ABSTRACT
Introduction
Evidence indicates an increase in the prevalence of enuresis in individuals with sickle cell disease. The present study aims to evaluate the prevalence and impact of enuresis on quality of life in individuals with sickle cell disease.
Materials and Methods
This cross-sectional study evaluated individuals with sickle cell disease followed at a reference clinic, using a questionnaire designed to evaluate the age of complete toilet training, the presence of enuresis and lower urinary tract, and the impact on quality of life of these individuals.
Results
Fifty children presenting SCD (52% females, mean age ten years) were included in the study. Of those, 34% (17/50) presented as HbSC, 56% with HbSS (28/50), 2% Sα-thalassemia (1/5) and 8% the type of SCD was not determined. The prevalence of enuresis was 42% (21/50), affecting 75% of subjects at five years and about 15% of adolescents at 15 years of age. Enuresis was classified as monosymptomatic in 33.3% (7/21) and nonmonosymptomatic in 66.6% (14/21) of the cases, being primary in all subjects. Nocturia was identified in 24% (12/50), urgency in 20% (10/50) and daytime incontinence 10% (5/50) of the individuals. Enuresis had a significant impact on the quality of life of 67% of the individuals.
Conclusion
Enuresis was highly prevalent among children with SCD, and continues to be prevalent throughout early adulthood, being more common in males. Primary nonmonosymptomatic enuresis was the most common type, and 2/3 of the study population had a low quality of life.
Keywords: Quality of Life, Sickle Cell Trait, Enuresis
INTRODUCTION
Sickle cell disease (SCD) is an autosomal recessive hereditary disease in which hemoglobin S is present (1, 2). According to the type of alteration present in hemoglobin, SCD can be classified in different clinical forms: homozygous form SS (referred to as sickle cell anemia-HbSS), and heterozygous forms, represented by associations of HbS with other hemoglobin defects ( SC, HbS/β0 thalassemia, HbS/β + thalassemia, S/α thalassemia) (1). SCD is the most common congenital hemoglobinopathy and affects mainly Africans or their descendants in America, being responsible for more than 300.000 live births per year (3, 4). In Brazil, 60.000 to 100.000 cases of the disease are currently estimated. SCD requires multi-professional approach for early diagnosis and management, due to its physical, psychological and socioeconomic impact, with high morbidity and mortality (1).
Lower urinary tract symptoms (LUTS) are also common in children occurring in about 14.7 to 21.8% (5, 6). LUTS is characterized by abnormal urine storage and/or bladder emptying in the absence of urinary tract infections, neurological or anatomical abnormalities (7, 8). Enuresis is both a symptom and a condition of intermittent incontinence that occurs during periods of sleep after the age of five years. According to symptoms, enuresis is classified as monosymptomatic, when no other symptom is present and non-monosymptomatic when associated with LUTS (8). Prevalence of enuresis in children aged 6 to 13 years varies from 9.5% to 12.9% (9, 10). Enuresis has adverse emotional and social effects that affect children’s quality of life (11).
The prevalence of enuresis in children with SCD is approximately 32% (12, 13) and its etiopathogenesis is still controversial (14). It has been related to nocturnal polyuria induced by hyposthenuria (13, 15, 16) and low functional bladder capacity (14, 17). Hyposthenuria is one of the earliest organic manifestations of SCD occurring as early as 12 months of age (13, 18). However, in a more recent study, Eneh et al. have shown that enuresis in children with SCD appears not to be related to hyposthenuria, but to other causal factors that apply to the general population (14).
The present cross-sectional study was conducted to estimate the prevalence of enuresis in individuals with SCD and its impact in the quality of life. We have hypothesized that enuresis is more prevalent in children, adolescents and young adults with SCD than described for general population, and that it greatly impacts the quality of life of this population.
MATERIALS AND METHODS
This cross-sectional study was carried out from July 2016 to September 2017. During this period, 50 consecutive patients (children, adolescent, and young adults) with SCD, aged 5 to 24 years, who regularly attended the regional outpatient reference center for SCD, were evaluated. Patients with current urogenital disorder, current use of medications or diseases known to interfere with bladder or sphincter function, such as urinary tract infection and severe intellectual disability, and those not yet toilet trained were not included in the study.
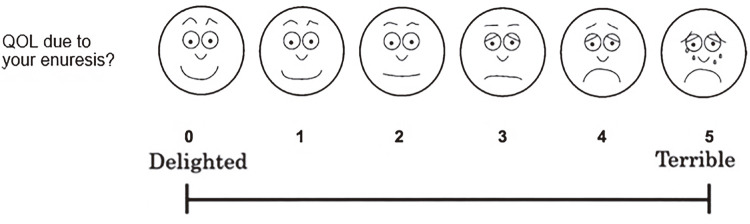
A questionnaire was developed for this study and applied to the participants and/or their caregivers. Questions related to sociodemographic characteristics, hemoglobin electrophoresis for detect different hemoglobin genotypes, age of acquisition of complete toilet training, presence of LUTS and enuresis (including frequency and classification: monosymptomatic or non-monosymptomatic) were addressed. The Visual Analogue Scale (VAS), adapted from Ushijima et al., 2006 (19), with appropriate facial expressions throughout the VAS was used to analyze the quality of life. The VAS used in this study was a 10cm line ranging from delighted at the left end of the line up to terrible at the right end of the line. The subjects were asked to assign a score of 0 to 5 according to the intensity with which the enuresis affected their well-being with faces graphic expressing each note. The higher the score, the more uncomfortable the impact and the subject was in a situation (19, 20) (Figure-1).
Figure 1. Visual Analog Scale.
Visual Analogue Scale (VAS) adapted from Ushijima et al., 2006
VAS questionnaire to assess discomfort or satisfaction regarding patient’s quality of life (QOL). How would you rate your discomfort with enuresis? The face scales were demostrated to help understanding, includinf: 1) laughing face to represent delighted or pleased above left end of VAS, 2) smiling face to represent mostly satisfied above left side to center of VAS, 3) face with neutral expression to represent neither satisfied and dissatisfied above center of VAS, 4) face in trouble to represent mostly dissatisfied above righr side to center of VAS and 5) crying face to represent unhappy or terrible above right end of VAS.
Quantitative variables, continuous or ordinal, were described by measures of central tendency (mean or median) and the respective dispersion measures (standard deviation, interquartile range or minimum and maximum values). Nominal or qualitative variables were described for their absolute values, percentages or proportions. To compare the differences of continuous variables, we used the Student t-test or the Mann-Whitney test. For comparison of categorical data, we used the chi-square test and its variants. The association between the parameters studied was expressed by the prevalence ratio (Odds Ratio). A 95% confidence intervals were used as measures of precision of the results and p values less than 0.05 (p <0.05) were considered significant. In the analysis, we used computational statistical software (GraphPad Prism, version 8.0.0, GraphPad Software, San Diego-CA, USA).
The study was approved by the institution ethics committee, (CEP-UEFS #1.440.239) and all participants or legal representative that agreed in participate in the study signed a free and informed consent.
RESULTS
In this study, a total of 50 patients with SCD were evaluated. The mean age at enrollment was of 10 years [7-15], being 52% (26/50) female. SCD genotypes diagnosed by hemoglobin electrophoresis at alkaline pH are shown in Table-1.
Table 1. General characteristics of the study population (n=50).
| Description | Percentage | |
|---|---|---|
| Gender | Female | 52 (26/50) |
| Male | 48 (24/50) | |
| Age (years) | 6-11 | 58(29/50) |
| 12-18 | 30(15/50) | |
| 19-24 | 12(06/50) | |
| Genotype | SS | 58(28/50) |
| SC | 34(17/50) | |
| Sα-thalassemia | 02(01/50) | |
| Undetermined | 08 (04/50) |
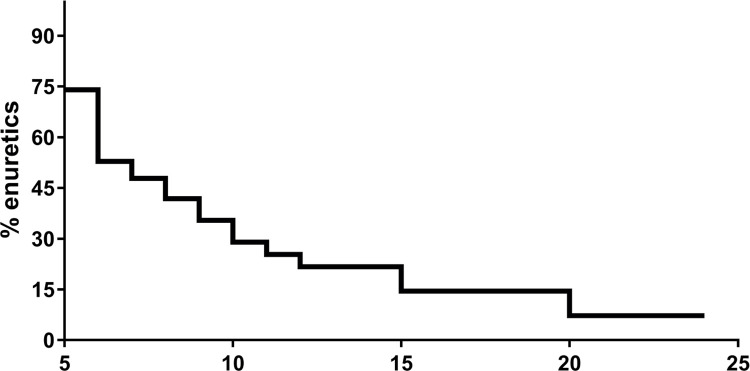
Of the 50 individuals evaluated, enuresis was identified in 42% (21/50) of the cases, affecting 75% of the subjects at five years and about 15% of the adolescents at 15 years of age (Figure-2). Enuresis was classified as monosymptomatic in 33.3% (7/21) and non-monosymptomatic in 66.6% (14/21) of the cases, being primary in 100% of all subjects. In 62% of subjects frequency, enuresis episodes were more than three times a week. Nocturia was identified in 24% (12/50), urgency in 20% (10/50) and daytime incontinence 10% (5/50) of the individuals.
Figure 2. Prevalence of enuresis according to age.
Enuresis appears to be more common in males (OR=1.89 [0.59-5.38]), but no statistical significance was found (p=0.395). Toilet training daytime was finished before completing two years of age in 32% (16/50) of patients, 24% (12/50) between two and three years, 20% (10/50) between three and four years, 14% (7/50) between four and five. The other five did not remember when toilet training was completed. No correlation was found between the age of completing toilet training daytime and the prevalence of enuresis.
Enuresis had a negative impact on the quality of life of those affected. Asymptomatic patients had significantly higher scores in VAS than symptomatic ones (9.26±0.92 and 7.22±3.44, respectively- p=0.015). According to VAS, 67% (14/21) of the study subjects considered that enuresis had a serious impact on their quality of life.
DISCUSSION
The present study is in agreement with previous studies that have shown a significantly increase in the prevalence of enuresis in subjects with SCD (13, 15, 17, 21), as evidenced in 42% of the population studied herein. A recent study recruited 243 children with SCD aged between 5 and 17 years and showed a prevalence of 49.4% of enuresis versus 29.6% in the control group (22). The inclusion of patients as old as 24 years of age is justified as these studies have shown that enuresis remains prevalent in adolescents and young adult with SCD (12, 16, 22).
Although some studies have reported hyposthenuria as the main determinant of enuresis in individuals with sickle cell disease (23), recent studies have not shown this association (14).
In the present study, there was a higher prevalence of monosymptomatic enuresis (66%) and all cases were primary enuresis. This finding is in agreement with Portacarrero et al. who showed that children and adolescents with SCD had non-monosymptomatic and primary enuresis in 58% and 86% of the cases, respectively. Another recent study also demonstrated an increased prevalence of primary non-monosymptomatic enuresis in SCD patients (24).
It is important to note that 15% of adolescents at 15 years of age had enuresis. These results are also similar to those of Portacarrero et al. that demonstrated the prevalence of 21% of enuresis in adolescents with sickle cell disease (15-18 years of age) (12). They are also compatible with findings from Field et al. and Esezobor et al. who documented persistently high rates of enuresis of 18% and 25% among adolescents over 14 years of age, respectively (22, 25). On the contrary, studies in healthy population have shown that the prevalence of enuresis decreases with age to around 1 to 3% at 15 years (11, 26). Therefore, the well-established decline in the prevalence of enuresis with age was less pronounced in individuals with sickle cell disease.
Although Mabiala et al. documented a higher prevalence of enuresis in females with SCD (27), our findings are in agreement with the majority of other studies (12, 16, 22) that have found a higher prevalence of enuresis in males. The reason for this preponderance in males is still unclear. No correlation between age of acquisition diurnal urinary control and the presence of enuresis and other lower urinary tract symptoms, as in Down syndrome patients was found (7).
An increased prevalence of urgency and daytime incontinence was present in this series as well as in other series (15). Portacarrero et al. demonstrated 33% of urgency and 23% of daytime incontinence in SCD (12). In addition to these LUTS, 24% of subjects in the present study had nocturia. Enuresis and nocturia are common in children with SCD being reported to be present in 68 to 79% of the patients (15, 23, 25) Further prospective studies are needed to clarify the pathophysiological mechanisms underlying the urinary manifestations of SCD.
In this study, enuresis negatively affected the quality of life in 67% of the individuals. SCD is characterized by systemic complications including thoracic syndrome, chronic lung disease, cardiomyopathy, splenic infarction, chronic liver disease, bone infarction, osteomyelitis and depression (28, 29). All these complications, with prolonged hospitalizations and frequent chronic pain, significantly reduce quality of life (29, 30). Although quality of life of all patients included in the study may be compromised by SCD, those presenting enuresis had an even worse score when compared to non-enuretic ones, showing that enuresis negatively impact quality of life, as previously demonstrated by Savaser et al. for general population (11).
This study has several limitations small sample size and absence of a control group, family history of enuresis was not investigated, the main complications of SCD, which may have negative impact patient quality of life, have not been addressed and presence of intestinal constipation did not was evaluated.
CONCLUSIONS
Enuresis was highly prevalent among children with SCD, and continues to be prevalent throughout early adulthood, especially in males generating a negative impact on quality of life. In all cases, enuresis was primary enuresis, and in the majority it was non-monosymptomatic. These findings are important to alert the parents and professionals involved in the follow-up of these patients about the need for diagnosing and treating this condition.
REFERENCES
- 1.1. Azar S, Wong TE. Sickle Cell Disease: A Brief Update. Med Clin North Am. 2017;101:375-93. [DOI] [PubMed]; Azar S, Wong TE. Sickle Cell Disease: A Brief Update. Med Clin North Am. 2017;101:375–393. doi: 10.1016/j.mcna.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 2.2. Lobitz S, Telfer P, Cela E, Allaf B, Angastiniotis M, Backman Johansson C, et al. Newborn screening for sickle cell disease in Europe: recommendations from a Pan-European Consensus Conference. Br J Haematol. 2018;183:648-60. [DOI] [PubMed]; Lobitz S, Telfer P, Cela E, Allaf B, Angastiniotis M, Backman Johansson C, et al. Newborn screening for sickle cell disease in Europe: recommendations from a Pan-European Consensus Conference. Br J Haematol. 2018;183:648–660. doi: 10.1111/bjh.15600. [DOI] [PubMed] [Google Scholar]
- 3.3. Serjeant G. World Sickle Cell Day: Lessons for India. Indian J Med Res. 2017;145:705-7. [DOI] [PMC free article] [PubMed]; Serjeant G. World Sickle Cell Day: Lessons for India. Indian J Med Res. 2017;145:705–707. doi: 10.4103/ijmr.IJMR_1208_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.4. Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311-23. [DOI] [PubMed]; Ware RE, Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 5.5. Mrad FCC, Figueiredo AA, Bessa J Jr, Bastos Netto JM. Prolonged toilet training in children with Down syndrome: a case-control study. J Pediatr (Rio J). 2018;94:286-92. [DOI] [PubMed]; Mrad FCC, Figueiredo AA, Bessa J, Jr, Bastos Netto JM. Prolonged toilet training in children with Down syndrome: a case-control study. J Pediatr (Rio J) 2018;94:286–292. doi: 10.1016/j.jped.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 6.6. Vaz GT, Vasconcelos MM, Oliveira EA, Ferreira AL, Magalhães PG, Silva FM, et al. Lima EM. Prevalence of lower urinary tract symptoms in school-age children. Pediatr Nephrol. 2012;27:597-603. [DOI] [PubMed]; Vaz GT, Vasconcelos MM, Oliveira EA, Ferreira AL, Magalhães PG, Silva FM, et al. Lima EM. Prevalence of lower urinary tract symptoms in school-age children. Pediatr Nephrol. 2012;27:597–603. doi: 10.1007/s00467-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 7.7. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. J Urol. 2014;191:1863-1865.e13. [DOI] [PubMed]; Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. J Urol. 2014;191:1863–1865.e13. doi: 10.1016/j.juro.2014.01.110. [DOI] [PubMed] [Google Scholar]
- 8.8. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471-81. [DOI] [PubMed]; Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471–481. doi: 10.1002/nau.22751. [DOI] [PubMed] [Google Scholar]
- 9.9. Sarici H, Telli O, Ozgur BC, Demirbas A, Ozgur S, Karagoz MA. Prevalence of nocturnal enuresis and its influence on quality of life in school-aged children. J Pediatr Urol. 2016;12:159.e1-6. [DOI] [PubMed]; Sarici H, Telli O, Ozgur BC, Demirbas A, Ozgur S, Karagoz MA. Prevalence of nocturnal enuresis and its influence on quality of life in school-aged children. J Pediatr Urol. 2016;12:159.e1–159.e6. doi: 10.1016/j.jpurol.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 10.10. Srivastava S, Srivastava KL, Shingla S. Prevalence of monosymptomatic nocturnal enuresis and its correlates in school going children of Lucknow. Indian J Pediatr. 2013;80:488-91. [DOI] [PubMed]; Srivastava S, Srivastava KL, Shingla S. Prevalence of monosymptomatic nocturnal enuresis and its correlates in school going children of Lucknow. Indian J Pediatr. 2013;80:488–491. doi: 10.1007/s12098-012-0870-1. [DOI] [PubMed] [Google Scholar]
- 11.11. Savaser S, Kizilkaya Beji N, Aslan E, Gozen D. The Prevalence of Diurnal Urinary Incontinence and Enuresis and Quality of Life: Sample of School. Urol J. 2018;15:173-9. [DOI] [PubMed]; Savaser S, Kizilkaya Beji N, Aslan E, Gozen D. The Prevalence of Diurnal Urinary Incontinence and Enuresis and Quality of Life: Sample of School. Urol J. 2018;15:173–179. doi: 10.22037/uj.v0i0.3982. [DOI] [PubMed] [Google Scholar]
- 12.12. Portocarrero ML, Portocarrero ML, Sobral MM, Lyra I, Lordêlo P, Barroso U Jr. Prevalence of enuresis and daytime urinary incontinence in children and adolescents with sickle cell disease. J Urol. 2012;187:1037-40. [DOI] [PubMed]; Portocarrero ML, Portocarrero ML, Sobral MM, Lyra I, Lordêlo P, Barroso U., Jr Prevalence of enuresis and daytime urinary incontinence in children and adolescents with sickle cell disease. J Urol. 2012;187:1037–1040. doi: 10.1016/j.juro.2011.10.171. [DOI] [PubMed] [Google Scholar]
- 13.13. Wolf RB, Kassim AA, Goodpaster RL, DeBaun MR. Nocturnal enuresis in sickle cell disease. Expert Rev Hematol. 2014;7:245-54. [DOI] [PubMed]; Wolf RB, Kassim AA, Goodpaster RL, DeBaun MR. Nocturnal enuresis in sickle cell disease. Expert Rev Hematol. 2014;7:245–254. doi: 10.1586/17474086.2014.892412. [DOI] [PubMed] [Google Scholar]
- 14.14. Eneh CI, Ikefuna AN, Okafor HU, Uwaezuoke SN. Nocturnal enuresis in school-aged children with sickle-cell anemia: Any relationship with hyposthenuria? Niger J Clin Pract. 2017;20:215-20. [DOI] [PubMed]; Eneh CI, Ikefuna AN, Okafor HU, Uwaezuoke SN. Nocturnal enuresis in school-aged children with sickle-cell anemia: Any relationship with hyposthenuria? Niger J Clin Pract. 2017;20:215–220. doi: 10.4103/1119-3077.187326. [DOI] [PubMed] [Google Scholar]
- 15.15. Claudino MA, Fertrin KY. Sickling cells, cyclic nucleotides, and protein kinases: the pathophysiology of urogenital disorders in sickle cell anemia. Anemia. 2012;2012:723520. [DOI] [PMC free article] [PubMed]; Claudino MA, Fertrin KY. Sickling cells, cyclic nucleotides, and protein kinases: the pathophysiology of urogenital disorders in sickle cell anemia. 723520Anemia. 2012;2012 doi: 10.1155/2012/723520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.16. Eneh CI, Okafor HU, Ikefuna AN, Uwaezuoke SN. Nocturnal enuresis: prevalence and risk factors among school-aged children with sickle-cell anaemia in a South-east Nigerian city. Ital J Pediatr. 2015;41:66. [DOI] [PMC free article] [PubMed]; Eneh CI, Okafor HU, Ikefuna AN, Uwaezuoke SN. Nocturnal enuresis: prevalence and risk factors among school-aged children with sickle-cell anaemia in a South-east Nigerian city. 66Ital J Pediatr. 2015;41 doi: 10.1186/s13052-015-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.17. Tewari S, Rees DC, Hannemann A, Gbotosho OT, Al Balushi HW, Gibson JS. Nocturnal enuresis and K+ transport in red blood cells from patients with sickle cell anemia. Haematologica. 2016;101:e469-e472. [DOI] [PMC free article] [PubMed]; Tewari S, Rees DC, Hannemann A, Gbotosho OT, Al Balushi HW, Gibson JS. Nocturnal enuresis and K+ transport in red blood cells from patients with sickle cell anemia. Haematologica. 2016;101:e469–e472. doi: 10.3324/haematol.2016.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.18. Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, Casella JF, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010;156:66-70.e1. [DOI] [PMC free article] [PubMed]; Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, Casella JF, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010;156:66–70.e1. doi: 10.1016/j.jpeds.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.19. Esezobor CI, Akintan P, Nwaogazie U, Akinwunmi E, Temiye E, Akinsulie A, et al. Enuresis in children and adolescents with sickle cell anaemia is more frequent and substantially different from the general population. PLoS One. 2018;13:e0201860. [DOI] [PMC free article] [PubMed]; Esezobor CI, Akintan P, Nwaogazie U, Akinwunmi E, Temiye E, Akinsulie A, et al. Enuresis in children and adolescents with sickle cell anaemia is more frequent and substantially different from the general population. PLoS One. 2018;13:e0201860. doi: 10.1371/journal.pone.0201860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.20. Ushijima S, Ukimura O, Okihara K, Mizutani Y, Kawauchi A, Miki T. Visual analog scale questionnaire to assess quality of life specific to each symptom of the International Prostate Symptom Score. J Urol. 2006;176:665-71. [DOI] [PubMed]; Ushijima S, Ukimura O, Okihara K, Mizutani Y, Kawauchi A, Miki T. Visual analog scale questionnaire to assess quality of life specific to each symptom of the International Prostate Symptom Score. J Urol. 2006;176:665–671. doi: 10.1016/j.juro.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 21.21. Preciado-Estrella DA, Kaplan SA, Iturriaga-Goyón E, Ramón-Trejo E, Mayorga-Gómez E, Auza-Benavides A. International Prostate Symptom Score and Gea Visual Analogue Scale® comparison for evaluating lower urinary tract symptoms. Rev Mex Urol 2017; 77:372-382.; Preciado-Estrella DA, Kaplan SA, Iturriaga-Goyón E, Ramón-Trejo E, Mayorga-Gómez E, Auza-Benavides A. International Prostate Symptom Score and Gea Visual Analogue Scale® comparison for evaluating lower urinary tract symptoms. Rev Mex Urol. 2017;77:372–382. [Google Scholar]
- 22.22. Ekinci O, Celik T, Ünal <bold><italic>Ş</italic></bold>, Oktay G, Toros F, Ozer C. Nocturnal enuresis in sickle cell disease and thalassemia major: associated factors in a clinical sample. Int J Hematol. 2013;98:430-6. Noll JB, Newman AJ, Gross S. Enuresis and nocturia in sickle cell disease. J Pediatr. 1967;70:965-7. [DOI] [PubMed]; Ekinci O, Celik T, <bold><italic>Ş</italic></bold> Ünal, Oktay G, Toros F, Ozer C. Nocturnal enuresis in sickle cell disease and thalassemia major: associated factors in a clinical sample. Int J Hematol. 2013;98:430–436. doi: 10.1007/s12185-013-1422-9. Noll JB, Newman AJ, Gross S. Enuresis and nocturia in sickle cell disease. J Pediatr. 1967;70:965-7. [DOI] [PubMed] [Google Scholar]
- 23.23. Noll JB, Newman AJ, Gross S. Enuresis and nocturia in sickle cell disease. J Pediatr. 1967;70:965-7. [DOI] [PubMed]; Noll JB, Newman AJ, Gross S. Enuresis and nocturia in sickle cell disease. J Pediatr. 1967;70:965–967. doi: 10.1016/s0022-3476(67)80273-7. [DOI] [PubMed] [Google Scholar]
- 24.24. Elawad Ahmed F.: Nocturnal Enuresis in Children and Adolescent with Sickle cell Anemia. Med Surg Urol. 2017; 6:119.; Elawad Ahmed F. Nocturnal Enuresis in Children and Adolescent with Sickle cell Anemia. 119Med Surg Urol. 2017;6 [Google Scholar]
- 25.25. Field JJ, Austin PF, An P, Yan Y, DeBaun MR. Enuresis is a common and persistent problem among children and young adults with sickle cell anemia. Urology. 2008;72:81-4. [DOI] [PMC free article] [PubMed]; Field JJ, Austin PF, An P, Yan Y, DeBaun MR. Enuresis is a common and persistent problem among children and young adults with sickle cell anemia. Urology. 2008;72:81–84. doi: 10.1016/j.urology.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.26. Ozden C, Ozdal OL, Altinova S, Oguzulgen I, Urgancioglu G, Memis A. Prevalence and associated factors of enuresis in Turkish children. Int Braz J Urol. 2007;33:216-22. [DOI] [PubMed]; Ozden C, Ozdal OL, Altinova S, Oguzulgen I, Urgancioglu G, Memis A. Prevalence and associated factors of enuresis in Turkish children. Int Braz J Urol. 2007;33:216–222. doi: 10.1590/s1677-55382007000200013. [DOI] [PubMed] [Google Scholar]
- 27.27. Mabiala Babela JR, Loumingou R, Pemba-Loufoua A, Londjongo W, Nzingoula S, Senga P. [Enuresis in children with sickle cell disease]. Arch Pediatr. 2004;11:1168-72. [DOI] [PubMed]; Mabiala Babela JR, Loumingou R, Pemba-Loufoua A, Londjongo W, Nzingoula S, Senga P. [ Enuresis in children with sickle cell disease] Arch Pediatr. 2004;11:1168–1172. doi: 10.1016/j.arcped.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 28.28. Wang MX, Pepin EW, Verma N, Mohammed TL. Manifestations of sickle cell disease on thoracic imaging. Clin Imaging. 2018;48:1-6. [DOI] [PubMed]; Wang MX, Pepin EW, Verma N, Mohammed TL. Manifestations of sickle cell disease on thoracic imaging. Clin Imaging. 2018;48:1–6. doi: 10.1016/j.clinimag.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 29.29. Graves JK, Hodge C, Jacob E. Depression, Anxiety, and Quality of Life In Children and Adolescents With Sickle Cell Disease. Pediatr Nurs. 2016;42:113-9. [PubMed]; Graves JK, Hodge C, Jacob E. Depression, Anxiety, and Quality of Life In Children and Adolescents With Sickle Cell Disease. Pediatr Nurs. 2016;42:113–119. [PubMed] [Google Scholar]
- 30.30. Lim CS, Karlson C, Edmond SN, Welkom JS, Osunkwo I, Cohen LL. Emotion-Focused Avoidance Coping Mediates the Association Between Pain and Health-Related Quality of Life in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2019;41:194-201. [DOI] [PMC free article] [PubMed]; Lim CS, Karlson C, Edmond SN, Welkom JS, Osunkwo I, Cohen LL. Emotion-Focused Avoidance Coping Mediates the Association Between Pain and Health-Related Quality of Life in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2019;41:194–201. doi: 10.1097/MPH.0000000000001429. [DOI] [PMC free article] [PubMed] [Google Scholar]