Abstract
Background
Although vascular dementia is the second most common cause of dementia globally, evidence‐based treatments are still lacking. Cerebrolysin is a porcine brain‐derived preparation that is said to have neurotrophic and neuroprotective activity. In many parts of the world Cerebrolysin, given as a series of daily intravenous infusions, is used as a potential intervention for vascular dementia. A previous Cochrane Review on Cerebrolysin in vascular dementia yielded inconsistent results. We wished to update the review to add new studies from the international literature and employ contemporary methods for appraising the strength of the evidence.
This is the first update of a review first published in 2013.
Objectives
Primary: to assess the effect of Cerebrolysin on cognitive function, global function, and all‐cause mortality in people living with vascular dementia.
Secondary: to assess the adverse effects of Cerebrolysin and to assess the effect of Cerebrolysin on quality of life and caregiver burden.
Search methods
We searched ALOIS, MEDLINE, Embase, PsycINFO, CINAHL, ISI Web of Knowledge, LILACS, the Cochrane Library, ClinicalTrials.gov, and the WHO ICTRP on 16 June 2017, 9 May 2018, and 9 May 2019. We expanded the search by adding four Chinese databases, searched from 1 January 2012 to 19 May 2019. We checked bibliographies of relevant papers identified and contacted pharmaceutical companies, trial authors, and experts in the field to identify any additional published or unpublished data.
Selection criteria
We included all randomised controlled trials of Cerebrolysin used in people living with vascular dementia. We applied no language restriction.
Data collection and analysis
Two review authors independently selected trials for inclusion and evaluated their methodological quality. Data were extracted and analysed using mean differences (MDs) or standardised mean differences (SMDs) with 95% confidence intervals (95% CI) for continuous outcomes. We reported dichotomous outcomes as risk ratio (RR) with 95% CI. We assessed the strength of the available evidence using the GRADE approach.
Main results
We identified six randomised controlled trials with a total of 597 participants that were eligible for inclusion in the 2013 review. No new studies were eligible for inclusion in this update. Participants in the included studies, where dementia severity was reported, had mild to moderate severity of vascular dementia (four trials). The included studies tested varying doses and duration of Cerebrolysin treatment. Follow‐up ranged from 15 days to three years. Five of included studies were conducted in China (three studies), Russia (one study), and Romania (one study), while relevant information of other study was unclear. Where details of funding were available, all studies were supported by the pharmaceutical industry (three studies).
Cognitive function was measured using the Mini‐Mental State Examination (MMSE) or Alzheimer's Disease Assessment Scale Cognitive Subpart, extended version (ADAS‐cog+). Combining the MMSE and ADAS‐cog+ data (three studies, 420 people), there was a beneficial effect of Cerebrolysin (SMD 0.36, 95% CI 0.13 to 0.58; very low‐quality evidence).
Global function was measured by Clinician's Interview‐Based Impression of Change plus Caregiver Input (CIBIC+) or Investigator's Clinical Global Impression (CGI). We assessed response rates on these measures (the proportion of participants with a CIBIC+ score of < 3; or at least moderate improvement of the CGI rating at the last visit). There was a beneficial effect of Cerebrolysin (two studies, 379 participants, RR 2.69, 95% CI 1.82 to 3.98; very low‐quality evidence).
Only one trial described mortality and reported no deaths. Four studies reported adverse events; data from two studies (379 people) were in a format that permitted meta‐analysis, and there was no difference in rates of adverse effects (RR 0.91, 95% CI 0.29 to 2.85; very low‐quality evidence). No studies reported on quality of life or caregiver burden.
Authors' conclusions
Courses of intravenous Cerebrolysin improved cognition and general function in people living with vascular dementia, with no suggestion of adverse effects. However, these data are not definitive. Our analyses were limited by heterogeneity, and the included papers had high risk of bias. If there are benefits of Cerebrolysin, the effects may be too small to be clinically meaningful. There have been no new studies of Cerebrolysin in vascular dementia since the last Cochrane Review. Cerebrolysin continues to be used and promoted as a treatment for vascular dementia, but the supporting evidence base is weak. Adequately powered, methodologically robust trials are needed to properly assess the effects of Cerebrolysin in vascular dementia.
Plain language summary
Cerebrolysin for vascular dementia
Background
Vascular dementia is a common form of dementia caused by poor blood flow to the brain. There are currently no proven and licenced treatments for vascular dementia. Cerebrolysin is a medicine that is made from pig's brains and given as an injection. In some countries, Cerebrolysin is used as a treatment for vascular dementia. A previous summary from 2013 considered all studies of Cerebrolysin and vascular dementia. A definitive conclusion as to whether Cerebrolysin was beneficial could not be made.
Purpose of this review
We wanted to learn whether Cerebrolysin can benefit people living with vascular dementia. We were particularly interested in the effects of the medication on memory and thinking; daily functioning; side effects; and and quality of life for people with vascular dementia and their caregivers. As some time has passed since the last review of Cerebrolysin, we wanted to update the review with a search for any new studies.
What we did
We searched for studies that described the effect of Cerebrolysin in people living with vascular dementia. We searched databases of scientific studies, including resources from countries where Cerebrolysin is commonly used. To be included in our review, the studies had to randomly decide whether people were treated with Cerebrolysin or a comparison. We combined the results of the included studies to estimate the effect of Cerebrolysin. We also assessed how well the studies were conducted and how credible the results were. The evidence is current to May 2019.
What we found
We found six studies including a total of 597 people living with vascular dementia. The method of Cerebrolysin treatment differed across studies, with varying strengths of Cerebrolysin and treatment durations. The studies reported that Cerebrolysin had beneficial effects on memory and thinking and on daily functioning. There was no reported risk of side effects with treatment. None of the studies described quality of life of people living with vascular dementia or their caregivers.
Although the studies suggested a benefit of Cerebrolysin treatment, the results are not definitive. The included studies had several issues that may have led to misleading results. Even if the benefit reported in the studies is real, the effect was modest and may not be important to people living with dementia. There is a need for a large, well‐conducted study to better understand if Cerebrolysin is a useful treatment for people living with vascular dementia.
Summary of findings
Summary of findings for the main comparison. Cerebrolysin for vascular dementia.
| Cerebrolysin for vascular dementia | ||||||
| Patient or population: vascular dementia Setting: hospitals and clinics Intervention: Cerebrolysin Comparison: placebo or routine treatment | ||||||
| Continuous outcomes | Score with placebo (mean) | Mean improvement in change score between Cerebrolysin and placebo | SMD (95% CI) meta‐analysis findings | № of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Cognitive function (MMSE or ADAS‐cog+) follow‐up: 4 weeks to 24 weeks |
Mean MMSE score at baseline was 19.9. Mean change from baseline of MMSE was 2.6. | MD 1.1 higher (0.37 higher to 1.82 higher) | 0.36 (0.13 to 0.58) | 420 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Analysed as standardised mean difference (Analysis 1.1) |
| Quality of life | See comment | See comment | See comment | See comment | See comment | No data on quality of life during the treatment and follow‐up periods were available from any of the included trials. |
| Caregiver burden | See comment | See comment | See comment | See comment | See comment | No data on evaluation of caregiver burden were available from the included trials. |
| Binary outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or routine treatment | Risk with Cerebrolysin | |||||
| Improvement in cognitive function reported as responder rates (MMSE, ADAS‐cog+, and HDS) follow‐up: 15 days to 3 years |
554 per 1000 | 809 per 1000 (610 to 1000) | RR 1.46 (1.10 to 1.94) | 338 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 5 | RR and control group risk from 3 studies (Analysis 1.4) |
| Improvement in global function reported as response rates (CIBIC+ and CGI) follow‐up: 4 weeks to 24 weeks |
144 per 1000 | 388 per 1000 (263 to 575) | RR 2.69 (1.82 to 3.98) | 379 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 6 7 | RR and control group risk from 2 studies (Analysis 2.1) |
| Non‐serious adverse events follow‐up: 4 weeks to 24 weeks | 80 per 1000 | 73 per 1000 (23 to 229) | RR 0.91 (0.29 to 2.85) | 379 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 6 7 | RR and control group risk from 2 studies (Analysis 3.2) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐cog+: Alzheimer's Disease Assessment Scale Cognitive Subpart, Extended Version; CGI: Clinical Global Impression; CI: confidence interval; CIBIC+: Clinician's Interview‐Based Impression of Change plus Caregiver Input; HDS: Hasegawa Dementia Scale; MD: mean difference; MMSE: Mini‐Mental State Examination; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to serious concern about indirectness: the dementia care provided as comparator was not always consistent with contemporary, guideline‐based best care and so there were issues with indirectness. 2Downgraded two levels due to very serious concern about risk of bias: two of the trials were at unclear risk of selection bias and unclear risk of reporting bias, and one trial was at unclear risk of detection bias. Most of the 'Risk of bias' judgements were unclear risk of bias. 3Downgraded one level due to serious concern about imprecision: modest sample size. 4Downgraded two levels due to very serious concern about risk of bias: two of the trials were at unclear risk of selection bias; one trial was at unclear risk of reporting bias; one trial was at unclear of other bias; one trial was at high risk of performance bias, high risk of detection bias, and high risk of reporting bias. There was a substantial risk of bias across the body of evidence. 5Downgraded one level due to serious concern about imprecision: modest sample size and few events. 6Downgraded two levels due to very serious concern about risk of bias: one of the trials was at unclear risk of selection bias, unclear risk of detection bias, and unclear risk of reporting bias. Most of the 'Risk of bias' judgements were unclear risk of bias. 7Downgraded one level due to serious concern about imprecision: modest sample size and wide confidence intervals.
Background
Description of the condition
Definition
Vascular dementia (VaD) is the second most common form of dementia after Alzheimer's disease (AD). The term 'vascular dementia' refers to a constellation of cognitive and functional impairments all caused by disordered blood flow to the brain. Vascular dementia can be considered a subset of the larger syndrome of vascular cognitive impairment (VCI), that is all cognitive syndromes associated with a cerebrovascular brain injury (Aggarwal 2007). As an aetiologic category, VaD includes dementia caused by ischaemic or haemorrhagic cerebrovascular diseases (CVD) or by ischaemic‐hypoxic brain lesions of cardiovascular origin (Román 2002).
Diagnosis and classification
There are several diagnostic criteria for VaD, such as the Diagnostic and Statistical Manual of Mental Disorders, third edition, third revised, fourth, and fifth edition (DSM‐III, DSM III‐R, DSM‐IV, DSM‐V) (APA 1980; APA 1987; APA 1994; APA 2013), Alzheimer's Disease Diagnostic and Treatment Centers (ADDTC) criteria (Chui 1992), International Statistical Classification of Diseases, 10th revision (ICD‐10) (WHO 1992), and National Institute of Neurological Disorders and Stroke ‐ Association Internationale Pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria (Román 1993). All of these criteria are based on two major requirements: clinical diagnosis of dementia and determination of its vascular origin. The latter requirement is problematic because of the frequent overlap between cerebrovascular and degenerative disorders, particularly in older people (Benisty 2008). As a result, the current diagnostic criteria for VaD are not concordant, which could make data analysis and comparisons difficult (Moorhouse 2008).
Within the VaD rubric, the following has been proposed as a classification of the subtypes of VaD (O'Brien 2015):
(i) multi‐infarct dementia (cortical VaD);
(ii) small‐vessel dementia (subcortical VaD);
(iii) strategic infarct dementia;
(iv) hypoperfusion dementia;
(v) haemorrhagic dementia;
(vi) AD with cerebrovascular disease (CVD);
(vii) hereditary vascular dementia (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)).
This classification is based on clinical differences and underlying pathologic changes. Although each category is well defined, in many cases it can be difficult to classify a patient into one definitive subtype. Subtypes are usually not pure. Rather, mixtures of pathologies frequently combine to contribute to cognitive impairment (Moorhouse 2008).
Vascular dementia and stroke disease are closely linked, but the terms VaD and poststroke dementia (PSD) are not synonymous (Mijajlović 2017). Although most PSD cases are pathologically confirmed as VaD, some have been reported to be other dementia‐related pathologies, such as AD (Ihara 2014).
Assessment
A wide range of instruments have been used to assess cognitive function in individuals with VaD (Korczyn 2012). However, since the most typical expression of VaD is executive dysfunction, the widely used Mini‐Mental State Examination (MMSE) may underestimate the cognitive decline in individuals with VaD. Mini‐Mental State Examination was designed to assess for Alzheimer’s disease and contains few items related to executive function (Folstein 1975; Moorhouse 2008). Other cognitive assessment tools such as the Montreal Cognitive Assessment (MoCA) may be more appropriate in such cases (Nasreddine 2005; Korczyn 2012).
Global burden of disease
Vascular dementia has traditionally received less attention than AD, yet international epidemiological data suggest a substantial global burden from VaD. The prevalence rate of VaD has been estimated to double every 5.3 years, compared with every 4.5 years for AD (O'Brien 2003). In North America, AD accounts for 44% to 70% of all dementia, while VaD accounts for 14.5% to 20% (Plassman 2007; Goodman 2017). Studies in the UK have estimated the incidence rate of AD as 1.59/1000 person‐years, whilst the incidence rate of VaD was 0.99 cases/1000 person‐years (Imfeld 2013). The prevalence of VaD among individuals aged 65 years and older was 1.50% in China between 2008 and 2009, while AD was the leading cause of dementia (3.21%) (Jia 2014). Although earlier studies in Japanese populations demonstrated a greater prevalence of VaD than AD, recent studies have shown that the trend has shifted with no changes in VaD prevalence and increases in AD prevalence over time (Ohara 2017).
Treatment
Despite its prevalence and societal impact, there is no definitive, proven pharmacological treatment for VaD. Potential interventions may be considered at a number of levels: primary prevention, secondary prevention, symptomatic treatments, and disease‐modifying or curative approaches (O'Brien 2006). The currently available data on the treatment of vascular risk factors does not prove a benefit in primary and secondary prevention (Moorhouse 2008). Trials of monotherapies such as antiplatelet agent or statins for the prevention or treatment of VaD are also equivocal (McGuinness 2014; O'Brien 2015; Chu 2018). Recent meta‐analysis of longitudinal trials and randomised clinical trials have shown, albeit far from conclusively, that hypertension is a potential risk factor for VaD and that antihypertensive medication use may decrease the risk of VaD (Shah 2009; Ligthart 2010; Chang 2011; Sharp 2011). Although the effects of cardiovascular drugs on VaD per se are unproven, these vascular primary and secondary prevention strategies have proven efficacy in preventing stroke, which will in turn contribute to vascular cognitive decline.
Potential symptomatic treatment for VaD, such as vasodilators, nootropics, antioxidants (O'Brien 2006; Moorhouse 2008; Perez 2012), and some traditional Chinese herbal medicines (Wu 2007; Hao 2009; Xu 2018), have been evaluated in multiple trials and systematic reviews with disappointing results. The effects of drugs used to treat AD are modest when used in people living with VaD (Moorhouse 2008): rivastigmine and galatimine provide only modest benefit of uncertain clinical significance and higher rate of side effects (Craig 2006; Birks 2013).
Description of the intervention
Cerebrolysin is a neuropeptide preparation produced by standardised enzymatic breakdown of porcine brain proteins. It is a mixture of 80% low‐molecular‐weight peptides and 20% free amino acids. Cerebrolysin is said to have potential neurotrophic and neuroprotective properties (Windisch 1998; Álvarez 2006; Álvarez 2011b).
Cerebrolysin is currently used for the treatment of dementia in a number of European and Asian countries. As a potential treatment for dementia, Cerebrolysin is supplied as a solution for injection or infusion, and it is recommended to be given as intravenous infusion once daily. Treatment is usually given in cycles; a typical cycle would be treatment five days a week repeated for four weeks, with two to four treatment cycles per year ( Plosker 2009).
Cerebrolysin has also been reported as having beneficial effects on cognitive and physical function in various other neurological conditions, including stroke (Hong 2005; Ladurner 2005; Gharagozli 2017), AD (Álvarez 2006; Gauthier 2015), and traumatic brain injury (Álvarez 2003; Chen 2013a).
How the intervention might work
Various in vitro and in vivo studies have shown that Cerebrolysin has pharmacodynamic properties similar to those of endogenous neurotrophic factors. For example, it exerts nerve growth factor (NGF)‐like activity on neurons and increases levels of NGF (Ubhi 2013). Various animal models have been established to demonstrate the neurotrophic and pro‐cognitive effects of Cerebrolysin (Veinbergs 2000; Rockenstein 2005; Ubhi 2013).
Pleiotropic properties include promotion of neuroprotection, neuroplasticity, and neurogenesis. Suggested underlying mechanisms for these actions include the following.
(1) Reduced amyloid plaque formation by regulating amyloid precursor protein (APP) (Rockenstein 2006; Zhang 2015).
(2) Inhibition of calpain, caspase‐3, Bcl‐2 and upregulation of Bax, thus protecting neurons from apoptosis and degeneration (Akai 1992; Hartbauer 2001; Xing 2014).
(3) Induction of modifications in dendritic morphology by increasing the dendritic length, density, and spine density, ameliorating dendritic pathology and spine loss, whilst increasing the levels of plasticity‐related synaptic proteins, thereby improving structural synaptic plasticity (Juárez 2011; Alcántara‐González 2012; Vázquez‐Roque 2014; Liu 2017b).
(4) Attenuating the physiological apoptosis of neuroblasts, augmenting the survival of neural progenitor cells, and enhancing neural progenitor cell proliferation and neuronal differentiation, thus stimulating neurogenesis (Tatebayashi 2003; Rockenstein 2007; Zhang 2010; Bornstein 2012; Zhang 2013).
Why it is important to do this review
Cerebrolysin is widely used for the treatment of stroke, traumatic brain injuries, and dementia in Russia, Eastern European countries, China, and other Asian countries. Given the lack of treatments and global prevalence of VaD, if beneficial effects of Cerebrolysin are proven then this will have major implications for global healthcare policy and practice.
Systematic reviews of Cerebrolysin used in other neurological conditions have not reported convincing efficacy. These reviews have also highlighted concerns over adverse effects and the methodological quality of included studies. For example, the Cochrane Review of Cerebrolysin in acute ischaemic stroke reported no clinical benefits and found increased risk of serious adverse events (Ziganshina 2015). Another systematic review suggests that Cerebrolysin may have benefits in in traumatic brain injury, but the validity of the conclusion was weakened by imprecision and methodological flaws of the included studies (El Sayed 2018). The previous Cochrane Review of Cerebrolysin in VaD(Chen 2013b) did not find sufficient evidence to support Cerebrolysin treatment, citing low numbers of trials, heterogeneity between trials, and risk of bias.
As clinical and research interest in VaD is increasing (Burns 2019), it seemed an appropriate time to update a review of the literature on Cerebrolysin and VaD. There were other motivating factors for updating the review. Since the last review, methods for assessing quality of evidence, particularly the use of GRADE methods, have evolved, and an updated review would permit a synthesis that uses these approaches. As Cerebrolysin is mostly used in Eastern countries, an updated review that included Chinese language databases may also yield additional information.
Objectives
Primary: to assess the effect of Cerebrolysin on cognitive function, global function, and all‐cause mortality in people living with vascular dementia.
Secondary: to assess the adverse effects of Cerebrolysin and to assess the effect of Cerebrolysin on quality of life and caregiver burden.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials (RCTs), without any language restrictions.
We included the following potential study designs. (1) Cerebrolysin alone compared with placebo. (2) Cerebrolysin alone compared with no treatment. (3) Cerebrolysin plus another treatment compared with placebo plus the same other treatment. (4) Cerebrolysin plus another treatment compared with the same other treatment alone.
We thus excluded studies where Cerebrolysin was used in both groups as a routine treatment and studies where another treatment was used in only one trial arm.
We excluded non‐RCT study designs, for example non‐randomised concurrent control trials, before‐after studies, and cohort studies.
Types of participants
We included participants of all ages and both sexes with a diagnosis of vascular dementia. To be included, diagnosis should have been made using accepted, validated criteria, such as the DSM, APA 2013, or VaD‐specific criteria such as those proposed by the NINDS‐AIREN (Román 1993).
We included studies involving a broader population of dementia or vascular disease if the data for relevant participants with VaD could be extracted.
We excluded studies of stroke unless the stroke group, or a subgroup, also had a confirmed diagnosis of VaD.
Types of interventions
We included Cerebrolysin given at any dose and for any duration (length of cycle and number of cycles).
Types of outcome measures
We included data from validated, cognitive, and functional assessment scales if possible. In cases where the outcome was evaluated by a less established measure, we searched the paper bibliography and reference texts to identify source information about the properties of the test or scale in question. We used this information to make a group decision as to whether or not to accept the measure as valid.
Primary outcomes
(1) Cognitive function (measured by multidomain tools such as MMSE).
(2) Global function (measured by tools such as Clinician Interview Based Impression of Change (CIBIC)).
(3) All‐cause death.
Secondary outcomes
(1) Adverse events.
(2) Quality of life (measured by tools such as Dementia Quality of Life Questionnaire (DEMQOL)).
(3) Caregiver burden (measured by tools such as the Zarit Burden Interview (ZBI)).
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialized Register, on 9 May 2019.
The search terms used were: Cerebrolysin, Cere, FPF1070, FPF‐1070.
ALOIS is maintained by the Information Specialists for the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy individuals. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Science Information database);
monthly searches of a number of trial registers: ISRCTN, UMIN (Japan's Trial Register), the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (which covers US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings, Index to Theses, Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed in the 'methods used in reviews' section within the editorial information about the Dementia and Cognitive Improvement Group.
In order to ensure that the search for the review was as up‐to‐date and comprehensive as possible, we conducted an additional search including: MEDLINE, Embase, PsycINFO, CINAHL, ISI Web of Knowledge, LILACS, CENTRAL, ClinicalTrials.gov, and the WHO ICTRP. The search strategies used are shown in Appendix 1.
In this updated review, we expanded the search by adding a search of the four most comprehensive and authoritative Chinese databases from 1 January 2012 to 19 May 2019: China National Knowledge Infrastructure, China Science and Technology Journal Database, Wanfang Data Knowledge Service Platform, and China Biology Medicine disc. The review authors translated the original search terms into Chinese.
Searching other resources
We checked the references of all relevant studies to identify additional trials. We also contacted the authors of published trials and known experts in the field to identify any additional published or unpublished data. We approached the medical information department of the relevant companies (EVER Neuro Pharma GmbH; Hebei Zhitong Pharmaceutical Group Co. Ltd) with a request for copies of all materials that described clinical studies with Cerebrolysin.
Data collection and analysis
Selection of studies
Two review authors (SH Cui and N Chen) independently scrutinised the titles and abstracts identified by the search. The two review authors obtained the full text of all studies deemed potentially relevant and independently assessed these against the inclusion criteria. Any disagreements on inclusion were resolved through discussion or by consulting a third review author (L He) to arbitrate as needed.
Data extraction and management
Two review authors (SH Cui and N Chen) independently extracted data from the trials to populate a data extraction form. We included information on: study name, design, participant characteristics, severity of VaD, duration of treatment, dosing of treatment, number of participants at baseline, number of participant withdrawals, participants analysed in the different treatment groups, inclusion and exclusion criteria, and outcomes. We also assessed for funding source. One review author (SH Cui) entered data into Review Manager 2014 (Review Manager 2014), and a second review author (N Chen) checked the data entry.
Assessment of risk of bias in included studies
Two review authors (N Chen and M Yang) independently assessed risk of bias of the included trials using the Cochrane 'Risk of bias' tool for the following domains (Higgins 2017).
• Sequence generation (selection bias)
• Allocation concealment (selection bias)
• Blinding of participants and personnel (performance bias)
• Blinding of outcome assessment (detection bias)
• Incomplete outcome data (attrition bias)
• Selective reporting (reporting bias)
• Any other bias
Any disagreements were resolved by discussion or by consulting a third assessor (L He).
We assigned a summary label of low risk of bias only when all individual domains were assessed as low risk of bias. We assigned a summary label of high risk of bias when any domain was assessed as high risk of bias. An issue with the previous Cerebrolysin review (Chen 2013b) was that poor reporting in the primary studies forced a label of 'unclear' for many of the 'Risk of bias' domains. In this review, where a domain was considered to be unclear even after we had contacted the study authors, we considered it likely that risk of bias was present, and this informed our GRADE assessment.
Measures of treatment effect
We analysed the data using Cochrane Review Manager 5 software. We expressed the results as risk ratio (RR) with 95% confidence intervals (95% CI) for dichotomous outcomes, and mean differences (MD) or standardised mean differences (SMD) with 95% CI for continuous outcomes. If a paper only reported an outcome with accompanying standard error, we used the Review Manager 5 generic inverse variance feature to permit inclusion of the data in summary analyses.
Unit of analysis issues
We considered only participant‐level outcomes. In order to avoid unit of analysis errors resulting from combining results from more than one time point, we used final time point data from each trial. We planned to analyse subgroups based on treatment duration where possible (Deeks 2017). For studies that compared more than two groups, we selected the paired comparison most relevant to the review question or combined intervention groups as appropriate.
Dealing with missing data
For all included studies, we extracted and presented numbers of participants lost to follow‐up, with reasons, in Characteristics of included studies. Where these data were not clear in the published paper, we requested the information from the study authors. We analysed data on an intention‐to‐treat (ITT) basis, reporting any imputation methods used in the primary studies (Characteristics of included studies). In the case of missing data for non‐completers, the study authors used 'last observation carried forward' data, which we included in our analyses of treatment outcomes.
Assessment of heterogeneity
We assessed statistical heterogeneity between trials using the Chi2 test with a 10% level of statistical significance (P < 0.1) and I2 > 50% (Higgins 2002; Higgins 2003). If significant heterogeneity was present, we performed a cause analysis, and then undertook subgroup and sensitivity analyses. We also planned to explore possible causes of heterogeneity by conducting meta‐regression analysis where applicable.
Assessment of reporting biases
We planned to use a funnel plot to investigate the possibility of publication bias if necessary, and then to evaluate and express the possible reporting biases. We would only perform tests for funnel plot asymmetry if more than 10 studies were included, which is thought to to give sufficient power to distinguish chance from real asymmetry (Sterne 2011).
Data synthesis
We used standard Cochrane methods to create forest plots and perform meta‐analysis. We used fixed‐effect models for analyses with homogeneity. When we considered that there was important clinical heterogeneity between studies, or when the quantitative measure of heterogeneity was greater than 50%, we used a random‐effects model.
For trials that were too clinically heterogeneous or presented insufficient information for pooling, we performed a descriptive analysis (Deeks 2017).
Subgroup analysis and investigation of heterogeneity
Where possible, we carried out subgroup analyses to explore potential sources of heterogeneity.
Sensitivity analysis
We undertook a sensitivity analysis on the basis of methodological quality, omitting those trials with unclear or high risk of bias.
Presentation of results and 'Summary of findings' tables
Within our cognition outcome, we were able to present data on change in scores from multidomain cognitive assessment tools and on domain‐specific tests.
Within our general function outcome, we were able to describe data as percentage change in functional status.
We used the five GRADE considerations (study limitations, inconsistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence (studies that contribute data for the prespecified outcomes) (Ryan 2016). We downgraded the evidence from 'high quality' by one level when one of these factors was present to a serious degree, and two levels if very serious. As only RCTs were eligible for inclusion in the review, reasons for upgrading (large effect, dose response, and plausible confounding factors) were not applicable. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017; Schünemann 2017). We used GRADEpro GDT(GRADEpro GDT). We justified all decisions to downgrade the quality of the evidence using footnotes and made comments to aid reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 39 potentially eligible records in this update. We excluded 33 references for 32 trials after full‐text screening. We incorporated 17 of these excluded studies into this update review. Six trials fulfilled our inclusion criteria. We identified no new studies for inclusion in this update. We attempted to contact authors of the included trials, but have received no response. A flow chart illustrating study selection is shown in Figure 1.
1.

Study flow diagram.
Included studies
We included six trials with a total of 597 participants in this updated review (Vereschagin 1991; Xiao 1999; Liang 2001; Zhang 2003; Muresanu 2008a; Guekht 2011).
All included studies were RCTs. Two were multicentre trials conducted in Russia, Guekht 2011, and China, Xiao 1999, while the others were a single‐centre trials. Sample sizes differed, ranging from 29 to 242 participants (each multicentre study had more than 100 participants).
Inclusion and exclusion criteria were explicitly stated in all studies, except the Vereschagin trial (Vereschagin 1991). Despite our attempts we could not obtain full details of this trial. Common exclusion criteria across four trials included: patients with significant concomitant neurological or psychiatric disorders; any significant systemic illness or unstable medical condition; and patients who had recently received other drugs that might impact on cognitive function (Xiao 1999; Zhang 2003; Muresanu 2008a; Guekht 2011). Relevant information of patients' exclusion was not available for the other two trials (Vereschagin 1991; Liang 2001).
Two trials recruited patients with a confirmed diagnosis of VaD (both probable and possible VaD, Guekht 2011) or probable VaD, Muresanu 2008a, according to the NINDS‐AIREN criteria (Román 1993); two trials, Xiao 1999; Zhang 2003, enrolled VaD patients using DSM‐IV criteria (APA 1994); and the other trial (Liang 2001)included patients with multi‐infarct dementia based on DSM‐III‐R, APA 1987, and ICD‐10, WHO 1992, criteria. All trials confirmed the VaD diagnosis using computed tomography (CT) or magnetic resonance imaging (MRI) neuroimaging scored according to the validated Hachinski ischaemic score, Hachinski 1975, or a modified version of this neuroimaging scale, Pantoni 1993. Relevant information of VaD' diagnosis was not available for the Vereschagin trial (Vereschagin 1991). Four trials required that baseline dementia severity be mild to moderate, as assessed using either MMSE, Xiao 1999; Muresanu 2008a; Guekht 2011, or Clinical Dementia Rating scores, Zhang 2003, but the range of eligible MMSE scores was not uniform (10 to 24 in Guekht 2011; 9 to 26 in Muresanu 2008a; and 15 to 25 in Xiao 1999). The remaining two trials did not report severity of VaD. The mean age of participants in each study ranged from 60.4 to 70.8 years. Three trials described mean duration of dementia (Liang 2001; Zhang 2003; Muresanu 2008a), which ranged from 4.6 months to 4.3 years.
In all the included trials, Cerebrolysin was administered as an intravenous infusion, diluted in physiologic saline or glucose injection. Four trials described comparing Cerebrolysin with placebo (Vereschagin 1991; Xiao 1999; Muresanu 2008a; Guekht 2011), whilst the other two trials compared Cerebrolysin with routine treatment. Routine treatments used in the trials differed and were not standard treatments recommended in international guidelines (Liang 2001 used mannitol, energy mixture, and Naomingjing; Zhang 2003 used intravenous Xuesaitong). The dose of Cerebrolysin ranged from 10 mL to 30 mL daily. The length of treatment also varied amongst trials. Three studies administered the intervention five days a week and repeated for four continuous weeks as one cycle. Using this approach, two studies treated participants for one cycle only (Xiao 1999; Muresanu 2008a), whilst one study repeated two cycles with an interval of eight weeks between cycles (Guekht 2011). Two trials administered Cerebrolysin continuously for 15 days, Liang 2001, and 28 days, Vereschagin 1991. The remaining trial had the longest period of treatment with Cerebrolysin (Zhang 2003), administering in cycles of 10 days, repeated for two cycles per year over three years. One study had three comparison arms (Cerebrolysin 10 mL versus 30 mL versus physiologic saline) (Muresanu 2008a).
The duration of follow‐up varied from 15 days to 3 years after enrolment. All six trials reported losses to follow‐up and withdrawals. Only one trial performed an ITT analysis based on the 'last observation carried forward' method (Guekht 2011).
The most common outcome included in trials was change of cognitive function from baseline, and some form of cognitive assessment was reported in all the included trials. The scales used to quantify this change differed between trials, including MMSE, Alzheimer's Disease Assessment Scale Cognitive Subpart, Extended Version (ADAS‐cog+), Hasegawa Dementia Scale (HDS), and Wechsler Adult Intelligence Scale (WAIS). In addition to multidomain tests, single‐domain tests (Trail‐Making Test A) and short screening tests (Clock‐Drawing Test) were also included in the trial outcomes. Three studies reported participant global function before and after treatment (Vereschagin 1991; Xiao 1999; Guekht 2011), evaluated by Clinician's Interview‐Based Impression of Change plus Caregiver Input (CIBIC+), Clinician's Interview‐Based Impression of Severity (CIBIS+), Activities of Daily Living (ADL), Investigator's Clinical Global Impression (CGI), Sandoz Clinical Assessment ‐ Geriatric (SCAG), or Nuremberg Activities Inventory (NAI). Four included trials reported adverse events associated with Cerebrolysin in detail (Xiao 1999; Zhang 2003; Muresanu 2008a; Guekht 2011), whilst the remaining two studies provided no relevant information (Vereschagin 1991; Liang 2001). None of the trials described data on quality of life or caregiver burden.
Three trials were supported by the pharmaceutical industry (EVER Neuro Pharma GmbH) (Xiao 1999; Muresanu 2008a; Guekht 2011). One of these studies was simultaneously funded by the Society for the Study of Neuroprotection and Neuroplasticity (Romania) (Muresanu 2008a). The funding of one trial was unclear (Zhang 2003), although we know that the Cerebrolysin used in the trial was produced by EVER Neuro Pharma GmbH. We were unable to obtain funding information from the published paper or authors of the other two trials (Vereschagin 1991; Liang 2001)
Excluded studies
We excluded 32 potentially relevant studies for one or more reasons as follows: not an RCT according to the full text (Meng 2001; Damulin 2008; Tapu 2009; Suvorova 2010; Shprakh 2011; Gavrilova 2017); validated diagnostic criteria not used to identify patients with VaD (Zheng 1999; Cao 2000; Vereschagin 2001; Damulin 2008; Han 2015; Gavrilova 2017); another treatment was used in the control group but not the intervention group (Jia 1991; Li 1996; Wang 1996; Pan 1999; Wang 2003; Chen 2006; Dai 2011; Alkebaiker 2014; Liu 2015; Wang 2015; Wang 2016; Xue 2016; Wang 2018); or used in the intervention group but not the control group (Hao 2015; Xu 2016; Wang 2017); Cerebrolysin was used in both groups as a routine treatment (Xian 2004; Chen 2014; Zhao 2015; Liu 2017a; Zhao 2017); study only involved a subset of participants with VaD, and the data for relevant participants could not be extracted (Li 2014); all outcomes were evaluated with invalidated scales (Meng 2001).
Risk of bias in included studies
According to the summary assessment for risk of bias (Higgins 2017), we rated one of the trials as low risk of bias (Guekht 2011); four as unclear risk of bias (Vereschagin 1991; Xiao 1999; Zhang 2003; Muresanu 2008a); and one as high risk of bias (Liang 2001) (see Characteristics of included studies; Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included trials stated they allocated participants to each study group randomly, but only two of them provided information about the random sequence generation (Zhang 2003; Guekht 2011): Guekht 2011 used computer software to generate randomisation codes, whilst Zhang 2003 allocated participants by drawing lots. The Guekht study also specified that the random allocation sequence was concealed throughout the trial, using sealed, sequentially numbered, identical cardboard boxes containing blinded study medication according to the allocation sequence (Guekht 2011). Descriptions in the reports of the other trials were insufficient to permit a judgement as to whether allocation concealment was adequate (Vereschagin 1991; Xiao 1999; Liang 2001; Zhang 2003; Muresanu 2008a).
Blinding
Five trials used a double‐blind design (Vereschagin 1991; Xiao 1999; Zhang 2003; Muresanu 2008a; Guekht 2011), of which three clearly stated that all participants and personnel involved in therapy performance and outcome assessment were blinded to treatment assignment during the entire study period (Zhang 2003; Muresanu 2008a; Guekht 2011). The Xiao study (Xiao 1999), reported to be double‐blind, used yellow opaque infusion bottles containing Cerebrolysin or placebo to secure blinding, which would indicate that the participants and personnel involved in treatment performance might be blinded to participant assignment, but whether the efficacy evaluators were also blinded could not be judged according to the reports. Vereschagin 1991 referred to blinding method only as "double‐blind", with no other relevant information available as to who was blinded. The remaining trial was an unblinded study according to the full text (Liang 2001): Cerebrolysin was an additional medication to routine treatment for ischaemic stroke in the intervention group, whereas the control group was treated with routine treatment only without any placebo or blank solution, which made it impossible to perform blinding.
Incomplete outcome data
All included studies reported the duration of follow‐up. Follow‐up varied substantially between trials. Three studies followed up participants less than one month after starting treatment (15 days in Liang 2001 and 4 weeks in Vereschagin 1991 and Xiao 1999). There were no dropouts in the first two studies, whilst one participant withdrew in the third trial because of refusal of treatment (data were not included in the analyses) (Xiao 1999). We found two reports of results in different phases of the Muresanu 2008a trial: the pilot phase evaluated the effects of Cerebrolysin after a four‐week treatment, when there had been no participant withdrawal (Muresanu 2008b); the extension study investigated efficacy outcomes through 12 weeks after the end of treatment, but eight participants (three and four in the two Cerebrolysin groups, respectively, and one in the placebo group) were lost to the follow‐up visit, with balanced numbers and reasons between groups. Data on the remaining 33 participants were assessed for the paper. The Guekht 2011 trial, with the largest sample size (242 participants in total), lost 25 participants during the 24‐week study, with similar numbers and reasons for discontinuation in the two groups. Those withdrawn were included in ITT analyses based on the 'last observation carried forward' data. Participants included in the ITT analyses had to have received at least one dose of study medication and have baseline plus at least one postbaseline assessment for both primary efficacy measures. As a result, four and six enrolled participants in each group were excluded from analysis. Although two participants were withdrawn because of adverse events, we judged the risk of attrition bias due to incomplete outcome data to be low. The sixth trial (Zhang 2003), with only 29 participants in a single centre, reported no attrition over three years.
Selective reporting
The study protocol was available for only one included trial (Guekht 2011), on ClinicalTrials.gov. All of the study's prespecified (both primary and secondary) outcome measures that were of interest in the current review were reported as per protocol, resulting in a judgement of low risk of bias due to selective reporting. We judged one trial as at high risk of bias for this domain (Liang 2001), as it did not mention the results for safety evaluation, which would be expected to have been reported as a key outcome for such an intervention study. We assessed the remaining four trials as at unclear risk of bias for this domain because protocols were unavailable and insufficient information could be obtained to make a definitive judgement(Vereschagin 1991; Xiao 1999; Zhang 2003; Muresanu 2008a).
Other potential sources of bias
Another potential risk of performance bias might come from a relaxed design of the intervention in the Liang 2001 study. Participants in both groups were treated with other medicine according to their condition, but which drugs were used was not recorded. Whether these drugs were balanced between groups or would impact the results was not specified in the article. We found no other potential bias in four trials (Xiao 1999; Zhang 2003; Muresanu 2008a; Guekht 2011), and insufficient information could be obtained from the remaining trial (Vereschagin 1991).
Effects of interventions
See: Table 1
Primary outcomes
Cognitive function
General cognitive function was evaluated as a primary efficacy parameter in all the included trials, but the instruments used and the type of data varied between trials.
For the overall evaluation of the effect of Cerebrolysin on cognitive function, validated tools of cognitive function included MMSE, ADAS‐cog+, and WAIS. Three studies reported response rates or partial data from scales that were not detailed enough to permit inclusion in meta‐analysis (Vereschagin 1991; Liang 2001; Zhang 2003). We thus included 3 studies involving 420 participants, of which 223 received Cerebrolysin (Xiao 1999; Muresanu 2008a; Guekht 2011). Where more than one measure of cognitive function was used in a study, data from the most common or the most extensive measure (in all cases MMSE) were included in the meta‐analysis.
The meta‐analysis suggested a beneficial effect of Cerebrolysin (standardised mean difference 0.36, 95% confidence interval (CI) 0.13 to 0.58; P = 0.002; Analysis 1.1; Figure 3). We assessed the quality of the evidence as very low due to concerns related to imprecision, indirectness, and serious risk of bias (Table 1).
1.1. Analysis.
Comparison 1 Cognitive function, Outcome 1 Change of cognitive function measured by MMSE or ADAS‐cog+.
3.

Forest plot of comparison: 1 Cognitive function, outcome: 1.1 Change of cognitive function measured by MMSE or ADAS‐cog+.
Two trials with 379 participants evaluated the effect of Cerebrolysin on general cognitive function measured by the MMSE score change from baseline (Xiao 1999; Guekht 2011). The Xiao 1999 trial reported means but not standard deviations for change from baseline. However, the study also reported the P value (0.028) for the comparison between groups, and so we calculated the missing change‐from‐baseline standard deviation using the method described in Section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The Zhang 2003 study also used the MMSE, but only reported response rates and not raw or change scores, and so could not be included in the meta‐analysis. Previous studies reported a change in MMSE test scores of at least two to four points as indicating a reliable change at the 90% confidence level (Hensel 2007; Stein 2012). Smaller changes may not be reliable or clinically meaningful.
The meta‐analysis suggested a beneficial effect of Cerebrolysin on general cognitive function measured with the MMSE (mean difference (MD) 1.10, 95% CI 0.37 to 1.82; P = 0.003; Analysis 1.2; Figure 4). We assessed the quality of the evidence as very low due to imprecision, indirectness, and serious risk of bias.
1.2. Analysis.
Comparison 1 Cognitive function, Outcome 2 Change of general cognitive function measured by MMSE.
4.
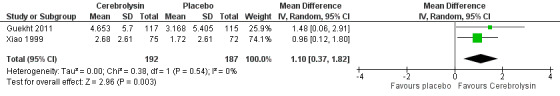
Forest plot of comparison: 1 Cognitive function, outcome: 1.2 Change of general cognitive function measured by MMSE.
The effect on global cognitive function was also evaluated by the change of ADAS‐cog+ scores in two trials with 273 participants (Muresanu 2008a; Guekht 2011). ADAS‐cog+ is more sensitive in mild cognitive impairment than the original ADAS‐cog version, since it covers more cognitive domains (visual attention, executive function, and delayed recall). Impairments in these domains may be particularly relevant in people with vascular cognitive impairment (Mohs 1997). For the Muresanu 2008a study, we only included data from the four‐week pilot study, although relevant outcomes were measured through a 12‐week extension, because the extension was open‐label, and nearly 20% of participants (8/41 in total; 3/16, 4/15, and 1/10 from the 10 mL Cerebrolysin, 30 mL Cerebrolysin, and placebo groups, respectively) were lost to follow‐up. We combined the two experimental intervention groups treated with different doses of Cerebrolysin into a single group using the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Past studies have considered a 3‐ to 4‐point change on the ADAS‐cog to be clinically relevant (Molnar 2009; Schrag 2012).
The meta‐analysis suggested a beneficial effect of Cerebrolysin (MD −4.21, 95% CI −7.87 to −0.56; P = 0.02; Analysis 1.3; Figure 5). We judged the quality of the evidence to be very low due to imprecision and serious risk of bias.
1.3. Analysis.
Comparison 1 Cognitive function, Outcome 3 Change of general cognitive function measured by ADAS‐cog+ score.
5.
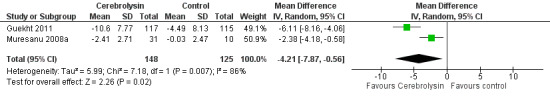
Forest plot of comparison: 1 Cognitive function, outcome: 1.3 Change of general cognitive function measured by ADAS‐cog+ score.
The authors of the Zhang 2003 trial reported WAIS scores at the end of three‐year follow‐up. Superiority of Cerebrolysin to control was reported in most of the verbal and performance subtests, including information, comprehension, arithmetic, similarities, digit span, vocabulary, digit symbol, picture completion, and picture arrangement (all P < 0.05), but the full intelligence quotient (IQ) was not given or compared between groups (Zhang 2003).
In addition to continuous data, some trials presented dichotomised data describing the number of responders at a certain threshold score. For studies using the ADAS‐cog+, responders were defined as those with at least a 4‐point improvement from baseline to the end of follow‐up (Guekht 2011), and for the HDS and MMSE, responders were those with at least a 2‐point increase compared to baseline (Liang 2001; Zhang 2003). We pooled data on response rates from these three trials with a total of 338 participants (Liang 2001; Zhang 2003; Guekht 2011). The meta‐analysis suggested a benefit of Cerebrolysin (risk ratio (RR) 1.46, 95% CI 1.10 to 1.94; P = 0.008; Analysis 1.4; Figure 6). We assessed the quality of the evidence to be very low due to concerns related to imprecision, indirectness, and serious risk of bias (Table 1).
1.4. Analysis.
Comparison 1 Cognitive function, Outcome 4 Improvement of general cognitive function reported as responder rates.
6.

Forest plot of comparison: 1 Cognitive function, outcome: 1.4 Improvement of general cognitive function reported as responder rates.
Two included trials evaluated executive function, one of the specific cognitive functions commonly impaired in VaD, using the Trail‐Making Test (Xiao 1999; Guekht 2011). Meta‐analysis suggested a beneficial effect of Cerebrolysin on time taken to complete the test (MD −24.75 s, 95% CI −40.46 to −9.03 s; P = 0.002; Analysis 1.5; Figure 7). We judged the quality of the evidence to be very low due to concerns related to imprecision, indirectness, and serious risk of bias.
1.5. Analysis.
Comparison 1 Cognitive function, Outcome 5 Change of executive function measured by the Trail‐Making Test.
7.

Forest plot of comparison: 1 Cognitive function, outcome: 1.5 Change of executive function measured by the Trail‐Making Test.
One trial used the Clock‐Drawing Test (Guekht 2011). The authors reported a beneficial effect of Cerebrolysin of 0.92 points (95% CI 0.45 to 1.39; P<0.001). We judged the quality of the evidence to be low due to concerns related to imprecision and indirectness.
The remaining trial stated that Cerebrolysin treatment resulted in significant improvements in memory, abstract thinking, and reaction time, confirmed by EEG‐mapping, but detailed data were unavailable for any outcomes to permit inclusion in meta‐analysis (Vereschagin 1991).
Global function
Improvement in global function was reported as response rates in two trials: in one trial investigators used the CIBIC+ scale (Guekht 2011), and CGI was used in the other trial (Xiao 1999). For pooled analysis, we defined responders as those with a CIBIC+ score of < 3 or those judged as at least moderately improved using the CGI rating at the last visit. The meta‐analysis suggested a benefit of Cerebrolysin (RR 2.69, 95% CI 1.82 to 3.98; P<0.001; Analysis 2.1; Figure 8). We assessed the quality of the evidence as very low due to concerns related to imprecision, indirectness, and serious risk of bias. (Table 1)
2.1. Analysis.
Comparison 2 Global function, Outcome 1 Improvement of global function reported as responder rates.
8.
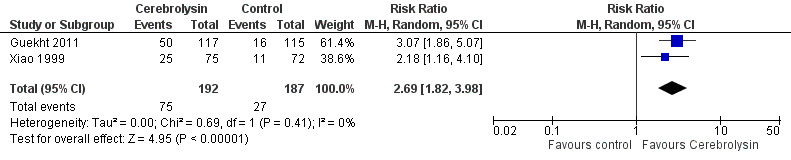
Forest plot of comparison: 2 Global function, outcome: 2.1 Improvement of global function reported as responder rates.
Change in global functional performance was measured by various scales across two included trials (Xiao 1999; Guekht 2011). The scales measured different constructs and were not considered suitable for meta‐analysis. In one trial, there was a beneficial effect of Cerebrolysin on ADL measures (MD 6.32, 95% CI 4.20 to 8.45; P<0.001; Analysis 2.2; Figure 9) (Guekht 2011). We judged the quality of the evidence to be low due to imprecision and indirectness. The other study used various scales and found no effect on global function for any measure (ADL: MD 0.77, 95% CI −0.93 to 2.47, P = 0.38; NAI: MD −0.75, 95% CI −2.33 to 0.83, P = 0.35; SCAG: MD −0.45, 95% CI −3.42 to 2.52, P = 0.77) (Xiao 1999). We judged the quality of the evidence to be very low due to concerns related to imprecision, indirectness, and serious risk of bias.
2.2. Analysis.
Comparison 2 Global function, Outcome 2 Change of global function measured by ADCS‐ADL score.
9.

Forest plot of comparison: 2 Global function, outcome: 2.2 Change of global function measured by ADCS‐ADL score.
All‐cause death
Only one trial reported mortality and specified that no deaths were reported during the experimental and follow‐up periods (Guekht 2011). No specific data on all‐cause mortality were available from the other included trials, although three trials reported no severe adverse effects in either group (Xiao 1999; Zhang 2003; Muresanu 2008a). We judged the quality of the evidence to be very low due to concerns related to imprecision, indirectness, and serious risk of bias.
Secondary outcomes
Adverse events
Adverse events were categorised as 'serious' or 'not serious'. Serious adverse events were those that led to death; were life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability; or was any important medical event that might have jeopardised the participant or required intervention to prevent it. All other adverse events were considered to be non‐serious (ICHEWG 1997).
Four included trials assessed safety by a mix of reporting adverse events, performing physical examinations or laboratory tests (Xiao 1999; Zhang 2003; Muresanu 2008a; Guekht 2011). The relationship between adverse events and Cerebrolysin therapy was judged by investigators. The remaining two trials did not mention adverse events (Vereschagin 1991; Liang 2001).
Three serious adverse events occurred during the Guekht 2011 trial: acute pyelonephritis, malignant lung neoplasm, and rectosigmoid cancer (3/117 and 0/115 in the Cerebrolysin and placebo groups, respectively). The adverse events were considered to be unrelated to the experimental treatment, and there was no significant difference between groups (RR 6.88, 95% CI 0.36 to 131.75; P = 0.20; Analysis 3.1; Figure 10). We assessed the quality of the evidence to be low due to concerns related to imprecision and indirectness. Three included trials reported no severe adverse effects in either group (Xiao 1999; Zhang 2003; Muresanu 2008a). We judged the quality of the evidence to be very low due to concerns related to imprecision, indirectness, and serious risk of bias.
3.1. Analysis.
Comparison 3 Adverse events, Outcome 1 Serious adverse events.
10.

Forest plot of comparison: 3 Adverse events, outcome: 3.1 Serious adverse events.
The most commonly reported non‐serious adverse events were headache, asthenia, dizziness, hypertension, and hypotension. Two trials provided sufficient data on adverse events by treatment arm to permit meta‐analysis (Xiao 1999; Guekht 2011). The meta‐analysis suggested no difference between groups in rates of adverse events (RR 0.91, 95% CI 0.29 to 2.85; P = 0.87; Analysis 3.2; Figure 11). We judged the quality of the evidence as very low due to concerns related to imprecision, indirectness, and serious risk of bias (Table 1). The Guekht 2011 trial also reported withdrawals due to adverse events, which were balanced between groups (1.7% for the Cerebrolysin group and 2.5% for the control group, P = 0.67) (Analysis 3.3). The Muresanu 2008a trial only stated that no severe adverse events had occurred, and no significant differences in non‐serious adverse events were found between groups, but relevant quantitative data were unavailable. Zhang and colleagues specified that no non‐serious adverse events were found during the three‐year experimental period (Zhang 2003).
3.2. Analysis.
Comparison 3 Adverse events, Outcome 2 Non‐serious adverse events.
11.

Forest plot of comparison: 4 Adverse events, outcome: 4.1 Non‐serious adverse events.
3.3. Analysis.
Comparison 3 Adverse events, Outcome 3 Withdrawals due to adverse events.
The Xiao 1999 and Guekht 2011 trials also reported the change in laboratory parameters, including haematology, clinical chemistry, urinalysis, and electrocardiogram, and revealed no relevant differences between groups.
Quality of life
No data on quality of life during the treatment and follow‐up periods were available from any of the included trials (Table 1).
Caregiver burden
No data on evaluation of caregiver burden were available from any of the included trials (Table 1).
Subgroup analyses
Significant statistical heterogeneity was present in the analysis of change in ADAS‐cog+ score (Analysis 1.3, I2 = 86%) and the analysis of rates of non‐serious adverse events (Analysis 3.2, I2 = 59%). Treatment duration differed between these and other studies, which could plausibly explain differential results. We were not able to perform a meta‐regression of treatment effect against the duration of intervention as the number of included trials was too small. We therefore undertook subgroup analyses defining categories of short‐ and longer‐term treatment duration.
Short‐term treatment duration (defined as only one treatment cycle or four weeks or less of treatment)
In four included trials, Cerebrolysin was given to participants for only one treatment cycle (five days a week repeated for four weeks), Xiao 1999; Muresanu 2008a, or four continuous weeks or less, Vereschagin 1991; Liang 2001. There was a beneficial effect of short‐term treatment with Cerebrolysin on general cognitive function measured by MMSE score in one study (MD 0.96, 95% CI 0.12 to 1.80; P = 0.03; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.1) and ADAS‐cog+ in one study (MD −2.38, 95% CI −4.18 to −0.58; P = 0.01; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.2). However, a continuous 15‐day treatment with Cerebrolysin had no effect on the response rate as measured by the HDS (RR 1.22, 95% CI 1.00 to 1.50; P = 0.05; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.3). In one study, Cerebrolysin had a beneficial effect on CGI after one cycle of treatment (RR 2.18, 95% CI 1.16 to 4.10; P = 0.02; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.4). The same study reported non‐serious side effects and found no difference between groups (RR 0.48, 95% CI 0.15 to 1.52; P = 0.21; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.5).
4.1. Analysis.
Comparison 4 Subgroup analyses , Outcome 1 Change of general cognitive function measured by MMSE.
4.2. Analysis.
Comparison 4 Subgroup analyses , Outcome 2 Change of general cognitive function measured by ADAS‐cog+ score.
4.3. Analysis.
Comparison 4 Subgroup analyses , Outcome 3 Improvement of general cognitive function reported as responder rates.
4.4. Analysis.
Comparison 4 Subgroup analyses , Outcome 4 Improvement of global function reported as responder rates.
4.5. Analysis.
Comparison 4 Subgroup analyses , Outcome 5 Non‐serious adverse events.
Longer treatment duration (defined as two or more treatment cycles or more than four weeks treatment)
Two trials had a longer treatment duration of 24 weeks, Guekht 2011, and three years, Zhang 2003. Cerebrolysin had a beneficial effect on MMSE (MD 1.48, 95% CI 0.06 to 2.91; P = 0.04; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.1), and a beneficial effect on ADAS‐cog+ (MD −6.11, 95% CI −8.16 to −4.06; P < 0.001; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.2) and responder rates (RR 1.66, 95% CI 1.23 to 2.24; P = 0.001; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.3). Two cycles of treatment with Cerebrolysin over 24 weeks also had a beneficial effect on clinically assessed global function (RR 3.07, 95% CI 1.86 to 5.07; P < 0.001; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.4). Longer‐term treatment resulted in no difference in the occurrence of non‐serious adverse events (RR 1.54, 95% CI 0.62 to 3.84; P = 0.35; very low‐quality evidence due to concerns related to imprecision, indirectness, and serious risk of bias; Analysis 4.5).
Sensitivity analysis
We undertook sensitivity analyses based on 'Risk of bias' assessment, limiting to those studies at low risk of bias. Since only one included study was rated as at low risk of bias (Guekht 2011), we repeated our analyses limited to data from this study. The overall results were unchanged (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6).
5.1. Analysis.
Comparison 5 Sensitivity analyses, Outcome 1 Change of general cognitive function measured by MMSE.
5.2. Analysis.
Comparison 5 Sensitivity analyses, Outcome 2 Change of general cognitive function measured by ADAS‐cog+ score.
5.3. Analysis.
Comparison 5 Sensitivity analyses, Outcome 3 Improvement of general cognitive function reported as responder rates.
5.4. Analysis.
Comparison 5 Sensitivity analyses, Outcome 4 Change of executive function measured by the Trail‐Making Test.
5.5. Analysis.
Comparison 5 Sensitivity analyses, Outcome 5 Improvement of global function reported as responder rates.
5.6. Analysis.
Comparison 5 Sensitivity analyses, Outcome 6 Non‐serious adverse events.
Discussion
Summary of main results
We found six eligible and relevant RCTs evaluating the efficacy of Cerebrolysin for VaD (Vereschagin 1991; Xiao 1999; Liang 2001; Zhang 2003; Muresanu 2008a; Guekht 2011), with five trials providing full data suitable for meta‐analysis (Xiao 1999; Liang 2001; Zhang 2003; Muresanu 2008a; Guekht 2011). No new studies were eligible for inclusion for this update.
Based on our summary data, Cerebrolysin may improve cognitive and general function with few adverse effects in people living with VaD. Beneficial effects on cognitive function were consistent across various assessment scales and various methods of analysing these data. Benefits were seen in both global cognitive function and in specific aspects of cognition said to be more impaired in VaD, namely executive function. However, there were substantial limitations in the published studies, and these data are not definitive evidence of efficacy or safety.
The summary effect sizes reported were often smaller than what is considered the minimal important clinical difference for the relevant scale. This may relate in part to the relatively short length of follow‐up across most of the studies. If the effect of Cerebrolysin is to slow the cognitive decline of VaD, it would be surprising to see clinically important differences in cognitive scores with studies that follow‐up participants for weeks only.
There were substantial differences in terms of dosage, frequency, and duration of administration of Cerebrolysin. We were able to perform a subgroup analysis that suggested increased benefit with longer duration of therapy. This result is not based on direct comparisons of differing treatment regimens and so must be interpreted with caution. Our review does not permit a recommendation for the optimum treatment schedule.
There is a degree of treatment burden associated with Cerebrolysin administration. Treatment requires frequent intravenous infusions in the hospital setting. We thought that assessing quality of life of both people living with vascular dementia and their caregivers would be important, but no studies reported these outcomes.
Overall completeness and applicability of evidence
Most included studies had data relevant to our prespecified outcomes of interest. However, reporting was often poor, and we could not always use these data in summary analyses. There were no data on quality of life despite the importance of this outcome to people living with dementia and their caregivers.
The study participants were representative of a population of adults with mild to moderate VaD. Ages were younger than would be seen in a contemporary, unselected vascular dementia cohort. Various pathologies were included under the vascular dementia rubric. Data were insufficient to draw any conclusions about differential effect based on subtype of VaD.
The studies were mostly from Eastern Europe and China, and some of the studies were rather old. Dementia care differs across international settings, and some of the treatments used as routine in the included studies are not recommended in European or North American guidelines. Best practice in dementia care has also changed over time, and the generalisability of studies that are decades old is also questionable.
Quality of the evidence
We assessed only one study as at low risk of bias. Issues common to the other included studies, such as lack of a published protocol, may reflect the historical nature of these trials. A registered protocol would now be mandatory for any published RCT of an interventional medicinal product. Reporting of study conduct was poor, and we had to assess several domains as unclear risk of bias. Again, the included studies pre‐date the era of mandatory use of reporting guidelines and checklists. Such reporting omissions should now be less frequent. Where details were not reported, such as for randomisation or allocation concealment, it seems likely that these aspects of good trial practice were not adhered to and that an overall assessment of high risk of bias could be made.
Applying the GRADE framework, we assessed the strength of evidence as low or very low. Issues related to risk of bias were common across the studies and represented a serious threat to the validity of the results. The dementia care provided as comparator was not always consistent with contemporary, guideline‐based best care, and so there were issues with indirectness. Sample sizes were modest, with corresponding wide confidence intervals suggesting lack of precision. The small number of studies did not permit us to assess publication bias detailed in our protocol. With a review of a commonly used intervention, where there are few studies and all are positive despite small numbers, the potential for publication bias remains.
Potential biases in the review process
We aimed for a comprehensive search, including published and unpublished studies. Recognising the international use of Cerebrolysin, we applied no language restrictions; searched Chinese language databases; and contacted investigators and industry as required to obtain additional information. However, we were unable to obtain further data from the authors or medical companies, and it remains possible that important studies were missed.
The small number of studies did not permit many of our planned subgroup and sensitivity analyses, and questions around patient selection and optimal treatment schedule remain.
Agreements and disagreements with other studies or reviews
Our review provides a summary of the clinical evidence regarding Cerebrolysin in VaD. A number of non‐systematic reviews describing Cerebrolysin in VaD are available (Plosker 2009; Plosker 2010; Allegri 2012). Most have concluded that Cerebrolysin appears to be a well‐tolerated and effective treatment option for VaD. Given our comprehensive and systematic literature search and robust quality assessment, we believe our review is a less biased synthesis of the clinical data than these other narrative reviews.
There are many pre‐clinical studies suggesting efficacy of Cerebrolysin in animal models of VaD (Solis‐Gaspar 2016; Liu 2017b). However, translating pre‐clinical benefits to clinical dementia trials is often difficult (IOM 2013; Banik 2015), and these data must be interpreted with caution.
Our interest was direct clinical outcomes. There are studies that have used surrogate outcomes and found a beneficial effect of Cerebrolysin in VaD. For example, electroencephalogram (EEG) has been suggested as a potential outcome marker in VaD (Renna 2003). Trials have demonstrated patterns of EEG activity that suggest beneficial effects of Cerebrolysin in VaD (Muresanu 2008a; Muresanu 2010). Again, whilst encouraging, these surrogate biomarkers are not sufficient to prove the benefits of a dementia treatment.
Data from other studies and reviews have suggested positive clinical effects and good tolerability of Cerebrolysin in the treatment of AD (Wei 2007; Álvarez 2011a; Álvarez 2011b), albeit with many of the limitations in the quality of evidence reported in this review. The distinction between AD and VaD is not always clear‐cut, and in older adults mixed pathologies are frequent (Chui 2015). If Cerebrolysin can be shown to provide benefit in AD, an effect in VaD would seem likely.
Authors' conclusions
Implications for practice.
The results of our review are encouraging, but we suspect that the strength of evidence will be insufficient to change practice in those countries where Cerebrolysin is not routinely used. Equally, whilst we have voiced some concerns about the quality of the evidence, our results are probably not sufficient to change practice in those centres that are already convinced about the clinical benefits of Cerebrolysin.
Although the available evidence suggests that Cerebrolysin is not associated with serious adverse events, there is a substantial burden and cost for a treatment that is given by frequent intravenous infusion. Information was insufficient to make informed decisions about the economics of this therapy. At the population level, we have no effective treatments for vascular dementia, and any treatment that reduces cognitive decline may have important effects. However, at the level of the individual patient, treatment effects may be modest at best and may not seem worthwhile.
Implications for research.
There remains equipoise regarding the use of Cerebrolysin in vascular dementia. We found no records of ongoing trials of Cerebrolysin in an exclusive vascular dementia population. Our review demonstrates the need for a new, well‐designed, randomised, placebo‐controlled trial to evaluate the efficacy, safety, and economics of Cerebrolysin in individuals with vascular dementia.
Based on this review, we can offer some guidance for a future Cerebrolysin randomised controlled trial. Future studies must:
follow the CONSORT statement for reporting of randomised controlled trials (Schulz 2010);
follow a prespecified design as described in a protocol that is publicly accessible;
employ a study design with sufficiently large sample size and long‐term follow‐up so that meaningful changes in cognitive scores can be assessed;
include outcomes that are important to people living with dementia;
have data to permit a health economic analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 9 May 2019 | New search has been performed | We performed top‐up searches on 16 June 2017, 9 May 2018, and 9 May 2019. We identified no new studies for inclusion. |
| 9 May 2019 | New citation required but conclusions have not changed | We performed top‐up searches on 16 June 2017, 9 May 2018, and 9 May 2019. We included no new studies. Content extensively revised. New author added. |
Acknowledgements
We are grateful for the assistance of the Cochrane Dementia and Cognitive Improvement Group, especially for the contribution of Terence Quinn (Joint Co‐ordinating Editor) for his advice and substantial editing.
We would like to thank peer reviewers Serge Gauthier and Azmil Abdul‐Rahim for their comments and feedback.
Appendices
Appendix 1. Sources searched and search strategies for updates between 2011 and 2018
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (alois.medsci.ox.ac.uk/) [Date of most recent search: 9 May 2019] |
Keyword search: cerebrolysin OR cere OR FPF1070 OR FPF (all dates) | Feb 2011: 20 Nov 2012: 19 Jun 2017: 1 May 2018: 0 May 2019: 0 |
| 2. MEDLINE In‐Process and Other Non‐Indexed Citations and MEDLINE 1950 to present (OvidSP) [Date of most recent search: 9 May 2019] |
1. cerebrolysin*.mp. 2. CERE.mp. 3. "FPF 1070".mp. 4. or/1‐3 5. Dementia, Vascular/ 6. Dementia, Multi‐Infarct/ 7. Delirium, Dementia, Amnestic, Cognitive Disorders/ or Dementia/ 8. Alzheimer Disease/ 9. Cerebrovascular Disorders/ 10. CADASIL/ 11. "cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy".mp. 12. dement*.mp. 13. alzheimer*.mp. 14. or/5‐13 15. 4 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi?ed.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 28. (2011* or 2012*).ed. 29. 27 and 28 |
Feb 2011: 51 Nov 2012: 13 Jun 2017: 22 May 2018: 2 May 2019: 3 |
| 3. Embase 1980 to 8 May 2019 (OvidSP) [Date of most recent search: 9 May 2019] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. cerebrolysin*.ti,ab. 25. CERE.mp. 26. "FPF 1070".mp. 27. FPF1070.ti,ab. 28. or/24‐27 29. 23 and 28 30. random*.ti,ab. 31. trial*.ti,ab. 32. randomized controlled trial/ 33. controlled clinical trial/ 34. placebo.ti,ab. 35. "control group".ab. 36. ("double‐blind*" or "double‐mask*" or "single‐blind*" or "single‐mask*" or "triple‐blind*" or "triple‐mask*").ti,ab. 37. or/30‐36 38. 29 and 37 39. (2011* or 2012*).em. 40. 38 and 39 |
Feb 2011: 69 Nov 2012: 18 Jun 2017: 44 May 2018: 19 May 2019: 8 |
| 4. PsycINFO 1806 to May week 2 2019 (OvidSP) [Date of most recent search: 9 May 2019] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. cerebrolysin*.ti,ab. 26. CERE.mp. 27. "FPF 1070".mp. 28. FPF1070.ti,ab. 29. or/25‐28 30. 24 and 29 31. (2011* or 2012*).up. 32. 30 and 31 |
Feb 2011: 15 Nov 2012: 11 Jun 2017: 23 May 2018: 0 May 2019: 1 |
| 5. CINAHL 1981 to 8 May 2019 (EBSCOhost) [Date of most recent search: 9 May 2019] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX cerebrolysin* S21 TX CERE S22 TX "FPF 1070" S23 TX FPF1070 S24 S20 or S21 or S22 or S23 S25 S19 and S24 S26 EM 2011 S27 EM 2012 S28 S26 OR S27 S29 S28 AND S25 |
Feb 2011:5 Nov 2012: 2 Jun 2017: 3 May 2018: 0 May 2019: 1 |
| 6. ISI Web of Knowledge [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] ‐ BIOSIS Previews NOT searched [Date of most recent search: 9 May 2019] |
Topic=(cerebrolysin* OR "FPF 1070" OR FPF1070) AND Topic=(dement* OR VaD OR "vascular cognitive impairment" OR VCI OR CADASIL OR cerebrovascular OR "subcortical VaD") AND Year Published=(2011‐2012) Timespan=All Years. Search language=English Lemmatization=On |
Feb 2011:100 Nov 2012: 16 Jun 2017: 48 May 2018: 9 May 2019: 5 |
| 7. LILACS (BIREME) [Date of most recent search: 9 May 2019] |
cerebrolysin OR FPF1070 OR CERE OR FPF [Words] | Feb 2011: 9 Nov 2012: 11 Jun 2017: 1 May 2018: 1 May 2019: 0 |
| 8. CENTRAL (the Cochrane Library) (Issue 5 of 12, 2019) [Date of most recent search: 9 May 2019] |
#1 cerebrolysin* OR CERE OR "FPF 1070" OR FPF1070 #2 dement* #3 VaD #4 "vascular cognit*" OR VCI #5 CADASIL #6 cerebrovascular #7 (#2 OR #3 OR #4 OR #5 OR #6) #8 (#1 AND #7) [limit to 2011‐2012] |
Feb 2011: 43 Nov 2012: 2 Jun 2017: 21 May 2018: 0 May 2019: 11 |
| 9. ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 9 May 2019] |
Interventional Studies | cerebrolysin OR CERE OR FPF1070 [rec:01/01/2011‐11/04/2012] | Feb 2011: 10 Nov 2012: 2 Jun 2017: 8 May 2018: 3 May 2019: 4 |
| 10. WHO ICTRP (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 9 May 2019] |
Interventional Studies | cerebrolysin OR CERE OR FPF1070 AND (dementia OR vascular) AND [rec:01/01/2011‐04/11/2012] | Feb 2011: 11 Nov 2012: 2 Jun 2017: 3 May 2018: 10 May 2019: 2 |
| TOTAL before de‐duplication | Feb 2011: 333 Nov 2012: 96 Jun 2017: 201 May 2018: 44 May 2019: 35 TOTAL: 709 |
|
Data and analyses
Comparison 1. Cognitive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of cognitive function measured by MMSE or ADAS‐cog+ | 3 | 420 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.13, 0.58] |
| 2 Change of general cognitive function measured by MMSE | 2 | 379 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.37, 1.82] |
| 3 Change of general cognitive function measured by ADAS‐cog+ score | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐7.87, ‐0.56] |
| 4 Improvement of general cognitive function reported as responder rates | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.10, 1.94] |
| 5 Change of executive function measured by the Trail‐Making Test | 2 | 526 | Mean Difference (IV, Random, 95% CI) | ‐24.75 [‐40.46, ‐9.03] |
Comparison 2. Global function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement of global function reported as responder rates | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [1.82, 3.98] |
| 2 Change of global function measured by ADCS‐ADL score | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 6.32 [4.20, 8.45] |
Comparison 3. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.88 [0.36, 131.75] |
| 2 Non‐serious adverse events | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.29, 2.85] |
| 3 Withdrawals due to adverse events | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.66] |
Comparison 4. Subgroup analyses .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of general cognitive function measured by MMSE | 2 | 379 | Mean Difference (IV, Random, 95% CI) | 1.10 [0.37, 1.82] |
| 1.1 Short‐term treatment | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 0.96 [0.12, 1.80] |
| 1.2 Long‐term treatment | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 1.48 [0.06, 2.91] |
| 2 Change of general cognitive function measured by ADAS‐cog+ score | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐7.87, ‐0.56] |
| 2.1 Short‐term treatment | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐4.18, ‐0.58] |
| 2.2 Long‐term treatment | 1 | 232 | Mean Difference (IV, Random, 95% CI) | ‐6.11 [‐8.16, ‐4.06] |
| 3 Improvement of general cognitive function reported as responder rates | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.10, 1.94] |
| 3.1 Short‐term treatment | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.50] |
| 3.2 Long‐term treatment | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.23, 2.24] |
| 4 Improvement of global function reported as responder rates | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [1.82, 3.98] |
| 4.1 Short‐term treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.16, 4.10] |
| 4.2 Long‐term treatment | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [1.86, 5.07] |
| 5 Non‐serious adverse events | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.29, 2.85] |
| 5.1 Short‐term treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.52] |
| 5.2 Long‐term treatment | 1 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.62, 3.84] |
Comparison 5. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of general cognitive function measured by MMSE | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 1.48 [0.06, 2.91] |
| 2 Change of general cognitive function measured by ADAS‐cog+ score | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐6.11 [‐8.16, ‐4.06] |
| 3 Improvement of general cognitive function reported as responder rates | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.29, 1.91] |
| 4 Change of executive function measured by the Trail‐Making Test | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐15.31 [‐30.19, ‐0.43] |
| 5 Improvement of global function reported as responder rates | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [1.86, 5.07] |
| 6 Non‐serious adverse events | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.62, 3.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Guekht 2011.
| Methods | Randomised: PROC PLAN in SAS version 8.2 (SAS Institute, Cary NC) was used for randomisation, and the random allocation sequence was concealed throughout the trial through the use of sealed, sequentially numbered, identical cardboard boxes containing blinded study medication according to the allocation sequence. Double‐blind Placebo‐controlled Duration: 24 weeks ITT analyses were performed, and the LOCF method was applied to account for missing data. |
|
| Participants | Country: multiple centres in the Russian Federation. Number of participants: 242 patients entered the study, and 232 (145 postmenopausal female, 87 male) were included in the ITT analyses. Age: average: 67.3 ± 8.0 years. Inclusion: mild to moderately severe VaD according to NINDS‐AIREN criteria, with an MMSE score of 10 to 24, a modified Hachinski ischaemic score of > 4, and a Hamilton Depression Rating Scale score of ≤ 15. Exclusion: patients with severe concomitant neurologic or psychiatric illnesses, any significant systemic illness or unstable medical condition that could lead to difficulty complying with the protocol, or a history of systemic cancer within the preceding 2 years. Baseline: the study groups had similar demographic and other baseline characteristics. |
|
| Interventions | Cere group: Cere 20 mL + physiological saline 80 mL Placebo group: physiological saline 100 mL i.v. infusions once daily, 5 days/week for 4 weeks, followed by a 2‐month treatment‐free interval (weeks 5 to 12) and then resumption of the 5‐day‐per‐week schedule (weeks 13 to 16), for a total of 40 infusions. |
|
| Outcomes | ADAS‐cog+ score CIBIC+ score CIBIS+ score MMSE ADCS‐ADL Trail‐Making Test A Clock‐Drawing Test Safety assessment |
|
| Notes | Supported by EVER Neuro Pharma GmbH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PROC PLAN in SAS version 8.2 (SAS Institute, Cary NC) was used for randomisation. |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed throughout the trial through the use of sealed, sequentially numbered, identical cardboard boxes containing blinded study medication according to the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and therapy providers were blinded to treatment assignment during the entire study period. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study personnel who assessed outcomes were also blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of missing data and reasons were reported and similar in each group, and ITT analyses were performed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all the prespecified outcomes that were of interest in the current review were reported. |
| Other bias | Low risk | No other potential bias was found. |
Liang 2001.
| Methods | Randomised: methods of randomisation and allocation concealment were not described. Non‐blind Blank‐controlled Duration: 15 days. ITT analysis: none were lost to follow‐up. |
|
| Participants | Country: single centre in China. Number of participants: 77 (23 female and 54 male). Age: average: 60.4 years in the Cere group, 61.2 years in the control group. Inclusion: VaD according to DSM‐III‐R and ICD‐10 criteria, with a Hachinski score of ≥ 7. Exclusion: not specified. The study groups had similar baseline characteristics. |
|
| Interventions | Cere group: Cere 20 mL + physiological saline 200 mL + routine treatment Control group: routine treatment Cere administered by i.v. infusions once daily for 15 days. |
|
| Outcomes | HDS | |
| Notes | Participants in both groups were treated with other medicine according to participant's condition, but whether those drugs were balanced between groups or would impact the results was not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding was used according to the report; placebos or blank controlled solutions were not used for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was used according to the report; placebos or blank controlled solutions were not used for blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the enrolled participants were lost to the study. |
| Selective reporting (reporting bias) | High risk | The study protocol is not available. The only outcome measure listed in the outcome section was reported, but safety results, which would be expected to have been reported for such a study, were not mentioned. |
| Other bias | Unclear risk | Participants in both groups were treated with other medicine according to participant's condition, but whether those drugs were balanced between groups or would impact the results was not specified. |
Muresanu 2008a.
| Methods | Randomised: 2:3:3 randomisation ratio (i.e. 31 participants were randomised to 3 groups: 10 in placebo group, 16 in Cere10 group, and 15 in Cere30 group); the method of randomisation and concealment was not described. Double‐blind Placebo‐controlled Duration: a total of 16 weeks (a 4‐week treatment period, followed by a 12‐week, open‐label extension); only data from the 4‐week treatment period were included in meta‐analyses of the current review. ITT analysis: not performed, although 8 participants (3, 4, and 1 from the Cere10, Cere30, and placebo groups, respectively) were lost to the follow‐up visit. |
|
| Participants | Country: Romania. Number of participants: 41 (21 postmenopausal female, 20 male). Age: average: 70.7 ± 1.6 years; range: 51 to 88 years. Inclusion: mild to moderately severe probable VaD according to NINDS‐AIREN criteria, MMSE between 9 and 26. Exclusion: non‐VaD dementia, current or past significant neurological diseases other than VaD, major depression, psychosis, substance dependence, laboratory abnormalities clinically relevant for dementia or significant concomitant illnesses. Baseline demographic data and disease characteristics did not differ significantly between groups. |
|
| Interventions | Cere10 group: Cere 10 mL + physiological saline 40 mL Cere30 group: Cere 30 mL + physiological saline 20 mL Control group: normal physiological saline 50 mL i.v. infusions once daily and 5 days/week for 4 weeks. |
|
| Outcomes | ADAS‐cog+ scores qEEG PR scores Adverse events |
|
| Notes | All randomised participants completed the study and were included in analysis. Supported by EVER Neuro Pharma GmbH (Austria) and the Society for the Study of Neuroprotection and Neuroplasticity (Romania). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described in the article. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants and implementers were blinded to the treatment assigned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The evaluators were blinded to the treatment assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed the 4‐week treatment and evaluation, and the number lost from the 12‐week extension study and reasons were similar in each group. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes listed in the treatment efficacy measures section were all reported, but as the study protocol was unavailable, there is insufficient information to permit a clear judgement. |
| Other bias | Low risk | No other potential bias was found. |
Vereschagin 1991.
| Methods | Randomised: methods of randomisation and allocation concealment were not described. Double‐blind Placebo‐controlled Duration: 4 weeks |
|
| Participants | Number of participants: 60 participants were divided equally into 2 groups. Inclusion: patients with a mild form of multi‐infarct dementia, but the diagnosis criteria were not described. Baseline: the study groups had similar baseline compared parameters. |
|
| Interventions | Cere group: Cere 15 mL (10 mL in the morning and 5 mL in the evening) + 0.85% NaCl solution 200 mL Placebo group: placebo 15 mL (10 mL in the morning and 5 mL in the evening) + 0.85% NaCl solution 200 mL i.v. infusions twice daily for 28 days. |
|
| Outcomes | Clinical evaluations (by means of a special scale) EEG Psychological test of Arnold‐Kohlmann Response times |
|
| Notes | Only an abstract was found. Despite our efforts, detailed information could not be obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Despite contacting study authors, detailed methods were not available. |
| Allocation concealment (selection bias) | Unclear risk | Despite contacting study authors, detailed methods were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Despite contacting study authors, detailed methods were not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Despite contacting study authors, detailed methods were not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Despite contacting study authors, details were not available. |
| Selective reporting (reporting bias) | Unclear risk | Despite contacting study authors, details were not available. |
| Other bias | Unclear risk | Despite contacting study authors, details were not available. |
Xiao 1999.
| Methods | Randomised: the methods of randomisation and allocation concealment were not described. Double‐blind: details were not provided. Placebo‐controlled Duration: 4 weeks ITT analysis: not performed; 1 participant who refused the treatment was excluded from the analyses. |
|
| Participants | Country: multiple centres in China. Number of participants: 148 participants were enrolled, 147 completed the study (45 females who were postmenopausal or who had been surgically sterilised prior to entry, 102 males). Age: average: 69.88 ± 6.33 years in the Cere group, 69.60 ± 7.22 years in the placebo group; range: 55 to 85 years. Inclusion: mild to moderately severe VaD according to DSM‐IV criteria, with an MMSE score of 15 to 25, a Hachinski score of ≥ 7, a GDS rating of 3 to 5, and a Hamilton Depression Rating Scale score of ≤ 15. Exclusion: patients with evidence of other psychiatric or neurological disorders and had clinically significant or active renal, hepatic, endocrine, or cardiovascular diseases; patients with severe heart disease, severe hypertension, and severe obstructive pulmonary disease, haematological or oncological disorders, or vitamin B12 or folate deficiency; patients receiving other nootropic agents, psychotropic drugs, hypnotics, drugs influencing cerebral blood flow, or stimulants. Baseline: the study groups had similar baseline characteristics. |
|
| Interventions | Cere group: Cere 30 mL + physiological saline 100 mL Placebo group: placebo 30 mL + physiological saline 100 mL i.v. infusions once daily, 5 days/week for 4 weeks. |
|
| Outcomes | MMSE Investigator's Clinical Global Impression Trail‐Making Test Sandoz Clinical Assessment ‐ Geriatric Activities of Daily Living Self‐reported Nuremberg Activities Inventory Adverse events |
|
| Notes | Supported by EVER Neuro Pharma GmbH (Austria). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described in the article. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not described in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind, and yellow, opaque infusion bottles containing Cerebrolysin or placebo were used, which indicates that the participants and personnel involved in administering treatment might be blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated as double‐blind, but details were not provided, so it remains unclear whether the outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant who refused the treatment was excluded, but the group to which this participant was allocated was not reported. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable, so information was insufficient information to make a definitive judgement. |
| Other bias | Low risk | No other potential bias was found. |
Zhang 2003.
| Methods | Randomised: enrolled participants were allocated randomly by drawing lots, but the method of allocation concealment was not described. Double‐blind: it was stated that participants and investigators were blinded during the interventions and evaluations. Blank‐controlled Duration: 3 years ITT analysis: no participants were lost to follow‐up. |
|
| Participants | Country: single centre in China. Number of participants: 29 (3 female and 26 male). Age: average: 70.80 ± 6.69 years in the Cere group, 69.51 ± 7.02 years in the control group. Inclusion: mild to moderately severe VaD according to DSM‐IV criteria, with a Hachinski score of ≥ 7. Dementia occurred within 3 months after the onset of confirmed cerebral vascular disease, and the manifestations persisted for more than 3 months. Exclusion: patients with evidence of other kinds of dementia, with severe cardiac, cerebral, or renal dysfunction, severe psychiatric disorders or diabetes mellitus, and patients who had recently received other treatments for dementia. Baseline: the study groups had similar baseline characteristics. |
|
| Interventions | Cere group: Cere 20 mL + Xuesaitong (a traditional Chinese patent medicine) 0.4 g + physiological saline 250 mL or 50 g/L glucose solution 250 mL Control group: Xuesaitong (a traditional Chinese patent medicine) 0.4 g + physiological saline 250 mL or 50 g/L glucose solution 250 mL i.v. infusions 60 to 120 min once daily, 10 days for each course and 1 course in each spring and autumn; in total 6 courses for the whole 3 years. |
|
| Outcomes | MMSE WAIS‐RC |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated randomly by drawing lots. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated that participants and investigators were blinded during the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated that participants and investigators were blinded during the evaluations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the 29 enrolled participants were lost to the study or follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable, so information was insufficient information to make a definitive judgement. |
| Other bias | Low risk | No other potential bias was found. |
ADAS‐cog+: Alzheimer's Disease Assessment Scale Cognitive Subpart, Extended Version; ADCS‐ADL: Alzheimer's Disease Cooperative Study – Activities of Daily Living; Cere: Cerebrolysin; CIBIC+: Clinician's Interview‐Based Impression of Change plus Caregiver Input; CIBIS+: Clinician's Interview‐Based Impression of Severity; DSM: Diagnostic and Statistical Manual of Mental Disorders; EEG: electroencephalogram; GDS: Global Deterioration Scale; HDS: Hasegawa Dementia Scale; ICD: International Classification of Disease; ITT: intention‐to‐treat; i.v.: intravenous injection; LOCF: last observation carried forward; MMSE: Mini‐Mental State Examination; NaCl: sodium chloride; NINDS‐AIREN: National Institute of Neurologic Disorders and Stroke – Association Internationale pour la Recherche et l'Enseignement en Neurosciences; PR: power ratio; qEEG: quantitative electroencephalogram; VaD: vascular dementia; WAIS‐RC: Wechsler Adult Intelligence Scale ‐ Revised for Chinese.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alkebaiker 2014 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Cao 2000 | This study enrolled participants with cerebrovascular disease, and information on participants with so‐called vascular dementia could be extracted, but there were no validated inclusion criteria for participant enrolment. Patients who were diagnosed with multiple infarcts or angiosclerosis in brain and with dementia could be enrolled, which we judged as an unacceptable criterion. |
| Chen 2006 | Cytidine diphosphate choline was used in the control group but not in the Cerebrolysin group. |
| Chen 2014 | Cerebrolysin was used in both groups as a routine treatment. |
| Dai 2011 | Cytidine diphosphate choline was used in the control group but not in the Cerebrolysin group. |
| Damulin 2008 | According to the full text this study was not a randomised controlled trial, and the enrolled participants were not diagnosed with dementia. |
| Gavrilova 2017 | According to the description this study was not a randomised controlled trial. |
| Han 2015 | There were neither clear diagnostic criteria for multi‐infarct dementia nor validated inclusion criteria for participant enrolment. |
| Hao 2015 | Citicoline was used in the Cerebrolysin group but not in the control group. |
| Jia 1991 | Cytidine diphosphate choline and piracetam were used in the control group but not in the Cerebrolysin group. |
| Li 1996 | Cytidine diphosphate choline was used in the control group but not in the Cerebrolysin group. |
| Li 2014 | This study only involved a subset of participants with vascular dementia, and the data for relevant participants could not be extracted. |
| Liu 2015 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Liu 2017a | Cerebrolysin was used in both groups. |
| Meng 2001 | We judged this study to not be a randomised controlled trial after reading the full text, and a non‐validated scale was used to evaluate the outcomes. |
| Pan 1999 | Piracetam was used in the control group but not in the Cerebrolysin group. |
| Shprakh 2011 | According to the description this study was not a randomised controlled trial. |
| Suvorova 2010 | According to the description this was not a randomised trial. |
| Tapu 2009 | According to the description this was not a randomised trial. |
| Vereschagin 2001 | The participants were individuals with cognitive disorders and arterial hypertension and atherosclerosis, but the diagnosis of dementia was not made. |
| Wang 1996 | Cytidine diphosphate choline and Cerebrolysin were used in the treatment group and control group, respectively. |
| Wang 2003 | Cytidine diphosphate choline was used in the control group but not in the Cerebrolysin group. |
| Wang 2015 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Wang 2016 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Wang 2017 | Citicoline was used in the Cerebrolysin group but not in the control group. |
| Wang 2018 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Xian 2004 | Cerebrolysin was used in both groups as a routine treatment. |
| Xu 2016 | Jiannaoyizhi decoction was used in the Cerebrolysin group but not in the control group. |
| Xue 2016 | Citicoline was used in the control group but not in the Cerebrolysin group. |
| Zhao 2015 | Cerebrolysin was used in both groups as a routine treatment. |
| Zhao 2017 | Cerebrolysin was used in both groups. |
| Zheng 1999 | Patients who were diagnosed with cerebrovascular disease and with dementia could be enrolled, which we judged as an unacceptable criterion. |
Differences between protocol and review
In the protocol, we intended to perform separate meta‐analyses using different periods of follow‐up to avoid the unit of analysis error. However, in consideration of the various follow‐up periods and small number of included trials, we decided to use final time point data from each trial and, in the presence of any evidence of heterogeneity, analysed subgroups based on treatment duration.
We planned subgroup analyses on the basis of ages, type and severity of vascular dementia, and route of administration. The data as presented in the included studies did not permit these analyses.
We planned to perform fixed‐effect analysis, except when there was unexplained heterogeneity, in which case we would conduct a random‐effects model. However, in this update of the review, we found obvious clinical heterogeneity in participants (e.g. mixed type and severity of vascular dementia) or interventions (e.g. various doses and duration of Cerebrolysin), and so used a random‐effects model for all pooled analyses. The quantitative data of this update review is thus different from that in the previous review.
Weighted mean difference or standardised mean difference were calculated for continuous data in previous versions of this review in accordance with our protocol. Since Section 9.2.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions was published (Deeks 2017), we changed the weighted mean difference to mean difference in the updated version.
We also updated the Assessment of risk of bias in included studies section according to the current Cochrane 'Risk of bias' tool (Higgins 2017).
We added a 'Summary of findings' table to the review and assessed the quality of evidence for each outcome according to GRADE criteria (Ryan 2016), which was not mentioned in the protocol.
Executive function is one of the specific cognitive functions commonly impaired in vascular dementia. We added executive function as part of the primary outcome of cognitive function and described it in detail in the Results section.
A new author was added so that the author order was rearranged.
Contributions of authors
Shuhui Cui and Ning Chen selected studies, extracted data, and assessed the quality of the evidence for the update. They also drafted the updated version of review. Ning Chen and Mi Yang assessed the risk of bias of the included studies. Jian Guo and Muke Zhou performed the searches for studies. Cairong Zhu carried out analyses. Li He supervised the review process and revised the draft.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Declarations of interest
Shuhui Cui ‐ none known.
Ning Chen ‐ none known.
Mi Yang ‐ none known.
Jian Guo ‐ none known.
Muke Zhou ‐ none known.
Cairong Zhu ‐ none known.
Li He ‐ none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Guekht 2011 {published data only}
- Guekht A, Doppler E, Moessler H, Gusev E. Cerebrolysin improves clinical outcome in moderate to moderately severe vascular dementia: results from a randomized, double‐blind, placebo‐controlled, multicenter trial. European Journal of Neurology 2009;16 (Suppl 3):391. [Google Scholar]
- Guekht AB, Moessler H, Novak PH, Gusev EI. Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double‐blind, placebo‐controlled multicenter trial. Journal of Stroke and Cerebrovascular Diseases 2011;20(4):310‐8. [DOI] [PubMed] [Google Scholar]
- NCT00947531. A clinical trial to evaluate the safety and efficacy of 20 ml Cerebrolysin in patients with Vascular Dementia (VascDem). clinicaltrials.gov/ct2/show/study/NCT00947531 Date first received: 28 July 2009.
Liang 2001 {published data only}
- Liang SY, Li ZJ, Yan HJ, Pei XM. The efficacy of Cerebrolysin in multi‐infarct dementia [脑活素治疗多发性脑梗塞性痴呆的疗效观察]. Heilongjiang Medicine and Pharmacy 2001;24(5):75. [Google Scholar]
Muresanu 2008a {published data only}
- Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, et al. A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. Journal of the Neurological Sciences 2008;267(1‐2):112‐9. [DOI] [PubMed] [Google Scholar]
- Muresanu DF, Alvarez XA, Moessler H, Novak PH, Stan A, Buzoianu A, et al. Persistence of the effects of Cerebrolysin on cognition and qEEG slowing in vascular dementia patients: results of a 3‐month extension study. Journal of the Neurological Sciences 2010;299(1‐2):179‐83. [DOI] [PubMed] [Google Scholar]
Vereschagin 1991 {published data only}
- Vereschagin NV, Nekrasova YM, Lebedeva NV, Suslina ZA, Solovyev OI, Piradov MA, et al. Mild forms of multi‐infarct dementia ‐ effectiveness of Cerebrolysin. Sovetskaya Meditsina 1991;11:6‐8. [PubMed] [Google Scholar]
Xiao 1999 {published data only}
- Xiao S, Yan H, Yao P. The efficacy of Cerebrolysin in patients with vascular dementia: results of a Chinese multicentre, randomised, double‐blind, placebo‐controlled trial. Hong Kong Journal of Psychiatry 1999;9(2):13‐9. [Google Scholar]
- Xiao S, Yan H, Yao PF, Wang lN, Tang HC, Jia JJ, et al. A multi‐center, double blind, placebo‐controlled trial of the efficacy of Cerebrolysin in treatment of vascular dementia. Journal of Clinical Psychological Medicine 1999;9(1):1‐3. [Google Scholar]
Zhang 2003 {published data only}
- Zhang LJ, Pan XH, Wang YH, Sheng HT. Cerebrolysin improves the intelligence of vascular dementia patients in clinic [脑活素改善血管性痴呆患者智能状况的临床研究]. Chinese Journal of Clinical Rehabilitation 2003;7(28):3850‐1. [Google Scholar]
References to studies excluded from this review
Alkebaiker 2014 {published data only}
- Alkebaiker JML, Guan YH, Li YB. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与施普善治疗脑血管疾病和血管性痴呆疗效对比]. Chinese Journal of Hypertension 2014;2(22):71‐2. [Google Scholar]
Cao 2000 {published data only}
- Cao G, Ma Y. Application of Cerebrolysin in ischaemic cerebrovascular disease [脑活素在缺血性脑血管病中的合理应用]. Clinical Focus 2000;21(15):986‐7. [Google Scholar]
Chen 2006 {published data only}
- Chen H. Efficacy of Cerebrolysin for vascular dementia [脑活素治疗老年血管性痴呆疗效观察]. Chinese Journal of Practical Medicine 2006;9(5):1019. [Google Scholar]
Chen 2014 {published data only}
- Chen RP. Efficacy of galanthamine in patients with vascular dementia [加兰他敏对血管性痴呆患者的临床治疗效果观察]. Journal of North Pharmacy 2014;8(11):72‐3. [Google Scholar]
Dai 2011 {published data only}
- Dai Y. Efficacy of Cerebrolysin in 41 patients with vascular dementia [施普善治疗血管性痴呆41例临床疗效观察]. Chinese and Foreign Medical Research 2011;15:44‐5. [Google Scholar]
Damulin 2008 {published data only}
- Damulin IV, Koberskaya NN, Mkhitaryan ÉA. Effects of Cerebrolysin on moderate cognitive impairments in cerebral vascular insufficiency (a clinical‐electrophysiological study). Neuroscience and Behavioral Physiology 2008;38(6):639‐45. [DOI] [PubMed] [Google Scholar]
Gavrilova 2017 {published data only}
- Gavrilova SI, Volpina OM, Kolykhalov IV, Fedorova YB, Selezneva ND, Ponomareva EV, et al. Therapeutic monitoring and prediction of the efficacy of neurotrophic treatment in patients with amnestic type of mild cognitive impairment. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova 2017;117(8):27‐38. [DOI] [PubMed] [Google Scholar]
Han 2015 {published data only}
- Han QS. The application value of cerebrolysin in multi‐infarction dementia [脑活素治疗多发性脑梗死性痴呆的临床应用价值评析]. Guide of China Medicine 2015;13(12):189. [Google Scholar]
Hao 2015 {published data only}
- Hao GM. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与脑活素治疗脑血管疾病和血管性痴呆的疗效对比]. China Continuing Medical Education 2015;12(7):164‐5. [Google Scholar]
Jia 1991 {published data only}
- Jia YP, Chen TW, Li YX. Application of Cerebrolysin in 30 patients of post stroke dementia [脑活素治疗卒中后痴呆30例]. New Drugs and Clinical Remedies 1991;10(3):129‐30. [Google Scholar]
Li 1996 {published data only}
- Li ZL, Huang W. Application of Cerebrolysin in 20 patients with vascular dementia [脑活素治疗血管性痴呆20例疗效观察]. Anhui Medicine 1996;5(17):41‐2. [Google Scholar]
Li 2014 {published data only}
- Li N, Li LW. Efficacy of cerebrolysin for elderly dementia [脑蛋白水解物注射液治疗老年期痴呆的疗效观察]. Guide of China Medicine 2014;36(12):195‐9. [Google Scholar]
Liu 2015 {published data only}
- Liu H, Liu W, Feng DX. Comparative study of citicoline and cerebrolysin for patients comorbid with cerebrovascular disease and vascular dementia [用胞磷胆碱和脑活素对合并血管性痴呆的脑血管疾病患者进行治疗的效果对比]. Contemporary Medical Symposium 2015;17(13):289‐90. [Google Scholar]
Liu 2017a {published data only}
- Liu Q, Fu BS, Zhong YX, Wang Y, Ding Y. Oxiracetam combined with cerebroprotein hydrolysate injection for treating cognitive dysfunction after stroke and its influence on serum Hcy and hs‐CRP levels [奥拉西坦联合脑蛋白水解物治疗脑卒中后认知功能障碍的临床疗效及对血清Hcy、hs‐CRP水平的影响]. Journal of Guangxi Medical University 2017;34(12):1748‐51. [Google Scholar]
Meng 2001 {published data only}
- Meng L, Zhang YJ, Zhou YM. The efficacy of Cerebrolysin for 62 old patients with vascular dementia [脑活素治疗老年血管性痴呆62例疗效观察]. Journal of Mudanjiang Medical College 2001;22(1):25‐6. [Google Scholar]
Pan 1999 {published data only}
- Pan AH. Efficacy of Cerebrolysin in 20 patients with vascular dementia [脑活素治疗老年血管性痴呆20例]. Herald of Medicine 1999;3(18):147. [Google Scholar]
Shprakh 2011 {published data only}
- Shprakh V, Suvorova I. Efficacy of prolonged therapy of vascular dementia [ЭФФЕКТИВНОСТЬ ДЛИТЕЛЬНОЙ ТЕРАПИИ СОСУДИСТОЙ ДЕМЕНЦИИ]. Klinicheskaia Meditsina 2011;89(5):57‐60. [PubMed] [Google Scholar]
Suvorova 2010 {published data only}
- Suvorova I. Clinical efficacy of prolonged course of cerebrolysin therapy in patients with poststroke vascular dementia. New Armenian Medical Journal 2010. [Google Scholar]
- Suvorova I, Shprakh V. Neuroprotective therapy in basic treatment of vascular dementia. European Neuropsychopharmacology 2010;20:S561. [Google Scholar]
Tapu 2009 {published data only}
- Tapu M, Bicu D, Tapu F, Stovicek O. The efficacy of cerebrolysin in vascular dementia. Journal of the Neurological Sciences 2009;283:286. [Google Scholar]
Vereschagin 2001 {published data only}
- Vereschagin NV, Suslina ZA, Timerbaeva SL, Kashina EM, Gnezditsky VV, Maksimova MY, et al. Treatment and prevention of cognitive dysfunction in patients with arterial hypertension and atherosclerosis: results of randomised double blind placebo‐controlled study of cerebrolysin. Terapevticheskii Arkhiv 2001;73:22‐7. [PubMed] [Google Scholar]
Wang 1996 {published data only}
- Wang XM, Sun JT, Chen HF, Han XL, He YB. Comparative study of cytidine diphosphate choline and cerebrolysin for cerebrovascular disease and vascular dementia [胞二磷胆碱与脑活素治疗脑血管疾病和血管性痴呆疗效对比]. New Drugs and Clinical Remedies 1996;15(4):244‐5. [Google Scholar]
Wang 2003 {published data only}
- Wang Y. Efficacy of cerebrolysin in patients with multi‐infarct dementia [脑活素治疗多发性脑梗死性痴呆的临床疗效观察]. Proceeding of Clinical Medicine 2003;12(5):362‐3. [Google Scholar]
Wang 2015 {published data only}
- Wang YN. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与脑活素治疗脑血管疾病和血管性痴呆的疗效对比]. Journal of Clinical Medicine 2015;10(2):1836‐7. [Google Scholar]
Wang 2016 {published data only}
- Wang J, Chen DX. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与脑活素治疗脑血管疾病和血管性痴呆疗效对比]. For All Health 2016;30(10):136. [Google Scholar]
Wang 2017 {published data only}
- Wang XH. Comparative study of citicoline and cerebrolysin for patients comorbid with cerebrovascular disease and vascular dementia [胞磷胆碱和脑活素对合并血管性痴呆的脑血管病患者进行治疗的效果对比评价]. Journal of Clinical Medicine 2017;4(75):14804‐6. [Google Scholar]
Wang 2018 {published data only}
- Wang YC. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与脑活素治疗脑血管疾病和血管性痴呆的疗效对比评价]. Inner Mongolia Medical Journal 2018;50(6):678‐9. [Google Scholar]
Xian 2004 {published data only}
- Xian MJ, Jiang XD, Yang Z, Li CM, Yuan L, Fang L, et al. Effect of galanthamine on memory and quality of life in patients with vascular dementia [加兰他敏对血管性痴呆患者记忆和生活质量的影响]. Chinese Journal of Clinical Rehabilitation 2004;8(16):3022‐3. [Google Scholar]
Xu 2016 {published data only}
- Xu JC. Jiannaoyizhi decoction combined with acupoint injection of cerebrolysin in treatment of senile vascular dementia randomized controlled study [健脑益智汤联合穴位注射脑蛋白水解物治疗老年血管性痴呆随机平行对照研究]. Journal of Practical Traditional Chinese Internal Medicine 2016;12(30):85‐8. [Google Scholar]
Xue 2016 {published data only}
- Xue H. Comparative study of citicoline and cerebrolysin for cerebrovascular disease and vascular dementia [胞磷胆碱与脑活素治疗脑血管疾病和血管性痴呆疗效对比]. World Latest Medicine Information 2016;22(16):95‐6. [Google Scholar]
Zhao 2015 {published data only}
- Zhao J. Efficacy of xingnaojing combined with venortuon and cerebrolysin in 60 patients with vascular dementia [醒脑静联合维脑路通及脑活素治疗血管性痴呆60例观察]. Journal of Practical Traditional Chinese Medicine 2015;12(31):1134. [Google Scholar]
Zhao 2017 {published data only}
- Zhao YM. Efficacy of Xuesaitong decoction combined with Cerebrolysin in 50 patients with poststroke dementia [自拟血塞通汤剂联合脑蛋白水解物辨治脑卒中认知功能障碍患者 50 例]. Global Traditional Chinese Medicine 2017;10(1):80‐2. [Google Scholar]
Zheng 1999 {published data only}
- Zheng YF, Xie JM, Li WD. Efficacy of cerebrolysin for vascular dementia [脑活素治疗脑血管性痴呆疗效观察]. Heilongjiang Medicine and Pharmacy 1999;22(3):103. [Google Scholar]
Additional references
Aggarwal 2007
- Aggarwal NT, Decarli C. Vascular dementia: emerging trends. Seminars in Neurology 2007;27(1):66‐77. [DOI] [PubMed] [Google Scholar]
Akai 1992
- Akai F, Hiruma S. Neurotrophic factor‐like effect of FPF1070 on septal cholinergic neurons after transections of fimbria‐fornix in the rat brain. Histology and Histopathology 1992;7(2):213‐21. [PubMed] [Google Scholar]
Alcántara‐González 2012
- Alcántara‐González F, Mendoza‐Perez CR, Zaragoza N, Juarez I, Arroyo‐García LE, Gamboa C, et al. Combined administration of cerebrolysin and donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse 2012;66(11):938‐49. [DOI] [PubMed] [Google Scholar]
Allegri 2012
- Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer's disease and vascular dementia. Drugs of Today 2012;48 Suppl A:25‐41. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Press, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition, revised. Washington, DC: American Psychiatric Press, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Banik 2015
- Banik A, Brown RE, Bamburg J, Lahiri DK, Khurana D, Friedland RP, et al. Translation of pre‐clinical studies into successful clinical trials for alzheimer's disease: what are the roadblocks and how can they be overcome?. Journal of Alzheimer's Disease 2015;47(4):815‐43. [DOI] [PubMed] [Google Scholar]
Benisty 2008
- Benisty S, Hernandez K, Viswanathan A. Diagnostic criteria of vascular dementia in CADASIL. Stroke 2008;39:838‐44. [DOI] [PubMed] [Google Scholar]
Birks 2013
- Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI] [PubMed] [Google Scholar]
Bornstein 2012
- Bornstein N, Poon WS. Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries. Drugs of Today 2012;48 Suppl A:43‐61. [DOI] [PubMed] [Google Scholar]
Burns 2019
- Burns A, Robert P, IDEAL steering group. Dementia care: international perspectives. Current Opinion in Psychiatry 2019;32(4):361‐5. [DOI] [PubMed] [Google Scholar]
Chang 2011
- Chang QH, Hui W, Chao MW, Zheng RW, Jun WG, Yong HL, et al. The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta‐analysis of longitudinal studies. International Journal of Clinical Practice 2011;65(12):1295‐305. [DOI] [PubMed] [Google Scholar]
Chen 2013a
- Chen CC, Wei ST, Tsaia SC, Chen XX, Cho DY. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double‐blind, placebo‐controlled, randomized study. British journal of neurosurgery 2013;27(6):803‐7. [DOI] [PubMed] [Google Scholar]
Chen 2013b
- Chen N, Yang M, Guo J, Zhou M, Zhu C, He L. Cerebrolysin for vascular dementia. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI] [PubMed] [Google Scholar]
Chu 2018
- Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, et al. Use of statins and the risk of dementia and mild cognitive impairment: a systematic review and meta‐analysis. Scientific reports 2018;8(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chui 1992
- Chui HC, Victoroff JI, Margolin D. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of California Alzheimer's disease diagnostic and treatment centers. Neurology 1992;42:473‐80. [DOI] [PubMed] [Google Scholar]
Chui 2015
- Chui HC, Ramirez‐Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimer's research & therapy 2015;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2006
- Craig D, Birks J. Cochrane Database of Systematic Reviews. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
El Sayed 2018
- Sayed I, Zaki A, Fayed AM, Shehata GM, Abdelmonem S. A meta‐analysis of the effect of different neuroprotective drugs in management of patients with traumatic brain injury. Neurosurgical Review 2018;41(2):427‐38. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Gauthier 2015
- Gauthier S, Proaño JV, Jia J, Froelich L, Vester JC, Doppler E. Cerebrolysin in mild‐to‐moderate Alzheimer's disease: a meta‐analysis of randomized controlled clinical trials. Dementia and geriatric cognitive disorders 2015;39(5‐6):332‐47. [DOI] [PubMed] [Google Scholar]
Gharagozli 2017
- Gharagozli K, Harandi AA, Houshmand S, Akbari N, Muresanu DF, Vester J, et al. Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo‐controlled, double‐blinded, multicenter clinical trial. Journal of medicine and life 2017;10(3):153‐60. [PMC free article] [PubMed] [Google Scholar]
Goodman 2017
- Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee‐for‐service beneficiaries, 2011‐2013. Alzheimer's & dementia 2017;13(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime).
Hachinski 1975
- Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral blood flow in dementia. Archives of Neurology 1975;9:632‐7. [DOI] [PubMed] [Google Scholar]
Hao 2009
- Hao Z, Liu M, Liu Z. Huperzine A for vascular dementia. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007365.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartbauer 2001
- Hartbauer M, Hutter‐Paier B, Windisch M. Effects of Cerebrolysin on the outgrowth and protection of processes of cultured brain neurons. Journal of Neural Transmission 2001;108:581‐92. [DOI] [PubMed] [Google Scholar]
Hensel 2007
- Hensel A, Angermeyer MC, Riedel‐Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini‐Mental State Examination. Journal of neurology, neurosurgery, and psychiatry 2007;78:1298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta‐analyses. British medical journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hong 2005
- Hong Z, Zhu GX, Chen HB, Chen SD, Yang QD, Zeng JS, et al. The clinical efficacy of Cerebrolysin in the treatment of acute ischemic stroke [施普善在缺血性脑卒中急性期的临床疗效研究]. Chinese Journal of Geriatric Heart Brain and Vessel Diseases 2005;7:331‐3. [Google Scholar]
ICHEWG 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. International Digest of Health Legislation 1997;48:231‐4. [PubMed] [Google Scholar]
Ihara 2014
- Ihara M, Kalaria RN. Understanding and preventing the development of post‐stroke dementia. Expert review of neurotherapeutics 2014;14(9):1067‐77. [DOI] [PubMed] [Google Scholar]
Imfeld 2013
- Imfeld P, Brauchli Pernus YB, Jick SS, Meier CR. Epidemiology, co‐morbidities, and medication use of patients with Alzheimer's disease or vascular dementia in the UK. Journal of Alzheimer's disease 2013;35(3):565‐73. [DOI] [PubMed] [Google Scholar]
IOM 2013
- IOM (Institute of Medicine). Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. Washington, DC: The National Academies Press, 2013. [PubMed] [Google Scholar]
Jia 2014
- Jia J, Wang F, Wei C, Zhou A, Jia X, Li F, et al. The prevalence of dementia in urban and rural areas of China. Alzheimer's & dementia 2014;10(1):1‐9. [DOI] [PubMed] [Google Scholar]
Juárez 2011
- Juárez I, González DJ, Mena R, Flores G. The chronic administration of cerebrolysin induces plastic changes in the prefrontal cortex and dentate gyrus in aged mice. Synapse 2011;65(11):1128‐35. [DOI] [PubMed] [Google Scholar]
Korczyn 2012
- Korczyn AD, Vakhapova V, Grinberg LT. Vascular dementia. Journal of the Neurological Sciences 2012;322(1‐2):2‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladurner 2005
- Ladurner G, Kalvach P, Moessler H, Cerebrolysin Study Group. Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of Neural Transmission 2005;112(3):415‐28. [DOI] [PubMed] [Google Scholar]
Ligthart 2010
- Ligthart SA, Moll van Charante EP, Gool WA, Richard E. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vascular health and risk management 2010, (6):775‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017b
- Liu Z, Hu M, Lu P, Wang H, Qi Q, Xu J, et al. Cerebrolysin alleviates cognitive deficits induced by chronic cerebral hypoperfusion by increasing the levels of plasticity‐related proteins and decreasing the levels of apoptosis‐related proteins in the rat hippocampus. Neuroscience letters 2017;651:72‐8. [DOI] [PubMed] [Google Scholar]
McGuinness 2014
- McGuinness B, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mijajlović 2017
- Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle‐Hansen HB, et al. Post‐stroke dementia ‐ a comprehensive review. BMC medicine 2017 Jan 18;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohs 1997
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Disease & Associated Disorders 1997;11(Suppl 2):S13‐21. [PubMed] [Google Scholar]
Molnar 2009
- Molnar FJ, Man‐Son‐Hing M, Fergusson D. Systematic review of measures of clinical significance employed in randomized controlled trials of drugs for dementia. Journal of the American Geriatrics Society 2009;57:536‐46. [DOI] [PubMed] [Google Scholar]
Moorhouse 2008
- Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurology 2008;7:246‐55. [DOI] [PubMed] [Google Scholar]
Muresanu 2008b
- Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, et al. A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. Journal of the Neurological Sciences 2008;267(1‐2):112‐9. [DOI] [PubMed] [Google Scholar]
Muresanu 2010
- Muresanu DF, Alvarez XA, Moessler H, Novak PH, Stan A, Buzoianu A, et al. Persistence of the effects of Cerebrolysin on cognition and qEEG slowing in vascular dementia patients: results of a 3‐month extension study. Journal of the Neurological Sciences 2010;299(1‐2):179‐83. [DOI] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society 2005;53(4):695‐9. [DOI] [PubMed] [Google Scholar]
O'Brien 2003
- O'Brien JT, Erkinjuntti T, Reisberg B. Vascular cognitive impairment. Lancet Neurology 2003;2(2):89–98. [DOI] [PubMed] [Google Scholar]
O'Brien 2006
- O'Brien JT. Vascular cognitive impairment. American Journal of Geriatric Psychiatry 2006;14:724‐33. [DOI] [PubMed] [Google Scholar]
O'Brien 2015
- O'Brien JT, Thomas A. Vascular dementia. Lancet 2015;386(10004):1698‐706. [PUBMED: 26595643] [DOI] [PubMed] [Google Scholar]
Ohara 2017
- Ohara T, Hata J, Yoshida D, Mukai N, Nagata M, Iwaki T, et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 2017;88(20):1925‐32. [DOI] [PubMed] [Google Scholar]
Pantoni 1993
- Pantoni L, Inzitari D. Hachinski's ischemic score and the diagnosis of vascular dementia: a review. Italian Journal of Neurological Sciences 1993;14:539‐46. [DOI] [PubMed] [Google Scholar]
Perez 2012
- Perez L, Heim L, Sherzai A, Jaceldo‐Siegl K, Sherzai A. Nutrition and vascular dementia. journal of nutrition, health & aging 2012;16(4):319‐24. [DOI] [PubMed] [Google Scholar]
Plassman 2007
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1‐2):125‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plosker 2009
- Plosker GL, Gauthier S. Cerebrolysin: a review of its use in dementia. Drugs and Aging 2009;26(11):893‐915. [DOI] [PubMed] [Google Scholar]
Plosker 2010
- Plosker GL, Gauthier S. Spotlight on cerebrolysin in dementia. CNS Drugs 2010;24(3):263‐6. [DOI] [PubMed] [Google Scholar]
Renna 2003
- Renna M, Handy J, Shah A. Low baseline Bispectral Index of the electroencephalogram in patients with dementia. Anesthesia and Analgesia 2003;96(5):1380‐5. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rockenstein 2005
- Rockenstein E, Adame A, Mante M. Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer's disease with the neurotrophic compound cerebrolysin. Journal of Neural Transmission 2005;112(2):269‐82. [DOI] [PubMed] [Google Scholar]
Rockenstein 2006
- Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, et al. Cerebrolysin decreases amyloid‐beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. Journal of Neuroscience Research 2006;83:1252‐61. [DOI] [PubMed] [Google Scholar]
Rockenstein 2007
- Rockenstein E, Mante M, Adame A, Crews L, Moessler H, Masliah E. Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta neuropathologica 2007;113(3):265‐75. [DOI] [PubMed] [Google Scholar]
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia. Diagnostic criteria for research studies: Report of the NINDS‐AIREN International Workshop. Neurology 1993;43:250‐60. [DOI] [PubMed] [Google Scholar]
Román 2002
- Román GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Medical Clinics of North America 2002;86(3):477‐99. [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. CCCG. Version 3.0. cccrg.cochrane.org/author‐resources. Melbourne: La Trobe University, (accessed prior to 11 September 2019).
Schrag 2012
- Schrag A, Schott JM, Alzheimer's Disease Neuroimaging Initiative. What is the clinically relevant change on the ADAS‐Cog?. Journal of neurology, neurosurgery, and psychiatry 2012;83:171‐3. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. British medical journal 2010;340:698‐702. [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Shah 2009
- Shah K, Qureshi SU, Johnson M, Parikh N, Schulz PE, Kunik ME. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. American journal of geriatric pharmacotherapy 2009;7(5):250‐61. [DOI] [PubMed] [Google Scholar]
Sharp 2011
- Sharp SI, Aarsland D, Day S, Sønnesyn H, Alzheimer's Society Vascular Dementia Systematic Review Group, Ballard C. Hypertension is a potential risk factor for vascular dementia: systematic review. International journal of geriatric psychiatry 2011;26(7):661‐9. [DOI] [PubMed] [Google Scholar]
Solis‐Gaspar 2016
- Solis‐Gaspar C, Vazquez‐Roque RA, Jesús Gómez‐Villalobos M, Flores G. Cerebrolysin improves memory and ameliorates neuronal atrophy in spontaneously hypertensive, aged rats. Synapse 2016;70(9):378‐89. [DOI] [PubMed] [Google Scholar]
Stein 2012
- Stein J, Luppa M, Maier W, Wagner M, Wolfsgruber S, Scherer M, et al. Assessing cognitive changes in the elderly: reliable change indices for the Mini‐Mental State Examination. Acta psychiatrica Scandinavica 2012;126:208‐18. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tatebayashi 2003
- Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke‐lqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer's disease. Acta Neuropathologica 2003;105(3):225‐32. [DOI] [PubMed] [Google Scholar]
Ubhi 2013
- Ubhi K, Rockenstein E, Vazquez‐Roque R, Mante M, Inglis C, Patrick C, et al. Cerebrolysin modulates pronerve growth factor/nerve growth factor ratio and ameliorates the cholinergic deficit in a transgenic model of Alzheimer's disease. Journal of neuroscience research 2013;91(2):167‐77. [DOI] [PubMed] [Google Scholar]
Veinbergs 2000
- Veinbergs I, Mante M, Mallory M. Neurotrophic effects of Cerebrolysin in animal models of excitotoxicity. Journal of Neural Transmission Supplement 2000;59:273‐80. [DOI] [PubMed] [Google Scholar]
Vázquez‐Roque 2014
- Vázquez‐Roque RA, Ubhi K, Masliah E, Flores G. Chronic cerebrolysin administration attenuates neuronal abnormalities in the basolateral amygdala induced by neonatal ventral hippocampus lesion in the rat. Synapse 2014;68(1):31‐8. [DOI] [PubMed] [Google Scholar]
Wei 2007
- Wei ZH, He QB, Wang H, Su BH, Chen HZ. Meta‐analysis: the efficacy of nootropic agent Cerebrolysin in the treatment of Alzheimer's disease. Journal of Neural Transmission 2007;114(5):629‐34. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The 10th Revision of the International Classification of Disease and Related Health Problems (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
Windisch 1998
- Windisch M, Gschanes A, Hutter‐Paier B. Neurotrophic activities and therapeutic experience with a brain derived peptide preparation. Journal of Neural Transmission Supplement 1998;53:289‐98. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu TX, Li QP, Yuan ZY. Yizhi capsule for vascular dementia. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005382.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xing 2014
- Xing S, Zhang J, Dang C, Liu G, Zhang Y, Li J, et al. Cerebrolysin reduces amyloid‐β deposits, apoptosis and autophagy in the thalamus and improves functional recovery after cortical infarction. Journal of the Neurological Sciences 2014;337(1‐2):104‐11. [DOI] [PubMed] [Google Scholar]
Xu 2018
- Xu QQ, Shan CS, Wang Y, Shi YH, Zhang QH, Zheng GQ. Chinese herbal medicine for vascular dementia: a systematic review and meta‐analysis of high‐quality randomized controlled trials. Journal of Alzheimer's disease 2018;62(1):429‐56. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang C, Chopp M, Cui Y, Wang L, Zhang R, Zhang L, et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of neuroscience research 2010;88(15):3275‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang L, Chopp M, Meier DH, Winter S, Wang L, Szalad A, et al. Sonic hedgehog signaling pathway mediates cerebrolysin‐improved neurological function after stroke. Stroke 2013;44(7):1965‐72. [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang Y, Chopp M, Meng Y, Zhang ZG, Doppler E, Winter S, et al. Cerebrolysin improves cognitive performance in rats after mild traumatic brain injury. Journal of neurosurgery 2015;122(4):843‐55. [DOI] [PubMed] [Google Scholar]
Ziganshina 2015
- Ziganshina LE, Abakumova T. Cerebrolysin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI] [PubMed] [Google Scholar]
Álvarez 2003
- Álvarez XA, Sampedro C, Pérez P, Laredo M, Couceiro V, Hernández Á, et al. Positive effects of Cerebrolysin on electroencephalogram slowing, cognition and clinical outcome in patients with postacute traumatic brain injury: an exploratory study. International Clinical Psychopharmacology 2003;18(5):271‐8. [DOI] [PubMed] [Google Scholar]
Álvarez 2006
- Álvarez XA, Cacabelos R, Laredo M. A 24‐week, double‐blind, placebo‐controlled study of three dosages of Cerebrolysin in patients with mild to moderate Alzheimer's disease. European Journal of Neurology 2006;13(1):43‐54. [DOI] [PubMed] [Google Scholar]
Álvarez 2011a
- Álvarez XA, Cacabelos R, Sampedro C, Aleixandre M, Linares C, Granizo E, et al. Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer's disease: results of a randomized, double‐blind, controlled trial investigating three dosages of Cerebrolysin. European Journal of Neurology 2011;18(1):59‐68. [DOI] [PubMed] [Google Scholar]
Álvarez 2011b
- Álvarez XA, Fuentes P. Cerebrolysin in Alzheimer's disease. Drugs Today (Barc) 2011;47(7):487‐513. [DOI] [PubMed] [Google Scholar]


