ABSTRACT
The gut microbiota plays a critical role in the anti-tumor immune response. There is increasing data showing that antibiotics (ATBs) change the composition of the gut microbiota and affect the efficacy of immune checkpoint inhibitors (ICIs). However, this is the first meta-analysis to evaluate the association between ATB use and ICI efficacy in cancer patients to provide a better understanding of the strength of this association. We performed a literature search for relevant studies that evaluated the relationship between ATB use and ICI efficacy using the PubMed, Embase, and conference databases. The primary outcomes consisted of overall survival (OS) and progression-free survival (PFS) measured by hazard ratios (HR) and corresponding 95% confidence intervals (CI). Subgroup and sensitivity analyses were also performed. A total of 19 eligible studies comprising 2,740 cancer patients treated with ICIs were included in the analysis. Our results indicated that ATB use was negatively associated with OS in cancer patients (HR = 2.37; 95% CI = 2.05–2.75; P < .001), without heterogeneity (I2 = 0.0%; P = .851). Moreover, ATB use significantly reduced PFS in patients treated with ICIs (HR = 1.84; 95% CI = 1.49–2.26; P < .001; I2 = 56.2%). Similar results were obtained in the subgroup analyses stratified by the time of ATB use and cancer type. Sensitivity analyses confirmed the stability of our results. Therefore, the findings of our meta-analysis indicated that ATB use is negatively associated with OS and PFS in cancer patients treated with ICI immunotherapy.
KEYWORDS: Antibiotics, immune checkpoint inhibitors, immunotherapy, overall survival, progression-free survival
Introduction
Immune checkpoint inhibitors (ICIs) that target programmed cell death-1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), have altered the therapeutic landscape and have become an attractive treatment strategy in several malignancies due to their high efficacy and lower toxicity compared with traditional cytotoxic drugs.1,2 However, in clinical practice, the clinical efficacy of ICIs is highly variable among cancer patients, and a considerable proportion of patients still progress with disease or relapse due to ICI resistance and limited efficacy.3,4 Recently, researchers have sought to identify prognostic and predictive biomarkers associated with the response to ICI immunotherapy (e.g., PD-L1 expression, tumor mutational burden, tumor infiltrating lymphocytes, mismatch repair deficiency, and high microsatellite instability); however, the predictive precision of such potential biomarkers remains limited.5,6 Thus, it is critical to explore reliable predictors to improve the clinical response to ICIs.
Evidence has shown that a balanced gut microbiota is an important regulator of the systemic immune system.7,8 Furthermore, preclinical studies have reported that the gut microbiota is involved in the response to ICI immunotherapy.9,10 Moreover, it has been is well-documented that antibiotics (ATBs) can alter the diversity and composition of the commensal gut microbiota.11 Thus, researchers hypothesize that dysbiosis of the gut microbiota caused by ATBs may be associated with resistance to ICI immunotherapy and have a negative impact on the efficacy of ICI immunotherapy. Although several studies have evaluated the association between ATB use and ICI efficacy, inter-study heterogeneity may exist between ATBs and ICI efficacy; thus, a pooled analysis may provide a greater understanding of the strength of this relationship.
The purpose of this study was to evaluate the association between ATB use and ICI efficacy in cancer patients treated with ICIs.
Results
Study selection and associated characteristics
A total of 719 studies were identified from our literature search, of which 658 studies were excluded after reviewing the titles and abstracts. The remaining 61 studies were further reviewed, and 42 studies were excluded based on the eligibility criteria. Finally, 19 studies were included in our quantitative analysis (Figure 1).10,12-29
Figure 1.
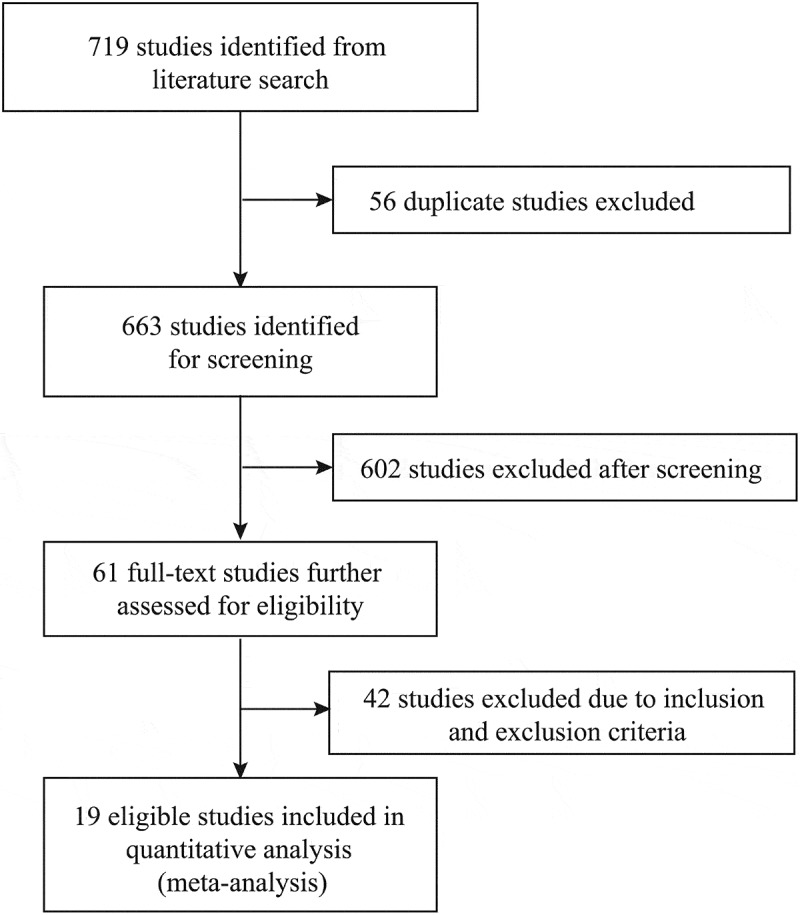
Literature search and study selection.
A total of 2,740 patients [mean: 144; median with range: 109 (30–360)] were included in our analysis. The included studies were published between 2017 and 2019 (17/19 studies, 89%, were published in 2018 and 2019) from the United States, the United Kingdom, China, Japan, Canada, Switzerland, Greece, Korea, Austria, and France. With regards to the time of ATB use, 11 studies provided results on the association between pre-therapy ATB use and ICI efficacy,13,15-19,21,23,24,26,28 nine studies provided data on the association between pre-therapy or post-therapy ATB use and ICI efficacy,10,12,14,20,22,24,25,27,29 and only one study provided results on the association between post-therapy ATB use and ICI efficacy.24 A total of 14 studies used anti-PD-1/PD-L1 inhibitors,10,12,13,15,16,18,20,22-25,27-29 three studies used anti-PD-1/PD-L1 inhibitors and/or anti-CTLA-4 inhibitors,17,21,26 and two studies did not report the type of ICI drug.14,19 Of the eligible studies, eight studies were complete cohort studies10,12,16,17,24,26,27,29 and 11 studies provided only an abstract.13-15,18-23,25,28 The main characteristics of the included studies are listed in Table 1.
Table 1.
The baseline characteristics of included studies.
| Author | Year | Country | Cancer type | Definition of antibiotics use | Treatment | Sample (Y/N) | Outcome |
|---|---|---|---|---|---|---|---|
| Zhao | 2019 | China | NSCLC | Within 1 month before or after initiation of anti-PD-1 therapy | PD-1 inhibitors alone or in combination with chemotherapy | 109(20/89) | OS, PFS |
| Hakozaki | 2019 | Japan | NSCLC | For ≥3 days within 30 days of nivolumab | Nivolumab monotherapy | 90(13/77) | OS, PFS |
| Elkrief | 2019 | Canada | Melanoma | Within 30 days prior to ICI initiation | PD-1 inhibitors or CTLA-4 inhibitors alone or in combination with chemotherapy | 74(10/64) | OS, PFS |
| Agarwal | 2019 | US | Urothelial carcinoma | Within 1 month before starting to during anti-PD1/PDL1 therapy | PD1/PD-L1 inhibitors | 101(26/75) | OS |
| Schett | 2019 | Switzerland | NSCLC | Within 2 months prior to start of therapy | PD-(L)1 inhibitors | 218(44/174) | OS, PFS |
| Rounis | 2019 | Greece | NSCLC | Within 30 days pre- or during therapy | ICI | 44(NR) | OS, PFS |
| Pinato | 2019 | United Kingdom | NSCLC and melanoma | Within 1 month prior to ICI or until ICI cessation | PD-1/PD-L1 inhibitors | 196(97/99) | OS |
| Routy | 2018 | France | NSCLC and urothelial carcinoma | Within 2 month before or 1 month after starting anti-PD-1 therapy | Nivolumab or durvalumab | 182(132/50) | OS, PFS |
| Tinsley | 2018 | United Kingdom | Melanoma, RCC and NSCLC | Within 2 weeks of ICI initiation or 6 weeks after | ICI | 303(94/209) | OS, PFS |
| Swami | 2018 | United Kingdom | Melanoma | Within 2 months before or after starting anti-PD-1 therapy | PD-1 inhibitors | 199(NR) | PFS |
| Sen | 2018 | United States | RCC, NSCLC, melanoma, sarcoma and gastrointestinal stromal tumors | during ICI use; within 30 days of ICI; 30–60 days prior to ICI | CTLA-4 or PD-1 inhibitors | 172(NR) | OS, PFS |
| Lalani | 2018 | United States | RCC | between 8-weeks pre- and 4-weeks post initiation of therapy | PD-1/PD-L1 inhibitors | 146(31/115) | OS, PFS |
| Kim | 2018 | Korea | Advanced cancer | Within 30 days of ICI initiation | Nivolumab, pembrolizumab or atezolizumab | 199(57/142) | OS |
| Huemer | 2018 | Austria | Non-squamous NSCLC | Within 1 month before or 1 month after ICI initiation | Nivolumab or pembrolizumab | 30(11/19) | OS, PFS |
| Do | 2018 | United States | Lung cancer | Within 30 days before nivolumab initiation to 30 days after the last dose of nivolumab | Nivolumab | 109(87/22) | OS |
| Derosa | 2018 | Canada | RCC and NSCLC | Within the 30 or 60 days before the start of anti-PD-(L)1 therapy | PD-(L)1 inhibitors alone or in combination with CTLA-4 inhibitors or bevacizumab | 360(90/270) | OS, PFS |
| Ahmed | 2018 | United States | Advanced cancer | Within 2 weeks prior to and after therapy initiation and within 10 weeks prior to disease progression | Nivolumab, pembrolizumab or atezolizumab | 60(17/43) | OS, PFS |
| Thompson | 2017 | United States | NSCLC | Within 6 weeks of initiating anti-PD-1 therapy | PD-1 inhibitors | 74(18/56) | OS, PFS |
| Kaderbhai | 2017 | France | NSCLC | Within 3 months before nivolumab initiation or during therapy | Nivolumab in monotherapy | 74(15/59) | PFS |
NOTE, ICI: Immune Checkpoint Inhibitors; NR: Not Reported; NSCLC: Non-Small-Cell Lung Cancer; OS: Overall Survival; PFS: Progression-Free Survival; RCC: Renal Cell Carcinoma; Y/N: antibiotics use/no antibiotics use
ATB use and overall survival (OS)
Our results indicated that ATB use was negatively associated with OS in cancer patients treated with ICIs (hazard ratios [HR] = 2.37, 95% confidence intervals [CI] = 2.05–2.75, P < .001; Figure 2), without obvious heterogeneity among the analyzed studies (I2 = 0.0%, P = .851). Sensitivity analyses using the leave-one-out approach confirmed the stability of our results, and no single study substantially dominated the results (Figure 3). Regarding the time of ATB use, pre-therapy ATB use had an unfavorable impact on OS without heterogeneity (HR = 2.29, 95% CI = 1.92–2.73, P < .001, I2 = 0.0%; Figure 4), and similar results were observed for patients who received pre-therapy or post-therapy ATBs without heterogeneity (HR = 2.56, 95% CI = 1.96–3.36, P < .001; I2 = 0.0%). Further analysis of pre-therapy ATB use indicated that the HR for ATB use within one month prior to ICI (HR = 2.23) was larger than that within two months before ICI (HR = 1.97), although the difference was small. Results for non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), and urothelial carcinoma (UC) were provided in 10, 2, and 2 studies, respectively. The pooled results also showed that patients treated with ATBs had poor OS without heterogeneity (NSCLC: HR = 2.68, 95% CI = 2.19–3.28, P < .001, I2 = 0.0%; RCC: HR = 1.68, 95% CI = 1.00–2.83, P = .052, I2 = 0.0%; UC: HR = 2.01, 95% CI = 1.23–3.29, P = .005, I2 = 0.0%). Moreover, these results were confirmed by a subgroup analysis based on the type of ICI drug (PD-1 inhibitors: HR = 2.45, 95% CI = 2.04–2.97, P < .001, I2 = 0.0%; PD-1/CTLA-4/PD-1+CTLA-4 inhibitors: HR = 2.23, 95% CI = 1.68–2.97, P < .001, I2 = 0.0%), sample size, publication country, and study type, which indicated that ATB use was associated with a decreased OS (Table 2).
Figure 2.
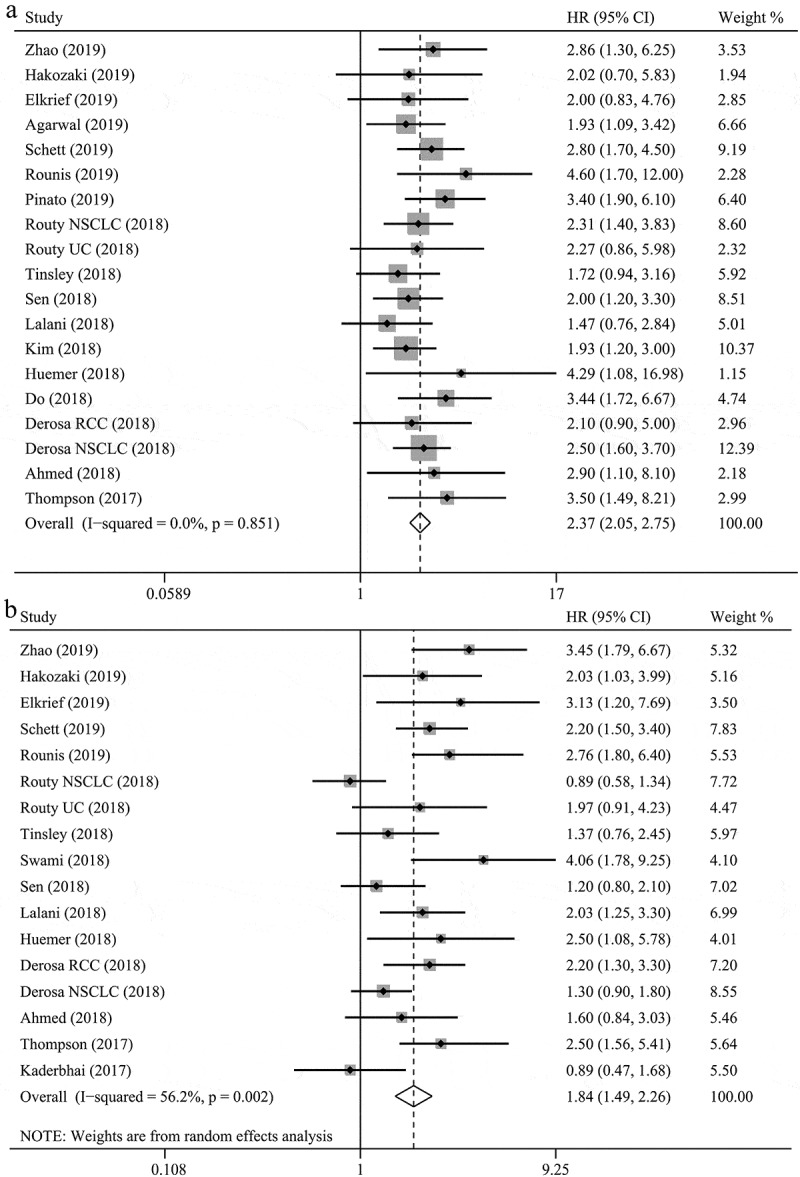
The associations between antibiotic use and overall survival (a) and progression-free survival (b) in cancer patients treated with immune checkpoint inhibitors.
Figure 3.
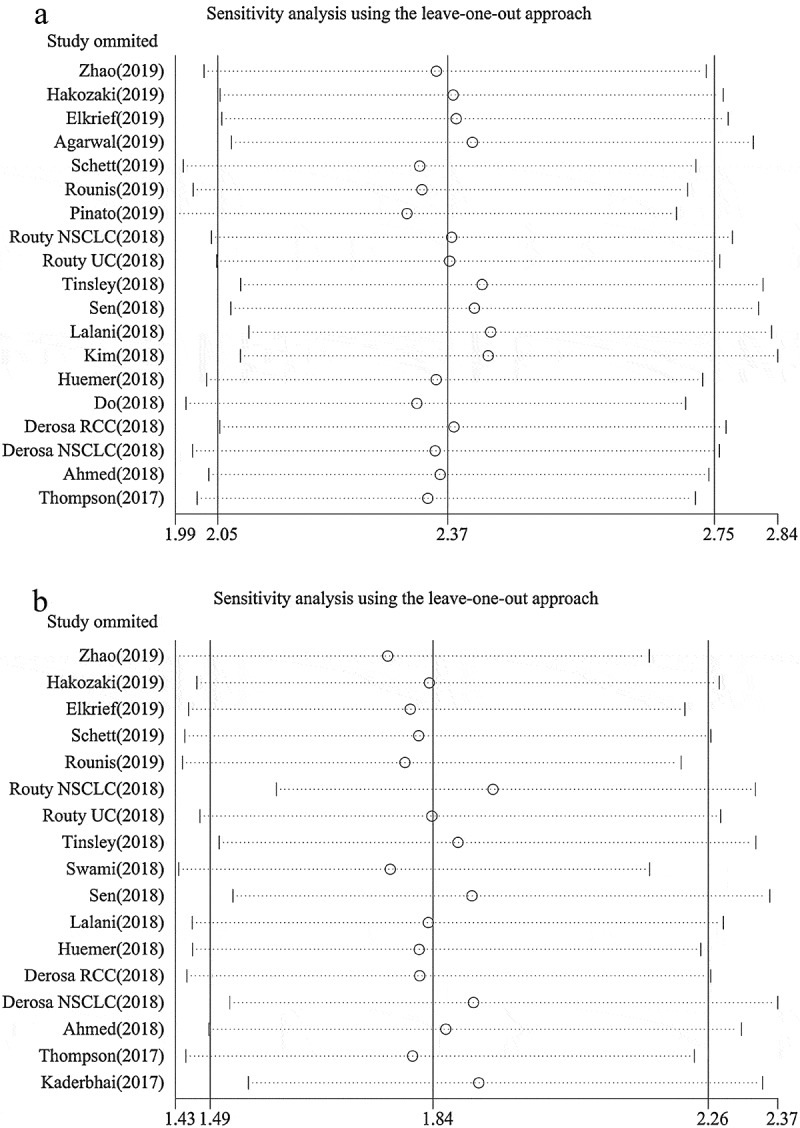
Sensitivity analysis of overall survival (a) and progression-free survival (b) based on leave-one-out approach.
Figure 4.
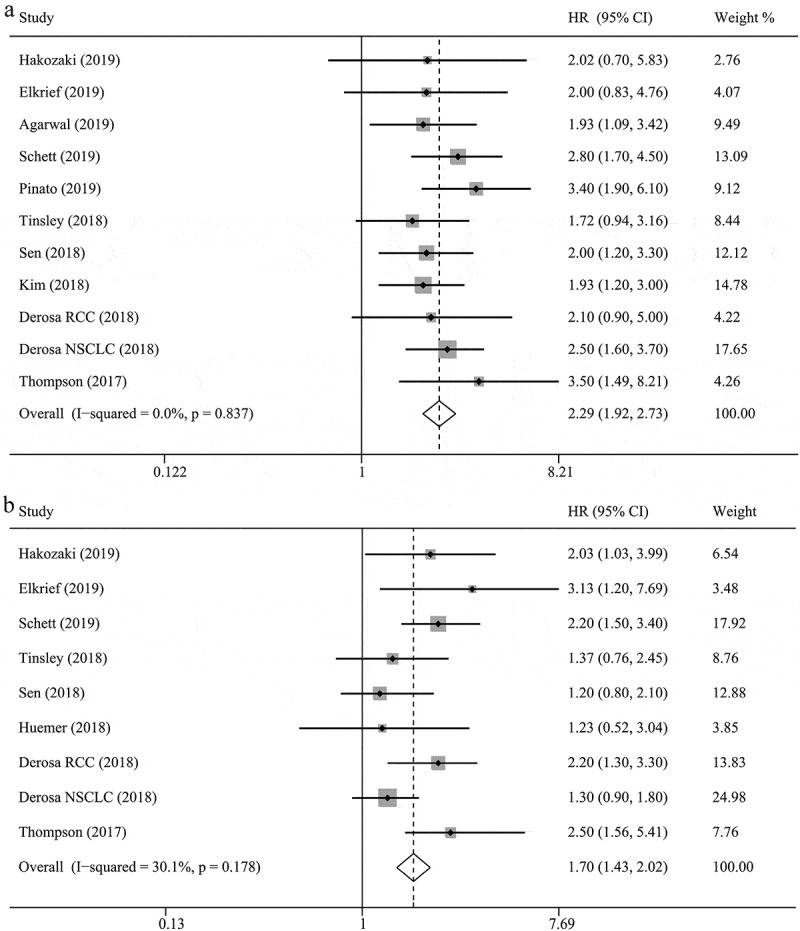
The associations between pre-therapy antibiotic use and overall survival (a) and progression-free survival (b) in cancer patients treated with immune checkpoint inhibitors.
Table 2.
The results for the association of antibiotics use and efficacy of immune checkpoint inhibitors.
| N | Hazard ratio | P for hazard ratio | Heterogeneity (P, I2) | Publication bias | |
|---|---|---|---|---|---|
| Overall survival | |||||
| Overall | 17 | 2.37(2.05–2.75) | < 0.001 | 0.851, 0.0% | Begg’s Test = 0.401; Egger’s test = 0.235 |
| Time of antibiotics use | |||||
| Before ICI initiation | 10 | 2.29(1.92–2.73) | < 0.001 | 0.837, 0.0% | Begg’s Test = 1.000; Egger’s test = 0.975 |
| One month before ICI initiation | 7 | 2.23(1.82–2.74) | < 0.001 | 0.865, 0.0% | Begg’s Test = 0.711; Egger’s test = 0.778 |
| Two month before ICI initiation | 2 | 1.97(1.49–2.59) | < 0.001 | 0.199, 38.0% | Begg’s Test = 1.000; Egger’s test = / |
| Before or after ICI initiation | 7 | 2.56(1.96–3.36) | < 0.001 | 0.572, 0.0% | Begg’s Test = 0.266; Egger’s test = 0.207 |
| Cancer type | |||||
| Non-small-cell lung cancer | 10 | 2.68(2.19–3.28) | < 0.001 | 0.911, 0.0% | Begg’s Test = 0.283; Egger’s test = 0.105 |
| Urothelial carcinoma | 2 | 2.01(1.23–3.29) | 0.005 | 0.778, 0.0% | Begg’s Test = 1.000; Egger’s test = / |
| Renal cell carcinoma | 2 | 1.68(1.00–2.83) | 0.052 | 0.518, 0.0% | Begg’s Test = 1.000; Egger’s test = / |
| ICI drug | |||||
| Anti-PD-1 | 12 | 2.45(2.04–2.97) | < 0.001 | 0.765, 0.0% | Begg’s Test = 0.669; Egger’s test = 0.316 |
| Anti-PD-1/CTLA-4/PD-1+ CTLA-4 | 3 | 2.23(1.68–2.97) | < 0.001 | 0.910, 0.0% | Begg’s Test = 0.734; Egger’s test = 0.398 |
| Treatment type | |||||
| ICI monotherapy | 5 | 2.90(1.80–4.68) | < 0.001 | 0.861, 0.0% | Begg’s Test = 0.806; Egger’s test = 0.494 |
| Sample size | |||||
| <100 | 6 | 2.97(1.98–4.44) | < 0.001 | 0.779, 0.0% | Begg’s Test = 1.000; Egger’s test = 0.617 |
| ≥100 | 11 | 2.29(1.95–2.68) | < 0.001 | 0.780, 0.0% | Begg’s Test = 0.855; Egger’s test = 0.983 |
| Country | |||||
| Asia | 3 | 2.12(1.46–3.07) | < 0.001 | 0.695, 0.0% | Begg’s Test = 1.000; Egger’s test = 0.647 |
| No-Asia | 14 | 2.41(2.06–2.84) | < 0.001 | 0.768, 0.0% | Begg’s Test = 0.499; Egger’s test = 0.323 |
| Progression-free survival | |||||
| Overall | 15 | 1.84(1.49–2.26) | < 0.001 | 0.002, 56.2% | Begg’s Test = 0.091; Egger’s test = 0.035 |
| Time of antibiotics use | |||||
| Before ICI initiation | 8 | 1.70(1.43–2.02) | < 0.001 | 0.178, 30.1% | Begg’s Test = 0.466; Egger’s test = 0.421 |
| Before or after ICI initiation | 8 | 1.91(1.31–2.78) | 0.001 | 0.001, 68.8% | Begg’s Test = 0.175; Egger’s test = 0.066 |
| Cancer type | |||||
| Non-small-cell lung cancer | 9 | 1.79(1.29–2.49) | < 0.001 | 0.001, 69.3% | Begg’s Test = 0.602; Egger’s test = 0.176 |
| Renal cell carcinoma | 2 | 2.12(1.51–2.96) | < 0.001 | 0.815, 0.0% | Begg’s Test = 1.000; Egger’s test = / |
| ICI drug | |||||
| Anti-PD-1 | 10 | 1.92(1.43–2.58) | < 0.001 | 0.003, 61.8% | Begg’s Test = 0.436; Egger’s test = 0.190 |
| Anti-PD-1/CTLA-4/PD-1+ CTLA-4 | 3 | 1.63(1.13–2.36) | 0.009 | 0.092, 53.4% | Begg’s Test = 0.734; Egger’s test = 0.317 |
| Treatment type | |||||
| ICI monotherapy | 5 | 1.94(1.20–3.13) | 0.007 | 0.076, 52.7% | Begg’s Test = 0.221; Egger’s test = 0.081 |
| Sample size | |||||
| <100 | 7 | 1.96(1.51–2.54) | < 0.001 | 0.157, 35.5% | Begg’s Test = 0.764; Egger’s test = 0.391 |
| ≥100 | 8 | 1.76(1.34–2.32) | < 0.001 | 0.002, 65.3% | Begg’s Test = 0.210; Egger’s test = 0.098 |
| Country | |||||
| Asia | 2 | 2.67(1.66–4.27) | < 0.001 | 0.271, 17.5% | Begg’s Test = 1.000; Egger’s test = / |
| No-Asia | 13 | 1.75(1.41–2.18) | < 0.001 | 0.004, 55.9% | Begg’s Test = 0.138; Egger’s test = 0.070 |
NOTE, CTLA-4: Cytotoxic T-Lymphocyte-associated Antigen 4; I:2 Degree of Heterogeneity; ICI: Immune Checkpoint Inhibitors; PD-1: Programmed cell death Drotein-1; PD-L1: Programmed cell Death-Ligand 1; “/”: Not applicable because Egger’s test cannot be performed when the number of studies was 2
ATB use and progression-free survival (PFS)
Our results indicated that ATB use significantly reduced the PFS of patients treated with ICIs (HR = 1.84, 95% CI = 1.49–2.26, P < .001; Figure 2), with heterogeneity among the studies (I2 = 56.2%, P = .002). Sensitivity analyses reported that the results were not dominated by any single study (Figure 3). Furthermore, a subgroup analysis based on the time of ATB use also revealed unfavorable levels of PFS in the group that received ATBs pre-therapy without heterogeneity (HR = 1.70, 95% CI = 1.43–2.02, P < .001, I2 = 30.1%; Figure 4), as well as those receiving pre-therapy or post-therapy ATBs (HR = 1.91, 95% CI = 1.31–2.78, P = .001, I2 = 68.6%). The subgroup analyses based on cancer type (NSCLC: HR = 1.79, 95% CI = 1.29–2.49, P < .001, I2 = 69.3%; RCC: HR = 2.12, 95% CI = 1.51–2.96, P < .001, I2 = 0.0%), type of ICI drug (PD-1 inhibitors: HR = 1.92, 95% CI = 1.43–2.58, P < .001, I2 = 61.8%; PD-1/CTLA-4/PD-1+CTLA-4 inhibitors: HR = 1.63, 95% CI = 1.13–2.36, P < .001, I2 = 53.4%), sample size, publication country, and study type obtained similar results, which indicated that ATB use was associated with a poor PFS (Table 2).
Assessment of publication bias
The results of Begg’s and Egger’s tests indicated that there was no significant publication bias, except in the overall analysis of PFS (PBegg’s = 0.091, PEgger’s = 0.035; Figure 5). Furthermore, the trim-and-fill analysis indicated that publication bias did not affect the PFS results (HR = 1.54, 95% CI = 1.36–1.74) and other subgroup analyses with low p values for Begg’s or Egger’s tests.
Figure 5.
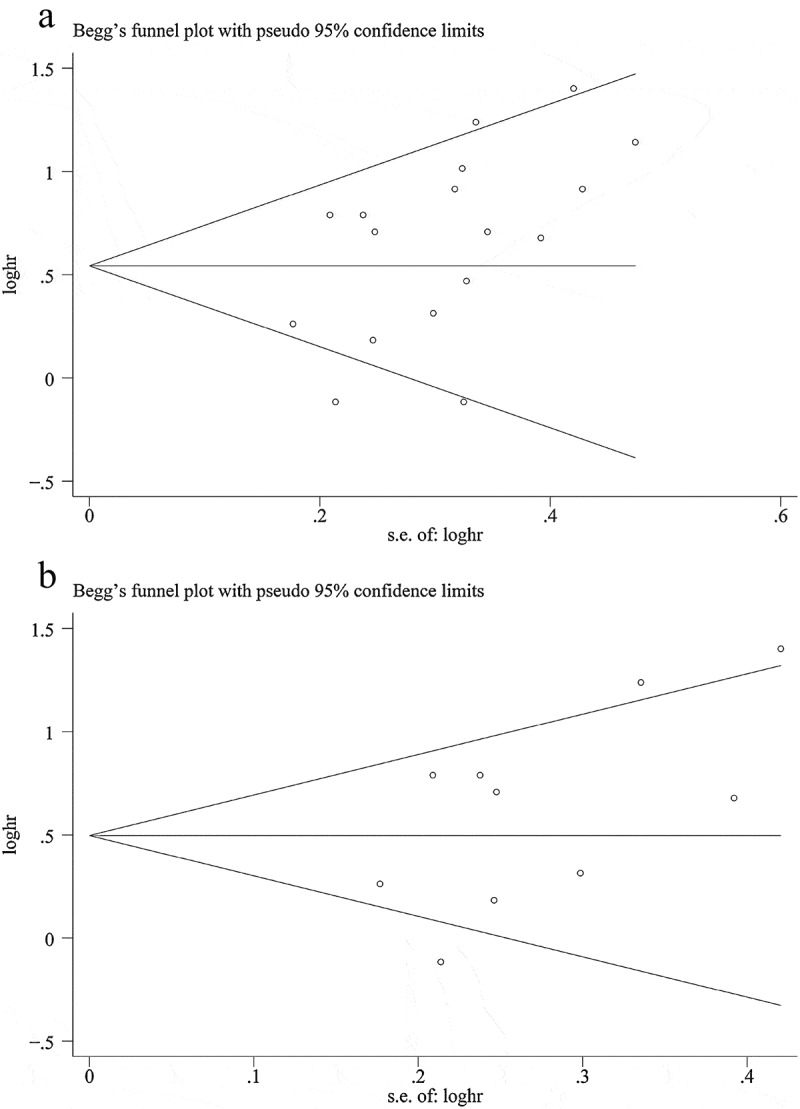
Funnel plot of progression-free survival. A: overall analysis; B: sample size ≥ 100 subgroup.
Discussion
ICI immunotherapy has revolutionized cancer therapy for several solid tumors, and the anti-tumor response to ICIs is enhanced by inhibiting the PD-1 or CTLA-4 pathways and subsequently re-activating the host’s immune function.30-32 Although the resistance and efficacy associated with ICI immunotherapy are greatly affected by the interaction between host and tumor factors, tumor factors alone cannot completely explain the differences in ICI efficacy. As a host factor, the gut microbiota plays a critical role in the anti-tumor response of ICI immunotherapy.9,10 Theoretically, ATBs, which can change the composition of the gut microbiota and lead to dysbiosis, may also affect the efficacy of ICI immunotherapy; however, the clinical data regarding the association between ATB use and ICI efficacy is limited.
To our knowledge, this is the first meta-analysis to systematically evaluate the association between ATB use and the clinical efficacy of ICIs. To assess the impact of ATB use on the clinical efficacy of ICI immunotherapy, this study included 19 eligible studies comprising 2740 cancer patients treated with ICIs. Our results indicated that ATB use is negatively associated with OS (HR = 2.37, 95% CI = 2.05–2.75, P < .001) and PFS (HR = 1.84, 95% CI = 1.49–2.26, P < .001) in cancer patients treated with ICIs. Similar results were obtained in the subgroup analysis of pre-therapy ATB use, NSCLC, and RCC. Furthermore, subgroup analyses based on sample size, publication country, and study type confirmed these results, indicating that ATB use was associated with unfavorable OS and PFS outcomes. In addition, sensitivity analyses revealed that no single study substantially dominated the results. Moreover, no significant publication bias was found, except in the overall analysis of PFS.
The negative association between ATB use and the clinical efficacy of ICI immunotherapy verified the results of previous studies reporting that the diversity and composition of the gut microbiota play a critical role in the immune response.7,10 Indeed, Matson et al. explored the association between the fecal microbial composition and clinical response in melanoma patients treated with anti-PD-1 or anti-CTLA-4, and found a greater abundance of bacterial species, including Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium, in the responders.9 Gopalakrishnan et al. and Routy et al. also found significant differences in gut microbiota diversity and composition between responders and non-responders (i.e., Ruminococcaceae family, Faecalibacterium, and Akkermansia muciniphila).10,33 In addition, several studies have reported that the anti-tumor effects of ICIs were improved in germ-free or ATB-treated mice receiving a fecal microbiota transplantation from responders rather than non-responders, and a favorable gut microbiota could enhance antigen presentation and T cell function associated with the systemic and anti-tumor immune response.9,10 Thus, ATB use may reduce the efficacy of ICI immunotherapy by altering the diversity and composition of the gut microbiota. Although perturbation of the gut microbiota is a highly plausible explanation for the detrimental effects of ATB exposure, this hypothesis has not been mechanistically and prospectively tested in cancer populations treated with ICI immunotherapy. Future large-scale, prospective studies are required to elucidate the mechanisms underlying the relationship between the perturbation of the gut microbiota caused by ATB and poor efficacy of ICI. Whether there are additional mechanisms for the detrimental effects that occur following ATB exposure should also be explored.
The time of ATB use was important for the assessment of ICI efficacy because the diversity and composition of the gut microbiota was temporally altered by ATBs, after which it recovered to baseline within a certain time period following the discontinuation of ATBs. However, in the clinical practice of ICI immunotherapy, no consistent and detailed definition was found for the time of ATB use. Various definitions were used among the included studies according authors’ preference. We found that most studies used a definition of 1 month before and/or after the initiation of ICI immunotherapy. This definition may be imprecise because the recovery time can differ greatly depending on the duration, route, and type of ATB used.34,35 Among the included studies, Derosa et al. explored the impact of time of ATB use (30 versus 60 days before therapy) on ICI efficacy in patients with NSCLC and RCC, and the results demonstrated that the impact of ATB use 60 days before therapy on efficacy was lower than that of ATB use 30 days before therapy. Indeed, our subgroup analyses also indicated that the HR for ATB use within 1 month before ICI was greater than that within 2 months before ICI. The reason for this finding may be that the gut microbiota partially recovered over a longer duration following ATB use.26 In addition, Kaderbhai et al. found that ATB use 3 months before nivolumab immunotherapy did not affect the clinical efficacy of ICI.29 It should be noted that the favorable results of ATB use 60 days prior to therapy could also be due to recall bias over the unavailability of ATB treatment data in retrospective studies. Thus, future large-scale, prospective studies are required to investigate the impact of the use time, duration, route, and drug type of ATBs on the clinical efficacy of ICI immunotherapy. Such findings may then lead to strategies that can help reduce this impact and improve ICI efficacy.
There were some limitations associated with this study: 1) this meta-analysis relied on published data from the included studies and we could not obtain detailed individual data on the tumor and host characteristics (i.e., PD-L1 expression, tumor mutational burden, tumor-infiltrating lymphocytes, mismatch repair deficiency, TNM stage, comorbidities, immune status, treatment strategies, and steroid use) that may influence the efficacy of ICIs; 2) our meta-analysis was not registered online. However, to prevent potential bias, the literature search strategy, inclusion and exclusion criteria, data extraction, and statistical analysis were defined prospectively prior to the initiation of this study; 3) our study could not assess the association between ATB use and immune-related adverse events due to the lack of eligible data. Thus, future studies are required to investigate this potential association; 4) PFS is a weaker endpoint in studies reporting outcomes from patients treated outside of trials given that the restaging interval is not standardized. Indeed, heterogeneity was observed among the included studies regarding PFS analysis. Moreover, the degree of heterogeneity could not be definitively eliminated in the subgroup analysis stratified by the time of ATB use, cancer type, sample size, type of ICI drug, publication country, and study type. This unexplained heterogeneity may result from differences in tumor and host characteristics, as well as other confounding factors; and 5) the limited number of subgroup analyses may affect the statistical power of the results.
In conclusion, the results of this meta-analysis indicated that ATB use was negatively associated with OS and PFS in cancer patients treated with ICI immunotherapy. Therefore, ATBs should be used with caution and strict indications to avoid unnecessary ATB use in cancer patients treated with ICIs. For patients who require ATB, careful ATB selection should be employed to avoid reducing the anti-tumor immune response. In addition to studying the underlying mechanism, future studies should identify which specific gut microbiota phenotypes can enhance or reduce anti-tumor immune responses, and elucidate whether it is feasible to modulate the gut microbiota to a more favorable phenotype to promote a synergistic effect with ICI immunotherapy through fecal microbiota transplantation, probiotic administration, or the reduction of unfavorable microbiota phenotypes through ABT use. Future multicenter randomized clinical studies are also required to explore which favorable interventions can promote gut microbiota recovery and resolve the deleterious effects of ATB-induced gut microbiota dysbiosis. Moreover, the association between ATB and chemo-immunotherapy combinations are also warranted in future studies.
Materials and methods
Literature search
We performed a systematical literature search for relevant studies that had evaluated the association between ATB use and ICI efficacy in cancer patients using the PubMed, Embase, American Society of Clinical Oncology, and European Society of Medical Oncology databases (up to May 2019) using the following search terms: “nivolumab”, “pembrolizumab”, “avelumab”, “atezolizumab”, “lambrolizumab”, “pidilizumab”, “durvalumab”, “ipilimumab”, “tremelimumab”, “immune checkpoint inhibitor”, “PD-1 inhibitor”, “PD-L1 inhibitors”, “CTLA-4 inhibitors”, “antibiotic”, “anti-infectious”, “anti-infection”, “cancer”, “tumor”, “neoplasm”, and “carcinoma”. Moreover, we manually searched the references of the relevant studies to identify other potentially eligible studies.
Eligibility criteria
Studies that met all of the following inclusion criteria were included in our meta-analysis: 1) patient: eligible patients were diagnosed with a solid cancer and treated with ICIs alone (PD-1, PD-L1, or CTLA-4 inhibitors) or in combination with systemic chemotherapy, whereas patients treated with ICIs alongside loco-regional therapy were excluded; 2) intervention: ATBs were administered before and/or after the initiation of ICI therapy, irrespective of the duration and dosage; 3) comparison: the control group did not receive treatment with ATBs; 4) outcome: the two primary outcomes were OS and PFS, and the outcome measures could be extracted. Furthermore, if there were several eligible duplicated studies identified, the most recent study was included in the meta-analysis.
Data extraction
The data from the included studies was independently reviewed and extracted by two authors (Xuan-Zhang Huang and Peng Gao). The following data were extracted from each included study: first author, publication year and country, study design, cancer type, definition of ATB use, type of ICI drug, sample size, age, and outcome measures. Any problems with the data extraction were resolved by discussion.
Statistical analysis
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Supplementary File 1).36 The primary outcome was OS and secondary outcome was PFS, and the association between ATB use and ICI efficacy was measured by HR with the corresponding 95% CI. The overall analysis was conducted by including all studies, and subgroup analyses were conducted based on the time of ATB use, cancer type, sample size, type of ICI drug, publication country, and study type.
We used I2 statistics and a Cochran Q test to evaluate the heterogeneity among the studies, and the heterogeneity was considered statistically significant when the I2 was greater than 50% and/or a P value less than 0.10. A random effect model was used to pool the HRs if the heterogeneity was significant; otherwise, a fixed effects model was used.37 Begg’s and Egger’s tests were used to evaluate publication bias, and a trim-and-fill analysis was performed to evaluate the effect of potential publication bias on an outcome if the p value for Begg’s or Egger’s tests was low (i.e., p < .15).38-40 To assess the bias risk of an individual study on the results and to investigate the stability and consistency of our results, sensitivity analyses were conducted to investigate whether a single study dominated the results by a leave-one-out approach (individually omitting each study).
All statistical analyses were performed using Stata software (Version 12.0, Stata Corporation, College Station, TX, USA). A two-sided P value of < 0.05 was considered statistically significant.
Funding Statement
This work was supported by the National Key R&D Program of China under Grant [MOST-2017YFC0908300], [MOST-2017YFC0908305], [MOST-2016YFC1303200] and [MOST-2016YFC1303202]; and the Program for Liaoning Innovative Research Team in University under Grant [LT2016005].
Acknowledgments
We thank the department of Surgical Oncology of First Hospital of China Medical University for technical assistance. The corresponding author had full access to all the data and analyses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Elias R, Morales J, Rehman Y, Khurshid H.. Immune checkpoint inhibitors in older adults. Curr Oncol Rep. 2016;18(8):1. doi: 10.1007/s11912-016-0534-9. [DOI] [PubMed] [Google Scholar]
- 2.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y.. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–10. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seto T, Sam D, Pan M. Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer. Med Sci. 2019;7(2):14. doi: 10.3390/medsci7020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prelaj A, Tay R, Ferrara R, Chaput N, Besse B, Califano R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer (oxford, England: 1990). 2019;106:144–159. doi: 10.1016/j.ejca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 11.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, Jia Y, He Y, Li A, Su C, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Schett A, Rothschild SI, Mauti LA, Schmid S, Appenzeller C, Curioni-Fontecedro A, Frueh M, Joerger M. Prognostic impact of the use of antibiotics in patients with advanced non-small cell lung cancer (NSCLC) receiving PD-(L)1 targeting monoclonal antibodies. Ann Oncol. 2019;30(Supplement_2). doi: 10.1093/annonc/mdz063.055. [DOI] [Google Scholar]
- 14.Rounis K, Papadaki C, Makrakis D, Monastirioti A, Vamvakas L, Kalbakis K, Mavroudis D, Aggelaki S. Correlation of various clinical, imaging and laboratory parameters with outcome in patients with metastatic non-small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors (ICIs): results from a prospective, observational, single institution study. Ann Oncol. 2019;30(Supplement_2):mdz063.064. doi:10.1093/annonc/mdz063.064. [Google Scholar]
- 15.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al. Antibiotic treatment prior to immune checkpoint inhibitor therapy as a tumor-agnostic predictive correlate of response in routine clinical practice. J Clin Oncol. 2019;37(8)(suppl 8; abstr 147):147. doi: 10.1200/JCO.2019.37.8_suppl.147. [DOI] [Google Scholar]
- 16.Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17(3):2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R, Letarte N, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8(4):e1568812. doi: 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Pond GR, Curran C, Nassar A, Nuzzo PV, Kumar V, McGregor BA, Wei XX, Harshman LC, Choueiri TK, et al. Impact of concurrent medications on outcomes with PD1/PDL1 inhibitors for metastatic urothelial carcinoma. J Clin Oncol. 2019;37(suppl 7S; abstr 435):435. doi: 10.1200/JCO.2019.37.7_suppl.435. [DOI] [Google Scholar]
- 19.Tinsley N, Zhou C, Villa S, Tan G, Lorigan P, Blackhall FH, Elliott T, Krebs M, Carter L, Thistlethwaite F, et al. Cumulative antibiotic use and efficacy of immune checkpoint inhibitors in patients with advanced cancer. J Clin Oncol. 2018;36(15):3010. doi: 10.1200/JCO.2018.36.15_suppl.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swami U, Monga V, Knutson T, Bossler AD, Zakharia Y, Milhem MM. Prognostic markers for progression free survival (PFS) to anti PD-1 therapies in metastatic melanoma. J Clin Oncol. 2018;36(15):e21527–e21527. doi: 10.1200/JCO.2018.36.15_suppl.e21527. [DOI] [Google Scholar]
- 21.Sen S, Carmagnani Pestana R, Hess K, Viola GM, Subbiah V. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann Oncol. 2018;29(12):2396–2398. doi: 10.1093/annonc/mdy453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalani AKA, Xie W, Lin X, Steinharter JA, Martini DJ, Duquette A, Bosse D, McKay RR, Simantov R, Wei XX, et al. Antibiotic use and outcomes with systemic therapy in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2018;36(6):607. doi: 10.1200/JCO.2018.36.6_suppl.607. [DOI] [Google Scholar]
- 23.Kim H, Kim IH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman K, Sabbatini R, Rha SY, Flaig TW, Page RD, et al. Clinical association of antibiotics in immune checkpoint inhibitors for advanced cancer treatment. Ann Oncol. 2018;29. doi: 10.1093/annonc/mdx807. [DOI] [Google Scholar]
- 24.Huemer F, Rinnerthaler G, Westphal T, Hackl H, Hutarew G, Gampenrieder SP, Weiss L, Greil R. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget. 2018;9(23):16512–16520. doi: 10.18632/oncotarget.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do TP, Hegde AM, Cherry CR, Stroud CRG, Sharma N, Cherukuri SD, Bowling M, Walker PR. Antibiotic use and overall survival in lung cancer patients receiving nivolumab. J Clin Oncol. 2018;36(15)(suppl; abstr e15109):e15109–e15109. doi: 10.1200/JCO.2018.36.15_suppl.e15109. [DOI] [Google Scholar]
- 26.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed J, Kumar A, Parikh K, Jin G, Su J, Chou JW, Hoadley KA, Print C, Knowlton N, Black MA, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7(11):e1507670. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson J, Szabo A, Arce-Lara C, Menon S. Microbiome & immunotherapy: antibiotic use is associated with inferior survival for lung cancer patients receiving PD-1 inhibitors. J Thoracic Oncol. 2017;12(11):S1998. doi: 10.1016/j.jtho.2016.09.002. [DOI] [Google Scholar]
- 29.Kaderbhai C, Richard C, Fumet JD, Aarnink A, Foucher P, Coudert B, Favier L, Lagrange A, Limagne E, Boidot R, et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37(6):3195–3200. doi: 10.21873/anticanres.11680. [DOI] [PubMed] [Google Scholar]
- 30.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 32.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discovery. 2016;15(4):235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 33.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12):e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62(Pt 1):97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (clinical Research Ed). 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 40.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


