Abstract
Lung transplant is a definitive treatment for several end-stage lung diseases. However, the high incidence of allograft rejection limits the overall survival following lung transplantation. Traditionally, alloimmunity directed against human leukocyte antigens (HLA) has been implicated in transplant rejection. Recently, the clinical impact of non-HLA lung-restricted antibodies (LRA) has been recognized and extensive research has demonstrated that they may play a dominant role in the development of lung allograft rejection. The immunogenic lung-restricted antigens that have been identified include amongst others, collagen type I, collagen type V, and k-alpha 1 tubulin. Pre-existing antibodies against these lung-restricted antigens are prevalent in patients undergoing lung transplantation and have emerged as one of the predominant risk factors for primary graft dysfunction which limits short-term survival following lung transplantation. Additionally, LRA have been shown to predispose to chronic lung allograft rejection, the predominant cause of poor long-term survival. This review will discuss ongoing research into the mechanisms of development of LRA as well as the pathogenesis of associated lung allograft injury.
Keywords: Lung transplant, antibodies, rejection
1. Introduction
Lung transplantation is a widely accepted treatment for advanced stage lung disease. Since the 1990s more than 62,000 lung transplants have been completed worldwide as indicated by the data from the International Society of Heart and Lung Transplantation (www.ishlt.org). Recent statistics on lung transplants show that 78% of patients survive the first year and 51% survive 5 years, which is significantly less compared to other solid organ transplants. For example, kidney and heart transplants have 85% and 75% survival at 5 years, respectively (https://optn.transplant.hrsa.gov).
Following transplant, lung allografts are subjected to various injury mechanisms such as ischemia-reperfusion, gastro-esophageal reflux, and microbial infection, all of which have been associated with the development of inflammatory milieu [1–5]. The long-term survival following lung transplant is limited by the development of chronic rejection known as bronchiolitis obliterans (BO). BO is characterized by inflammation of the airway epithelium which leads to fibroproliferation and scar tissue formation in the lung graft. Primary graft dysfunction (PGD), which occurs within the first 72 hours of transplant, has emerged as the predominant cause of early mortality and also a risk for BO [2, 6–9]. PGD is clinically characterized by hypoxemia and pulmonary infiltrates on chest radiograph and has a reported incidence of over 50% [10].
Both allo- and auto-immunity predispose to lung allograft rejection with their development and mechanism of lung injury being possibly interdependent. As such, administration of antibodies to lung-associated self-antigens (SAgs) can induce both cellular and humoral allo-immune responses. Conversely, alloimmunity can induce development of lung-restricted autoimmunity [11]. It has recently been shown that T cells specific for lung tissue-restricted SAgs are not deleted by the thymus, but are actively suppressed by thymus-derived, antigen-specific forkhead box P3 (Foxp3)1 regulatory T cells (Tregs) [12]. Loss of Tregs, for example, by respiratory viral infections, can lead to the expansion of lung tissue-restricted T cells and development of both cellular and humoral lung-restricted autoimmunity which in turn can promote alloimmunity as well as development of lung allograft rejection [1, 3, 9, 13–20]. In this review we will discuss the importance of non-HLA LRA in mediating lung allograft injury.
2. Discussion
2.1. Spectrum of disease caused by lung-restricted antibodies
2.1.1. Hyperacute rejection
PGD is a potential lethal syndrome which affects over 50% of lung transplant recipients within 72 hours and remains the leading cause of early post-transplant mortality [21, 22]. PGD is characterized by hypoxemic respiratory failure and has been associated with ischemia-reperfusion injury, neutrophil infiltration and diffuse alveolar damage [2, 4]. Intriguingly, the characteristic histological features of PGD such as neutrophil infiltration, alveolar edema, and capillaritis are also observed in antibody-mediated rejection (AMR), raising the suspicion that pre-formed antibodies may lead to allograft rejection which may mimic PGD in some patients [23–26]. Indeed, some patients with PGD demonstrate C4d deposition on the allograft even in the absence of any donor HLA-specific antibodies which also suggests activation of antibody-antigen complexes [27, 28].
In a prospective clinical study, we previously observed that about 30% of lung transplant recipients had one or more pre-formed autoantibodies which strongly predisposed to PGD [27]. Furthermore, PGD was associated with a very significant increase in pro-inflammatory cytokines which augmented development of donor-specific alloimmunity and chronic rejection [2]. Unlike histocompatibility antigens, the SAgs are conserved within species [29]. Ischemia-reperfusion injury can reveal epitopes of SAgs which typically serve as structural proteins in the lung [30]. One possible mechanism is activation of matrix metalloproteinases which can unmask SAgs leading to their exposure for about 30 days following transplant [31–34]. Exposure of the SAgs can then lead to the development of allograft rejection if LRA are already present in the recipient [35]. In murine models we found that pre-formed LRA can induce PGD in syngeneic lung grafts and break tolerance after allogeneic lung transplantation, without affecting other organs [18]. We also recently found that hyperacute rejection, classically mediated by pre-formed antibodies against donor HLA class I antigens, can also occur in human lung transplant recipients with pre-formed LRA [8]. Development of PGD associated with pre-existing LRA has also been demonstrated in the rat lung transplant (LTx) model. In a study by Iwata et al., rats who received Col-V antibodies prior to syngeneic graft transplantation developed a syndrome of PGD [34]. The authors showed that PGD associated with Col-V antibodies was histologically characterized with both antibodies and complement deposition. Together, these data indicate that the syndrome of PGD may represent two distinct forms of injury, one that is related to ischemia-reperfusion injury and the other to pre-formed LRA.
2.1.2. Chronic rejection.
Chronic lung allograft rejection remains the predominant cause of poor long-term survival following lung transplantation [36, 37]. Our prior reports suggest that pre-existing autoantibodies remain one of the most important risk factors for chronic lung allograft rejection [2, 27]. Additionally, over 90% of patients who do not have pre-existing autoantibodies at the time of transplant will develop LRA which also predisposes to chronic rejection [38]. While several risk factors have been identified for chronic rejection, lung-restricted autoimmunity has emerged as potentially the final common terminal pathway leading to chronic rejection from a variety of these risk factors. For example, alloimmunity predisposes to de novo lung-restricted autoimmunity and chronic rejection. Clinical studies demonstrated that therapy directed against alloimmunity only reduced the incidence of chronic rejection if autoantibodies were depleted and abrogation of alloimmunity alone was not associated with amelioration of chronic rejection [39, 40]. Similarly, gastroesophageal reflux which has been associated with chronic rejection is now known to induce de novo lung-restricted autoimmunity [19, 41]. Intriguingly, LRA, especially Col-V, are structural proteins located predominantly in the peri-capillary and peri-bronchiolar tissue where BO lesions are observed [42]. The repeated cycles of injury and repair that LTx patients undergo might lead to expansion of lung-restricted autoimmunity, possibly due to release of SAgs and epitope spreading [9]. It is well recognized that T cells play an important role in distinguishing self and non-self-antigens. Activated CD4+ T cells are known to differentiate into Th1, Th2, or Th17 phenotypes, characterized by the production of specific cytokines and different biological functions. Tregs are found in many different tissues and are crucial for immune tolerance by suppressing inflammation and autoimmune response [43]. Tregs suppress the development of autoimmunity in lung transplant recipient and their depletion is necessary for development of LRA [14, 17, 19]. Since Treg function and development is dependent on IL-2 [44], calcineurin inhibition in the long-term can also, paradoxically, promote the development of LRA by inducing loss of Tregs which can promote development of LRA.
The Major histocompatibility complex (MHC) class I-related chain A (MICA) is another non-histocompatibility antigen associated with chronic lung allograft rejection. Anti-HLA precedes de novo development of anti-MICA with peak titers of anti-MICA detected during chronic lung allograft rejection [45]. Taken together, lung-restricted autoimmunity, both pre-existing and de novo, are associated with lung allograft rejection..
2.2. Development of LRA: The two-hit hypothesis
Our group has identified an association between respiratory viral infections and de novo development of LRA following lung transplantation in humans and murine models [15–17]. Additionally, we found that respiratory viruses could induce apoptosis in Tregs, possible through the Fas-FasL pathway upon infecting epithelial cells [17]. Clinical and experimental studies implicate a pivotal role of Tregs in modulating tolerance against SAgs and preventing autoimmunity. Therefore, we proposed a “two-hit” mechanism where loss of Tregs, combined with the lung injury to expose the concealed SAgs, would be required to induce lung-restricted autoimmunity. Accordingly, we found that a combined intra-tracheal administration of anti-MHC class I antibodies with Sendai virus infection, which we have previously shown to induce Treg apoptosis, promotes humoral and cellular autoimmunity against lung-restricted SAgs which did not occur with administration of anti-HLA antibodies or sendai virus infection alone [17]. We replicated these results by inducing lung injury through the administration of hydrochloric acid, a murine model mimicking gastroesophageal reflux, in Foxp3DTR mice in which administration of diphtheria toxin can deplete Tregs [19]. These findings correlate with our observations that LTx patients who had gastro-esophageal reflux and developed respiratory infections and diminished Tregs levels established de novo lung-restricted autoimmunity following transplant [19, 46]. These same mechanisms might play a role in the development of pre-existing LRA in patients with end-stage lung disease undergoing lung transplantation. The end-stage lung disease leads to ongoing inflammation and when combined with mechanisms that lead to Treg apoptosis, such as respiratory viral infection common in this patient population, can induce pre-existing autoimmunity. These patients are predisposed to develop severe primary graft dysfunction immediately post-transplant and chronic lung allograft rejection in the long-term [5, 8, 9, 18]. Additionally, there is growing evidence to support the role of exosomes in the development of LRA which is discussed in a separate review in this issue of Human Immunology [46–50].
2.3. Mechanisms of LRA induced allograft injury
The mechanisms by which LRA induce lung allograft rejection and injury still remain unclear which has precluded the development of effective clinical interventions. However, there are several proposed hypothesis that alone or in combination could explain parts of the mechanism of LRA induced allograft injury.
2.3.1. Role of pulmonary endothelial permeabilization in extravasation of LRA.
Pre-existing LRA against Col-V and KAT can be detected in over a third of patients undergoing lung transplantation [27]. Unlike the major histocompatibility antigens, Col-V and KAT are non-polymorphic, and identical in all humans, raising three related questions: i) why circulating LRA do not bind to SAgs in the native lungs prior to transplantation; ii) why, in recipients of single lung transplantation, LRA-mediated injury spares the contralateral native lung; and iii) why PGD develops in only a subset of patients harboring LRA [8, 18, 19]. It is known that these SAgs are structural proteins which are localized to the extravascular space, likely preventing their interaction with circulating LRA during homeostasis [51–53]. We recently discovered that Ly6ChighCCR2+ spleen derived classical monocytes enter the lung allograft and produce IL1β, which is necessary to open endothelial tight junctions, increase vascular permeability and promote neutrophil extravasation [54].. We hypothesize that the opening of the endothelial tight junctions by IL1β allows the passage of LRA into the interstitium where they bind to cognate antigens to induce lung injury (Figure 1A). IL1β is crucial for a variety of host responses to injury [55–58], including hepatic [59] and renal [60] ischemia-reperfusion injury. IL1β is produced as an inactive precursor in response to cellular activation through pattern recognition receptors (PRRs). Inflammasome-dependent conversion of pro-caspase-1 zymogen to caspase-1 results in processing and secretion of biologically active IL1β [61, 62]. Since inhibition of both the inflammasome and IL1β are feasible, further elucidation of the role of these pathways has potential for rapid clinical translation for preventing LRA-mediated lung injury.
Figure 1.
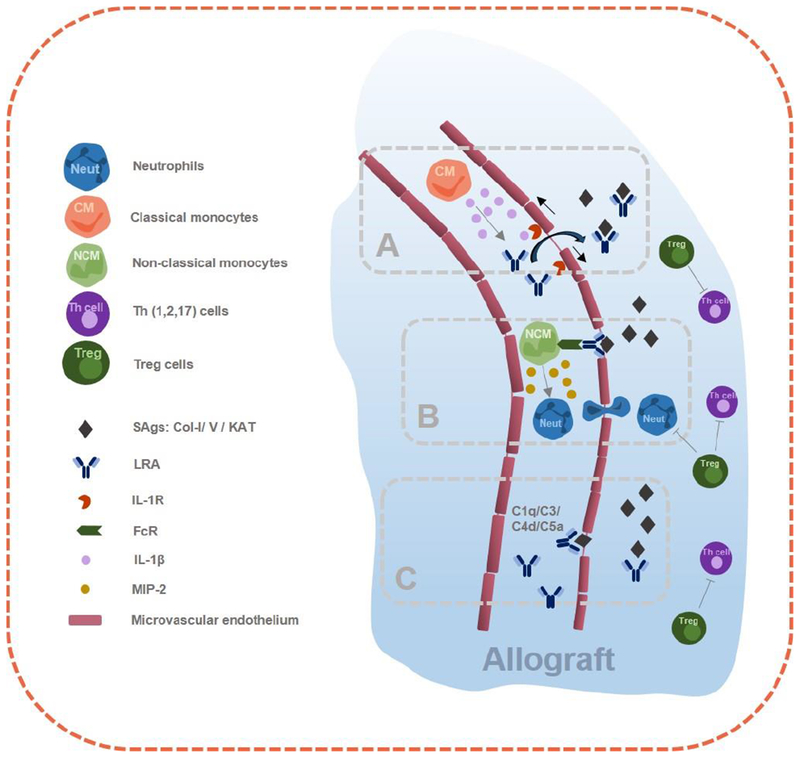
Complex immune interactions within the allograft triggered by lung-restricted antibodies. A) IL1β Secretion of classical-monocytes (CM) leads the opening of the endothelial junctions which facilitates LRA passage and binding to cognate antigens. B) Donor activated non-classical monocytes (NCM) produces neutrophil chemoattractant CXCL2/MIP-2. C) Complement activation facilitates LRA binding to cognate antigens.
2.3.2. Role of donor non-classical monocytes in LRA-induced lung allograft injury.
We reported the presence of pulmonary intravascular non-classical monocytes in donor lungs using a multi-channel flow-cytometry panel developed by our group [63–65]. These donor non-classical monocytes are activated during ischemia-reperfusion through toll-receptor signaling leading to the production of neutrophil chemoattractants (Figure 1B). Neutrophils recruited to the transplanted allograft then mediate reperfusion injury leading to PGD [2, 6, 8, 27]. Neutrophils extravasate after host splenic classical monocytes (recruited by donor non-classical monocytes) open the endothelial tight junctions [54] and initiate the process of NETosis, a regulated cell death program that generates neutrophils extracellular traps (NETS), extracellular elaborations of DNA complexed with histones and neutrophil granular proteins [5, 65, 66].
2.3.3. Complement activation.
Activation of the classical complement pathway is initiated by binding of plasma C1q to the Fc segments of immunoglobulins, while activation of the alternative complement pathway is mediated by complement Factor B-dependent hydrolysis of C3. Both of these pathways converge on C3 convertase which then activates the downstream complement cascade [67]. LRA are IgG antibodies capable of activating complement, and our published and preliminary data suggest that LRA-mediated lung allograft injury is associated with complement activation [8, 19]. However, which complement pathway (classic, alternate or both) contributes to the injury is not known. This uncertainty complicates pathologic observations of C4d staining in tissues of patients with graft dysfunction: expert guidelines caution that this finding is not sufficient to diagnose LRA-mediated allograft injury or to guide treatment [68]. Since complement inhibition is now possible with FDA approved drugs including Eculizumab [69], further studies to determine whether the activation of specific complement pathways plays a causal role in LRA-mediated lung injury could immediately impact clinical practice (Figure 1C).
Patients with PGD have higher plasma C5a levels post-transplantation [70] and inhibition of the complement pathway by C1 esterase and complement receptor type 1 inhibitors leads to mitigation of PGD [71, 72]. Also, complement subcomponents or by-products, including C3d and C4d, in the allograft are found to be associated with PGD and early BO. In a recent trial, short-term complement inhibition with a soluble complement receptor 1 inhibitor (TP10) resulted in a significant decrease in the number of days on a ventilator following lung transplantation [73]. This further emphasizes the potential for exploring complement inhibition in improving lung transplant outcomes.
Furthermore, IL-17 mediated regulation of immune response to SAgs is augmented by complement activation. In a study by Suzuki et al., the down-regulation of complement regulatory protein Complement receptor 1-related gene/protein y and up-regulation of C3a (complement activation marker) was noted in both human and murine BO [74]. Neutralizing IL-17 could improve complement regulatory protein expression in mice lung and reduce C3a production. Additionally, neutralizing C5 with a monoclonal antibody revoked progression of BO, showing that BO associated with lung-restricted autoimmunity might be complement dependent.. Given that LRA strongly predispose to BO, further studies are necessary to determine if complement activation and BO are linked through LRA. The complex immune interactions within the allograft triggered by LRA are summarized in Figure 1.
At our center, we screen patients undergoing lung transplant for pre-existing LRA and if they develop allograft injury we perform histological analysis, including complement analysis, through lung parenchymal or transbronchial biopsies. We find diffuse alveolar damage (DAD) more commonly in true ischemia-reperfusion mediated PGD while in LRA mediated PGD, DAD is lacking. While the role of complement analysis in detection of antibody-mediated lung allograft rejection is still evolving, we have successfully adopted this analysis in our clinical practice to treat antibody-mediated rejection. Based on that, we have successfully treated patients with LRA-induced allograft injury with complement inhibition combined with antibody-directed therapies (Figure 2).
Figure 2.
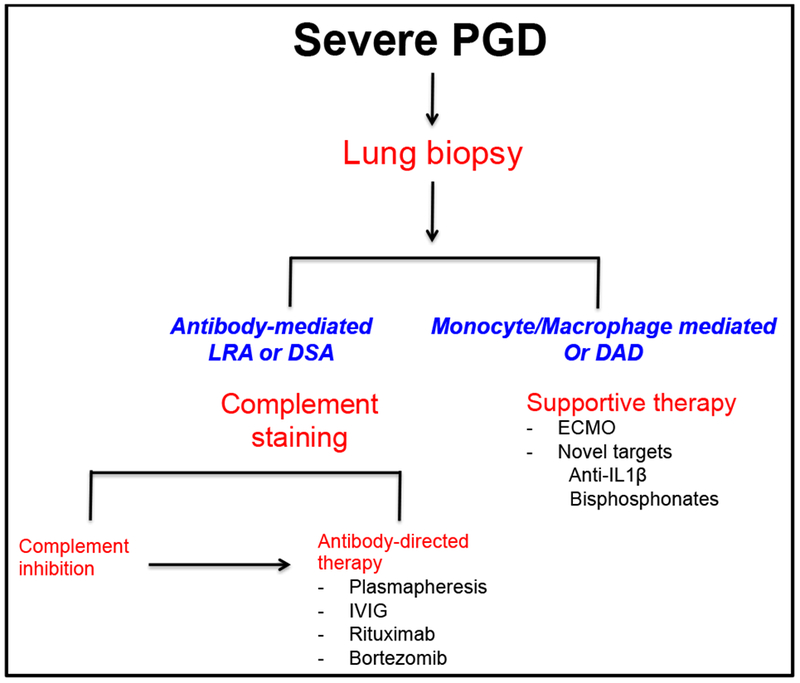
A practical approach to the treatment of primary graft dysfunction. PGD: Primary Graft Dysfunction, LRA: Lung-restricted antibodies, DAD: Diffuse alveolar damage, DSA: Donor specific antibodies, IVIG: Intravenous Immune Globulins
2.3.4. Pro-fibroblastic growth factors.
Antibodies against MHC class I antigens can stimulate airway obliteration by the release of various fibrogenic factors such as, basic fibroblast growth factor, granulocyte-monocyte colony-stimulating factor and transforming growth factor-beta (TGF-β) [9], as such, we found that administering anti-MHC I antibodies to different mice strain induced epithelial hyperplasia and fibrosis [11, 19]. Moreover, administration of MHC I antibodies increased IL-17 levels inducing T-cells against SAgs including KAT and Col-V [75]. In the context of LRA, we have demonstrated that binding of anti-KAT to epithelial cells can increase expression of the transcription factor TCF5, mediating inflammatory response genes and expression of signaling proteins such as vascular endothelial growth factor and TGF-β which could possibly affect the fibro-proliferation cascade leading to BO subsequent to lung transplantation [76]. KAT antibodies also increase expression of hypoxia-inducible factor (HIF-1α) and inhibition of the HIF-1α normalized the fibrotic growth factor levels [77]. Hence, it is suggested that induction of KAT antibodies upregulates HIF-1α mediated fibrogenesis. .
2.4. Novel immunogenic SAgs and tissue-restricted antibodies relevant in allograft rejection
2.4.1. Angiotensin-II type 1 receptor (AT1R) and endothelin type A receptor (ETAR).
Non-HLA antibodies specific for anti-AT1R and anti-ETAR (IgG1 and IgG3 subclasses) are linked to renal allograft injury [78]. These antibodies are often associated with C4d negative rejection phenotype. Hence, graft rejection can be initiated through endothelial injury mediated by these non-HLA antibodies [79].
2.4.2. Vimentin.
Vimentin, an intermediate filament protein found in mesenchymal cells, is integral to support organelles and cell shape maintenance. In a recent study, vimentin expression and antibodies increased significantly in patients with BO suggesting a role of anti-vimentin antibodies in the pathogenesis of chronic rejection [80].
2.4.3. Perlecan.
Perlecan (heparan sulfate proteoglycan 2) is a proteoglycan synthesized by several cells including leucocytes. It modulates cell growth, differentiation, death and lipid metabolism [81, 82]. Perlecan is a major component of the vessel wall. The C-terminal domain of perlecan, endorepellin, is best known for its anti-angiogenic properties and contains three laminin-like globular (LG) domains separated by two sets of epidermal growth factor-like repeats. Most of the antiangiogenic activity of endorepellin resides in the LG3 subdomain [83]. It has recently been shown that pre-transplant and post-transplant levels of LG3 antibodies are significantly higher in some transplant recipients with acute vascular rejection [84] and it has been postulated that LG3 autoantibodies can elicit endothelial cell and graft damage [85].
3. Conclusion:
Lung allografts, unlike other solid organs, experience stimuli from an open environment, which might augment the immunity against self-antigens. Conventional immunosuppression therapies strategies have not resulted in significant long-term improvements following lung transplantation. In view of the growing recent literature, integration of treatment of detection and treatment of LRA in clinical practice has the potential to favorably impact lung allograft survival by ameliorating both PGD and chronic rejection.
Acknowledgements
The work in this manuscript was supported by K08HL125940 and R01HL145478 to AB. The authors are thankful to Ms. Elena Susan for formatting and submission of the manuscript to the journal.
Abbreviations:
- AMR
Antibody-mediated rejection
- AT1R
Angiotensin-II type 1 receptor
- BO
Bronchiolitis obliterans syndrome
- Col-V
Collagen type V
- ETAR
endothelin type A receptor
- HLA
Human leukocyte antigen
- KAT
k-alpha 1 tubulin
- LRA
Lung restricted antibodies
- LTx
Lung transplantation
- MHC
Major histocompatibility complex
- MHC I
Major histocompatibility complex class I
- MICA
MHC class I-related chain A
- PGD
Primary graft dysfunction
- PRR
pattern recognition receptors
- SAgs
self-antigens
- TGF-β
transforming growth factor-beta
- Tregs
Regulatory T cells.
References
- [1].Bharat A, Mohanakumar T: Allopeptides and the alloimmune response. Cell Immunol 2007;248:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP et al. : Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg 2008;86:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bharat A, Mohanakumar T: Autoimmunity and lung transplantation. Front Biosci (Elite Ed) 2012;4:2378. [DOI] [PubMed] [Google Scholar]
- [4].Morrison MI, Pither TL, Fisher AJ: Pathophysiology and classification of primary graft dysfunction after lung transplantation. J Thorac Dis 2017;9:4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bharat A, Kreisel D: Immunopathogenesis of Primary Graft Dysfunction After Lung Transplantation. Ann Thorac Surg 2018;105:671. [DOI] [PubMed] [Google Scholar]
- [6].Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A et al. : Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation 2007;83:150. [DOI] [PubMed] [Google Scholar]
- [7].Morrell MR, Pilewski JM, Gries CJ, Pipeling MR, Crespo MM, Ensor CR et al. : De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant 2014;33:1288. [DOI] [PubMed] [Google Scholar]
- [8].Fernandez R, Chiu S, Raparia K, Garcha P, Farver C, Budev M et al. : Humoral Human Lung Allograft Rejection by Tissue-Restricted Non-HLA Antibodies. Ann Thorac Surg 2016;102:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bharat A, Mohanakumar T: Immune Responses to Tissue-Restricted Nonmajor Histocompatibility Complex Antigens in Allograft Rejection. J Immunol Res 2017;2017:6312514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F et al. : Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357. [DOI] [PubMed] [Google Scholar]
- [11].Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA et al. : Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol 2009;182:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ et al. : CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity 2015;43:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bharat A, Fields RC, Mohanakumar T: Regulatory T cell-mediated transplantation tolerance. Immunol Res 2005;33:195. [DOI] [PubMed] [Google Scholar]
- [14].Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T: Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol 2006;177:5631. [DOI] [PubMed] [Google Scholar]
- [15].Kuo E, Bharat A, Goers T, Chapman W, Yan L, Street T et al. : Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg 2006;82:1043. [DOI] [PubMed] [Google Scholar]
- [16].Kuo E, Bharat A, Shih J, Street T, Norris J, Liu W et al. : Role of airway epithelial injury in murine orthotopic tracheal allograft rejection. Ann Thorac Surg 2006;82:1226. [DOI] [PubMed] [Google Scholar]
- [17].Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP et al. : Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg 2010;90:1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bharat A, Chiu S, Zheng Z, Sun H, Yeldandi A, DeCamp MM et al. : Lung-Restricted Antibodies Mediate Primary Graft Dysfunction and Prevent Allotolerance after Murine Lung Transplantation. Am J Respir Cell Mol Biol 2016;55:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chiu S, Fernandez R, Subramanian V, Sun H, DeCamp MM, Kreisel D et al. : Lung Injury Combined with Loss of Regulatory T Cells Leads to De Novo Lung-Restricted Autoimmunity. J Immunol 2016;197:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Snyder ME, Finlayson MO, Connors TJ, Dogra P, Senda T, Bush E et al. : Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci Immunol 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D et al. : Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454. [DOI] [PubMed] [Google Scholar]
- [22].Porteous MK, Diamond JM, Christie JD: Primary graft dysfunction: lessons learned about the first 72 h after lung transplantation. Curr Opin Organ Transplant 2015;20:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ et al. : Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2016;35:397. [DOI] [PubMed] [Google Scholar]
- [24].Loupy A, Lefaucheur C: Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med 2018;379:1150. [DOI] [PubMed] [Google Scholar]
- [25].Roux A, Levine DJ, Zeevi A, Hachem R, Halloran K, Halloran PF et al. : Banff Lung Report: Current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR). Am J Transplant 2019;19:21. [DOI] [PubMed] [Google Scholar]
- [26].Calabrese F, Hirschi S, Neil D, Montero-Fernandez A, Timens W, Verbeken E et al. : Alveolar septal widening as an “alert” signal to look into lung antibody-mediated rejection: A multicenter pilot study. Transplantation 2019. [DOI] [PubMed] [Google Scholar]
- [27].Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA et al. : Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg 2010;90:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ngo C, Danel C, Duong-Quy S, Dauriat G, Castier Y, Lortat-Jacob B et al. : C4d detection and histological patterns in the diagnosis of antibody-mediated rejection after lung transplantation: a single-centre study. Histopathology 2019. [DOI] [PubMed] [Google Scholar]
- [29].Guerder S, Viret C, Luche H, Ardouin L, Malissen B: Differential processing of self-antigens by subsets of thymic stromal cells. Curr Opin Immunol 2012;24:99. [DOI] [PubMed] [Google Scholar]
- [30].Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ et al. : Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant 2006;6:724. [DOI] [PubMed] [Google Scholar]
- [31].Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI et al. : Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol 1993;121:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zheng L, Ward C, Snell GI, Orsida BE, Li X, Wilson JW et al. : Scar collagen deposition in the airways of allografts of lung transplant recipients. Am J Respir Crit Care Med 1997;155:2072. [DOI] [PubMed] [Google Scholar]
- [33].Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL et al. : IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 2007;117:3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iwata T, Chiyo M, Yoshida S, Smith GN Jr., Mickler EA, Presson R Jr.et al. : Lung transplant ischemia reperfusion injury: metalloprotease inhibition down-regulates exposure of type V collagen, growth-related oncogene-induced neutrophil chemotaxis, and tumor necrosis factor-alpha expression. Transplantation 2008;85:417. [DOI] [PubMed] [Google Scholar]
- [35].Wilkes DS: Autoantibody formation in human and rat studies of chronic rejection and primary graft dysfunction. Semin Immunol 2012;24:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F et al. : The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073. [DOI] [PubMed] [Google Scholar]
- [37].Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ et al. : The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170. [DOI] [PubMed] [Google Scholar]
- [38].Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T: Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant 2012;12:2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M et al. : Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant 2011;30:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA et al. : Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant 2010;29:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bobadilla JL, Jankowska-Gan E, Xu Q, Haynes LD, Munoz del Rio A, Meyer K et al. : Reflux-induced collagen type v sensitization: potential mediator of bronchiolitis obliterans syndrome. Chest 2010;138:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T: CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant 2006;6:1799. [DOI] [PubMed] [Google Scholar]
- [43].King C: A fine romance: T follicular helper cells and B cells. Immunity 2011;34:827. [DOI] [PubMed] [Google Scholar]
- [44].Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y et al. : An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 2016;17:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sumitran-Holgersson S: Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol 2008;20:607. [DOI] [PubMed] [Google Scholar]
- [46].Akbarpour M, Bharat A: Lung Injury and Loss of Regulatory T Cells Primes for Lung-Restricted Autoimmunity. Crit Rev Immunol 2017;37:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sharma M, Liu W, Perincheri S, Gunasekaran M, Mohanakumar T: Exosomes expressing the self-antigens myosin and vimentin play an important role in syngeneic cardiac transplant rejection induced by antibodies to cardiac myosin. Am J Transplant 2018;18:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sharma M, Ravichandran R, Bansal S, Bremner RM, Smith MA, Mohanakumar T: Tissue-associated self-antigens containing exosomes: Role in allograft rejection. Hum Immunol 2018;79:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bansal S, Sharma M, R R, Mohanakumar T: The role of exosomes in allograft immunity. Cell Immunol 2018;331:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T: Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection. J Immunol 2018;200:2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B et al. : Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol 2000;23:62. [DOI] [PubMed] [Google Scholar]
- [52].Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y et al. : Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol 2002;169:1542. [DOI] [PubMed] [Google Scholar]
- [53].Yamada Y, Sekine Y, Yoshida S, Yasufuku K, Petrache I, Benson HL et al. : Type V collagen-induced oral tolerance plus low-dose cyclosporine prevents rejection of MHC class I and II incompatible lung allografts. J Immunol 2009;183:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hsiao HM, Fernandez R, Tanaka S, Li W, Spahn JH, Chiu S et al. : Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1beta. J Clin Invest 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dinarello CA: Biologic basis for interleukin-1 in disease. Blood 1996;87:2095. [PubMed] [Google Scholar]
- [56].Sloand EM, Young NS, Sato T, Kim S, Maciejewski JP: Inhibition of interleukin-1beta-converting enzyme in human hematopoietic progenitor cells results in blockade of cytokine-mediated apoptosis and expansion of their proliferative potential. Exp Hematol 1998;26:1093. [PubMed] [Google Scholar]
- [57].Rega FR, Vanaudenaerde BM, Wuyts WA, Jannis NC, Verleden GM, Lerut TE et al. : IL-1beta in bronchial lavage fluid is a non-invasive marker that predicts the viability of the pulmonary graft from the non-heart-beating donor. J Heart Lung Transplant 2005;24:20. [DOI] [PubMed] [Google Scholar]
- [58].Andreasson ASI, Borthwick LA, Gillespie C, Jiwa K, Scott J, Henderson P et al. : The role of interleukin-1beta as a predictive biomarker and potential therapeutic target during clinical ex vivo lung perfusion. J Heart Lung Transplant 2017;36:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sadatomo A, Inoue Y, Ito H, Karasawa T, Kimura H, Watanabe S et al. : Interaction of Neutrophils with Macrophages Promotes IL-1beta Maturation and Contributes to Hepatic Ischemia-Reperfusion Injury. J Immunol 2017;199:3306. [DOI] [PubMed] [Google Scholar]
- [60].Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V et al. : Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1beta and IL-18 and Arrests CKD. J Am Soc Nephrol 2017;28:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lopez-Castejon G, Brough D: Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev 2011;22:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brough D, Rothwell NJ: Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 2007;120:772. [DOI] [PubMed] [Google Scholar]
- [63].Bharat A, Bhorade SM, Morales-Nebreda L, McQuattie-Pimentel AC, Soberanes S, Ridge K et al. : Flow Cytometry Reveals Similarities Between Lung Macrophages in Humans and Mice. Am J Respir Cell Mol Biol 2016;54:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zheng Z, Chiu S, Akbarpour M, Sun H, Reyfman PA, Anekalla KR et al. : Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bharat A, McQuattie-Pimentel AC, Budinger GRS: Non-classical monocytes in tissue injury and cancer. Oncotarget 2017;8:106171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V et al. : Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Afshar-Kharghan V: The role of the complement system in cancer. J Clin Invest 2017;127:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Berry G, Burke M, Andersen C, Angelini A, Bruneval P, Calabrese F et al. : Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J Heart Lung Transplant 2013;32:14. [DOI] [PubMed] [Google Scholar]
- [69].Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L: Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007;25:1256. [DOI] [PubMed] [Google Scholar]
- [70].Shah RJ, Emtiazjoo AM, Diamond JM, Smith PA, Roe DW, Wille KM et al. : Plasma Complement Levels Are Associated with Primary Graft Dysfunction and Mortality after Lung Transplantation. American Journal of Respiratory and Critical Care Medicine 2014;189:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sommer W, Tudorache I, Kuhn C, Avsar M, Salman J, Ius F et al. : C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation 2014;97:1185. [DOI] [PubMed] [Google Scholar]
- [72].Naka Y, Marsh HC, Scesney SM, Oz MC, Pinsky DJ: Complement activation as a cause for primary graft failure in an isogeneic rat model of hypothermic lung preservation and transplantation. Transplantation 1997;64:1248. [DOI] [PubMed] [Google Scholar]
- [73].Keshavjee S, Davis RD, Zamora MR, de Perrot M, Patterson GA: A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg 2005;129:423. [DOI] [PubMed] [Google Scholar]
- [74].Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL et al. : Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol 2013;191:4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Basha HI, Ramachandran S, Tiriveedhi V, Takenaka M, Subramanian V, Nath DS et al. : Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by Anti-MHC I antibodies. Transplantation 2013;95:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T: De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol 2008;180:4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tiriveedhi V, Gelman AE, Mohanakumar T: HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol 2012;273:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Banasik M, Boratynska M, Koscielska-Kasprzak K, Kaminska D, Zmonarski S, Mazanowska O et al. : Non-HLA antibodies: angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss. Transplant Proc 2014;46:2618. [DOI] [PubMed] [Google Scholar]
- [79].Günther J, Kill A, Becker MO, Heidecke H, Rademacher J, Siegert E et al. : Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Research & Therapy 2014;16:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang C, Niu Y, Yu L, Lv W, Xu H, Abuduwufuer A et al. : The role of epithelial-mesenchymal transition in the post-lung transplantation bronchiolitis obliterans. J Cardiothorac Surg 2017;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C et al. : Heparan Sulfate-Dependent Signaling of Fibroblast Growth Factor 18 by Chondrocyte-Derived Perlecan. Biochemistry 2010;49:5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rnjak-Kovacina J, Tang F, Whitelock JM, Lord MS: Silk biomaterials functionalized with recombinant domain V of human perlecan modulate endothelial cell and platelet interactions for vascular applications. Colloids and Surfaces B: Biointerfaces 2016;148:130. [DOI] [PubMed] [Google Scholar]
- [83].Iozzo RV, Sanderson RD: Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 2011;15:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cardinal H, Dieude M, Brassard N, Qi S, Patey N, Soulez M et al. : Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant 2013;13:861. [DOI] [PubMed] [Google Scholar]
- [85].Zhang Q, Reed EF: The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol 2016;12:484. [DOI] [PMC free article] [PubMed] [Google Scholar]


