ABSTRACT
A solid tumor consists of cancer and stromal cells, which comprise the tumor microenvironment (TME). Tumor-associated macrophages (TAMs) are usually abundant in the TME, contributing to tumor progression. In cases of peritoneal dissemination of gastric cancer (GC), the contribution of intraperitoneal TAMs remains unclear. Macrophages from peritoneal washings of GC patients were analyzed, and the link between intraperitoneal TAMs and GC cells was investigated to clarify the interaction between them in peritoneal dissemination. Macrophages were predominant among leukocytes constituting the microenvironment of the peritoneal cavity. The proportion of CD163-positive TAMs was significantly higher in stage IV than in stage I GC. Co-culture with TAMs potentiated migration and invasion of GC. IL-6 was the most increased in the medium of in vitro co-culture of macrophages and GC, and IL-6 elevation was also observed in the peritoneal washes with peritoneal dissemination. An elevated concentration of intraperitoneal IL-6 was correlated with a poor prognosis in clinical cases. In conclusion, intraperitoneal TAMs are involved in promoting peritoneal dissemination of GC via secreted IL-6. TAM-derived IL-6 could be a potential therapeutic target for peritoneal dissemination of GC.
KEYWORDS: Gastric cancer, tumor-associated macrophages, tumor microenvironment, peritoneal dissemination
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide, and the third leading cause of cancer-related deaths.1 Peritoneal metastasis is a frequent mode of metastasis of GC,2,3 which leads to a serious clinical condition, resulting in dismal consequences.4 Nevertheless, there is no definitive therapy for the peritoneal spread of GC, mainly because the mechanism of peritoneal metastasis has yet to be fully understood, and the appropriate target has not been identified. Thus, to overcome this disease entity, we still need to explore and understand the complexity of peritoneal metastasis to develop novel strategies.
In recent years, it has been reported that TME plays a pivotal role in cancer progression. TME consists of various components, including extracellular matrix, endothelial cells, fibroblasts, lymphocytes, and macrophages.5 Of them, tissue-infiltrating macrophages have been found to be correlated with a poor prognosis in several cancers.6–10 Such macrophages in the TME have been recognized as TAMs. Macrophages display remarkable plasticity in response to environmental cues and have a function between two phenotypes, the classical M1 and the alternative M2 macrophages,11 in their functional spectrum. The M1 macrophages are involved in the inflammatory response, pathogen clearance, and antitumor immunity. Conversely, the M2 macrophages are associated with an anti-inflammatory response, wound healing, and pro-tumorigenic properties.12 The evidence shows that TAMs are relatively skewed to the M2 type,13,14 and those in solid tumors modulate the TME to make it favorable for cancer, contributing to aggravation of their malignant phenotype and immunoregulation.15 Although it is now recognized that macrophages are plastic and versatile and the concept of M1-M2 extremes may not capture their whole spectrum,16–19 some studies have actually shown that TAMs promote the progression of GC.20,21
Given this evidence that the TME is associated with cancer progression, we have an interest in how the intraperitoneal microenvironment contributes to the development and progression of peritoneal metastasis. Since few reports have described the interaction between intraperitoneal TAMs and cancer cells, the molecular mechanisms underlying the tumor-promoting properties of TAMs remain unclear. Because of the difficulty of accessing the peritoneal cavity in humans, ways to explore the intraperitoneal microenvironment are limited. One of the available strategies is the investigation of cellular samples in the washes of the abdominal cavity obtained during surgery. Based on the fact that the presence of intraperitoneal free cancer cells is a strong factor related to an increased risk of peritoneal recurrence, peritoneal lavage cytology is incorporated into the staging of GC and routinely performed during surgery for patients with advanced GC.22,23 Using peritoneal lavage samples, the previously unappreciated numerous normal cells co-existing with cancer cells were investigated as components of the intraperitoneal microenvironment, focusing particularly on TAMs, and how the intraperitoneal microenvironment contributes to promote peritoneal dissemination was explored.
Results
Macrophages differentiated to CD163+ TAMs are predominant in the peritoneal cavity in the presence of GC cells
Intraperitoneal cellular components of a GC patient with positive cytology were investigated (Figure 1). Cancer cells were labeled with telomerase-specific replicative adenovirus, TelomeScan.24–26 As shown in Figure 1(a), it was confirmed that numerous CD45+ leukocytes existed in the peritoneal cavity along with GFP-positive cancer cells, and almost all of them were found to be CD14+ macrophages. Furthermore, when they were stained with anti-CD163 antibody, abundant CD163+ macrophages were present in the surroundings of the cancer cells (Figure 1(b)). The peritoneal wash was then further analyzed to compare the intraperitoneal microenvironment between stage I and IV GC patients focusing on the constituent rate of intraperitoneal macrophages by flow cytometry (Figure 1(c,d)). The proportion of macrophages positive for CD14 in CD45+ leukocytes (CD14/CD45) was significantly higher in stage IV patients than in stage I patients. Moreover, the proportion of CD163+ cells in CD14+ macrophages (CD163/CD14) was significantly higher in stage IV patients than in stage I patients. Of note, in the peritoneal wash from stage I GC patients, the number of cells was small in general, and most of them were stained by CD45, but not by CD14 (Figure S1). These data suggest that, in the presence of GC cells, numerous macrophages were deployed into the peritoneal cavity and differentiated to CD163+ TAMs.
Figure 1.
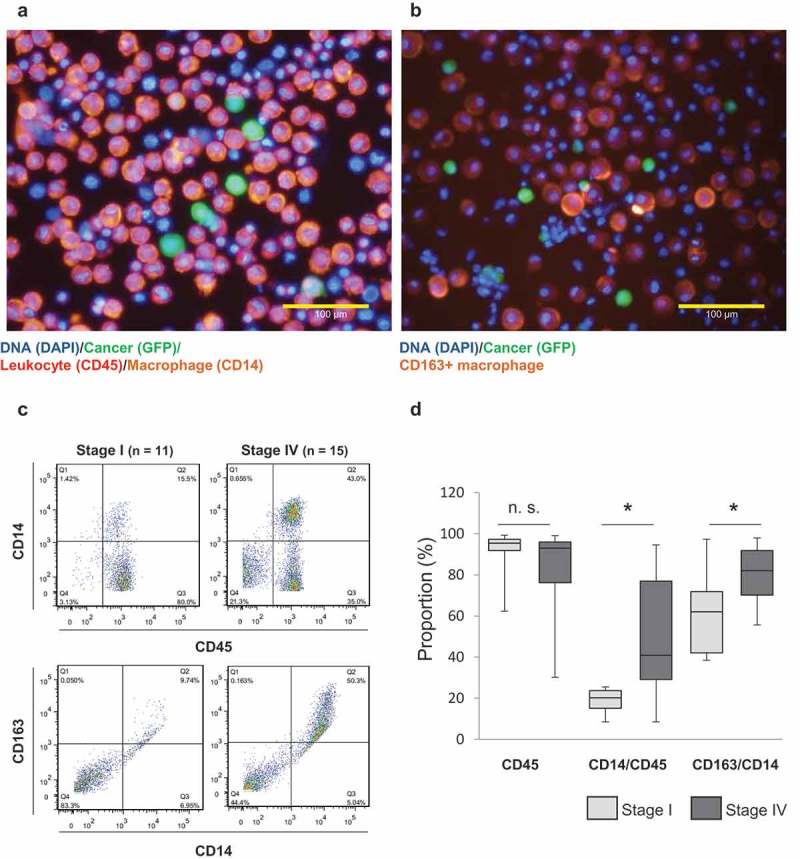
Most macrophages co-existing with cancer cells in the peritoneal cavity are positive for CD163.
(a) and (b) The cells in peritoneal washes were infected with cancer-imaging adenovirus, TelomeScan. Immunofluorescent staining was added to visualize intraperitoneal leukocytes (CD45), macrophages (CD14), and activated macrophages (CD163). (c) Representative figure of flow cytometric analysis. (d) Summarized data of flow cytometric analysis of Stage I and Stage IV samples. The values represent medians ± standard deviation, SD. (stage I: n = 11, stage IV: n = 15). (n.s.: not significant, *p < .01, Student’s t-test)
Upregulated migration and invasion ability of GC cells by co-culture with CD163-positive TAMs
Considering such an intraperitoneal TME in clinical samples of GC, the interaction between GC cells and TAMs was examined. To obtain TAMs, first, CD163+ macrophages were differentiated from THP-1 cells with IL-4 according to a differentiation protocol27 (Figure 2(a)). GC cells were co-cultured with those THP-1-derived macrophages (TDMs) using a biphasic chamber to mimic the intraperitoneal environment. Since TAMs are known to affect the malignant phenotype of cancer, 28–30 whether TDMs increased the migration and invasion abilities of GC cells was investigated. Consistent with the previous reports, the migration and invasion abilities of GCIY were significantly increased by TDMs compared with the non-co-cultured group (Figure 2(b)). Similar results were also observed in MKN45 and MKN1 cells (Figure S2A). Next, the effects of TDMs on the chemo-sensitivity of GC cells were investigated. After co-culture with TDMs, GC cells tended to be insensitive to chemotherapeutic agents including paclitaxel (Figure S3A) and 5-FU (Figure S3B), although the difference compared to control was not significant.
Figure 2.
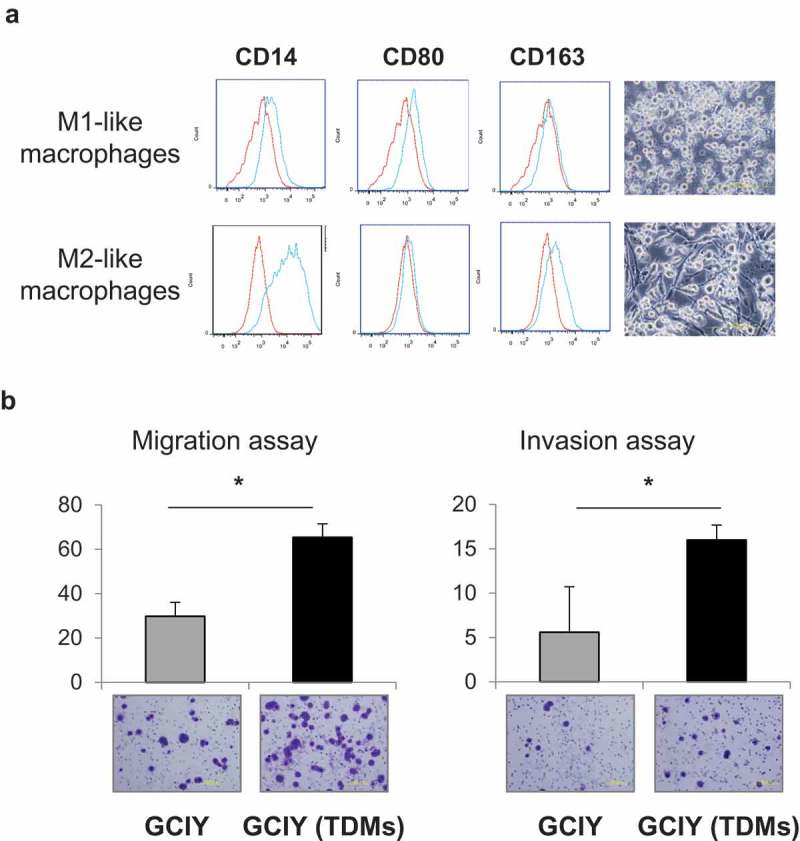
CD163+ macrophages intensify migration and invasion abilities.
(a) THP-1 cells were differentiated into macrophages. Polarized CD80+ or CD163+ macrophages were evaluated by flow cytometry. CD14: a pan-macrophage marker, CD80: an M1-type macrophage marker, and CD163: an M2-type macrophage marker. (b) GCIY cells were stimulated with TDMs. Subsequently, migration and invasion abilities were evaluated in comparison with the unstimulated cells. Values represent means ± SD. (n = 5 fields/well). (*p < .01, Student’s t-test)
These data confirmed that indirect co-culture with TDMs intensifies the malignant phenotype of GC. In addition, these phenomena suggest that some kinds of humoral factors were associated with the promotion of the malignant phenotype of GC cells.
Macrophage-derived IL-6 was increased by the interaction with GCIY
To explore how TAMs affect malignant phenotypes of GC, the supernatants from GCIY/TDM co-cultures were examined using an array for cytokines/chemokines in vitro. When the relative values of cytokines and chemokines in the supernatant were compared, IL-6 had the highest value, which was increased by more than 3-fold in the co-culture of TDMs and GCIY cells compared with the culture of TDMs alone (Figure 3(a)). With the quantification of IL-6 by ELISA, TDMs secreted more IL-6 on stimulation with GCIY than GCIY or TDMs in mono-culture (Figure 3(b)). The same results were obtained in MKN45 and NUGC3, although KATO-III did not stimulate macrophages to secrete IL-6 (Figure S4A). Even though TDMs themselves secreted a small amount of IL-6 depending on their cell number, TDMs were found to secrete more IL-6 when co-cultured with even a small amount of cancer cells (Figure 3(c)).
Figure 3.
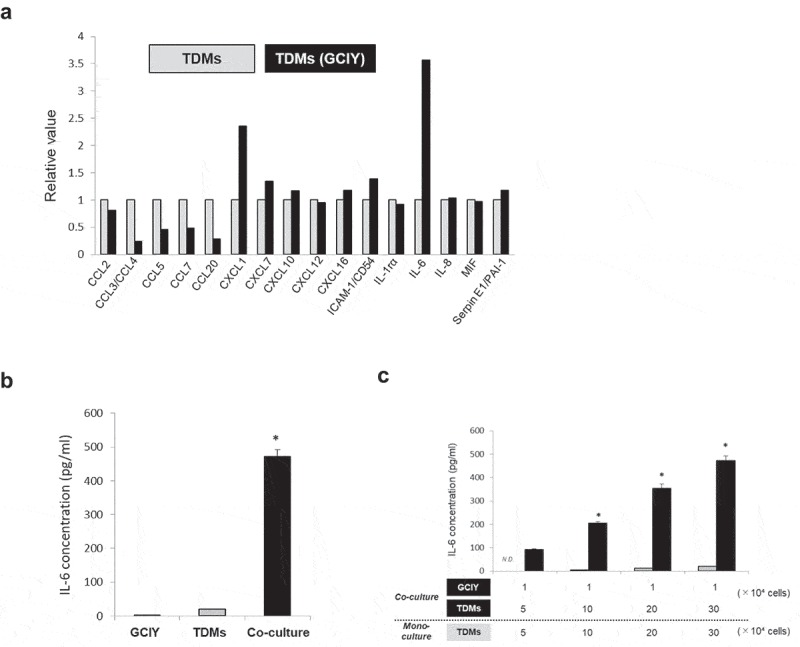
IL-6 is elevated with the co-culture of TDMs and GC cells.
(a) Cytokine and chemokine arrays of supernatants from transwell co-culture TDMs and GCIY cells. The values are normalized by TDMs mono-culture supernatants. (b) IL-6 concentration in TDMs/GCIY transwell co-culture supernatants were quantified by ELISA (n = 3). As a control, each mono-culture supernatant of GCIY or TDMs was analyzed. The values represent means ± SD. (*p < .01, Student’s t-test) (c) GCIY (1 × 104) cells were co-cultured with various numbers of TDMs (5, 10, 20, and 30 × 104). IL-6 concentrations of the co-culture supernatants were quantified. The values represent means ± SD. (*p < .01, Student’s t-test)
IL-6 mediated the JAK/STAT3 pathway and promoted the migration and invasion abilities of GC cells
Given the fact that IL-6 was the most upregulated cytokine secreted from macrophages by their interactions with GC cells, whether IL-6 directly promotes the migration and invasion of GC cells was examined. Both migration and invasion of GCIY were accelerated, and the effect depended on the concentration of IL-6 (Figure 4(a)). The same result was also obtained in MKN45 GC cells (Figure S2B). However, cell proliferation was not affected by IL-6 (Figure S4B). Moreover, along with this effect, STAT3 in GCIY was gradually phosphorylated when treated with IL-6 (Figure 4(b)) and co-cultured with TDMs (Figure 4(c)), in a dose or cell number-dependent manner. These data imply that IL-6 from TDMs upregulated the migration and invasion abilities of GC cells, which would be associated with the JAK/STAT3-mediated pathway.
Figure 4.
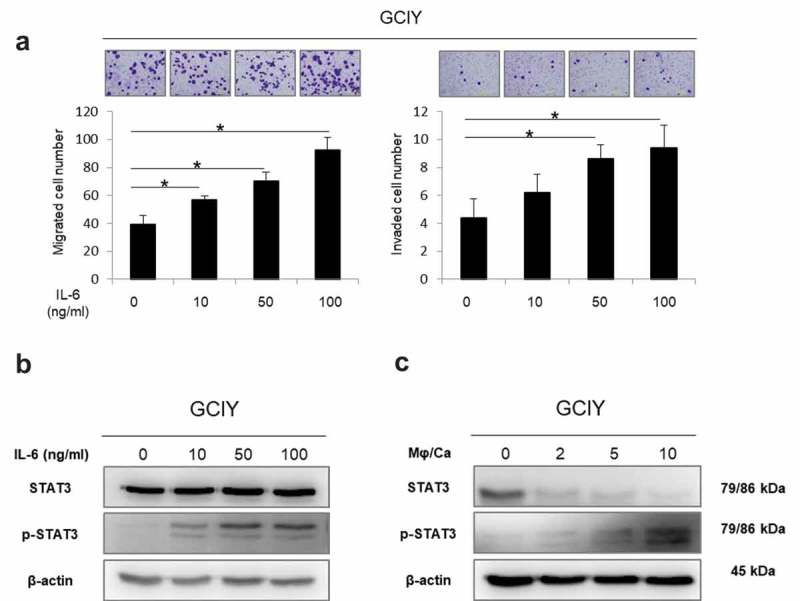
IL-6 potentiates migration and invasion of GC and induces phosphorylation of STAT3.
(a) Migration and invasion assay of GCIY cells treated with the medium containing IL-6 (0–100 ng/ml). Migrated and invaded cells were counted by staining with 0.5% crystal violet. The values represent means ± S. D. (*p < .01, Student’s t-test) (b) Western blotting analysis of GCIY cells treated with IL-6 at different doses (0–100 ng/ml). (c) Western blotting analysis of GCIY cells treated with CD163+ TDMs (ratios TDMs/GCIY of 0, 2, 5, and 10).
Intraperitoneal microenvironment in the peritoneal dissemination of the in vivo mouse model
GCIY-Luc or MKN45-Luc cells were injected into the peritoneal cavity of mice, and the mouse peritoneal dissemination models were established (Figure 5(a)). As with the clinical peritoneal washes, saline was injected into the mouse peritoneal cavity, and the peritoneal washes were obtained. When their cellular components were assessed by flow cytometry (Figure 5(b,c)), more CD14+ macrophages were induced into the peritoneal cavity in the presence of cancer cells, especially in GCIY-peritoneal dissemination, than in the mock-group. Moreover, most CD14+ macrophages were confirmed to express CD163, although the proportion of CD163+ macrophages was also high in the mock-group. Interestingly, the concentration of IL-6 was markedly elevated in the peritoneal dissemination mice compared to the control mice (Figure 5(d)). These results demonstrated that CD163+ TAMs were prevalent in the peritoneal cavity with dissemination of GC, and that IL-6 appears to be secreted by TAMs upon their interaction with cancer cells.
Figure 5.
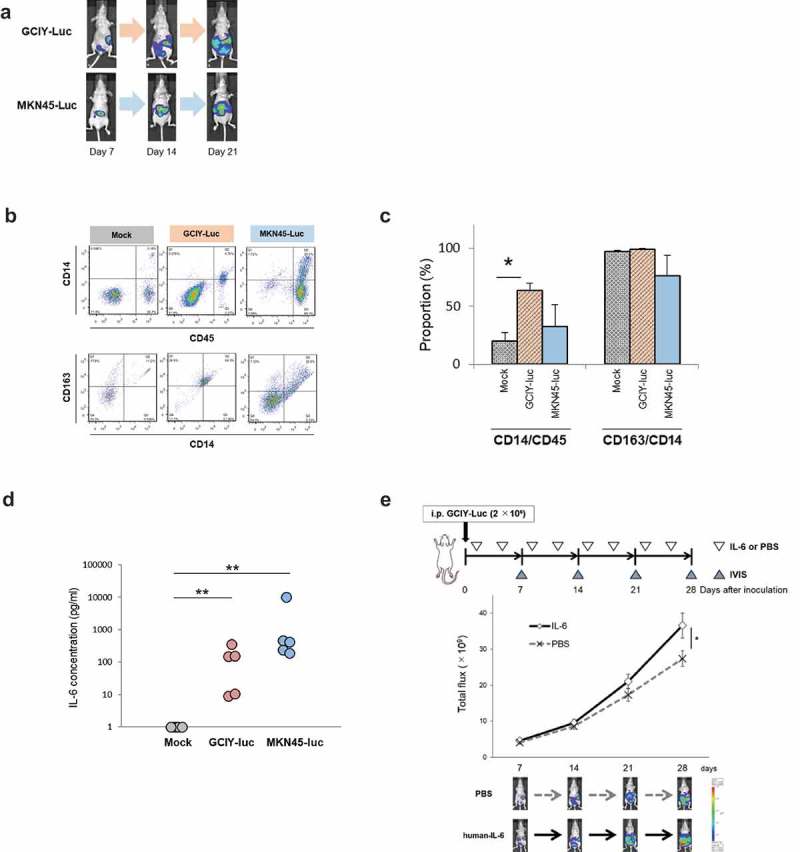
In vivo model of peritoneal dissemination.
(a) GCIY-Luc and MKN45-Luc cells were inoculated into the peritoneal cavity of BALB/C nu/nu mice (n = 5/group). Peritoneal dissemination was quantified by IVIS once a week. (b) Peritoneal washes were collected at 21 days after inoculation. Peritoneal macrophages were evaluated by flow cytometry. (c) The proportions of CD14/CD45 and CD163/CD14 are summarized. The values represent means ± SD. (*p < .01, Student’s t-test) (d) IL-6 in the peritoneal wash was quantified. The value of IL-6 at less than detectable sensitivity was defined as one. (*p < .01, Wilcoxon-test) (e) IL-6 promotes peritoneal dissemination in the GCIY-Luc xenografted model. GCIY-Luc cells were inoculated into the peritoneal cavity of BALB/C, nu/nu, mice (n = 9-10/group). Subsequently, human IL-6 recombinant protein (200 ng/200 μl/body) or PBS (200 μl/body) was inoculated twice a week, and peritoneal dissemination was quantified by IVIS once a week. The values represent means ± standard error of the mean, SEM. (*p < .05, Student’s t-test)
In vivo effect of IL-6 for the acceleration of peritoneal dissemination
Whether IL-6 is the factor that actually accelerates the development of peritoneal dissemination was further assessed. When GC cells were inoculated into the peritoneal cavity of mice along with IL-6 recombinant protein, peritoneal dissemination was significantly enhanced in the mice compared with the controls (Figure 5(e)). Whether neutralization of intraperitoneal IL-6 by an anti-mouse IL-6 antibody could inhibit the peritoneal dissemination of GC was then investigated (Figure S5). Blocking the IL-6-mediated pathway tended to suppress peritoneal dissemination, although the difference between the groups was not significant. To further investigate the intraperitoneal environment in the mouse peritoneal dissemination models, the peritoneal washes collected from GCIY-Luc and MKN45-Luc-inoculated mice were examined using an array for humoral factors. When the relative values of cytokines, chemokines, and angiogenic factors in the washes were compared between mock and peritoneally disseminated mice, elevations of various cytokines, chemokines, and angiogenic factors other than IL-6 were also observed (Figure S6). These data imply that IL-6 was in part associated with the promotion of peritoneal dissemination.
Clinical correlation between IL-6 and CD163-positive TAMs in the peritoneal cavity
Based on these observations, the peritoneal washes obtained from stage IV and stage I GC patients were again analyzed. The levels of IL-6 and the number of CD163+ macrophages per ml of peritoneal wash were quantified by ELISA and flow cytometry, respectively, and compared between stages IV and I GC patients. It was found that IL-6 was higher (Figure 6(a)) and CD163+ macrophages were significantly more numerous (Figure 6(b)) in stage IV patients than in stage I patients. Moreover, when they were compared in the peritoneal dissemination-positive (P1) group and the cytology-positive without peritoneal dissemination (CY1/P0) group in stage IV patients, IL-6 was even higher, and the number of CD163+ macrophages was even larger in the P1 group than in the CY1/P0 group (Figure 6(c,d)). Furthermore, when stage IV patients were divided by IL-6 positivity, a significantly larger number of CD163+ macrophages was detected in the IL-6-positive group (Figure 6(e)). Ultimately, the survival period of IL-6 positive patients was significantly shorter than that of IL-6-negative patients (Figure 6(f)).
Figure 6.
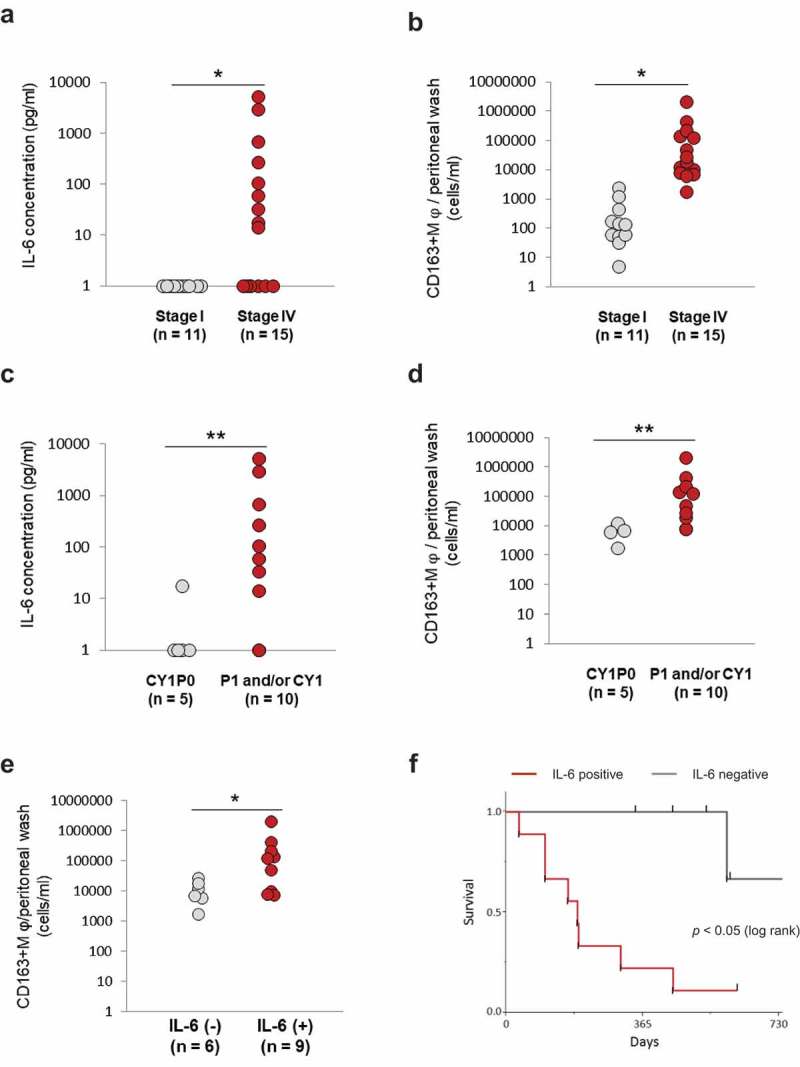
Clinical correlation between IL-6 and TAMs in the peritoneal cavity.
(a) and (b) The levels of IL-6 and CD163+ macrophages were evaluated in stage I and stage IV patients. (c) and (d) The levels of IL-6 and CD163+ macrophages were compared between cytology-positive only and peritoneal dissemination-positive patients.A value of IL-6 less than detectable sensitivity was defined as one. A value greater than 4 pg/ml, which is a serum reference value, was defined as positive. The values represent means ± SD. (*p < .01, ** p < .05, Pearson’s chi-squared test). The absolute value of CD163-positive TAMs per ml of peritoneal wash was also measured. Values represent means ± SD. (*p < .01, **p < .05 Wilcoxon test) Correlations of IL-6 and CD163+ macrophages with the prognosis of GC patients(e) Stage IV GC patients were divided into groups according to IL-6 positivity, and the numbers of intraperitoneal CD163+ macrophages were compared. A value greater than 4 pg/ml, which is a serum reference value, of IL-6 was defined as positive. (*p < .05, Wilcoxon test) (f) Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. p < .05 was considered significant.
Discussion
In this study, peritoneal washes from GC patients were examined, and it was found that the majority of the cellular components in stage IV patients were CD163+ macrophages, which led to the hypothesis that the intraperitoneal TAMs were associated with GC peritoneal dissemination. The indirect co-culture of TDMs potentiated the migration and invasion abilities of GC cells. Interestingly, among the various cytokines and chemokines, IL-6 was found to show the highest relative increase in the medium of in vitro co-culture of macrophages and GC. The concentration of IL-6 was higher in the ascites fluid of mice with peritoneal dissemination than in those without it. The results suggest that IL-6 secreted from TAMs must be one of the key mediators that accelerates the peritoneal dissemination of GC.
Macrophages have been recognized as regulators of the complex TME, where they exhibit either pro-tumor or anti-tumor functions. Considering macrophage phenotype classification by the M1-M2 paradigm, TAMs are generally thought to be much closer to M2-polarized macrophages, but macrophages actually show a spectrum of activation states rather than such dichotomous phenotypes.16 Since in vivo macrophages in particular do not so neatly divide into the M1 and M2 classification schemes, there is an effort to avoid the ambiguity of the M1-M2 characterization of TAMs.16–19 Therefore, in the present study, use of “M1” and “M2” for the macrophages observed in this study was avoided.
Although it has been reported that TAMs secrete some pro-tumor factors in the TME, 12 IL-6 can also be one.31,32 IL-6 is known to be a multifunctional cytokine, 33 and its stimulation induces homo-dimerization of gp130, phosphorylating JAKs and STAT3 after binding IL-6 receptor, and then phosphorylated STAT3 transfers the signals to its downstream .34 IL-6 would thus contribute to promoting some kinds of malignant phenotype in many cancers .35 Based on the result shown in Figure 3(c), it was assumed that the macrophages produced IL-6, because when TDMs were co-cultured with even a certain and small amount of cancer cells, more TDMs secreted more IL-6. The previous studies demonstrated that the main producers of IL-6 are the myeloid cells.32,36 However, autocrine IL-6 in epithelial cancer cells has also been documented.37,38 Thus, GC cells may also secrete IL-6 under certain conditions.
In the present experiment, by co-culture with CD163+ macrophages or with IL-6, the migration and invasion abilities (Figures 2(b) and 4(a), S2A, and S2B) of GC cells were potentiated. Phosphorylation of STAT3 in GCIY co-cultured with macrophages also suggested the involvement of IL-6 (Figure 4(b)). Other groups reported that IL-6 is involved in metastasis through inducing the epithelial mesenchymal transition (EMT) in many cancer tissues, such as pancreatic cancer, colorectal cancer, head and neck cancer, cervical cancer, and ovarian cancer, as well as GC.39–44 As we recently demonstrated in pancreatic cancer,30 the intensification of the malignant traits of intraperitoneal GC might be associated with the EMT.
In the mouse peritoneal dissemination model, CD163+ TAMs were predominant in the peritoneal cavity in the presence of cancer cells (Figure 5(a–c)). In even the mock group, peritoneal macrophages were also predominantly CD163+ cells (Figure 5(c)). According to the previous reports, the peritoneal microenvironment is supposed to be a hypoxic, lactic, and starved condition,45 which might affect the macrophages’ phenotype.46–48 In the clinical peritoneal washes, there was a certain percentage of CD163+ macrophages even in stage I, but the absolute numbers of macrophages were definitely higher in stage IV than in stage I (Figure 6(b)). Moreover, the striking difference in the peritoneal cavity between stage I and IV was the concentration of IL-6 (Figure 6(a)). These data suggest that more macrophages were induced into the peritoneal cavity by emerging cancer cells, and they preferentially differentiated to CD163+ TAMs, secreting a large amount of IL-6 through their interaction with them. Further supporting that, in stage IV patients, a significantly larger number of CD163+ TAMs and a larger amount of IL-6 were detected in the P1 group than in the CY1/P0 group (Figure 6(a,d)). Finally, the IL-6-positive group had a significantly larger number of CD163+ TAMs (Figure 6(e)) and a worse prognosis (Figure 6(f)) than the IL-6-negative group. Previous studies have reported that serum IL-6 is a prognostic biomarker in many cancers, such as GC,49 pancreatic cancer,50 lung cancer,51 prostate cancer,52 and renal cell carcinoma.53 The present data showing the correlation between intraperitoneal IL-6 and prognosis might be concordant with these previous reports. Collectively, these data imply that CD163+ TAMs contribute to promoting peritoneal dissemination by secreting IL-6 after their interaction with free cancer cells.
The in vivo effect of IL-6, as well as anti-IL-6 antibody, on the formation of peritoneal dissemination was evaluated in the mouse model. Recombinant IL-6 accelerated the expansion of peritoneal dissemination (Figure 5(e)), and anti-mouse IL-6 antibody tended to suppress the expansion of peritoneal dissemination (Figure S5). IL-6 was reported to be associated with the activation of natural killer T cells, 54 and, thus, it is possible that the correlation between IL-6 and the formation of peritoneal dissemination is related to the activity of natural killer T cells.55
This study has potential limitations that need to be addressed. First, THP-1 acute monocytic leukemia cells were used as a model for human monocytes.56 These cells have been widely used to investigate the function of monocytes and macrophages, but some differences relative to human peripheral blood monocytes have been identified.57,58 For example, the THP-1 cells express lower levels of CD14 and are less responsive to LPS than primary monocytes.59 Though THP-1-derived macrophages might not entirely mimic primary monocytes, the cells exhibit some of the functions of macrophages, as previous studies demonstrated. However, it is important to interpret the study results keeping in mind that the findings were obtained from THP-1-derived cells. It is desirable to validate the results in this study under other conditions to draw more definite conclusions. Second, “M2-like” CD163+ cells were mainly used, and M1-like cells were not investigated. M1-like polarized cells may also produce IL-6.19 The accumulating evidence shows that TAMs have a spectrum of different activation states and share phenotypes of both M1 and M2. Thus, the intraperitoneal TME would be more dynamic and complex than the experimental models. Third, common problems in cell culture experiments must be taken into account to interpret the results, such as genetic instability and contamination with microorganisms that propagating cell lines might have. The cells used in the present study were not tested for mycoplasma. However, only cells purchased directly from commercial providers were used, using cells of as low passages as possible, usually less than 10 passages. Therefore, these efforts would have minimized fluctuations in the experimental results.
Based on these results, macrophage-targeting anti-tumor treatment approaches might be potential candidates, and they have actually been under clinical investigation.60 The agents in clinical trials targeting macrophages in tumors include CSF-1R inhibitors, anti-CD47 antibodies, and a CCR2 antagonist, although few approaches targeting IL-6 are also clinically available. Of note, the anti-tumor efficacy of blockade of IL-6 signaling with tocilizumab, a drug approved for the treatment of rheumatoid arthritis, was demonstrated for pancreatic cancer61 and hepatocellular carcinoma.32 However, since tocilizumab has not yet been evaluated for its efficacy in peritoneal dissemination of GC, further study will be needed.
In conclusion, macrophages were skewed to CD163+ TAMs in the peritoneal cavity, and their co-existence with GC cells made them secrete IL-6. These TAMs accelerate the formation of peritoneal dissemination via an IL-6/STAT3-mediated pathway, and intraperitoneal IL-6 was found to be a prognostic factor for survival. Targeting intraperitoneal TAM-derived IL-6 might be a novel therapeutic strategy to overcome peritoneal dissemination in GC patients.
Material and methods
Clinical peritoneal washes
Peritoneal washes were obtained from GC patients during surgery performed in our hospital,30,62 including 15 stage IV patients and 11 stage I patients. The samples were prospectively collected from all patients who gave their informed consent to participate in the study, and those used in this study were selected retrospectively and almost consecutively from the latest one, in consideration of the stock status and clinical stages. The clinicopathological data were obtained from the medical records, and the cancers were staged in accordance with the Japanese Classification of Gastric Carcinoma: 3rd English edition.63 The clinicopathological characteristics of these patients are presented in supplementary tables A and B. The samples were centrifuged at 1500 rpm, 24°C for 3 minutes, and the supernatants were preserved at −80°C for the later experiments. For the cell sediment analysis, red blood cells were removed with RBC lysis buffer (BioLegend, #420301), and the other cellular components were analyzed by flow cytometry.
Cell lines and cell cultures
A human monocytic leukemia cell line, THP-1, was purchased from the American Type Culture Collection and cultured in RPMI-1640 medium (Sigma-Aldrich, #R8758) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). The macrophage-like state was obtained by treating THP-1 with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, #P8139) for 48 hours. Polarized, adherent cells were washed and rested for 24 hours in the culture medium to obtain the resting state of macrophages. These resting macrophages were primed for 24 hours with fresh medium supplemented with interferon-γ (20 ng/ml; Sigma-Aldrich, #I3265) + c (1 μg/ml; Sigma-Aldrich) to polarize into the M1 phenotype and with IL-4 (20 ng/ml; Sigma-Aldrich, #SRP4137) to polarize into the M2-like phenotype.27 The human GC cell line GCIY (poorly differentiated adenocarcinoma) was purchased from the Cell Engineering Division of RIKEN BioResource Center (RIKEN Cell Bank). GCIY-Luc, expressing luciferase, was established in our laboratory. GCIY cells were cultured in MEM (Sigma-Aldrich, #M4655) supplemented with 15% FBS and 1% P/S. GENETICIN (800 μg/ml) was additionally added in GCIY culture medium for GCIY-Luc. MKN45 (poorly differentiated adenocarcinoma), MKN45-Luc expressing luciferase, KATO-III (signet ring carcinoma), NUGC-3 (poorly differentiated adenocarcinoma), and MKN1 (adenosquamous carcinoma) cell lines were purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB). These cell lines were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% P/S. The molecular characteristics of the GC cell lines are summarized in supplementary table C.
Immunofluorescent staining
Cell components in peritoneal washes were re-suspended in RPMI-1640 supplemented with 10% FBS. Then, the total number of viable cells was counted, and the cells were infected with TelomeScan at 1 MOI for 24 hours at 37ºC to visualize cancer cells as GFP-positive cells.24–26 The cells were further stained with Alexa Fluor 647-conjugated anti-human CD45 antibody (BioLegend, 304056, RRID: AB_2564155), phycoerythrin (PE)-conjugated anti-human CD14 antibody (BioLegend, 301805, RRID:AB_31418), and PE-conjugated anti-human CD163 antibody (BioLegend, # 333606, RRID:AB_1134002) as markers of leukocytes, macrophages, and TAMs, respectively. Nuclei were stained with 4ʹ, 6-diamidino-2-phenylindole (DAPI). Immunofluorescent staining was observed under an inverted fluorescence microscope (IX71; Olympus).
Flow cytometry
The samples were washed with PBS containing 2% FBS and centrifuged at 300 × g, 4°C for 5 minutes. Dead cells were stained with APC-Cy7-conjugated Zombie NIR (BioLegend, #423105), and the cells were subsequently stained with the following surface markers for 30 minutes at 4°C: Alexa Fluor 647-conjugated anti-human CD45 antibody (BioLegend), FITC-conjugated anti-human CD14 antibody (BioLegend, #301803, RRID:AB_314185), and PE-conjugated anti-human CD163 antibody (BioLegend). As the controls, IgG-isotype control antibodies (BioLegend, #400112) were used. Cells were analyzed by flow cytometry (FACS Array, Becton Dickinson), and data were reanalyzed with Flow Jo software (Tree Star).
Migration and invasion assay
The migration assay was performed using transwell 24-well plates with 8-μm pore polyethylene terephthalate (PET) track-etched membranes (CORNING). Matrigel-coated filters (CORNING, BioCoat Matrigel invasion chamber) were used for the invasion assay. Cancer cells were indirectly co-cultured with or without TDMs (ratio of TDMs/GC cells, 2/1) using transwell 6-well plates with 4-μm pore PET track-etched membranes (CORNING) for 48 hours. Then, cells (GCIY: 5 × 10⁴, MKN45: 1 × 10⁵, MKN1: 1 × 10⁴) were plated in the upper chamber with 500 μl MEM or RPMI-1640 containing 0.1% FBS. The lower chamber was filled with MEM or RPMI-1640 containing 15% or 10%FBS, respectively. After incubation for 24 hours at 37°C and 5% CO2, migrating and invading cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The number of cells on the lower surface of the membrane in 5 random fields was counted using a bright field light microscope. To examine the effect of IL-6 on migration and invasion ability of GC cells, the same experiment was performed in the medium containing recombinant human IL-6 (R & D, Catalog # 206-IL) at a range of 0–100 ng/ml in the upper chamber (GCIY: 3 × 10⁴, MKN45: 3 × 10⁴) instead of co-culture with TDMs. After interaction for 48 hours, migrating and invading cells were counted in the same way.
XTT assay
The XTT assay (Cell proliferation kit II, Roche Mannheim) was performed to evaluate the sensitivity to chemotherapy. In the same way, after co-culture with TDMs, cancer cells were plated (3 × 103/well) in 96-well plates. The adherent cells were treated with paclitaxel (Nippon Kayaku Co., Ltd) or 5-FU (Kyowa Hakko Kirin Co., Ltd) for 48 hours at a range of 0–10 or 0–1000 μM, respectively, and cell viability was evaluated. Optical densities were measured at 450 nm and 690 nm. After treatment with the chemotherapeutic agents, dose-response curves were drawn, and the IC50 values were determined by GraphPad Prism 8 version 8.00 (GraphPad Software, Inc.).
Mouse peritoneal dissemination model and in vivo efficacy of IL-6
Female BALB/c (nu/nu) mice were purchased from CLEA and housed under sterile conditions. The experiment was started when the mice were 5 weeks of age. GC cells were inoculated into the peritoneal cavity, and 3 groups (n = 5/group), the Mock-group (non-treated), the GCIY-Luc-group (5 × 10⁶), and the MKN45-Luc-group (5 × 10⁶), were compared. Three weeks after inoculation, peritoneal washes were collected by washing the peritoneal cavity with 2 ml of PBS. Then, the samples were treated the same as patients’ samples and analyzed by flow cytometry and ELISA for IL-6. Recombinant human IL-6 protein (R & D, Catalog # 206-IL) at a concentration of 200 ng/200 μl/body or PBS at a volume of 200 μl/body was injected into the peritoneal cavity to assess whether IL-6 accelerates the development of peritoneal dissemination. GCIY-Luc (2 × 10⁶) was inoculated into the peritoneal cavity, and reagents were inoculated (n = 9 – 10/group) twice a week from the next day. Peritoneal dissemination was measured by IVIS once a week. To assess the effect of blockade of IL-6 from mouse macrophages, GCIY-Luc (5 × 10⁶) was inoculated into the peritoneal cavity, and anti-mouse IL-6 antibody (Bio X Cell: clone MP5-20F3, # BE0046, RRID:AB_1107709) or rat IgG-isotype control (Bio X Cell: clone HRPN, # BE0088, RRID:AB_1107775) was peritoneally injected at a concentration of 200 μg/200 μl/body (n = 8 – 9/group) twice a week and evaluated by IVIS as well.
Cytokine and chemokine assays
TDMs (3 × 10⁵) were indirectly co-cultured with GCIY (1 × 10⁵) for 72 hours using transwell 6-well plates with 4-μm pore PET track-etched membranes (Corning), and then the cell culture supernatant mixture was collected by centrifugation. As a control, the supernatant of the TDM mono-culture (3 × 10⁵) was used. The assay was performed according to the manufacturer’s protocol (Human Cytokine/Chemokine Array, catalog # ARY005B, #ARY017, R&D Systems).
To investigate the humoral factors in the peritoneal cavity, the peritoneal washes were obtained from mice with or without peritoneal dissemination of GC, as described above. The washes were analyzed with Mouse Cytokine Array Kit (R&D Systems, #ARY006), Mouse Chemokine Array Kit (R&D Systems, #ARY020), and Mouse Angiogenesis Array Kit (R&D Systems, #ARY015).
ELISA for human IL-6
The IL-6 levels of the cell culture supernatant were determined using enzyme-linked immunosorbent assay (ELISA) kits (Human IL-6, catalog # D6050, R&D Systems) according to the manufacturer’s instructions. Each measurement was repeated in triplicate, and the average value was recorded (pg/mL). In the in vitro experiment, to mimic the intraperitoneal status, a large number of TDMs (3 × 10⁵) was stimulated with a small number of GCIY (1 × 10⁴) for 72 hours. Then, the IL-6 concentration in the cell culture supernatant mixture was determined and compared with the mono-culture of TDMs or GCIY. Other GC cell lines (KATO-III, MKN45, and NUGC3) were also subjected to the same experiment. Furthermore, to evaluate the effect of macrophages depending on cell number, macrophages (5, 10, 20, and 30 × 10⁴) were co-cultured with GCIY (1 × 10⁴). The cell culture supernatant mixture was then collected, and the assay was performed. Moreover, in the in vivo experiment, mouse peritoneal wash was analyzed for IL-6 (Mouse IL-6 Quantikine ELISA Kit, R&D Systems, # M6000B). A value of IL-6 less than detectable sensitivity was defined as one, and a value of more than 4 pg/ml, which is a serum reference value, was defined as positive.
Western blotting
GCIY was co-cultured with TDMs (ratio of TDMs/GCIY: 0, 2, 5, 10) or cultured in medium containing recombinant human IL-6 at a range of 0–100 ng/ml for 48 hours. Cells were washed with cold PBS and lysed with the SDS buffer containing protease inhibitors (cOmplete Mini, Roche Diagnostics GmbH, #11836153001). Equivalent amounts of protein from whole-cell lysates were loaded into each lane of 10% SDS–polyacrylamide gel and electrophoretically transferred to Hybond-polyvinylidene difluoride transfer membranes (GE Healthcare UK Ltd.). Membranes were incubated with the following primary antibodies overnight at 4°C: anti-signal transducer and activator of transcription 3 (STAT3; rabbit monoclonal IgG, diluted 1:1000; Cell Signaling Technology, # 14801, RRID:AB_2798618) and anti-phosphorylated STAT 3 (p-STAT3; Rabbit monoclonal IgG, diluted 1:2000; Cell Signaling Technology, #9145, RRID:AB_2491009). Equal loading of samples was confirmed by probing with anti–β-actin antibody (Sigma-Aldrich, #A5441). Membranes were subsequently incubated with secondary antibodies for 1 hour. Peroxidase activity of secondary antibodies was detected using an ECL prime Western Blotting Detection Reagent (GE Healthcare UK Ltd., #RPN2232) and visualized using an Amersham Imager 600 (GE Healthcare UK Ltd.) according to the manufacturer’s protocol.
Statistical analysis
Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. P values < .05 were considered significant. Statistical analysis was performed with JMP® software (JMP version 11, SAS Institute). Patient clinicopathological data were obtained from medical records and analyzed by Student’s t-test and Pearson’s chi-squared test. The analysis for IC50 was performed using GraphPad Prism 8 (GraphPad Software, Inc).
Funding Statement
This work was supported by Japan Society for the Promotion of Science [KAKENHI Grant Numbers JP15K15193, JP16H05416 JP18K08679], and Ministry of Health, Labor and Welfare [14525167].
Abbreviations
- GFP
green fluorescent protein
- TAMs
tumor-associated macrophages
- TDMs
THP-1-derived macrophages differentiated with IL-4
- EMT
epithelial-to-mesenchymal transition
- FBS
fetal bovine serum
Acknowledgments
The authors would like to thank Tomoko Sueishi, Yuko Hoshijima, Noriko Imagawa, and Tae Yamanishi for excellent technical support.
Competing interests
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were approved by the institutional review board of Okayama University Graduate School (KEN1507-031), and informed consent was obtained from all patients. All procedures were in accordance with the Helsinki Declaration. The animal care and experimental procedures were conducted in accordance with the regulations of the Animal Care and Use Committee of Okayama University.
Supplementary material
Supplemental data for this article can be accessed publisher’s website.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):1–13. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Shiozaki H, Elimova E, Slack RS, Chen HC, Staerkel GA, Sneige N, Shimodaira Y, Sagebiel T, Lee JH, Bhutani MS, et al. Prognosis of gastric adenocarcinoma patients with various burdens of peritoneal metastases. J Surg Oncol. 2016;113(1):29–35. doi: 10.1002/jso.24087. [DOI] [PubMed] [Google Scholar]
- 3.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS.. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 4.Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, De Stefano A. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90(9):1113–1119. doi: 10.1002/bjs.4164. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, Xu X, He S, Chen S, Shi Z, et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res. 2016;2016:9720912. doi: 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao Y-W, Wei Y-Q, Hoque MO. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Wang F, Wang L, Huang L, Wang J, Zhang B, Zhang Y. CD163+ tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patients. Oncotarget. 2015;6(12):10592–10603. doi: 10.18632/oncotarget.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi H, Tanaka M, Tanaka A, Tsunemi A, Yamamoto H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol Lett. 2016;11(5):3403–3408. doi: 10.3892/ol.2016.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 12.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerriero JL. Macrophages: the Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med. 2018;24(5):472–489. doi: 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A. Reflections on immunological nomenclature: in praise of imperfection. Nat Immunol. 2016;17(3):215–216. doi: 10.1038/ni.3354. [DOI] [PubMed] [Google Scholar]
- 18.Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immun. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18(4):740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19(4):1052–1065. doi: 10.1007/s10120-015-0579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, Rowsell C, Coburn NG. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S27–37. doi: 10.1007/s10120-011-0071-z. [DOI] [PubMed] [Google Scholar]
- 23.Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12(5):347–353. doi: 10.1245/ASO.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12(10):1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 25.Kojima T, Hashimoto Y, Watanabe Y, Kagawa S, Uno F, Kuroda S, Tazawa H, Kyo S, Mizuguchi H, Urata Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest. 2009;119(10):3172–3181. doi: 10.1172/JCI38609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigeyasu K, Tazawa H, Hashimoto Y, Mori Y, Nishizaki M, Kishimoto H, Nagasaka T, Kuroda S, Urata Y, Goel A, et al. Fluorescence virus-guided capturing system of human colorectal circulating tumour cells for non-invasive companion diagnostics. Gut. 2015;64(4):627–635. doi: 10.1136/gutjnl-2014-306957. [DOI] [PubMed] [Google Scholar]
- 27.Chanput W, Mes JJ, Savelkoul HF, Wichers HJ. Characterization of polarized THP-1 macrophages and polarizing ability of LPS and food compounds. Food Funct. 2013;4(2):266–276. doi: 10.1039/c2fo30156c. [DOI] [PubMed] [Google Scholar]
- 28.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 30.Kuwada K, Kagawa S, Yoshida R, Sakamoto S, Ito A, Watanabe M, Ieda T, Kuroda S, Kikuchi S, Tazawa H, et al. The epithelial-to-mesenchymal transition induced by tumor-associated macrophages confers chemoresistance in peritoneally disseminated pancreatic cancer. J Exp Clin Cancer Res. 2018;37(1):307. doi: 10.1186/s13046-018-0981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 32.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterol. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33(11):571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Howlett M, Menheniott TR, Judd LM, Giraud AS. Cytokine signalling via gp130 in gastric cancer. Biochim Biophys Acta. 2009;1793(11):1623–1633. doi: 10.1016/j.bbamcr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375(1):51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 36.Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK, Nguyen PM, Preaudet A, Farid R, Edwards KM, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24(2):257–271. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo K, Oka M, Murase K, Soda H, Isomoto H, Takeshima F, Mizuta Y, Murata I, Kohno S. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J Int Med Res. 2003;31(2):69–75. doi: 10.1177/147323000303100202. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8(13):20741–20750. doi: 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada S, Masamune A, Yoshida N, Takikawa T, Shimosegawa T. IL-6/STAT3 plays a regulatory role in the interaction between pancreatic stellate cells and cancer cells. Dig Dis Sci. 2016;61(6):1561–1571. doi: 10.1007/s10620-015-4001-5. [DOI] [PubMed] [Google Scholar]
- 41.Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124(4):1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9(12):1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 2015;356(2Pt B):321–331. doi: 10.1016/j.canlet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, Ackland L, Ahmed N. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100(1):134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22(30):6829–6840. doi: 10.3748/wjg.v22.i30.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P, et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7(49):80521–80542. doi: 10.18632/oncotarget.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu AS, Huang Y-J, Wu M-S. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008;14(2):428–434. doi: 10.1158/1078-0432.CCR-07-1032. [DOI] [PubMed] [Google Scholar]
- 50.Chen I, Dehlendorff C, Jensen BV, Pfeiffer P, Nielsen SE, Hollander NH, Yilmaz MK, Johanse J. Serum interleukin-6 as a prognostic biomarker for survival in patients with unresectable pancreatic cancer. Ann Oncol. 2016;27(suppl_6). doi: 10.1093/annonc/mdw141. [DOI] [Google Scholar]
- 51.Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, Huang MS, Chen HL, Li YJ, Yang PC, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132(9):1977–1985. doi: 10.1002/ijc.27892. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 53.Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, Mercatello A, Ravault A, Tourani JM, Moskovtchenko JF. Serum level of interleukin-6 as a prognosis factor in metastatic renal-cell carcinoma. Cancer Res. 1992;52:3317–3322. [PubMed] [Google Scholar]
- 54.Gallagher G, Stimson WH, Findlay J, al-Azzawi F. Interleukin-6 enhances the induction of human lymphokine-activated killer cells. Cancer Immunol Immunother. 1990;31(1):49–52. doi: 10.1007/bf01742495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luger TA, Krutmann J, Kirnbauer R, Urbanski A, Schwarz T, Klappacher G, Köck A, Micksche M, Malejczyk J, Schauer E. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. 1989;143:1206–1209. [PubMed] [Google Scholar]
- 56.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 57.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23(1):37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221(1):2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Bosshart H, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4(21):438. doi: 10.21037/atm.2016.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goumas FA, Holmer R, Egberts JH, Gontarewicz A, Heneweer C, Geisen U, Hauser C, Mende -M-M, Legler K, Röcken C, et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int J Cancer. 2015;137(5):1035–1046. doi: 10.1002/ijc.29445. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe M, Kagawa S, Kuwada K, Hashimoto Y, Shigeyasu K, Ishida M, Sakamoto S, Ito A, Kikuchi S, Kuroda S, et al. Integrated fluorescent cytology with nano-biologics in peritoneally disseminated gastric cancer. Cancer Sci. 2018;109(10):3263–3271. doi: 10.1111/cas.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Japanese Gastric Cancer A Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


