Abstract
Glutamate is the major excitatory neurotransmitter in the central nervous system, and related signaling involves both AMPA and NMDA subtype receptors. The expression of glutamate receptors is dynamically regulated during development. Recent studies showed that the dysregulation of glutamate receptor expression and function is associated with neurodevelopmental disorders including intellectual disability. Previously, a Noonan syndrome (NS)-associated SHP2 mutation (SHP2D61G) was shown to increase the synaptic delivery of AMPA receptor, subsequently impairing synaptic plasticity and learning in adult mice. However, how the mutant SHP2 affects glutamate receptor expression during development is not known. Here, we found that the SHP2D61G differentially regulates the expression of AMPA and NMDA receptors depending on the stage of neuronal maturation. In cultured neurons (immature stage; DIV 6), overexpression of SHP2D61G significantly increased the average size and the number of NMDA receptor-containing particles, but not those with AMPA receptors. In early matured neurons (DIV 12), SHP2D61G significantly increased only the average size of AMPA receptor particles, and subsequently increased their number in matured neurons (DIV 18). Importantly, all the changes described above for SHP2D61G neurons were reversed by inhibiting MAPK. These data demonstrate that the increased activation of MAPK signaling pathway by SHP2D61G could deregulate the surface expression of synaptic receptors during neuronal development, which likely contributes to cognitive impairments in NS patients.
Keywords: AMPA receptor, NMDA receptor, developmental disorder, MAPK
1. Introduction
Excitatory synaptic transmission in the central nervous system is mainly mediated by two subtypes of glutamate receptors: AMPA and NMDA receptors. The composition of glutamate receptors at the synapse is dynamically regulated both in developing and in the adult brain. In early developmental stages, NMDA receptors appear first and AMPA receptor levels gradually increase [1]. The composition of NMDA receptors also changes during development. While the GluN2B subtype is highly expressed in early developmental stages, its expression is significantly decreased in the adult [2]. Activity-dependent changes in the surface expression of AMPA receptors are critically involved in synaptic plasticity in multiple brain regions [3, 4].
Receptor expression and trafficking can be regulated by multiple signaling pathways. The Ras-MAPK pathway modulates the transport of AMPA receptors to postsynaptic sites [5]. Accordingly, deregulation of Ras-MAPK signaling disrupts synaptic AMPA receptor composition and subsequently impairs synaptic plasticity. Synaptic Ras GTPase-activating protein 1 (SYNGAP1) haploinsufficiency increases Ras activation and enhances AMPA receptor-mediated currents in the developing brain [6]. Previously, we showed that a Noonan syndrome (NS)-associated SHP2 mutation (SHP2D61G) enhances both Ras-MAPK signaling and the expression of postsynaptic AMPA receptors in fully matured cultured neurons [day in vitro (DIV) 21] and in the adult hippocampus [7]. NS is a relatively common developmental disorder associated with cardiac defects, growth delay, facial abnormalities and learning disabilities [8, 9]. Although NS-associated mutations can have a large impact on neural development [10], and SHP2 is expressed in both mitotic and postmitotic neurons in the developing brain [11], how NS-associated mutations affect glutamate receptor expression in early developmental states is not known. In this study, we investigated the effect of overexpressing SHP2D61G on the cell surface expression of glutamate subtypes at different stages of development in vitro (DIV 6, 12, and 18). We found that mutant SHP2 increases the level of NMDA receptors at the neuronal membrane surface at early stage, whereas it increases the surface level of AMPA receptors at later stage. Importantly, all these changes in composition of glutamate receptors could be reversed by inhibiting MAPK signaling. Thus, our data imply that NS-associated SHP2 mutations disrupt the expression dynamics of glutamate receptor subtypes during neuronal maturation.
2. Materials and Methods
2.1. Neuron culture
Dissociated hippocampal neuron cultures were prepared from 1-day-old rat pups, plated at ~10,000 neurons/coverslip, and maintained with Neurobasal-A medium (Invitrogen, Carlsbad, CA) supplemented with B27 (Invitrogen) before use as previously reported [12].
2.2. Sindbis virus
Construction and packaging of the Sindbis virus expressing SHP2D61G has been previously described [7]. After the addition of viral particles to media, cultures were incubated for 11 hrs for transgene expression.
2.3. Immunohistochemistry and confocal imaging
Immunohistochemistry has been performed as previously described [13]. Briefly, cultured neurons were fixed with 4% paraformaldehyde solution and subjected to immunostaining without permeabilization. The following antibodies were used: polyclonal anti-GluN1 (extracellular) antibody (#AGC-001, alomone labs, Jerusalem, Israel), polyclonal anti-GluA1 (extracellular) antibody (#AGC-004, alomone labs), Cy™3-conjugated goat anti-rabbit IgG antibody (#111–165-003, Jackson ImmunoResearch Lab, West Grove, PA).0Images were acquired by using confocal microscope (Zeiss 710, Carl Zeiss, Oberkochen, Germany) and analyzed by using NIH ImageJ software (ver. 1.47v). All procedures of image acquisition and analysis were performed by experimenters blind to experimental condition.
2.4. Drug treatment
Cultured neurons were infected with the Sindbis virus expressing SHP2D61G at the day indicated, and incubated for an additional 11 hours followed by 10 μM PD98059 (Tocris, Avonmouth, UK) or dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO) treatment for 1 hour. Then, cultures were immediately fixed and subjected to immunostaining.
2.5. Statistical analyses
Statistical analyses were performed with GraphPad Prism 5 software (La Jolla, CA). Unpaired t-tests were used to determine statistical significance between groups. Two-way analyses of variance (ANOVA) were used to determine the effect of drug treatment. Error bars indicate the standard error (SEM).
3. Results
3.1. Mutant SHP2 overexpression at DIV 6 increases the surface expression of NMDA receptors
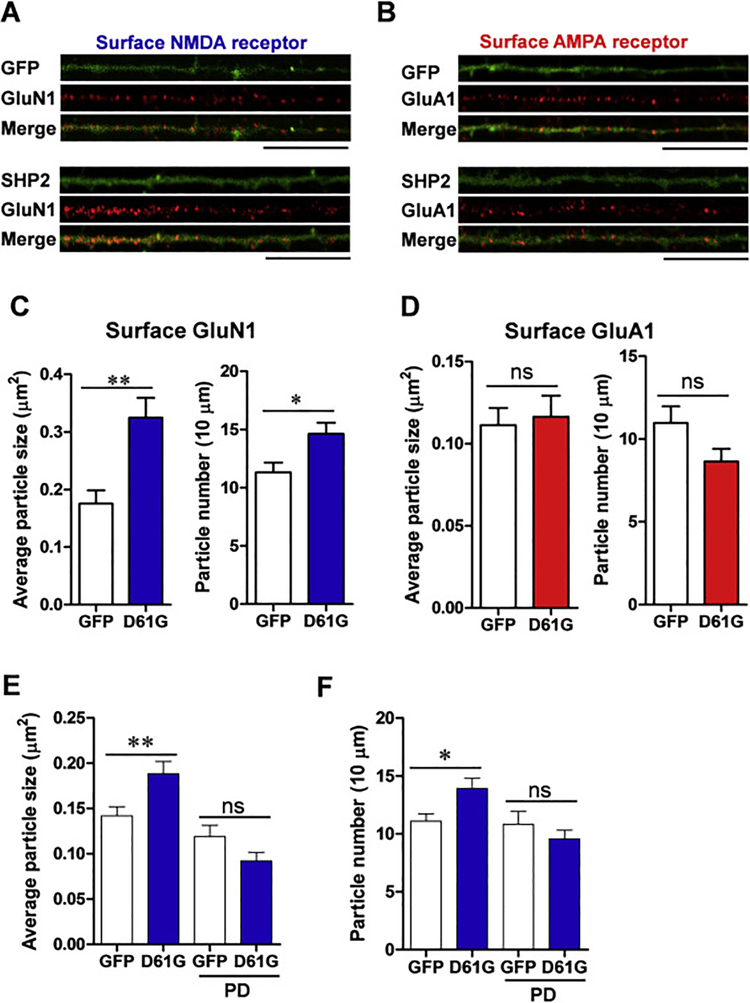
We examined the effect of expressing SHP2D61G on the surface expression of glutamate receptor subtypes in cultured hippocampal neurons at different stages of maturation: DIV 6 for immature, DIV 12 for early mature, and DIV 18 for fully mature neurons [14, 15] We overexpressed SHP2D61G in cultured hippocampal neurons DIV 6 by using a bicistronic Sindbis viral vector co-expressing enhanced green fluorescent protein (EGFP) as a reporter, and analyzed the surface expression of glutamate receptors using the antibodies that detect the extracellular N-terminus for GluN1 and GluA1 for NMDA and AMPA receptors, respectively. The overexpression of SHP2D61G did not affect gross morphology of neurons compared to that of GFP expressing control neurons. In addition, SHP2D61G did not affect the expression of other non-target genes such as Rab-4 or actin (Supplementary Fig. 1). Surprisingly, the surface expression of GluN1 was significantly increased by SHP2D61G expression. Both the size and the number GluN1-containing clusters were significantly increased in SHP2D61G-expressing neurons compared to EGFP-expressing control neurons (Fig. 1A, C; GluN1 particle size: SHP2D61G, 0.32 ± 0.0 μm2, n = 15 neurons, 1,450 μm of dendrites; EGFP, 0.18 ± 0.0 μm2, n = 20 neurons, 1,803 μm of dendrites; unpaired t-test, ** p < 0.01; GluN1 particle number per 10 μm: SHP2D61G, 14.62 ± 1.0, n = 15 neurons, 1,450 μm of dendrites; EGFP, 11.31 ± 0.8, n = 20 neurons, 1,803 μm of dendrites; unpaired t-test, * p < 0.05). In contrast, SHP2D61G overexpression did not affect the surface expression of GluA1 at DIV 6 (Fig. 1B, D; GluA1 particle size: SHP2D61G, 0.12 ± 0.1 μm2, n = 21 neurons, 1,894 μm of dendrites; EGFP, 0.11 ± 0.0 μm2, n = 16 neurons, 1,394 μm of dendrites; unpaired t-test, p = 0.768; GluA1 particle number per 10 μm: SHP2D61G, 8.66 ± 0.8, n = 21 neurons, 1,894 μm of dendrites; EGFP, 10.97 ± 1.0, n = 16 neurons, 1,394 μm of dendrites; unpaired t-test, p = 0.069). Importantly, the total expression level of GluN1 was not affected by the expression of SHP2D61G (Supplementary Fig. 1).
Fig. 1. SHP2D61G overexpression at DIV 6 increases surface NMDA receptor expression.
(A-B) Representative images of surface GluN1 (A) or GluA1 (B) receptor immunostaining. GFP alone (upper) or SHP2D61G and GFP (lower) were expressed in cultured hippocampal neuron at DIV6 by using a bicistronic Sindbis virus vector. Scale bar, 20 μm. (C) Bar graph showing the average size (left) or the number (right) of GluN1-stained particles per 10 μm of dendrites. Unpaired t-test, ns, not significant. (D) Bar graph showing the average size (left) or the number (right) of GluA1-stained particles per 10 μm of dendrites. Unpaired t-test, * P < 0.05, ** P < 0.01. (E-F) Effect of PD98059 (PD: 10 μM) treatment on the average particle size (E) and the number (F) of surface GluN1. Two-way ANOVA followed by Bonferroni posttests. * P < 0.05, ** P < 0.01. Error bars represent standard error of the mean (S.E.M.).
The majority of the NS-associated mutations in SHP2, including SHP2D61G, are gain-of-function mutations that enhance Ras-MAPK activation [7, 16]. Consistently, we found that the SHP2D61G-expressing neurons treated with MAPK/Erk kinase (MEK) inhibitor PD98059 (10 μM, 1 hr) showed unchanged number and size of surface GluN1 particles compared to EGFP controls (Fig. 1E, GluN1 particle size: SHP2D61G, 0.19 ± 0.0 μm2, n = 25 neurons, 2,378 μm of dendrites; EGFP, 0.14 ± 0.0 μm2, n = 24 neurons, 2,207 μm of dendrites; SHP2D61G + PD98059, 0.09 ± 0.0 μm2, n = 21 neurons, 2,012 μm of dendrites; EGFP + PD98059, 0.12 ± 0.0 μm2, n = 15 neurons, 1,451 μm of dendrites; Two-way ANOVA, interaction between genotype and treatment, F1, 81 = 9.506, P = 0.003, Bonferroni posttest, ** P < 0.01; Fig. 1F, GluN1 particle number: SHP2D61G, 13.93 ± 0.9, n = 25 neurons, 2,378 μm of dendrites; EGFP, 11.10 ± 0.6, n = 24 neurons, 2,207 μm of dendrites; SHP2D61G + PD98059, 9.58 ± 0.7, n = 21 neurons, 2,012 μm of dendrites; EGFP + PD98059, 10.83 ± 1.1, n = 15 neurons, 1,451 μm of dendrites; Two-way ANOVA, interaction between genotype and treatment, F1, 81 = 5.756, P = 0.019, Bonferroni posttest, * P < 0.05). It is worthy to note that 10 μM PD98059 was shown to be a subthreshold dose which inhibits the excessively activated MAPK in cultured neuron without affecting neurotrophin-induced changes in dendritic spines [17]. This result demonstrates that the mutant SHP2 increases the surface expression of NMDA receptors in a Ras-MAPK pathway dependent manner.
3.2. Mutant SHP2 overexpression at DIV 12 increases the size of AMPA receptor clusters
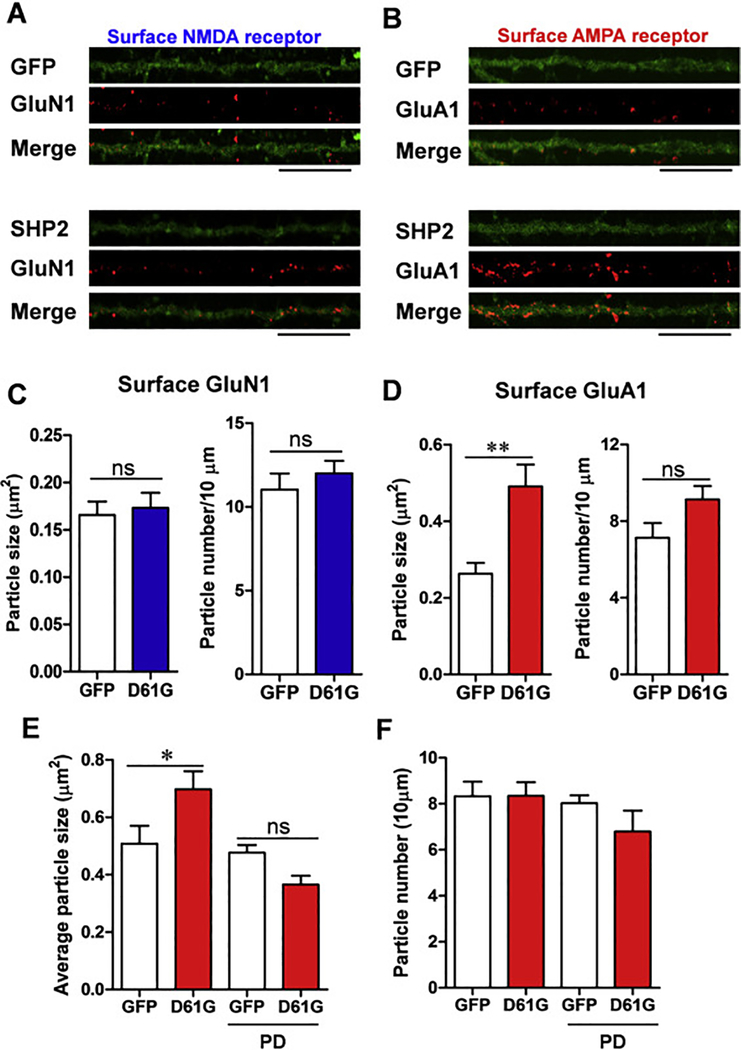
We infected cultured neurons with the Sindbis viruses encoding SHP2D61G or EGFP at DIV 12 and analyzed the surface expression of glutamate receptors. In contrast to DIV 6, neurons infected at DIV 12 did not show significant differences in NMDA receptor expression in either the size or number of GluN1 clusters (Fig. 2A, C; GluN1 particle size: SHP2D61G, 0.17 ± 0.0 μm2, n = 16 neurons, 1,418 μm of dendrites; EGFP, 0.17 ± 0.0 μm2, n = 15 neurons, 1,323 μm of dendrites; unpaired t-test, p = 0.727; GluN1 particle number per 10 μm: SHP2D61G, 12.01 ± 0.7, n = 16 neurons, 1,418 μm of dendrites; EGFP, 11.03 ± 1.0, n = 15 neurons, 1,323 μm of dendrites; unpaired t-test, p = 0.422). However, we found that the size of GluA1 receptor clusters were significantly increased in SHP2D61G-expressing neurons compared to EGFP controls (Fig. 2B, D; GluA1 particle size: SHP2D61G, 0.49 ± 0.1 μm2, n = 14 neurons, 1,245 μm of dendrites; EGFP, 0.26 ± 0.0 μm2, n = 14 neurons, 1,220 μm of dendrites; unpaired t-test, ** p < 0.01). Interestingly, the numbers of GluA1 clusters were not statistically different between groups, although there was a trend toward an increase in the SHP2D61G group (Fig. 2B, D; GluA1 particle number per 10 μm: SHP2D61G, 9.14 ± 0.7, n = 14 neurons, 1,245 μm of dendrites; EGFP, 7.14 ± 0.8, n = 14 neurons, 1,220 μm of dendrites; unpaired t-test, p = 0.067). Importantly, PD98059 treatment completely normalized the size of GluA1 clusters in SHP2D61G-expressing neurons, whereas it did not affect the number of GluA1 clusters (Fig. 2E, GluA1 particle size: SHP2D61G, 0.70 ± 0.1 μm2, n = 12 neurons, 1,167 μm of dendrites; EGFP, 0.51 ± 0.1 μm2, n = 12 neurons, 1,239 μm of dendrites; SHP2D61G + PD98059, 0.37 ± 0.0 μm2, n = 13 neurons, 1,336 μm of dendrites; EGFP + PD98059, 0.48 ± 0.0 μm2, n = 13 neurons, 1,229 μm of dendrites; Two-way ANOVA, interaction between genotype and treatment, F1, 46 = 10.080, P = 0.0027, Bonferroni posttest, * P < 0.05; Fig. 2F, GluA1 particle number: SHP2D61G, 8.34 ± 0.6, n = 12 neurons, 1,167 μm of dendrites; EGFP, 8.37 ± 0.6, n = 12 neurons, 1,239 μm of dendrites; SHP2D61G + PD98059, 6.80 ± 0.9, n = 13 neurons, 1,336 μm of dendrites; EGFP + PD98059, 8.02 ± 0.3, n = 13 neurons, 1,229 μm of dendrites; Two-way ANOVA, interaction between genotype and treatment, F1, 46 = 0.911, P = 0.345), suggesting that SHP2D61G-mediated hyperactivation of the Ras-MAPK signaling pathway in moderately matured hippocampal neurons specifically increases the size of surface AMPA receptor clusters, without affecting NMDA receptor containing clusters. In addition, the total expression level of GluA1 was not changed by the expression of SHP2D61G (Supplementary Fig. 1).
Fig. 2. SHP2D61G overexpression at DIV 12 increases surface AMPA receptor expression.
(A-B) Representative images of surface GluN1 (A) or GluA1 (B) receptor immunostaining. GFP alone (upper) or SHP2D61G and GFP (lower) were expressed in cultured hippocampal neuron at DIV 12. Scale bar, 20 μm. (C) Bar graph showing the average size (left) or the number (right) of GluN1-stained particles per 10 μm of dendrites. Unpaired t-test, ** P < 0.01. (D) Bar graph showing the average size (left) or the number (right) of GluA1-stained particles per 10 μm of dendrites. Unpaired t-test, ns, not significant. (E-F) Effect of PD98059 (PD: 10 μM) treatment on the average particle size (E) and the number (F) of surface GluA1. Two-way ANOVA followed by Bonferroni posttests. * P < 0.05. Error bars represent S.E.M.
3.3. Mutant SHP2 overexpression at DIV 18 increases both the number and the size of AMPA receptor clusters
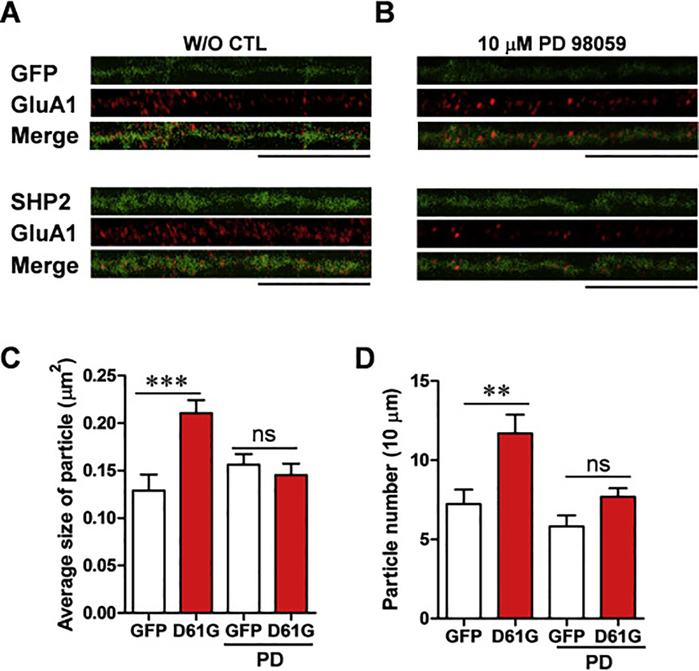
We examined the effect of SHP2D61G overexpression on AMPA receptor expression in developing neurons at later stages. In fully mature neuron (DIV 18), both the size and the number GluA1-containing receptor clusters were significantly increased in SHP2D61G-expressing neurons compared to EGFP-expressing control neurons (Fig. 3A, C–D). Consistently, SHP2D61G-expressing neurons treated with PD98059 showed comparable size and number of GluA1 clusters to those in the EGFP control group (Fig. 3A–C; GluA1 particle size: SHP2D61G, 0.21 ± 0.0 μm2, n = 10 neurons, 862 μm of dendrites; EGFP, 0.13 ± 0.0 μm2, n = 9 neurons, 896 μm of dendrites; SHP2D61G + PD98059, 0.15 ± 0.0 μm2, n = 15 neurons, 1,324 μm of dendrites; EGFP + PD98059, 0.16 ± 0.0 μm2, n = 11 neurons, 1,058 μm of dendrites; Two-way ANOVA, interaction between genotype and treatment, F1, 41 = 11.62, P = 0.0015, Bonferroni posttest, *** P <0.001; Fig. 3D, GluA1 particle number: SHP2D61G, 11.70 ± 1.2, n = 10 neurons, 862 μm of dendrites; EGFP, 7.23 ± 0.9, n = 9 neurons, 896 μm of dendrites; SHP2D61G + PD98059, 7.70 ± 0.5, n = 15 neurons, 1,324 μm of dendrites; EGFP + PD98059, 5.82 ± 0.7, n = 11 neurons, 1,058 μm of dendrites; Two-way ANOVA, effect of treatment, F1, 41 = 10.940, P = 0.002, Bonferroni posttest, ** P < 0.01), demonstrating that SHP2D61G expression facilitates the surface expression of AMPA receptors in both the size and the number of GluA1-containing receptor clusters.
Fig. 3. SHP2D61G overexpression at DIV 18 increases both the size and the number of the surface AMPA receptor clusters.
(A-B) Representative images of surface GluA1 receptor staining in control (A) or PD98059-treated (B) cultured neurons. GFP alone (upper) or SHP2D61G and GFP (lower) were expressed in cultured hippocampal neuron at DIV 18. Scale bar, 20 μm. (C-D) Effect of PD98059 (10 μM) treatment on the average particle size (C) and the number (D) of surface GluA1. Two-way ANOVA followed by Bonferroni post tests. ns, not significant, ** P < 0.01, *** P < 0.001. Error bars represent S.E.M.
The results presented above demonstrate the differential effect of SHP2D61G overexpression on glutamate receptor expression during development and show that decreasing MAPK signaling reverses abnormalities in the size and number of GluA1 and GluN1 clusters in SHP2D61G-expressing neurons (Table 1).
Table 1.
Differential effects of SHP2D61G during maturation
| Premature (DIV 6) | Mature (> DIV 12) | Adult (> DIV 21) | ||||
|---|---|---|---|---|---|---|
| Size | Number | Size | Number | Size | Number | |
| GluN1 particle | ↑ | ↑ | — | — | — | — |
| GluA1 particle | — | — | ↑ | ↑ | — | ↑ |
↑, increase; —, no effect
4. Discussion
Mutations in the components of Ras-MAPK signaling pathway are closely associated with a group of genetic disorders, collectively called Rasopathies, that includes NS, neurofibromatosis, cardio-facio-cutaneous syndrome, LEOPARD syndrome and others [18]. Mutations in PTPN11, which encodes a non-receptor tyrosine phosphatase SHP2, account for ~50 % of NS cases. In the developing brain, SHP2 plays a pivotal role in determining the fate of neural progenitor cells [10, 19]. SHP2 is also involved in the generation of oligodendrocyte progenitor cells [20, 21]. We have reported that a NS-associated SHP2D61G mutation impairs synaptic plasticity and subsequently disrupt hippocampal learning in adult mice, which can be reversed by inhibiting the activation of Ras-MAPK pathway [7]. We showed that AMPA receptor-mediated currents were significantly enhanced in adult NS mutants and that the surface expression of AMPA receptors was enhanced in SHP2D61G-expressing cultured neurons at DIV 21 [7]. Consistently, a recent study also showed that SHP2 is critically involved in the surface expression of AMPA receptors using cultured neuron at DIV 18 [22]. In the present study, we also found that surface AMPA receptor expression is significantly increased in SHP2D61G-expressing cultured neurons at DIV 18, which can be reversed by inhibiting MAPK signaling (Fig. 3). It is interesting that SHP2D61G first increased the size of GluA1 (AMPA receptor) clusters at DIV 12 and later increased the number of GluA1 clusters at DIV 18 (Fig. 2 and 3). In the previous study, SHP2D61G increased only the number of GluA1 clusters, but not the size in fully matured neurons at DIV 21 [7]. In this study, we choose DIV 18 to determine if changes in GluA1 expression were present prior to DIV 21. Altogether, the mutant SHP2 first increases the size of AMPA receptor clusters, presumably within functional spines, which may then affect multiple spines and subsequently increase the number of AMPA receptor clusters in fully matured neurons (Table 1).
Unexpectedly, we found that SHP2D61G affects the surface expression of GluN1-containing NMDA receptor clusters only in premature neurons at DIV 6, but not in early maturing neurons at DIV 12 (Table 1). It is well known that NMDA receptor-mediated currents are larger than AMPA receptor currents in early developmental stages and that the number of synaptic AMPA receptors increase relative to the number of synaptic NMDARs during development [23]. Our data suggest that SHP2D61G first increases NMDA receptor expression in a MAPK-dependent manner. Although NMDA receptor trafficking and expression can be regulated by the phosphorylation of receptor subunits [24], it is not yet clear how Ras-MAPK activation facilitates the surface expression of NMDA receptors. It has been reported that SHP2 is involved in BDNF-mediated upregulation of GluN2B NMDA receptors in the spinal dorsal horn after nerve ligation [25]. However, it is unknown whether BDNF/SHP2/GluN2B signaling also requires MAPK activation. It is well established that MAPK is critical for the regulation of activity-induced translation [26]. One possibility is that SHP2D61G–mediated MAPK activation enhances the synaptic translation of NMDA receptor genes in premature neurons. Alternatively, SHP2D61G may block the processes involved in either the endocytosis or the degradation of NMDA receptors. Further studies are required to clarify the underlying mechanism for the upregulation of NMDA receptor expression by SHP2D61G in premature neurons. In addition, it is not clear whether the changes in GluN1 at DIV 6 may have any impact on the changes in GluA1 levels after DIV 12.
In summary, we found that NS-associated mutant SHP2 D61G differentially disrupt the composition of postsynaptic glutamate receptor subtypes over development, which may interfere with the formation of normal functional connectivity in the brain and eventually contribute to the cognitive deficits in NS.
Supplementary Material
Highlights.
A Noonan syndrome-associated mutation in SHP2 increases NMDA receptor expression in premature neurons.
This mutation increases the size of AMPA receptor clusters in early maturing neurons.
The SHP2 mutation also increases both the size and the number of AMPA receptor clusters in mature neurons.
The altered expressions of glutamate receptors by mutant SHP2 can be reversed by inhibiting the MAPK signaling pathway.
Acknowledgments
We thank Soonil Pyo for assistance with editing the manuscript. This work was supported by the intramural research grant of Chungbuk National University in 2015, by the overseas dispatch program of Chungbuk National University in 2016 to H.K.K and NRF-2016R1A4A1008035 to S.R and Y.-S.L and by R01 MH084315 to A.J.S.
Footnotes
Conflicts of interest
The authors declare that they have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL, GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’, Trends Neurosci 20 (1997) 523–529. [DOI] [PubMed] [Google Scholar]
- [2].Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY, Changing subunit composition of heteromeric NMDA receptors during development of rat cortex, Nature 368 (1994) 144–147. [DOI] [PubMed] [Google Scholar]
- [3].Malinow R, Malenka RC, AMPA receptor trafficking and synaptic plasticity, Annu Rev Neurosci 25 (2002) 103–126. [DOI] [PubMed] [Google Scholar]
- [4].Su C, D’Amour J, Lee M, Lin HY, Manders T, Xu D, Eberle SE, Goffer Y, Zou AH, Rahman M, Ziff E, Froemke RC, Huang D, Wang J, Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens, Mol Brain 8 (2015) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R, Ras and Rap control AMPA receptor trafficking during synaptic plasticity, Cell 110 (2002) 443–455. [DOI] [PubMed] [Google Scholar]
- [6].Clement JP, Aceti M, Creson TK, Ozkan ED, Shi Y, Reish NJ, Almonte AG, Miller BH, Wiltgen BJ, Miller CA, Xu X, Rumbaugh G, Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses, Cell 151 (2012) 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee YS, Ehninger D, Zhou M, Oh JY, Kang M, Kwak C, Ryu HH, Butz D, Araki T, Cai Y, Balaji J, Sano Y, Nam CI, Kim HK, Kaang BK, Burger C, Neel BG, Silva AJ, Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome, Nat Neurosci 17 (2014) 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pierpont EI, Tworog-Dube E, Roberts AE, Learning and memory in children with Noonan syndrome, Am J Med Genet A 161 (2013) 2250–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, Roberts AE, Robinson W, Takemoto CM, Noonan JA, Noonan syndrome: clinical features, diagnosis, and management guidelines, Pediatrics 126 (2010) 746–759. [DOI] [PubMed] [Google Scholar]
- [10].Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD, Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome, Neuron 54 (2007) 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Servidei T, Bhide PG, Huang Z, Moskowitz MA, Harsh G, Reeves SA, The protein tyrosine phosphatase SHP-2 is expressed in glial and neuronal progenitor cells, postmitotic neurons and reactive astrocytes, Neuroscience 82 (1998) 529–543. [DOI] [PubMed] [Google Scholar]
- [12].Oh JY, Kwon A, Jo A, Kim H, Goo YS, Lee JA, Kim HK, Activity-dependent synaptic localization of processing bodies and their role in dendritic structural plasticity, J Cell Sci 126 (2013) 2114–2123. [DOI] [PubMed] [Google Scholar]
- [13].Kim H, Oh JY, Choi SL, Nam YJ, Jo A, Kwon A, Shin EY, Kim EG, Kim HK, Down-regulation of p21-activated serine/threonine kinase 1 is involved in loss of mesencephalic dopamine neurons, Mol Brain 9 (2016) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaech S, Banker G, Culturing hippocampal neurons, Nat Protoc 1 (2006) 2406–2415. [DOI] [PubMed] [Google Scholar]
- [15].Craig AM, Banker G, Neuronal polarity, Annu Rev Neurosci 17 (1994) 267–310. [DOI] [PubMed] [Google Scholar]
- [16].Neel BG, Gu H, Pao L, The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling, Trends Biochem Sci 28 (2003) 284–293. [DOI] [PubMed] [Google Scholar]
- [17].Alonso M, Medina JH, Pozzo-Miller L, ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons, Learn Mem 11 (2004) 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tidyman WE, Rauen KA, The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation, Curr Opin Genet Dev 19 (2009) 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS, Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality, Mol Cell Biol 27 (2007) 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ehrman LA, Nardini D, Ehrman S, Rizvi TA, Gulick J, Krenz M, Dasgupta B, Robbins J, Ratner N, Nakafuku M, Waclaw RR, The protein tyrosine phosphatase Shp2 is required for the generation of oligodendrocyte progenitor cells and myelination in the mouse telencephalon, J Neurosci 34 (2014) 3767–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu Y, Park J, Hu X, Zheng K, Li H, Cao Q, Feng GS, Qiu M, Control of oligodendrocyte generation and proliferation by Shp2 protein tyrosine phosphatase, Glia 58 (2010) 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang B, Du YL, Lu W, Yan XY, Yang Q, Yang W, Luo JH, Increased Activity of Src Homology 2 Domain Containing Phosphotyrosine Phosphatase 2 (Shp2) Regulates Activity-dependent AMPA Receptor Trafficking, J Biol Chem 291 (2016) 18856–18866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hsia AY, Malenka RC, Nicoll RA, Development of excitatory circuitry in the hippocampus, J Neurophysiol 79 (1998) 2013–2024. [DOI] [PubMed] [Google Scholar]
- [24].Chen BS, Roche KW, Regulation of NMDA receptors by phosphorylation, Neuropharmacology 53 (2007) 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding X, Cai J, Li S, Liu XD, Wan Y, Xing GG, BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation, Neurobiol Dis 73 (2015) 428–451. [DOI] [PubMed] [Google Scholar]
- [26].Kelleher RJ 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S, Translational control by MAPK signaling in long-term synaptic plasticity and memory, Cell 116 (2004) 467–479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.