ABSTRACT
Pleomorphic dermal sarcoma (PDS) is one of the most common sarcoma of the skin. Currently, limited treatment options exist for advanced stages of the disease. While immune checkpoint inhibitors (CPIs) have revolutionized cancer treatment options–their efficacy in PDS has not been explored yet. Here, we present two advanced PDS cases that showed response to anti-PD-1 therapy. Patient A had a locally metastasized PDS and reached a complete remission of the disease after eight cycles of Pembrolizumab. Patient B developed an inoperable relapse of PDS with a complete remission of the disease 4 months after treatment with Pembrolizumab in combination with radiotherapy. To our knowledge, this is the first report of two individuals with advanced PDS that successfully underwent anti-PD1 treatment. By comparing the immune micromilieu to a previously published cohort, we show that the two cases are representative for PDS tumors – potentially making these results more generalizable.
KEYWORDS: Pleomorphic dermal sarcoma (PDS), sarcoma of the skin, PD-1 inhibition, immunotherapy, Pembrolizumab
Introduction
Pleomorphic dermal sarcoma (PDS) is a tumor that commonly occurs in UV-exposed regions, mostly in elderly individuals. Although its prevalence remains largely unknown, PDS cases are observed frequently within the group of cutaneous sarcomas. Although the majority of these tumors can be treated by curative excisions, local recurrences occur in about 30% of cases, and distant metastases have been described in up to 20%, respectively.1–3 In case of unresectable or metastatic disease, there are no effective systemic treatment options available until now. There is also only limited evidence about the effectiveness of primary or adjuvant radiation therapy (RT) in this entity.
Recently, we have reported that the majority of PDS cases exhibited high amount of CD8+ tumor-infiltrating lymphocytes (TILs) and they showed positive correlation with HLA class I and II molecule expression. They also had high infiltration of PD-L1- and LAG-3-expressing immune cells, which are associated with response to immune checkpoint inhibitors (CPIs).4 In different soft tissue sarcomas, both tumor cells and TILs can also express PD-L1.5–7 Based on these results, we hypothesized that PDS tumors with a similar immune background could respond to CPI treatment. Here, we present two patients with locally recurring PDS, who were treated with Pembrolizumab (anti-PD-1). We discuss the molecular characteristics and immune profiles-potentially guiding future therapeutic options. To our knowledge, this is the first report of an effective treatment with CPI in advanced PDS.
Materials and methods
Macrodissection and DNA/RNA isolation
Ten micrometers thin sections were cut from FFPE tissue blocks for DNA/RNA extraction. Three to six sections were macrodissected from unstained slides using a marked hematoxylin-eosin (H&E) stained slide as a reference. Maxwell 16 FFPE Plus LEV DNA Purification Kit was used with Maxwell 16 Instrument for DNA isolation and Maxwell RSC RNA FFPE Kit was used with the Maxwell RSC Instrument for RNA isolation according to manufacturer’s instruction, including DNAse digestion (Promega, WI, U.S.A.). Quantification of DNA was done using Qubit BR DNA assay and RNA was measured using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, MA, U.S.A.).
TruSight oncology 500 assay (Illumina)
Forty nanograms DNA were quantified with the Qubit dsDNA HS Assay (Thermo Fisher Scientific) on the Qubit 2.0 Fluorometer (Thermo Fisher Scientific) and sheared using the LE220-plus Focused-Ultrasonificator (Covaris, Woburn, MA, USA). The TruSight Oncology 500 (Illumina) assay was used for the library preparation following the manufacturer’s protocol. For quality control before sequencing, fragment analysis was performed using the Tapestation with the High Sensitivity D1000 ScreenTape (Agilent). For sequencing, DNA libraries were normalized, pooled, diluted and sequenced on the NextSeq 500 (Illumina) with a NextSeq 500/550 Mid Output Cartridge V2.5 reagent kit (Illumina) following manufacturer’s recommendations. For data analysis of the TMB, variant calling and interpretation was performed using the TruSight Oncology 500 Local App Version 1.3.0.39 (Illumina).
Targeted next-generation sequencing
We performed targeted next-generation sequencing in primary PDS of the two patients using a panel that encompassed exons of 17 characteristically mutated genes in UV-induced tumors as described earlier:8,9 BRAF exons 11, 15; CDK4 exon 2; CDKN2A exons 1, 2; GNA11 exon 5; GNAQ exon 5; HRAS exons 2–4; IDH1 exon 4; KIT exons 9, 11, 13, 17, 18; KNSTRN exon 1; KRAS exons 2–4; NRAS exons 2–4; OXA1L exon 1, PDGFRA exons 12, 14, 18; PIK3CA exons 9, 20; PTEN exons 1–7, RAC1 exon 2, TP53 exons 5–9.
RNA expression profiles using nanostring
Profiling of immune-related gene expression was conducted using the NanoStringPanCancer IO360 Profiling as previously described.4 In short, isolated total RNA was hybridized for 20 h at 65°C and counted. Fifteen reference genes with low variance were used for normalization using the nsolver 4.0 software (NanoString Technologies Inc., WA, U.S.A.). Subsequent statistical analyses were done using R, the R-Project (Vienna, Austria).
Immunohistochemistry (IHC)
Immunohistochemical staining of CD4 (4B12, Thermofisher), CD8 (CD8 Dako), MHC class I (EPR1394y, Abcam), PD-L1 (28–8, Abcam, Rabbit, IgG, EDTA; 1:100), PD-1 (ab5, Abcam, Mouse, IgG1, EDTA; 1:200), CD68 (PG-M1, Dako) and FoxP3 (236A/E7, Abcam) was performed on full tumor sections using the BOND MAX from Leica (Leica, Germany) according to the protocol of the manufacturers. Slides were scanned using a NanoZoomer S360 (Hamamatsu Photonics) slide scanner.
Ethical approval and ethical standards
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by the approval of the institution’s human research review committee (Ethics Committee of the Medical Faculty of University of Cologne: registration no. 15–307).
Informed consent
All patients gave written informed consent to the use of their tumors and their data for research.
Availability of data
Additional data are available from the corresponding author upon reasonable request.
Results
Patient A
A male patient aged 77 years had PDS on his right upper forehead, which was initially excised with 1 cm safety margins. Six months later, he developed a local relapse of PDS on the right upper forehead as well as a cutaneous metastasis in the upper parietal region, which were both excised with safety margins of 1 cm. The patient further received adjuvant radiotherapy with 66Gy on the primary tumor as well as on the cutaneous metastasis site. Five months after these interventions, the patient developed multiple cutaneous metastases in addition to a relapse of PDS at the primary site (Figure 1a). Histologically, the tumor presented with a morphology typical of PDS (Figure 1e). A moderate infiltration of PD-L1+ lymphocytes could be observed on the invasion front of the tumor (Figure 1f). Furthermore, few CD4+ cells could be found, in addition to CD8+ and CD68+ cells (Figure 1(i-k)). The tumor showed infiltration by FOXP3+ and PD-1+ cells and a strong expression of MHC-I (Figure 1(l-n)).
Figure 1.
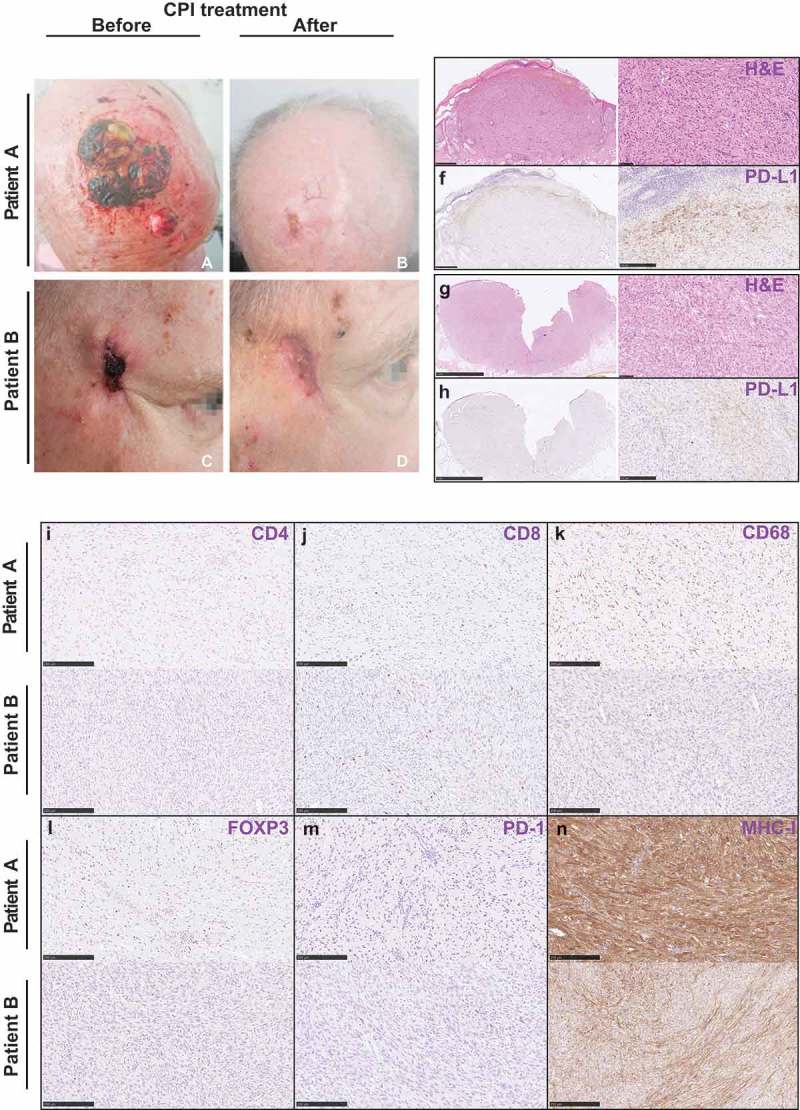
Clinical images and histology of patient A and patient B.
Clinical images of patient A (a, b) and patient B (c, d) before and after immunotherapy. Hematoxylin-Eosin stains (e, g) and immunohistochemical pictures of various markers of immune cells for patient A and patient B (f, h, i-n).
Targeted next-generation sequencing (NGS) revealed that his PDS tumors harbor TP53c.534_535delinsTT (p.H179Y) mutation and PIK3CA c.3194A>T (p.H1065L) mutation with a tumor mutation burden (TMB) of 63.162/MB (Table 1). In addition, using multiplex gene expression analysis, we discovered that patient A harbors similar immune phenotype to the previously published cohort except showing higher expression level for CD47, CDC25C, and CD276 (Figure 2b). Regarding the expression level of the checkpoint molecules/ligands, he mostly displayed lower than median expression level compared to our previously published PDS cohort (Figure 2b). However, CD40, HLA-C, ITGAE, KIR3DL1, PDCD1 (encodes PD-1), TNFSF18, and TNFSF9 were similar to the median value (Figure 2a). In addition, principle component analysis based on the results of the multiplex gene expression assay revealed that patient A showed similar expression to the previously published cohort (Figure 2c).
Table 1.
Summary of genetic characteristics of patient A and patient B.
| Description | Patient A | Patient B |
|---|---|---|
| TP53 | p.H179Y (pathogenic) | p.R282W (pathogenic) |
| PIK3CA | p.H1065L (unknown) | - |
| CDKN2A | - | p.N42K (unknown) |
| KIT | - | p.K499R (unknown) |
| TMB (per Megabase) | 63.162 | 77.997 |
Overview of the genetic alterations found in both PDS tumors with help of targeted sequencing. Pathogenic and unknown mutations are highlighted.
Figure 2.
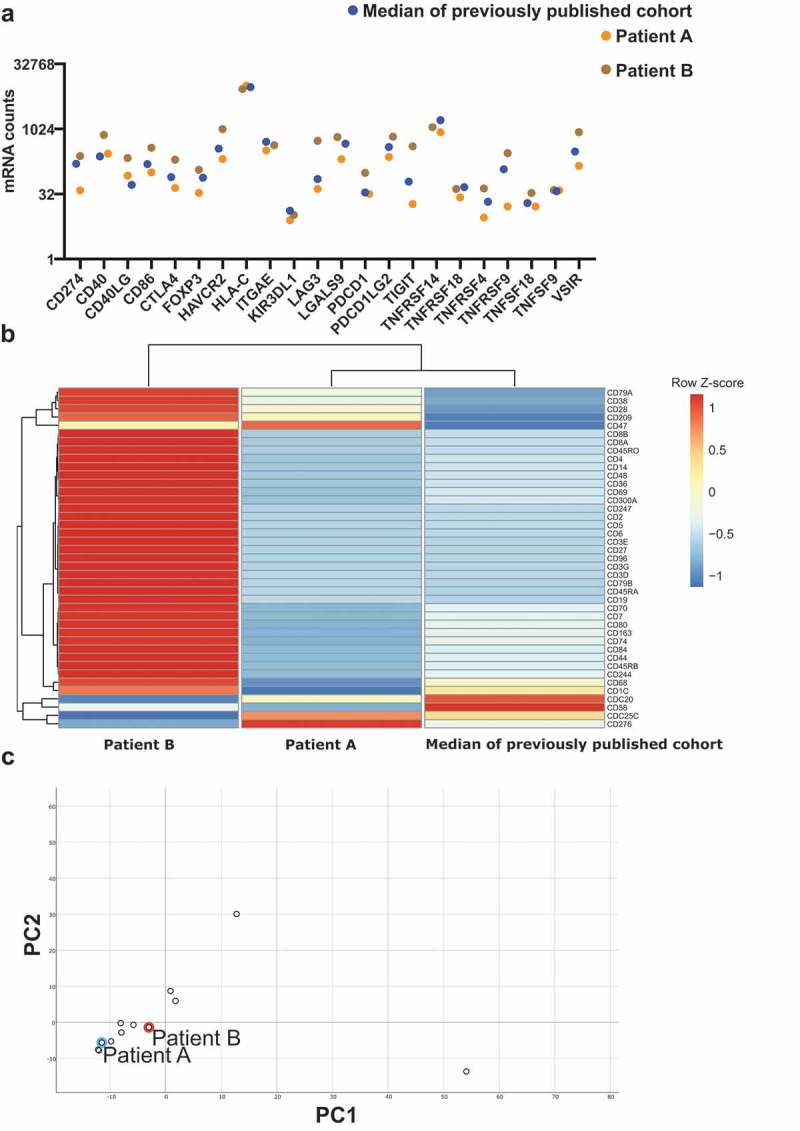
Immune profile of two patients with advanced PDS treated with CPI.
(a) RNA expression of various immune checkpoint molecules and ligands. Cases are color-coded: Patient A (orange), patient B (brown), median (blue). Median signifies the median value from our previously published study (N = 9). (b) Heatmap of all CD molecules included in the Nanostring IO360 panel. Columns represent patients and rows represent genes. Median signifies the median value from our previously published study (N = 9).4 Expression levels have been centered and scaled using z-scores within rows. Rows and columns have been grouped using an unsupervised clustering approach. (c) Principal component analysis of all genes from the IO360 Nanostring panel. Patient A and patient B are both highlighted.
Based on these findings and our preliminary studies, the patient was enrolled in PD-1 inhibitor therapy.4 Almost 1 year ago, he started receiving 2 mg/kg Pembrolizumab every 3 weeks and he reached a complete remission of the disease after eight cycles. Currently, he is still continuing the treatment every 3 weeks. Until now, a sustained complete remission and good tolerability could be seen (Figure 1b).
Patient B
An 88-year-old male patient presented PDS in the right temporal region which was excised with a safety margin of 1 cm. Two years later, the patient developed an inoperable local relapse, which was histologically confirmed (Figure 1(c,g)). In contrast to limited expression of PD-L1 in immune cells, it was also detected within the tumor (Figure 1h). As for CD4, CD8, CD68, FOXP3, PD-1, and MHC-I, similar histological results to patient A could be observed (Figure 1(i-n)).
NGS analysis of the primary PDS uncovered three missense mutations: TP53c.844C>T mutation (p.R282W), CDKN2A c.126T>A mutation (p.N42K), and KIT c.1496A>G mutation (p.K499R), and illustrated a TMB of 77.997/MB (Table 1). Regarding the immune contexture, patient B mostly exhibited higher than median expression level of multiple checkpoint molecules/ligands, such as CD40LG, CTLA-4, HAVCR2/TIM-3, LAG-3, PDCD1, and TIGIT, than our previous PDS cohort (Figure 2a). We also discovered that he expressed higher levels of most CD molecules when compared to patient A and 10 other PDS samples from our previous study (Figure 2b). These multiplex gene expression assay revealed that patient B also displayed a similar gene expression profile to patient A and our previously published cohort (Figure 2c).
After interdisciplinary discussion, the patient agreed to receive Pembrolizumab 200 mg every 3 weeks combined with local RT in 2 Gy fractions and a total dose of 70 Gy using 6 MeV electrons in a linear accelerator. After 4 months of combined therapy, the skin tumor showed complete remission (Figure 1d).
Overview of the genetic alterations found in both PDS tumors with help of targeted sequencing. Pathogenic and unknown mutations are highlighted.
Discussion
CPI targeting the programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) have demonstrated promising and durable antitumor activity in several tumor entities, including other UV-induced skin tumors, such as malignant melanoma (MM). This has led to the approval of PD-1 inhibitors – Nivolumab (Opdivo®, Bristol-Myers-Squibb, NY, USA) and Pembrolizumab (KEYTRUDA®, MSD, NY, USA) – for the treatment of MM.10,11 Elevated mutational burden, the amount of tumor-infiltrating CD8 + T cells or the expression of PD-L1 on the surface of tumor cells or tumor-associated immune cells has been shown to correlate with CPI treatment response.12–16 We recently reported that majority of PDS cases present with an elevated immune cell infiltration and an upregulation of genes being involved in immune responses, including HLA class I and II molecules (corresponds to MHC class I and II) and checkpoint molecules, such as PD-L1 and LAG-3. Moreover, we revealed that only a minority of our PDS being investigated showed reduced MHC-I (2 out of 13) or aberrant MHC-II (4 out of 13) expression, underlining a proficient antigen-presenting machinery in PDS tumors.4 Other recent studies showed that PD-1 expression correlates with FOXP3+ TIL density and CD8+ TIL density, but not with CD4+ TIL density.17 Moreover, low PD-1 incidence among CD8+ cells was a distinctive feature of nivolumab-treated NSCLC patients, showing clinical benefit with a prolonged progression-free survival.18 Nonetheless, no studies have been conducted to test the efficacy of CPI in advanced PDS cases.
Similar to other skin malignancies, PDS tumors are highly mutated (Table 1) with typical UV-signatures. In addition to characteristic UV-induced loss-of function TP53 mutations that can be found in almost all cases of PDS, a minority of PDS harbors other UV-induced activating PIK3CA or RAS mutations as potentially targetable genetic alterations.8,9 Likewise, we have observed a PIK3CA p.H1065L mutation in patient A, which lies in the kinase domain, and a CDKN2A and KIT mutation in patient B.
Patient A showed a decreased immune signature of various lymphocyte subtypes, including several CD molecules (Figure 2a). In detail, CD8, a marker for cytotoxic T-cells, as well as CD4, a marker for T-Helper cells, and CD19 as a marker for B-cells, were lower expressed comparing to median and patient B. Still, patient A responded to CPI treatment.
Therefore, although there are differences in the inflammatory status of PDS tumors, they generally appear to have a pro-inflammatory microenvironment (Figure 2b) – qualifying them for CPI treatment in this study.
By virtue of study results in other tumor entities that radiotherapy (RT) can enhance infiltration of tumors with TILs and relieve a given myeloid-suppressive tumor microenvironment,19–21 we decided to recommend patient B to receive a combination treatment. Indeed, RT has been demonstrated to stimulate T-cell activation and proliferation by releasing tumor antigens and to modulate the expression of immune checkpoint ligands, including PD-L1 on tumor cells.22 Moreover, it is correlated with an abscopal effect, a phenomenon in which tumor regression occurs in non-irradiated lesions.23 However, we cannot exclude that the response was caused by radiotherapy alone.
In conclusion of these data–despite the varying molecular backgrounds, immune signatures, and markers of immune response in the two patients–we successfully treated two patients with advanced PDS with the PD-1 inhibitor Pembrolizumab. Considering the evident similarity between the gene expression of those two cases and a previously published cohort through a PCA analysis, we support the idea that the entity of PDS should more generally be considered for immunotherapy. We believe that these results from the representative advanced PDS cases demonstrating a pro-inflammatory microenvironment and may encourage clinicians to initiate this treatment option in future cases of metastatic PDS.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft through the [SFB829] (Z4 to Doris Helbig and Cornelia Mauch) and the Else Kröner-Fresenius-Stiftung [EKFS-2014-A06 and 2016_Kolleg.19].
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Tardio JC, Pinedo F, Aramburu JA, Suarez-Massa D, Pampin A, Requena L, Santonja C.. Pleomorphic dermal sarcoma: a more aggressive neoplasm than previously estimated. J Cutan Pathol. 2016;43:1–6. doi: 10.1111/cup.12603. [DOI] [PubMed] [Google Scholar]
- 2.Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol. 2012;36:1317–1326. doi: 10.1097/PAS.0b013e31825359e1. [DOI] [PubMed] [Google Scholar]
- 3.Persa OD, Loquai C, Wobser M, et al. Extended surgical safety margins and ulceration are associated with an improved prognosis in pleomorphic dermal sarcomas. J Eur Acad Dermatol Venereol. 2019. doi: 10.1111/jdv.15493. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Mauch C, Wagener-Ryczek S, Schoemmel M, Buettner R, Quaas A, Helbig D. Immune-phenotyping of pleomorphic dermal sarcomas suggests this entity as a potential candidate for immunotherapy. Cancer Immunol Immunother. 2019;68:973–982. doi: 10.1007/s00262-019-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertucci F, Finetti P, Perrot D, et al. PDL1 expression is a poor-prognosis factor in soft-tissue sarcomas. Oncoimmunology. 2017;6:e1278100. doi: 10.1080/2162402X.2016.1278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boxberg M, Steiger K, Lenze U, Rechl H, von Eisenhart-Rothe R, Wortler K, Weichert W, Langer R, Specht K. PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue - prognostic implications and rationale for immunotherapy. Oncoimmunology. 2018;7:e1389366. doi: 10.1080/2162402X.2017.1389366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack SM, He Q, Yearley JH, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123:3291–3304. doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helbig D, Ihle MA, Putz K, Tantcheva-Poor I, Mauch C, Buttner R, Quaas A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2016:7. doi: 10.18632/oncotarget.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helbig D, Quaas A, Mauch C, et al. Copy number variations in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2017;8:109457–109467. doi: 10.18632/oncotarget.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with Pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SP, Kurzrock R. PD-l1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 15.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Arakawa Y, Yokogawa R, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13:e0194594. doi: 10.1371/journal.pone.0194594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res. 2018;24:407–419. doi: 10.1158/1078-0432.CCR-17-2156. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016;76:5994–6005. doi: 10.1158/0008-5472.CAN-16-0549. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini JL. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017;40:200–211. doi: 10.1016/j.bj.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 22.Parker JJ, Jones JC, Strober S, Knox SJ. Characterization of direct radiation-induced immune function and molecular signaling changes in an antigen presenting cell line. Clin Immunol. 2013;148:44–55. doi: 10.1016/j.clim.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data are available from the corresponding author upon reasonable request.


