. doi: 10.1177/0884533610379851. [DOI] [PubMed] [Google Scholar]
- 12.Baker A. M., Haeri S., Camargo C. A., Jr., Espinola J. A., Stuebe A. M. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. The Journal of Clinical Endocrinology & Metabolism. 2010;95(11):5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson C. J., Alanis M. C., Wagner C. L., Hollis B. W., Johnson D. D. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. American Journal of Obstetrics & Gynecology. 2010;203(4):366.e1–366.e6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azar M., Basu A., Jenkins A. J., Nankervis A. J., Hanssen K. F., Scholz H., Henriksen T., Garg S. K., Hammad S. M., Scardo J. A., Aston C. E., Lyons T. J. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: A longitudinal study. Diabetes Care. 2011;34(6):1258–1264. doi: 10.2337/dc10-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wikström Shemer E., Marschall H. Decreased 1,25-dihydroxy vitamin D levels in women with intrahepatic cholestasis of pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(11):1420–1423. doi: 10.3109/00016349.2010.515665. [DOI] [PubMed] [Google Scholar]
- 16.Dawodu A., Nath R. High prevalence of moderately severe vitamin D deficiency in preterm infants. Pediatrics International. 2011;53(2):207–210. doi: 10.1111/j.1442-200X.2010.03209.x. [DOI] [PubMed] [Google Scholar]
- 17.Morley R., Carlin J. B., Pasco J. A., Wark J. D. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. The Journal of Clinical Endocrinology & Metabolism. 2006;91(3):906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 18.Merewood A., Mehta S. D., Chen T. C., Bauchner H., Holick M. F. Association between vitamin D deficiency and primary cesarean section. The Journal of Clinical Endocrinology & Metabolism. 2009;94(3):940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensel K. J., Randis T. M., Gelber S. E., Ratner A. J. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. American Journal of Obstetrics & Gynecology. 2011;204(1):41.e1–41.e9. doi: 10.1016/j.ajog.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Dror D. K. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Current Opinion in Obstetrics & Gynecology. 2011;23(6):422–426. doi: 10.1097/GCO.0b013e32834cb791. [DOI] [PubMed] [Google Scholar]
- 21.Rethman MP. Pregnancy and periodontal disease. J Am Dent Assoc. 1939:142–484. doi: 10.14219/jada.archive.2011.0208. [DOI] [PubMed] [Google Scholar]
- 22.Boggess K. A., Espinola J. A., Moss K., Beck J., Offenbacher S., Camargo C. A., Jr. Vitamin D status and periodontal disease among pregnant women. Journal of Periodontology. 2011;82(2):195–200. doi: 10.1902/jop.2010.100384. [DOI] [PubMed] [Google Scholar]
- 23.Mehta S., Giovannucci E., Mugusi F. M., Spiegelman D., Aboud S., Hertzmark E., Msamanga G. I., Hunter D., Fawzi W. W. Vitamin D status of HIV-infected women and its association with hiv disease progression, anemia, and mortality. PLoS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008770.e8770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake Y., Sasaki S., Tanaka K., Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. European Respiratory Journal. 2010;35(6):1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 25.Swamy G. K., Garrett M. E., Miranda M. L., Ashley-Koch A. E. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. American Journal of Medical Genetics Part A. 2011;155(6):1264–1271. doi: 10.1002/ajmg.a.33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodnar L. M., Catov J. M., Zmuda J. M., Cooper M. E., Parrott M. S., Roberts J. M., Marazita M. L., Simhan H. N. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. Journal of Nutrition. 2010;140(5):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leffelaar E. R., Vrijkotte T. G., van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. British Journal of Nutrition. 2010;104(1):108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 28.Pasco J. A., Wark J. D., Carlin J. B., Ponsonby A., Vuillermin P. J., Morley R. Maternal vitamin D in pregnancy may influence not only offspring bone mass but other aspects of musculoskeletal health and adiposity. Medical Hypotheses. 2008;71(2):266–269. doi: 10.1016/j.mehy.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Krishnaveni G. V., Veena S. R., Winder N. R., Hill J. C., Noonan K., Boucher B. J., Karat S. C., Fall C. H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. American Journal of Clinical Nutrition. 2011;93(3):628–635. doi: 10.3945/ajcn.110.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahon P., Harvey N., Crozier S., Inskip H., Robinson S., Arden N., Swaminathan R., Cooper C., Godfrey K. Low maternal vitamin D status and fetal bone development: Cohort study. Journal of Bone and Mineral Research. 2010;25(1):14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viljakainen H. T., Saarnio E., Hytinantti T., Miettinen M., Surcel H., Mäkitie O., Andersson S., Laitinen K., Lamberg-Allardt C. Maternal Vitamin D Status Determines Bone Variables in the Newborn. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 32.Viljakainen H. T., Korhonen T., Hytinantti T., Laitinen E. K., Andersson S., Mäkitie O., Lamberg-Allardt C. Maternal vitamin D status affects bone growth in early childhood—a prospective cohort study. Osteoporosis International. 2011;22(3):883–891. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner C. L., Greer F. R., American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 34.Ganmaa D., Holick M. F., Rich-Edwards J. W., Frazier L. A., Davaalkham D., Ninjin B., Janes C., Hoover R. N., Troisi R. Vitamin D deficiency in reproductive age Mongolian women: A cross sectional study. The Journal of Steroid Biochemistry and Molecular Biology. 2014;139:1–6. doi: 10.1016/j.jsbmb.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundqvist A., Sandström H., Stenlund H., Johansson I., Hultdin J., Slominski A. T. Vitamin D status during pregnancy: a longitudinal study in swedish women from early pregnancy to seven months postpartum. PLoS ONE. 2016;11(3):e0150385. doi: 10.1371/journal.pone.0150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marjamäki L., Niinistö S., Kenward M. G., Uusitalo L., Uusitalo U., Ovaskainen M.-L., Kronberg-Kippilä C., Simell O., Veijola R., Ilonen J., Knip M., Virtanen S. M. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010;53(8):1599–1607. doi: 10.1007/s00125-010-1734-8. [DOI] [PubMed] [Google Scholar]
- 37.Chi A., Wildfire J., Mcloughlin R., Wood R. A., Bloomberg G. R., Kattan M., Gergen P., Gold D. R., Witter F., Chen T., Holick M., Visness C., Gern J., O'Connor G. T. Umbilical cord plasma 25-hydroxyvitamin D concentration and immune function at birth: the Urban Environment and Childhood Asthma study. Clinical & Experimental Allergy. 2011;41(6):842–850. doi: 10.1111/j.1365-2222.2011.03712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rochat M. K., Ege M. J., Plabst D., Steinle J., Bitter S., Braun-Fahrländer C., Dalphin J.-., Riedler J., Roponen M., Hirvonen M.-., Büchele G., Renz H., Lauener R., Krauss-Etschmann S., Von Mutius E. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clinical & Experimental Allergy. 2010;40(5):786–794. doi: 10.1111/j.1365-2222.2009.03428.x. [DOI] [PubMed] [Google Scholar]
- 39.Belderbos M. E., Houben M. L., Wilbrink B., Lentjes E., Bloemen E. M., Kimpen J. L. L., Rovers M., Bont L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127(6):e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 40.Camargo C. A., Jr., Ingham T., Wickens K., Thadhani R., Silvers K. M., Epton M. J., Town G. I., Pattemore P. K., Espinola J. A., Crane J., Dench C., Duignan M., Fishwick D., Fitzharris P., Irvine V., Kelly R., Lampshire P., Lane J., Leadbitter P., MacDonald C., McCartin F., McLeod S., Nicholson A., Roff K., Sawyer G., Siebers R., Wilson H., Withell K. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 41.Murthi P., Davies-Tuck M., Lappas M., Singh H., Mockler J., Rahman R., Lim R., Leaw B., Doery J., Wallace E. M., Ebeling P. R. Maternal 25-hydroxyvitamin D is inversely correlated with foetal serotonin. Clinical Endocrinology. 2017;86(3):401–409. doi: 10.1111/cen.13281. [DOI] [PubMed] [Google Scholar]
- 42.Dawodu A., Wagner C. L. Mother-child vitamin D deficiency: an international perspective. Archives of Disease in Childhood. 2007;92(9):737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unger M. D., Cuppari L., Titan S. M., Magalhães M. C., Sassaki A. L., dos Reis L. M., Jorgetti V., Moysés R. M. Vitamin D status in a sunny country: Where has the sun gone? Clinical Nutrition. 2010;29(6):784–788. doi: 10.1016/j.clnu.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Bassil D., Rahme M., Hoteit M., Fuleihan G. E. Hypovitaminosis D in the Middle East and North Africa. Dermato-Endocrinology. 2014;5(2):274–298. doi: 10.4161/derm.25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Hajj Fuleihan G. Vitamin D deficiency in the Middle East and its health consequences for children and adults. Clinical Reviews in Bone and Mineral Metabolism. 2009;7(1):77–93. doi: 10.1007/s12018-009-9027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basile L. A., Taylor S. N., Wagner C. L., Quinones L., Hollis B. W. Neonatal vitamin D status at birth at latitude 32°72′: Evidence of deficiency. Journal of Perinatology. 2007;27(9):568–571. doi: 10.1038/sj.jp.7211796. [DOI] [PubMed] [Google Scholar]
- 47.Aly Y. F., El Koumi M. A., Abd El Rahman R. N. Impact of maternal vitamin D status during pregnancy on the prevalence of neonatal vitamin D deficiency. Pediatric Reports. 2013;5(1):24–27. doi: 10.4081/pr.2013.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawodu A., Akinbi H. Vitamin D nutrition in pregnancy: current opinion. International Journal of Women's Health. :333. doi: 10.2147/IJWH.S34032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saraf R., Morton S. M. B., Camargo C. A., Grant C. C. Global summary of maternal and newborn vitamin D status – a systematic review. Maternal & Child Nutrition. 2016;12(4):647–668. doi: 10.1111/mcn.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter G. D. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Current Drug Targets. 2011;12(1):19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 51.Hollis B. W. Vitamin D requirement during pregnancy and lactation. Journal of Bone and Mineral Research. 2007;22(supplement 2):V39–V44. doi: 10.1359/jbmr.07s215. [DOI] [PubMed] [Google Scholar]
- 52.Tsiaras W. G., Weinstock M. A. Factors influencing vitamin d status. Acta Dermato-Venereologica. 2011;91(2):115–124. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- 53.Dror D. K., King J. C., Durand D. J., Allen L. H. Association of Modifiable and Nonmodifiable Factors with Vitamin D Status in Pregnant Women and Neonates in Oakland, CA. Journal of the Academy of Nutrition and Dietetics. 2011;111(1):111–116. doi: 10.1016/j.jada.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Al-Shaikh G. K., Ibrahim G. H., Fayed A. A., Al-Mandeel H. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: a cross-sectional study. BMC Pregnancy and Childbirth. 2016;16(1) doi: 10.1186/s12884-016-0901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Faris N. High Prevalence of Vitamin D Deficiency among Pregnant Saudi Women. Nutrients. 2016;8(2):77. doi: 10.3390/nu8020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alfaleh K. M., Al-Manie A. M., Al-Mahmoud H. F., Al-Razqan H. M., Al-Mutlaq A. B., Al-Rumaih S. A., Hasanato R. M., Al-Mandeel H. M. Prevalence of vitamin D deficiency in Saudi newborns at a tertiary care center. Saudi Medical Journal. 2014;35(2):178–182. [PubMed] [Google Scholar]
- 57.Aljebory H. D. The Correlation between Maternal and Newborn Serum 25 Hydroxy Vitamin D in a Sample of Iraqi Women. Middle East Journal of Internal Medicine. 2013;6(5):3–12. doi: 10.5742/MEJIM.2013.65355. [DOI] [Google Scholar]
- 58.Al Kalbani M., Elshafie O., Rawahi M., Al-Mamari A., Al-Zakwani A., Woodhouse N. Vitamin D status in pregnant Omanis: A disturbingly high proportion of patients with low vitamin D stores. Sultan Qaboos University Medical Sciences Journal. 2011;11(1):52–55. [PMC free article] [PubMed] [Google Scholar]
- 59.Khalessi N., Kalani M., Araghi M., Farahani Z. The relationship between maternal vitamin D deficiency and low birth weight neonates. J Fam Reprod Health. 2015;9:113–117. [PMC free article] [PubMed] [Google Scholar]
- 60.Ainy E., Ghazi A. A. M., Azizi F. Changes in calcium, 25(OH) vitamin D3 and other biochemical factors during pregnancy. Journal of Endocrinological Investigation. 2006;29(4):303–307. doi: 10.1007/bf03344100. [DOI] [PubMed] [Google Scholar]
- 61.Asemi Z., Taghizade M., Sarahroodi S., Jazayeri S., Tabasi Z., Seyyedi F. Assessment of the relationship of vitamin D with serum antioxidant vitamins E and A and their deficiencies in Iranian pregnant women. Saudi Medical Journal. 2010;31(10):1119–1123. [PubMed] [Google Scholar]
- 62.Zahediasl S., Eynie. The relationship between calcium vitamin D and vitamins A and E in pregnant Tehranian women. Iranian J Endocrinol Metabol. 2004:6. [Google Scholar]
- 63.Pehlivani Hatus S., Aydogan M., Babaoglu K., Turker G., Gokalp AS. Maternal serum vitamin D levels in the third trimester of pregnancy. Turkish J Med Sci. 2002;32:237–241. [Google Scholar]
- 64.Slevin M., Farrington N., Duffy G., Daly L., Murphy J. F. A. Altering the NICU and measuring infants' responses. Acta Paediatrica. 2000;89(5):577–581. doi: 10.1111/j.1651-2227.2000.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 65.Salek M., Hashemipour M., Aminorroaya A., Gheiratmand A., Kelishadi R., Ardestani P., Nejadnik H., Amini M., Zolfaghari B. Vitamin D Deficiency among Pregnant Women and Their Newborns in Isfahan, Iran. Experimental and Clinical Endocrinology & Diabetes. 2008;116(06):352–356. doi: 10.1055/s-2008-1042403. [DOI] [PubMed] [Google Scholar]
- 66.Maghbooli Z., Hossein-Nezhad A., Karimi F., Shafaei A.-R., Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes/Metabolism Research and Reviews. 2008;24(1):27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- 67.Maghbooli Z., Hossein-Nezhad A., Shafaei A. R., Karimi F., Madani F. S., Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy and Childbirth. 2007;7 doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Narchi H., Kochiyil J., Zayed R., Abdulrazzak W., Agarwal M. Maternal vitamin D status throughout and after pregnancy. Journal of Obstetrics & Gynaecology. 2010;30(2):137–142. doi: 10.3109/01443610903315652. [DOI] [PubMed] [Google Scholar]
- 69.Gür G., Abaci A., Köksoy A. Y., Anik A., Çatli G., Kişlal F. M., Akin K. O., Andiran N. Incidence of maternal vitamin D deficiency in a region of Ankara, Turkey: a preliminary study. TURKISH JOURNAL OF MEDICAL SCIENCES. 2014;44(4):616–623. doi: 10.3906/sag-1304-107. [DOI] [PubMed] [Google Scholar]
- 70.Bener A., Al-Hamaq A., Saleh N. Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. International Journal of Women's Health. :523. doi: 10.2147/IJWH.S51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molla A. M., Al Badawi M., Hammoud M. S., Molla A. M., Shukkur M., Thalib L., Eliwa M. S. Vitamin D status of mothers and their neonates in Kuwait. Pediatrics International. 2005;47(6):649–652. doi: 10.1111/j.1442-200x.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 72.Güven A., Ecevit A., Sözer O., Tarcan A., Tarcan A., Özbek N. Correlation between the cord vitamin D levels and regulatory T cells in newborn infants. European Journal of Pediatrics. 2012;171(8):1161–1166. doi: 10.1007/s00431-012-1688-6. [DOI] [PubMed] [Google Scholar]
- 73.Kelishadi R., Sharifi-Ghazvini F., Poursafa P., Mehrabian F., Farajian S., Yousefy H., Movahedian M., Sharifi-Ghazvini S. Determinants of hypovitaminosis D in pregnant women and their newborns in a sunny region. International Journal of Endocrinology. 2013;2013 doi: 10.1155/2013/460970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kazemi A., Sharifi F., Jafari N., Mousavinasab N. High Prevalence of Vitamin D Deficiency among Pregnant Women and their Newborns in an Iranian Population. Journal of Women's Health. 2009;18(6):835–839. doi: 10.1089/jwh.2008.0954. [DOI] [PubMed] [Google Scholar]
- 75.Asadi M., Saeidifard F., Qorbani M., Adabi K. Vitamin D deficiency and mode of delivery: A study in Tehran women general hospital. Tehran University Medical Journal. 2015;73(6):442–446. [Google Scholar]
- 76.Rostami M., Tehrani F. R., Simbar M., Panah F. H., Majd S. H. A. Prevalence of vitamin d deficiency and related factors among pregnant women referred to masjed soleimam health centers in 2014. Iranian Journal of Obstetrics, Gynecology and Infertility. 2015;18(164):1–10. [Google Scholar]
- 77.Rahbar N., Rajabi M., Mirmohammadkhani M. 25-hydroxy Vitamin d serum level in pregnant women with 8-12 gestational weeks in semnan city and its association with Fasting Blood Sugar and Body Mass Index. Iranian Journal of Obstetrics, Gynecology and Infertility. 2015;18(167):1–8. [Google Scholar]
- 78.Akhlaghi F., Vakili R., Khorasani E. Evaluation of umbilical cord vitamin D level and maternal factors effective on it in three hospitals of Emam Reza, Ghaem & Omol Banin during 2013-2014. Iranian Journal of Obstetrics, Gynecology and Infertility. 2014;17(134):1–7. [Google Scholar]
- 79.Abbasian M., Chaman R., Amiri M., Ajami M. E., Jafari-Koshki T., Rohani H., Taghavi-Shahri S. M., Sadeghi E., Raei M. Vitamin D Deficiency in Pregnant Women and Their Neonates. Global Journal of Health Science. 2015;8(9):83. doi: 10.5539/gjhs.v8n9p83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khosravi N., Entekhabi S. Correlation between vitamin D status in mothers and their neonates in an Iranian population. Int J Child Adolescents. 2016;2:14–17. [Google Scholar]
- 81.Mirbolouk F., Pakseresht S., Asgharnia M., Farjadmand B. M., Kazemnezhad E. Study of Vitamin D Status in Pregnant Women in North of Iran. International Journal of Women's Health and Reproduction Sciences. 2016;4(4):176–180. doi: 10.15296/ijwhr.2016.39. [DOI] [Google Scholar]
- 82.Gur E. B., Gokduman A., Turan G. A., Tatar S., Hepyilmaz I., Zengin E. B., Eskicioglu F., Guclu S. Mid-pregnancy vitamin D levels and postpartum depression. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;179:110–116. doi: 10.1016/j.ejogrb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 83.Al-Ajlan A., Krishnaswamy S., Alokail M. S., Aljohani N. J., Al-Serehi A., Sheshah E., Alshingetti N. M., Fouda M., Turkistani I. Z., Al-Daghri N. M. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Pregnancy and Childbirth. 2015;15(1) doi: 10.1186/s12884-015-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aydogmus S., Kelekci S., Aydogmus H., Eriş S., Desdicioğlu R., Yilmaz B., Sağlam G. High prevalence of vitamin D deficiency among pregnant women in a Turkish population and impact on perinatal outcomes. The Journal of Maternal-Fetal & Neonatal Medicine. 2014;28(15):1828–1832. doi: 10.3109/14767058.2014.969235. [DOI] [PubMed] [Google Scholar]
- 85.Gur E. B., Turan G. A., Tatar S., Gokduman A., Karadeniz M., Celik G., Genc M., Guclu S. The effect of place of residence and lifestyle on vitamin D deficiency in pregnancy: Comparison of eastern and western parts of Turkey. Journal of the Turkish German Gynecological Association. 2014;15(3):149–155. doi: 10.5152/jtgga.2014.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halicioglu O., Aksit S., Koc F., Akman S. A., Albudak E., Yaprak I., Coker I., Colak A., Ozturk C., Gulec E. S. Vitamin D deficiency in pregnant women and their neonates in spring time in western Turkey. Paediatric and Perinatal Epidemiology. 2012;26(1):53–60. doi: 10.1111/j.1365-3016.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- 87.Hatami G., AhmadiS Motamed N., Eghbali SS., Amirani S. 25-OH Vitamin D serum level in pregnant women in Bushehr-2012. ISMJ. Vol. 16. 16: 410-418; 2014. [Google Scholar]
- 88.Jafarzadeh L., Motamedi A., Behradmanesh M., Hashemi R. A Comparison of Serum Levels of 25-hydroxy Vitamin D in Pregnant Women at Risk for Gestational Diabetes Mellitus and Women Without Risk Factors. Materia Socio Medica Journal. 2015;27(5):318. doi: 10.5455/msm.2015.27.318-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shor D. B., Barzel J., Tauber E., Amital H. The effects of maternal vitamin D on neonatal growth parameters. European Journal of Pediatrics. 2015;174(9):1169–1174. doi: 10.1007/s00431-015-2517-5. [DOI] [PubMed] [Google Scholar]
- 90.Al Emadi S., Hammoudeh M. Vitamin D study in pregnant women and their babies. Qatar Medical Journal. 2013;2013(1):7. doi: 10.5339/qmj.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuhur S. S., Erol R. S., Kuzu I., Altuntas Y. The relationship between low maternal serum 25- hydroxyvitamin D levels and gestational diabetes mellitus according to the severity of 25-hydroxyvitamin D deficiency. Clinics. 2013;68(5):658–664. doi: 10.6061/clinics/2013(05)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yildiz O., Colak Aydogdu A., Coker I., Turkon H. Vitamin D correlation between mother and baby during pregnancy in the winter. Turkish Journal of Biochemistry. 2012;37(2):146–149. doi: 10.5505/tjb.2012.30602. [DOI] [Google Scholar]
- 93.Parlak M., Kalay S., Kalay Z., Kirecci A., Guney O., Koklu E. Severe vitamin D deficiency among pregnant women and their newborns in Turkey. The Journal of Maternal-Fetal & Neonatal Medicine. 2014;28(5):548–551. doi: 10.3109/14767058.2014.924103. [DOI] [PubMed] [Google Scholar]
- 94.Gunduz S., Kosger H., Aldemir S., Akcal B., Tevrizci H., Hizli D., Celik H. T. Sleep deprivation in the last trimester of pregnancy and inadequate vitamin D: Is there a relationship? Journal of the Chinese Medical Association. 2016;79(1):34–38. doi: 10.1016/j.jcma.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Ustuner I., Keskin H. L., Tas E. E., Neselioglu S., Sengul O., Filiz Avsar A. Maternal serum 25(OH)D levels in the third trimester of pregnancy during the winter season. The Journal of Maternal-Fetal & Neonatal Medicine. 2011;24(12):1421–1426. doi: 10.3109/14767058.2011.566768. [DOI] [PubMed] [Google Scholar]
- 96.Kılıcaslan A. Ö., Kutlu R., Kilinc I., Ozberk D. I. The effects of vitamin D supplementation during pregnancy and maternal vitamin D levels on neonatal vitamin D levels and birth parameters. The Journal of Maternal-Fetal and Neonatal Medicine. 2018;31(13):1727–1734. doi: 10.1080/14767058.2017.1326897. [DOI] [PubMed] [Google Scholar]
- 97.Yılmaz B., Aygün C., Çetinoğlu E. Vitamin D levels in newborns and association with neonatal hypocalcemia. The Journal of Maternal-Fetal & Neonatal Medicine. 2017;31(14):1889–1893. doi: 10.1080/14767058.2017.1331430. [DOI] [PubMed] [Google Scholar]
- 98.Khuri-Bulos N., Lang R. D., Blevins M., Kudyba K., Lawrence L., Davidson M., Faouri S., Halasa N. B. Vitamin D Deficiency among Newborns in Amman, Jordan. Global Journal of Health Science. 2013;6(1) doi: 10.5539/gjhs.v6n1p162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Narchi H., Kochiyil J., Zayed R., Abdulrazzak W., Agarwal M. Longitudinal study of vitamin D status in the 1st 6 months of life. Annals of Tropical Paediatrics. 2013;31(3):225–230. doi: 10.1179/1465328111Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 100.Ergur A. T., Berberoglu M., Atasay B., Siklar Z., Bilir P., Arsan S., Soylemez F., Ocal G. Vitamin D Deficiency in Turkish Mothers and Their Neonates and in Women of Reproductive Age. Journal of Clinical Research in Pediatric Endocrinology. 2009;1(6):266–269. doi: 10.4274/jcrpe.v1i6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ates S., Sevket O., Ozcan P., Ozkal F., Kaya M. O., Dane B. Vitamin D status in the first-trimester: effects of Vitamin D deficiency on pregnancy outcomes. African Health Sciences. 2016;16(1):36. doi: 10.4314/ahs.v16i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dawodu A., editor. Vitamin D status of Arab mothers and infants. J Arab Neonatol Forum. Vol. 1. 1: 15-22; 2004. [Google Scholar]
- 103.Karras S. N., Anagnostis P., Annweiler C., Naughton D. P., Petroczi A., Bili E., Harizopoulou V., Tarlatzis B. C., Persinaki A., Papadopoulou F., Goulis D. G. Maternal vitamin D status during pregnancy: the Mediterranean reality. European Journal of Clinical Nutrition. 2014;68(8):864–869. doi: 10.1038/ejcn.2014.80. [DOI] [PubMed] [Google Scholar]
- 104.Sachan A., Gupta R., Das V., Agarwal A., Awasthi P. K., Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. American Journal of Clinical Nutrition. 2005;81(5):1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 105.Judkins A., Eagleton C. Vitamin D deficiency in pregnant New Zealand women. NZ Med. 2005:119–U2144. [PubMed] [Google Scholar]
- 106.van der Meer I. M., Karamali N. S., Boeke A. J., Lips P., Middelkoop B. J., Verhoeven I., Wuister J. D. High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. American Journal of Clinical Nutrition. 2006;84(2):350–353. doi: 10.1093/ajcn/84.2.350. [DOI] [PubMed] [Google Scholar]
- 107.Irvine J., Onyett H., Saylor K., Schroter H., Young M., Wong S., Carson J., Godel J. C., Dedam-Montour D., Ford E., Gagnon E., Qurd G., Harrison C., Langlois K., Moore K., Morningstar M., Vides E. Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatrics & Child Health. 2007;12(7):583–598. doi: 10.1093/pch/12.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Del Valle HB., Yaktine AL., Taylor CL., Ross AC. Dietary reference intakes for calcium and vitamin D. Institute of Medicine. Washington DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 109.Hollis B. W., Wagner C. L. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition. 2004;80(6):1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 110.Hollis B. W., Johnson D., Hulsey T. C., Ebeling M., Wagner C. L. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research. 2011;26(10):2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balasubramanian S., Ganesh R. Vitamin D deficiency in exclusively breast-fed infants. Indian J Med Res. 2008:127–250. [PubMed] [Google Scholar]
- 112.Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., Gordon C. M., Hanley D. A., Heaney R. P., Murad M. H., Weaver C. M. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 113.Dawson-Hughes B., Heaney R. P., Holick M. F., Lips P., Meunier P. J., Vieth R. Estimates of optimal vitamin D status. Osteoporosis International. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 114.Walker V. P., Zhang X., Rastegar I., Liu P. T., Hollis B. W., Adams J. S., Modlin R. L. Cord Blood Vitamin D Status Impacts Innate Immune Responses. The Journal of Clinical Endocrinology & Metabolism. 2011;96(6):1835–1843. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hollis BW., Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 116.Mulligan M. L., Felton S. K., Riek A. E., Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. American Journal of Obstetrics & Gynecology. 2010;202(5):429.e1–429.e9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagner C. L., McNeil R., Hamilton S. A., Winkler J., Rodriguez Cook C., Warner G., Bivens B., Davis D. J., Smith P. G., Murphy M., Shary J. R., Hollis B. W. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. American Journal of Obstetrics & Gynecology. 2013;208(2):137.e1–137.e13. doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pawley N., Bishop N. J. Prenatal and infant predictors of bone health: the influence of vitamin D. American Journal of Clinical Nutrition. 2004;80(6):1748S–1751S. doi: 10.1093/ajcn/80.6.1748S. [DOI] [PubMed] [Google Scholar]
- 119.Nicolaidou P., Hatzistamatiou Z., Papadopoulou A., Kaleyias J., Floropoulou E., Lagona E., Tsagris V., Costalos C., Antsaklis A. Low vitamin D status in mother-newborn pairs in Greece. Calcified Tissue International. 2006;78(6):337–342. doi: 10.1007/s00223-006-0007-5. [DOI] [PubMed] [Google Scholar]
- 120.Bodnar L. M., Simhan H. N., Powers R. W., Frank M. P., Cooperstein E., Roberts J. M. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. Journal of Nutrition. 2007;137(2):447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khallaf M. The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources. InTech; 2011. [DOI] [Google Scholar]
- 122.Webb A. R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Progress in Biophysics and Molecular Biology. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 123.Misra M., Pacaud D., Petryk A., Collett-Solberg P. F., Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 124.Wagner C. L., Howard C. R., Hulsey T. C., Lawrence R. A., Ebeling M., Shary J., Smith P. G., Morella K., Taylor S. N., Hollis B. W. Maternal and infant vitamin D status during lactation: Is latitude important? Health. 2013;05(12):2004–2013. doi: 10.4236/health.2013.512271. [DOI] [Google Scholar]
- 125.Çuhacı-Çakır B., Demirel F. Effects of seasonal variation and maternal clothing style on vitamin d levels of mothers and their infants. The Turkish Journal of Pediatrics. 2015;56(5):475–481. [PubMed] [Google Scholar]
- 126.Mishal A. A. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporosis International. 2001;12(11):931–935. doi: 10.1007/s001980170021. [DOI] [PubMed] [Google Scholar]
- 127.Micka A. Vitamin D status among Bangladeshi women of reproductive age [Masters theses] [Masters, thesis] Amherst: University of Massachusetts Amherst; 2009. [Google Scholar]
- 128.Okonofua F., Houlder S., Bell J., Dandona P. Vitamin D nutrition in pregnant Nigerian women at term and their newborn infants. Journal of Clinical Pathology. 1986;39(6):650–653. doi: 10.1136/jcp.39.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fields J., Trivedi N. J., Horton E., Mechanick J. I. Vitamin D in the Persian Gulf: Integrative Physiology and Socioeconomic Factors. Current Osteoporosis Reports. 2011;9(4):243–250. doi: 10.1007/s11914-011-0071-2. [DOI] [PubMed] [Google Scholar]
- 130.Kanan R. M., Al Saleh Y. M., Fakhoury H. M., Adham M., Aljaser S., Tamimi W. Year-round vitamin D deficiency among Saudi female out-patients. Public Health Nutrition. 2013;16(3):544–548. doi: 10.1017/S1368980012002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mabry R. M., Reeves M. M., Eakin E. G., Owen N. Gender differences in prevalence of the metabolic syndrome in Gulf Cooperation Council Countries: a systematic review. Diabetic Medicine. 2010;27(5):593–597. doi: 10.1111/j.1464-5491.2010.02998.x. [DOI] [PubMed] [Google Scholar]
- 132.Pettifor J. M. Nutritional rickets: deficiency of vitamin D, calcium, or both? American Journal of Clinical Nutrition. 2004;80(6):1725S–1729S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 133.Agarwal K. S., Puliyel J. M., Mughal M. Z., Upadhyay P., Berry J. L., Mawer E. B. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Archives of Disease in Childhood. 2002;87(2):111–113. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barnard W., Saxena V., Wenny B., DeLuisi J. Daily Surface UV Exposure and Its Relationship to Surface Pollutant Measurements. Journal of the Air & Waste Management Association. 2012;53(2):237–245. doi: 10.1080/10473289.2003.10466134. [DOI] [PubMed] [Google Scholar]
- 135.Baïz N., Dargent-Molina P., Wark J. D., Souberbielle J.-C., Slama R., Annesi-Maesano I. Gestational exposure to urban air pollution related to a decrease in cord blood vitamin D levels. The Journal of Clinical Endocrinology & Metabolism. 2012;97(11):4087–4095. doi: 10.1210/jc.2012-1943. [DOI] [PubMed] [Google Scholar]
- 136.Kelishadi R., Moeini R., Poursafa P., Farajian S., Yousefy H., Okhovat-Souraki A. Independent association between air pollutants and vitamin D deficiency in young children in Isfahan, Iran. Paediatrics and International Child Health. 2013;34(1):50–55. doi: 10.1179/2046905513Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 137.Hosseinpanah F., pour S. H., Heibatollahi M., Moghbel N., Asefzade S., Azizi F. The effects of air pollution on vitamin D status in healthy women: A cross sectional study. BMC Public Health. 2010;10(1) doi: 10.1186/1471-2458-10-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manicourt D., Devogelaer J. Urban Tropospheric Ozone Increases the Prevalence of Vitamin D Deficiency among Belgian Postmenopausal Women with Outdoor Activities during Summer. The Journal of Clinical Endocrinology & Metabolism. 2008;93(10):3893–3899. doi: 10.1210/jc.2007-2663. [DOI] [PubMed] [Google Scholar]
- 139.Wagner CL., Taylor SN., Hollis BW. New insights into vitamin D during pregnancy, lactation, early infancy. Hale Pub. Plano: TX; 2010. [Google Scholar]
- 140.Bailey B. A., Manning T., Peiris A. N. The Impact of Living in Rural and Urban Areas: Vitamin D and Medical Costs in Veterans. The Journal of Rural Health. 2012;28(4):356–363. doi: 10.1111/j.1748-0361.2012.00407.x. [DOI] [PubMed] [Google Scholar]
- 141.Jain V., Gupta N., Kalaivani M., Jain A., Sinha A., Agarwal R. Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: Seasonal variation & determinants. Indian Journal of Medical Research. 2011;133(3):267–273. [PMC free article] [PubMed] [Google Scholar]
- 142.ORiordan M., Kiely M., Higgins J., Cashman K. O’Riordan M. Vol. 101. 101: 240-242; 2008. Prevalence of suboptimal vitamin D status during pregnancy. Ir Med J; pp. 240–242. [PubMed] [Google Scholar]
- 143.Basile L. A., Taylor S. N., Wagner C. L., Quinones L., Hollis B. W. Neonatal vitamin D status at birth at latitude 32°72′: evidence of deficiency. Journal of Perinatology. 2007;27(9):568–571. doi: 10.1038/sj.jp.7211796. [DOI] [PubMed] [Google Scholar]
- 144.Webb A. R., Engelsen O. Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status. Photochemistry and Photobiology. 2006;82(6):1697. doi: 10.1562/2006-09-01-RA-670. [DOI] [PubMed] [Google Scholar]
- 145.Dawodu A., Davidson B., Woo J., Peng Y., Ruiz-Palacios G., Guerrero M., Morrow A. Sun Exposure and Vitamin D Supplementation in Relation to Vitamin D Status of Breastfeeding Mothers and Infants in the Global Exploration of Human Milk Study. Nutrients. 2015;7(2):1081–1093. doi: 10.3390/nu7021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Holick M. F., Chen T. C. Vitamin D deficiency: a worldwide problem with health consequences. American Journal of Clinical Nutrition. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 147.Keegan R. H., Lu Z., Bogusz J. M., Williams J. E., Holick M. F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermato-Endocrinology. 2014;5(1):165–176. doi: 10.4161/derm.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. Molecular, Clinical and Environmental Toxicology. Vol. 101. Basel, Switzerland: Springer; 2012. Heavy metal toxicity and the environment; pp. 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Food and Nutrition Board: Institue of Medicine. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC, USA: National Academies Press; 2005. [DOI] [Google Scholar]
- 150.Scientific Advisory Committee on Nutrition (SACN) Vitamin D and health report. 2016. Available from: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report.
- 151.Kumaratne M., Early G., Cisneros J. Vitamin D Deficiency and Association With Body Mass Index and Lipid Levels in Hispanic American Adolescents. Global Pediatric Health. 2017;4:2333794X1774414. doi: 10.1177/2333794X17744141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ross AC., Manson JE., Abrams SA., Aloia JF., Brannon PM., Clinton SK., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metabol. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heaney R. P., Davies K. M., Chen T. C., Holick M. F., Janet Barger-Lux M. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition. 2003;77(1):204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 154.Saadi H. F., Dawodu A., Afandi B. O., Zayed R., Benedict S., Nagelkerke N. Efficacy of daily and monthly high-dose calciferol in vitamin D-deficient nulliparous and lactating women. American Journal of Clinical Nutrition. 2007;85(6):1565–1571. doi: 10.1093/ajcn/85.6.1565. [DOI] [PubMed] [Google Scholar]
- 155.Dawodu A., Saadi H. F., Bekdache G., Javed Y., Altaye M., Hollis B. W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. The Journal of Clinical Endocrinology & Metabolism. 2013;98(6):2337–2346. doi: 10.1210/jc.2013-1154. [DOI] [PubMed] [Google Scholar]
- 156.Hollis B. W., Wagner C. L. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcified Tissue International. 2013;92(2):128–139. doi: 10.1007/s00223-012-9607-4. [DOI] [PubMed] [Google Scholar]
- 157.Grant C. C., Stewart A. W., Scragg R., Milne T., Rowden J., Ekeroma A., Wall C., Mitchell E. A., Crengle S., Trenholme A., Crane J., Camargo C. A., Jr. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133(1):e143–e153. doi: 10.1542/peds.2013-2602. [DOI] [PubMed] [Google Scholar]
- 158.Hollis B. W., Wagner C. L. Vitamin D requirements and supplementation during pregnancy. Current Opinion in Endocrinology & Diabetes and Obesity. 2011;18(6):371–375. doi: 10.1097/MED.0b013e32834b0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wagner CL., Howard CR., Hulsey T., Ebeling M., Shary J., Smith P. Results of NICHD two-site maternal vitamin D. Washington, DC: Pediatric Academic Societies; 2013. supplementation randomized controlled trial during lactation [abstract; platform presentation] [Google Scholar]
- 160.Al-Garawi A., Carey V. J., Chhabra D., Mirzakhani H., Morrow J., Lasky-Su J., Qiu W., Laranjo N., Litonjua A. A., Weiss S. T., Roemer K. The Role of Vitamin D in the Transcriptional Program of Human Pregnancy. PLoS ONE. 2016;11(10):e0163832. doi: 10.1371/journal.pone.0163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kimball S., Fuleihan G. E., Vieth R. Vitamin D: A Growing Perspective. Critical Reviews in Clinical Laboratory Sciences. 2008;45(4):339–414. doi: 10.1080/10408360802165295. [DOI] [PubMed] [Google Scholar]
- 162.McDonnell S. L., Baggerly K. A., Baggerly C. A., Aliano J. L., French C. B., Baggerly L. L., Ebeling M. D., Rittenberg C. S., Goodier C. G., Mateus Niño J. F., Wineland R. J., Newman R. B., Hollis B. W., Wagner C. L. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE. 2017;12(7) doi: 10.1371/journal.pone.0180483.e0180483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wagner C. L., Taylor S. N., Johnson D. D., Hollis B. W. The Role of Vitamin D in Pregnancy and Lactation: Emerging Concepts. Women's Health Journal (WHJ) 2012;8(3):323–340. doi: 10.2217/WHE.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mithal A., Kalra S. Vitamin D supplementation in pregnancy. Indian Journal of Endocrinology and Metabolism. 2014;18(5):593–596. doi: 10.4103/2230-8210.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vanlint S. Vitamin D and obesity. Nutrients. 2013;5(3):949–956. doi: 10.3390/nu5030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bodnar L. M., Catov J. M., Roberts J. M., Simhan H. N. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. Journal of Nutrition. 2007;137(11):2437–2442. doi: 10.1093/jn/137.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tzotzas T., Papadopoulou F. G., Tziomalos K., Karras S., Gastaris K., Perros P., Krassas G. E. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. The Journal of Clinical Endocrinology & Metabolism. 2010;95(9):4251–4257. doi: 10.1210/jc.2010-0757. [DOI] [PubMed] [Google Scholar]


