ABSTRACT
Introduction: PD-1 inhibitors have been approved for the treatment of dMMR patients with metastatic colorectal cancer, but the efficacy of neoadjuvant treatment with PD-1 in dMMR locally advanced rectal cancer (LARC) patients has not yet been defined.
Patients and methods: Two patients with LARC received Nivolumab as neoadjuvant treatment in July 2017. Whole-exome sequencing (WES) and multiplex immunofluorescence analysis were performed.
Results: Of the two patients, one achieved pathological complete response after six cycles of nivolumab followed by surgery. The other patient was confirmed to be clinical complete response after six cycles of nivolumab. “Watch and wait” strategy was performed for anal preservation. WES showed high tumor mutation burden. Multiplex immunofluorescence analysis showed immune microenvironment alternation between pretreatment specimen and post-treatment specimen.
Conclusion: Neoadjuvant nivolumab induced complete response in both of the two patients with LARC. Immunotherapy might be an alternative strategy for neoadjuvant treatment for dMMR/MSI rectal cancer.
KEYWORDS: Locally advanced rectal cancer, neoadjuvant treatment, nivolumab, complete response
Introduction
Neoadjuvant chemoradiotherapy was still the standard treatment for locally advanced rectal cancer. However, due to the low incidence, we do not have much data to evaluate the efficacy of neoadjuvant treatment in rectal cancer patients with mismatch repair gene deficiency (dMMR) or microsatellite instability (MSI). PD-1 blockade in tumors with dMMR metastatic colorectal cancer showed tremendous efficacy.1 The response rate was 30–40% in previously treated patients.2 And the checkpoint inhibitors, both pembrolizumab and nivolumab, had been recommended as salvage treatment for metastatic colorectal cancer patients with dMMR/MSI-H. Even in first-line treatment, checkpoint inhibitor was also an option for dMMR/MSI-H population who is not appropriate for intensive therapy. However, there is no study to support neoadjuvant treatment with pembrolizumab or nivolumab in locally advanced rectal cancer due to low incidence of dMMR or MSI-H in rectal cancer.3
To our knowledge, there is no prior report describing neoadjuvant with PD-1 antibody in LARC. Here, we report two local advanced rectal cancer patients with MSI-H who had a complete response to treatment with single-agent nivolumab.
Methods
This is a retrospective study and received institutional review board approval from The Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Both of the two participants provided written informed consent for the treatment and biomarker study.
Results
Two patients with locally advanced rectal cancer were confirmed to be dMMR and lynch syndrome. Two cases received PD-1 antibody (nivolumab) as neoadjuvant treatment, which had not been previously reported in detail. The clinicopathologic characteristic of the two patients is listed in Table 1.
Table 1.
The clinicopathologic and characteristic of the two patients.
| Item | Patient 1 | Patient 2 |
|---|---|---|
| Gender | Male | Female |
| Age (years) | 27 | 62 |
| ECOG PS | 0 | 1 |
| cTNM stage | cT4bN2M0 | cT4bN2M0 |
| MRF | Positive | negative |
| EMVI | Positive | Positive |
| DTAV (cm) | 6.4 | 1.0 |
| Tumor length (cm) | 8.0 | 5.0 |
| MMR | MSH2(-), MSH6(-) | MSH2(-), MSH6(-) |
| MSI (NGS) | MSI-H | MSI-H |
| KRAS | Wild type | Mutation (p.G12D) |
| BRAF | Wild type | Wild type |
| Previous treatment | None | FOLFOXIRI |
| ypTNM | pCR | ccR |
Abbreviation: ECOG PS, ECOG performance status; MRF, Mesorectal fascia; EMVI, Extramural vascular invasion; DTAV, Distance from anal verge; MMR, mismatch repair gene; MSI, microsatellite instability; pCR, pathological complete response; cCR, clinical complete response
Cases
Patient 1, a 27-year-old man, was diagnosed as having locally advanced rectal cancer. The clinical stage was cT4bN2 with MRI examination. Left seminal vesicle gland and the posterior wall of the bladder were invaded by the large mass in the middle part of the rectum, with positive mesorectal fascia (MRF). The distance from the inferior margin of tumor to the anal verge was 6.4 cm and the longitudinal diameter was 8.0 cm (Figure 1A). Multiple enlarged lymph nodes was observed next to the mesorectal and superior rectal artery. No distant metastasis was found with chest abdomen and pelvic enhanced CT scan. The pathology examination was moderate differentiated adenocarcinoma. And the immunohistochemistry for protein of mismatch repair gene showed deficiency of MSH2 and MSH6, which was dMMR. The blood tumor markers were all normal. The patient was diagnosed with ascending colon cancer 5 years ago. He had undergone operation once and the pathologic result proved to be stage III mucinous carcinoma. He refused adjuvant chemotherapy and regular and surveillance after surgery. And about the family history, the patient’s mother was diagnosed with rectal cancer and endometrial carcinoma, and the uncle on the mother’s side was also diagnosed with colon cancer. According to Amsterdam criteria, the patient was diagnosed as Lynch syndrome. For this patient, the standard treatment should be chemoradiotherapy. However, the young patient refused chemotherapy and radiotherapy. Besides, he wanted to preserve the fertility function. To relieve incomplete obstruction, intestinal stent was placed first. Since dMMR was confirmed, we gave him anti-PD-1 antibody as neoadjuvant treatment (nivolumab 3mg/kg, every 2 weeks) with the approval of institutional review board and written informed consent of the patient. No significant adverse event was observed except grade 2 fatigue. After six cycles of treatment, tremendous effect was observed on MRI evaluation (Figure 1A). And then, total mesorectal excision was performed. The pathologic result showed complete response. After surveillance for more than 1 year, no recurrence was found.
Figure 1.
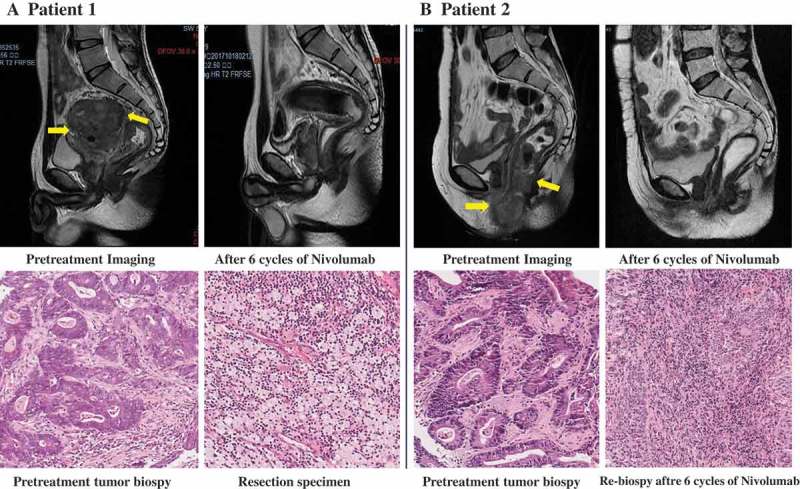
Radiologic and pathological response to neoadjuvant treatment with nivolumab.
Panel A shows pelvic MRI of patient 1 with stage cT4bN2M0 rectal cancer before and after the administration of nivolumab. In the upper row, the pretreatment scan shows a large mass in the upper middle of rectum was adhered to left seminal vesicle gland, the posterior wall of the bladder and the presacral space (arrow). The distance from inferior margin of tumor to the anal verge was 6.4 cm and the tumor length was 8.0 cm. A scan performed before surgery shows greatly shrinkage of the tumor. In the lower row, shown are representative sections of tumor specimens obtained from patient 1 before the administration of nivolumab (left) and after the administration (right) (hematoxylin and eosin staining). This patient had 100% pathological regression of the primary tumor. Panel B shows Pelvic MRI of patient 2 with stage cT4bN2M0 rectal cancer, who received six cycles of nivolumab as neoadjuvant treatment. In the upper row, before the infusion of nivolumab (left), the tumor grows out of intestine and invaded the vaginal inferior wall, the perineal shallow area, the posterior wall of the vaginal vestibule, the anal canal, and the right levator ani muscle (arrow). After six cycles of nivolumab, no extraluminal infiltration was observed. The lesion was significantly shrunk and scarring. In the lower row, shown are representative sections of tumor specimens from patient 2 before the administration of nivolumab (left) and after administration (right) (hematoxylin and eosin staining). Tumor cells are present throughout the pretreatment specimen, whereas in the post-treatment biopsy specimen, there were no viable cancer cells.
Patient 2, a 62-year-old woman, received Dixon surgery 20 years ago after diagnosis with rectal cancer, and underwent hysterectomy and bilateral oophorectomy 9 years ago after diagnosis with endometrial carcinoma. Now the patient was diagnosed as recurrence locally advanced low-lying rectal cancer with anal pain and bloody stool. The tumor located below the rectal anastomosis, and the lesion was involved in the vaginal inferior wall, the posterior wall of the vaginal vestibule, the anal canal, and the right levator ani muscle. The clinical stage was cT4bN2M0 with the pelvic MRI (Figure 1B) and chest abdomen and pelvic CT evaluation. The pathology examination was adenocarcinoma. And the immunohistochemistry showed CDX2 positive, deficiency of MSH2 and MSH6, which was dMMR. According to the family history, lynch syndrome was also diagnosed. In order to shrink the primary tumor and relieve the pain as soon as possible, mFOLFOXIRI regimen was performed as neoadjuvant chemotherapy for four cycles. The main adverse event was grade 3 fatigue and grade 3 neutropenia. The MRI evaluation showed stable disease after four cycles of treatment. The patients refused further chemotherapy and radiotherapy. Given the dMMR status, anti-PD-1 antibody (nivolumab 3 mg/kg, every 2 weeks) was administered to the patient. No grade 3/4 adverse event was observed. After six cycles of treatment, the tumor shrank greatly and clinical complete response (cCR) was considered. On MRI evaluation, only scarring was observed without obvious infiltration in the perineal area (Figure 1B). The pathological assessment of the biopsy was also negative. For anal sphincter preservation, the patient refused miles surgery. Hence, watch and wait strategy was chosen. With 1-year follow-up, endoscopic examination and pelvic MRI every 3 months, the patient was still disease free.
Genomic analysis
In order to examine the landscape of genomic alterations, predicted neoantigens, tumor mutation burden and their association with treatment response, whole-exome sequencing (WES) was performed for these two patients with pretreatment biopsy tissue. Of the two patients, 2143 and 2793 somatic mutations per tumor were detected. In patient 1, MSH2 and MSH6 mutation were detected, and MSI-high was also confirmed. Besides, POLD1 mutation was detected in this patient, which plays a critical role in DNA replication and repair and contributed to the high tumor mutation burden. ATR, PMRM1, ARID2 and SMARCA4 mutation were noted, which were all positive predictor for immunotherapy. In patient 2, the mismatch repair gene MLH1 mutation was found. And the positive biomarker for immunotherapy included BRCA1, ATR, ATM, BRIP1, ARID2, SMARCA4. In addition, no immune-related negative predictor was observed. There were some other driver genes with a mutation of unknown significance in WES.
Multiplex immunofluorescence analysis
Both of the two patients achieved complete response after neoadjuvant administration of nivolumab. One patient had underwent total mesorectal excision and the pathological result showed no residual viable tumor cells. The other patient showed clinical complete response in imaging evaluation and no tumor cells was observed in post-treatment biopsy. To explore the alternation of microenvironment in the tumor, multiplex immunofluorescence staining was performed for the two patients with pre- and post-treatment specimen (Figure 2). The pre-treatment specimen contained PD-L1 positive tumor cells, CD68+ macrophages abutting PD-1 positive, CD8 + T cells. And the post-treatment specimen contained an influx of CD8 + T cells and high expression of PD-L1 on immune cells, which was consistent with an adaptive PD-L1 up-regulation mechanism.
Figure 2.
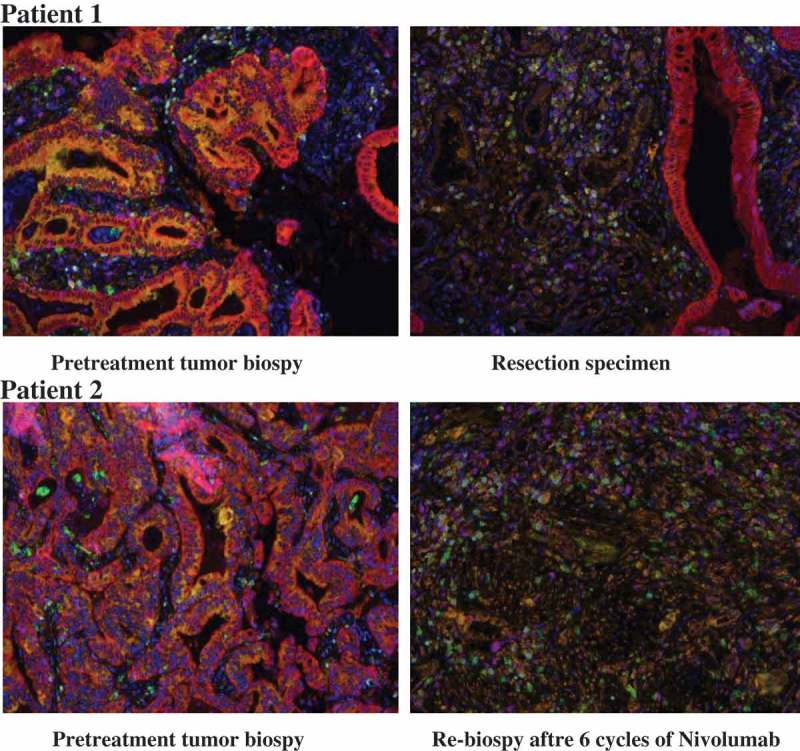
Pathological assessment of response to neoadjuvant blockade of PD-1.
Multiplex immunofluorescence staining was performed on specimens obtained from patient 1 (upper row) and patient 2 (lower row) before (left) and after (right) neoadjuvant administration of nivolumab. With this staining technique, visible structures include cytokeratin (CK) positive tumor cells (magenta), DAPI positive cells (dark blue), CD68+ macrophages (green), FoxP3+ regulatory T cells (light-blue), CD8 + T cells (purple), PD-1 + cells (yellow-green), and PD-L1 + cells (orange). In the pretreatment specimen, PD-L1 positive and CD68+ macrophages abutting PD-1 positive, CD8 + T cells. There are multiple foci where PD-L1 and PD-1 are expressed in close proximity to each other. After administration of nivolumab, CD8+ and PD-1+ immune cells infiltration was observed. PD-L1 expression was observed on macrophages and other infiltrating immune cells.
Discussion
We observed that neoadjuvant administration of nivolumab in dMMR/MSI patients with LARC was associated with few adverse events and led to complete response in both of the two patients, with one pathologic complete response and one clinical complete response. Most importantly, the one who achieved clinical complete response avoided miles’ surgery and preserved the anal function. This is the first report with anti-PD-1 antibody monotherapy as neoadjuvant treatment in dMMR rectal cancer.
In LARC, there was about 6% of patients with dMMR or MSI.3,4 Most of the patients were young. The standard treatment for LARC was fluoropyrimidine with concurrent radiotherapy followed by total mesorectal excision (TME) surgery.5,6 But for this small population, the efficacy of neoadjuvant treatment with 5FU-based chemoradiotherapy was unknown. In our previous study,7 the pCR rate with 5FU-based chemoradiotherapy was only about 10% in this subgroup of patients, while performing neoadjuvant chemotherapy alone, the pCR rate was 0. Another concern about chemoradiotherapy was the toxicities caused by radiation, such as higher rates of incontinence, sexual dysfunction, and fertility dysfunction.8,9 New treatment strategy was warranted for this kind of patients, especially in young patients.
As is known, anti-PD-1 antibody has been approved in metastatic dMMR/MSI solid tumor as salvage treatment.1,2 However, the incidence of dMMR or MSI was only about 5% in metastatic colorectal cancer.10 Whether immunotherapy is effective in another 10% of early dMMR/MSI colorectal cancer patients has not yet been fully evaluated. NICHE study had showed that neoadjuvant treatment with short-term ipilimumab plus nivolumab resulted in major pathological responses in 100% of dMMR tumors and did not compromise surgery.11 Besides, in patients with resectable lung cancer, neoadjuvant nivolumab also induced a major pathological response in 45% of resected tumors.12 In our study, the two patients had complete response to the neoadjuvant immunotherapy with nivolumab alone. One of the patients avoided radiotherapy and protected the fertility function. The other patient avoided miles’ surgery and preserved anal function. Hence, exploring the efficacy of neoadjuvant immunotherapy in LARC with dMMR is necessary and meaningful.
Another issue that needs to be concerned was the duration and efficacy evaluation of neoadjuvant immunotherapy.13 In resectable melanoma, a single dose of neoadjuvant PD-1 blockade led to complete or major pathologic response in 30% of the patients.14 While in early stage colon cancer, one dose of ipilimumab and two dose of nivolumab resulted major pathological responses in 100% of dMMR tumors.11 Due to the large size of the primary tumor of the two patients in our study, we gave six cycles of nivolumab and achieved complete response. The duration of neoadjuvant immunotherapy has not yet been determined. Distinguished from chemotherapy, the radiological evaluation is more complicated with immunotherapy. A heterogeneity of responses might appear in patients receiving checkpoint blockade, including pseudoprogression or hyperprogression.15 In resectable lung cancer with neoadjuvant PD-1 blockade, some of the patients with tumor increasing in size were found to have minimal or no residual tumor in resected specimen.12 This process occurs because of immune-cell infiltration into the tumor rather than true tumor growth.16 We have to avoid either premature withdrawal of the treatment or prolonging ineffective treatment. In the contrast, the potential risk of hyperprogression might turn resectable tumor into unresectable, for whom beginning the treatment should be obviated. However, the predictive biomarkers of hyperprogression have been largely unknown in colorectal cancer. Further genomic analysis was necessary.
We performed WES in these two patients. High tumor mutation burden (TMB) was detected, which was in accordance with the MSI-high status. Besides, we also found other gene mutation that might contribute to the high TMB, such as POLD1, ATR, ATM, PMRM1, and ARID2 mutation. The potential risk with immunotherapy was hyperprogression, a phenomenon reflecting a very rapid tumor progression following immunotherapy, which had been reported in lung cancer.17–19 Fortunately, no immune-resistance-related gene were detected in these two patients. Furthermore, with multiplex immunofluorescence staining, we observed the microenvironment alternation after administration of PD-1 antibody. PD-1 blockade worked directly unleash intratumoral T-cell killing, which might enhance the systemic priming of anti-tumor T cells, thereby potentially eliminating micrometastatic cancer that otherwise might cause relapse.20 This concept had been underpinned in preclinical study.21
The limitations of our study include the small number of patients enrolled and the short postoperative follow-up period. Further studies are needed to compare the efficacy of neoadjuvant treatment with PD-1 blockade versus standard chemoradiotherapy in LARC patients with dMMR. And the most effective duration of neoadjuvant therapy has to be determined.
Conclusions
Neoadjuvant immunotherapy in dMMR or MSI LARC patients led to complete response, which protect the fertility function and preserved the anal function. Further, larger studies are needed to determine the best predictive biomarkers of response and the most effective duration of neoadjuvant therapy and to correlate the pathological response resulting from neoadjuvant immunotherapy with overall survival. Long-term follow-up was also necessary to define the efficacy of PD-1 blockade in reducing recurrence in LARC.
References
- 1.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):1–5. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, Yu L, Roychowdhury S. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostwal V, Pande NS, Engineer R, Saklani A, deSouza A, Ramadwar M, Sawant S, Mandavkar S, Shrirangwar S, Kataria P, et al. Low prevalence of deficient mismatch repair (dMMR) protein in locally advanced rectal cancers (LARC) and treatment outcomes. J Gastrointest Oncol. 2019;10(1):19–29. doi: 10.21037/jgo.2018.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khrizman P, Niland JC, Ter Veer A, Milne D, Bullard Dunn K, Carson WE, Engstrom PF, Shibata S, Skibber JM, Weiser MR, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol. 2013;31(1):30–38. doi: 10.1200/JCO.2011.40.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson AB 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen Y-J, Cooper HS, Engstrom PF, Enzinger PC, Fenton MJ, Fuchs CS, et al. Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:719–728;quiz 728. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Zhang J.. The efficacy of neoadjuvant treatment in locally advanced rectal cancer with dMMR. J Clin Oncol. 2019;37(suppl4,abstr615):615. doi: 10.1200/JCO.2019.37.4_suppl.615. [DOI] [Google Scholar]
- 8.Kilic D, Yalman D, Aksu G, Atasoy BM, Igdem S, Dincbas FO, Yalcin S.. Impact of adjuvant chemoradiotherapy for rectal cancer on the long-term quality of life and late side effects: a multicentric clinical evaluation by the turkish oncology group. Asian Pac J Cancer Prev. 2012;13(11):5741–5746. doi: 10.7314/apjcp.2012.13.11.5741. [DOI] [PubMed] [Google Scholar]
- 9.Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, Carlsen E, Tveit KM. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76(4):1005–1011. doi: 10.1016/j.ijrobp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 11.Grootscholten C, Voest EE, Chalabi M, Cosgriff T, Srimuninnimit V, Pittman K, Sabbatini R, Rha SY, Flaig TW, Page RD, et al. LBA37_PRNeoadjuvant ipilimumab plus nivolumab in early stage colon cancer. Ann Oncol. 2018;29(suppl_8). doi: 10.1093/annonc/mdx807. [DOI] [Google Scholar]
- 12.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15(23):7116–7118. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 14.Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125–135. doi: 10.1016/j.intimp.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al. Evaluation of Immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria J-C, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated With PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44(4):924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, Young A, O’Donnell JS, Allen S, Smyth MJ, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]


