Abstract
Objectives
The community-based organisation Treatment Action Group has established an online listing of HIV cure-related trials and observational studies derived from trial registries. Our objective was to use the listing as a basis for a landscape analysis of the current status of HIV cure-related clinical research.
Methods
Trials and observational studies listed as of August 2018 formed the sample set. Survey questions were developed on trial development, trial design, recruitment, enrolment, study completion and dissemination plans. A survey was sent to the contact(s) for each study. Supplemental information was collected from clinicaltrials.gov. The full dataset was then analysed.
Results
A total of 99 interventional trials and 29 observational studies were included. Diverse interventions are under evaluation, including combinations of experimental candidates. Current studies plan to enrol over 7000 participants. Projected completion dates for ~90% of the sample fell between the fourth quarter of 2018 and the end of 2020. Potential obstacles to enrolment that were reported included concerns over invasive procedures and lack of potential benefit to participants. Data on the sex and ethnicity of enrollees were limited but sufficient to note a significant under-representation of women.
Conclusions
A considerable amount of HIV cure-related clinical research is under way. The results from these studies, which should help shape the future of the field, will become available over the next 2–4 years. Diversity both geographically and in terms of enrollees remains limited, particularly in terms of the participation of women, a concern that could significantly affect the generalisability of the findings.
Keywords: HIV cure, participant diversity, obstacles to enrolment, trial development, invasive procedures, clinical trial registries
Introduction
The research effort to develop a cure for HIV infection has ramped up significantly over the past decade [1]. The field has been spurred by the case of Timothy Ray Brown, an individual who appears to have been cured of HIV after receiving stem cell transplants from a donor homozygous for the CCR5Δ32 mutation as part of a series of treatments for concomitant acute myelogenous leukaemia [2], as well as a number of reports of transient HIV remission [3–6] and post-treatment containment of viral load [7,8].
A review of HIV research funding by the National Institutes of Health conducted in 2014 identified the pursuit of a cure as one of three key priorities [9,10]. Total global financial support has increased substantially in the period 2012–2017, from $88 million to $2.8 billion [11].
Since 2014, the Treatment Action Group (TAG) has published a regularly updated online listing of HIV cure-related clinical research [12]. The information is largely drawn from clinical trial registries, with clinicaltrials.gov as the primary source. Phase I trials are not required to be registered by the US Code of Federal Regulations [13], but in our experience, the majority of these studies are entered into clinicaltrials.gov voluntarily.
In August 2018, the Bill & Melinda Gates Foundation contracted TAG to conduct a more detailed evaluation of the status of the clinical trials included in our listing. This paper presents a summary of the results.
Methods
TAG's ‘Research Toward a Cure Trials’ [12] provided the starting point for this landscape analysis. The listing is populated through regular searches on clinicaltrials.gov and at the time of the analysis also included studies sourced from the UK Central Portfolio Management System (CPMS) (since changed to the UK Clinical Trials Gateway [14]) and the website for the EPIC4 study in Canada [15]. Criteria for designating a trial or observational study as being HIV cure related include any of the following:
-
•
Any explicit articulation in the registry entry that the study is related to HIV cure research
-
•
Inclusion of relevant endpoints, such as measures of the HIV reservoir or other parameters connected to HIV persistence
-
•
Evaluations of immune responses that may have a role in controlling viral replication
-
•
Assessments of viral load rebound after antiretroviral therapy interruption
HIV cure-related clinical trials and observational studies listed as of August 2018 that met these criteria formed the sample set for this landscape analysis. Our sample included N = 128 HIV cure-related trials, obtained via clinicaltrials.gov (n = 125), the UK CPMS (n = 2) and the website for the EPIC4 study in Canada (n = 1).
Cross-sectional survey questions were developed related to the following domains: (1) trial development (five questions), (2) trial design (seven questions), (3) recruitment (two questions), (4) enrolment (eight questions), (5) study completion (one question) and (6) dissemination plans (three questions). The survey was semi-structured, and some questions asked respondents to elaborate on answers. The survey is included in the Appendix. Respondents were encouraged to provide open-ended responses when appropriate. We sent the survey link via email to all contacts listed for each study in clinicaltrials.gov and to principal investigators for studies not included in the clinicaltrials.gov registry. Of the 128 study contacts, 73 (57.0%) completed the survey, 7 (5.5%) declined to complete the survey and 48 (37.5%) did not return a response.
Instructions that accompanied the survey explained that it was to be completed by study principal investigators or their representatives. The link was first sent at the end of August 2018, with a request for responses by 5 October 2018. Email reminders were sent to those who had not yet completed the survey at the end of September 2018 and again in early October 2018.
The relevant registry listings were subsequently reviewed for all studies in the listing to identify the countries in which trial is being conducted, invasive study procedures specified on the listing, primary study sponsors and estimated study completion date. Invasive procedures were defined as high-volume blood draws/leukapheresis, gut-associated lymphoid tissue (GALT) biopsies, lumbar punctures, lymph node biopsies and analytic treatment interruptions (ATIs). Survey respondents were asked to provide estimated participant demographic information for open and enrolling studies. Demographic information was available for completed studies in clinicaltrials.gov or associated publications.
We prepared a master database in Excel. We performed a summary descriptive analysis on the full dataset composed of survey responses and additional information from registry entries in Excel using descriptive statistics (i.e. mean, median, count and range). We identified key trends in development speed, enrolment speed and participant diversity based on trial category, location, sponsor, invasive procedures and other factors. We compiled frequencies and percentages for key variables (i.e. study types, number of participants, study location, enrolment status, participant sex/gender information and study sponsor) to characterise the current landscape of HIV cure-related research. This early descriptive analysis did not test a hypothesis about relationships between these domains; rather, we sought to describe the overall landscape of HIV cure research and to identify areas for future research statistical analyses.
Ethics statement
Study procedures were reviewed by the University of Maryland, Baltimore County Office of Research Protections and Compliance and were determined not to be human subjects research as defined by US Department of Health and Human Services regulations 46.102(7)(l) or 46.102(e)(1).
Results
Description of data
At the time of this analysis (August 2018), a total of 128 HIV cure-related studies were grouped into 24 different categories, with observational (29 studies), combinations (17 studies) and antibodies (13 studies) being the most common (Table 1). In the online listing, ‘combinations’ refers to trials combining interventions from multiple categories; the category of the individual components in these trials is not counted in Table 1 but is reported in Appendix Table 1A. Note that some studies in individual categories involve multiple variations of the same approach, for example, dual broadly neutralising antibodies or prime-boost immunisation regimens, including more than one therapeutic vaccine. The number of categories indicates the diversity of approaches currently under investigation in HIV cure-related research.
Table 1.
Overview of study characteristics
| Category | Studies n | Average number of participants n | Range | Total participants N |
|---|---|---|---|---|
| Adoptive immunotherapy | 1 | 12 | — | 12 |
| Anti-inflammatory | 3 | 66 (median 60) | 30–110 | 200 |
| Antiproliferative | 1 | 5 | — | 5 |
| Antibodies | 13 | 38 (median 34) | 12–68 | 500 |
| Antifibrotic | 2 | 42 (median 42) | 21–63 | 84 |
| Antiretroviral therapy | 1 | 36 | — | 36 |
| Cannabinoids | 1 | 26 | — | 26 |
| Combinations | 17 | 40 (median 30) | 5–192 | 680 |
| Cytokines | 2 | 15 (median 15) | 10–20 | 30 |
| Dual-affinity retargeting molecules | 1 | 26 | — | 26 |
| Gene therapies | 8 | 16 (median 12) | 6–40 | 132 |
| Gene therapies for HIV-positive people with cancers | 8 | 8 (median 7) | 3–18 | 69 |
| Gonadotropin-releasing hormone agonists | 1 | 52 | — | 52 |
| Hormones | 1 | 22 | — | 22 |
| Imaging studies | 2 | 7 (median 7) | 5–10 | 15 |
| Immune checkpoint inhibitors | 4 | 46 (median 40) | 20–84 | 184 |
| Latency-reversing agents | 3 | 32 (median 28) | 9–60 | 97 |
| Mammalian target of rapamycin inhibitors | 2 | 16 (median 16) | 10-22 | 32 |
| Observational | 29 | 88 (median 50) | 10–536 | 2571 |
| Proteasome inhibitors | 1 | 17 | — | 17 |
| Stem cell transplantation | 4 | 13 (median 12) | 5–25 | 55 |
| Therapeutic vaccines | 8 | 46 (median 39) | 26–105 | 374 |
| Toll-like receptor agonists | 2 | 50 (median 50) | 28–72 | 100 |
| Treatment intensification/early treatment | 10 | 68 (median 65)
205 (median 72) |
15–905 | 2054 |
| Total | 7373 |
Appendix Table 1A.
Categories of interventions in Combination Trials
| Category | No. of combination trials |
|---|---|
| Adoptive immunotherapy | 2 |
| Antibodies | 5 |
| Antiproliferative | 2 |
| CCR5 inhibitors | 2 |
| Cytokines | 5 |
| Gene therapies | 2 |
| Latency-reversing agents | 8 |
| Mammalian target of rapamycin inhibitors | 1 |
| Routine vaccines | 1 |
| Selective oestrogen receptor modulator | 1 |
| Therapeutic vaccines | 5 |
Based on data provided to registries, the current portfolio of HIV cure research will enrol over 7000 participants; recruitment targets for individual trials range from 5 to 905 (Table 1).
Enrolment targets for two trials of HIV treatment in newborns (totalling 1505 participants) represent the number of pregnant HIV-positive women at risk of vertical transmission that will be enrolled. The primary aim of these studies was to treat the small subset of newborns diagnosed with HIV infection, which is likely to be in the range of 5%–10% of the enrolment target total. Most newborns will be uninfected and will receive standard preventive HIV treatment, exiting the trials 4–6 weeks postpartum. These two trials are included in the ‘treatment intensification/early treatment’ category.
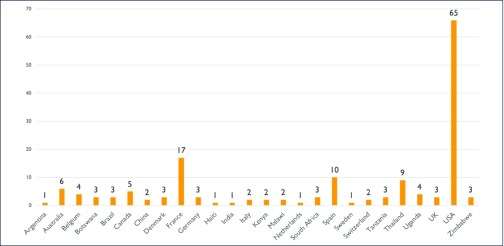
The majority of the studies (N = 118) are targeted to adults, nine involve neonates, infants or young children, and one is limited to adolescents. Most (65 of 128) plans to enrol participants in the USA, although HIV cure efforts are increasingly global: studies are taking place in 26 countries on six continents (Figure 1). Importantly, while the geographic breadth of cure research is relatively wide, the average number of studies taking place in countries in Africa (n = 20), South America (n = 4) and Asia (n = 10) is substantially lower than the number of studies taking place in the North America (n = 71) and Europe (n = 46).
Figure 1.
Location of cure trials
We received survey responses from 73 studies representing 19 of the 24 cure-related study categories. The studies represented in the responses will enrol 3936 of the 7373 total participants anticipated in the entire dataset.
Trial completion
Projected completion dates for ~90% of the sample fall between the fourth quarter of 2018 and the end of 2020 (range: 1 April 2017–1 June 2034; median 1 November 2019). These projected completion dates reflect those trials that are included in clinicaltrials.gov and the TAG listing; studies that are early in development are likely not included in the sample.
Study development and trial design
Across all survey responses, the average length of study development was 20 months (median = 18 months, range = 4–60 months). While 21 respondents (28%) reported no obstacles during development, the majority of respondents reported at least one challenge. Respondents could select multiple challenges from prepopulated responses and indicate additional challenges as open-ended responses. A total of 94 obstacles during study development were reported. Local regulatory obstacles were the most frequently cited challenge during development (n = 25, 26%), followed by securing funding (n = 19, 20%) and study team concerns over intensity of participation (n = 16, 17%).
A total of 38 of the 73 respondents specified that community representatives or advisory bodies were involved in protocol development and had the opportunity to provide feedback. The majority of these respondents (n = 22) noted that the community was enthusiastic about the trial or concept, while only 4 respondents noted community representatives’ concerns. Reported community concerns largely centred on ATIs and the potential for drug resistance.
The concerns of study teams regarding the intensity of HIV cure trials were primarily related to the number of complex or potentially invasive procedures required in many protocols. Of the 128 studies in the full dataset, at least 32 (25%) across 9 categories required participants to undergo an ATI. An additional 67 required invasive procedures, such as GALT biopsies, lymph node biopsies, lumbar punctures, leukapheresis and/or stem cell transplants. Because information on study procedures was collected from clinicaltrials.gov, it is possible that there are additional invasive procedures that were not submitted to the registry record by study contacts. In addition to ATIs and invasive sampling procedures, survey respondents expressed concerns over the ability of potential trial participants to meet strict inclusion/exclusion criteria and the required duration of study participation.
Factors impacting enrolment
Survey respondents were asked to select all applicable obstacles to enrolment from prepopulated responses and to indicate additional challenges as open-ended responses. A total of 51 survey respondents indicated at least one obstacle to enrolment with 82 obstacles to enrolment reported across all responses. The most commonly reported obstacle to enrolment was participants’ reluctance to undergo invasive procedures (n = 21), followed by ‘study has no benefit to participants’ (n = 15). In the open-ended answers provided to the survey question about enrolment obstacles, respondents noted strict inclusion/exclusion criteria (n = 12) and complex study visit schedules/lengthy follow-up periods (n = 10) as obstacles to enrolment. Several survey respondents commented on study procedures (stigma around lumbar punctures, fear around ATIs and the frequent site visits required) and discussed the need to strengthen relationships with providers who could provide referrals. Of note, 19 respondents (26%) reported no obstacles to enrolment (Table 2).
Table 2.
Obstacles to enrolment
| Prepopulated obstacle | Count | Survey respondents selecting this answer (%) |
|---|---|---|
| Participants are hesitant to undergo study procedures. | 21 | 28 |
| None | 19 | 26 |
| Study has no benefit for participants. | 15 | 20 |
| Participants are nervous about jeopardising current health. | 7 | 9 |
| Providers are unwilling to refer participants to the study. | 4 | 5 |
| Challenges developing effective recruitment materials | 4 | 5 |
| Difficulties meeting demographic enrolment targets | 3 | 4 |
|
28 | 38 |
Despite the intensity of participation, the vast majority (90%) of survey respondents who provided a projected completion date expected their studies to be completed by 2020 (range 2018–2034). These survey responses were in line with projected completion dates in the full dataset (which included the projected completion dates provided to clinicaltrials.gov for all studies). Survey respondents were also asked to indicate their trial's current enrolment status: 55% of studies are currently more than halfway enrolled. Although 30% of studies reported they were less than 25% enrolled, that group included studies that have not yet opened to enrolment.
The pace of enrolment across studies was highly variable (range 3–84 months), with an overall average by study of 21.4 months. The average anticipated time for different categories of study to fully enrol varied from 6 months or less (categories = imaging studies, antiproliferative and hormones) to longer than 2 years (categories = treatment intensification/early treatment, observational, immune checkpoint inhibitors and stem cell transplantation), with an average length of enrolment by category of 18.3 months (median = 18 months). Time to enrolment is likely influenced in part by a study's planned number of participants in addition to study procedures and other factors. Of the 10 studies that planned to enrol 10 or fewer participants, the average reported length of enrolment was 19 months (range 3–60). Of the 10 studies planning to enrol 100 or more participants, the average reported length of enrolment was 24 months (range 12–36).
Regarding invasive procedures, studies involving ATIs expected to enrol in an average of 22.86 months, studies with GALT/colorectal biopsies reported an average expected enrolment of 24.7 months, and studies with lumbar punctures and lymph node biopsies reported average expected enrolment times of 26.68 and 28 months, respectively.
Participant demographics
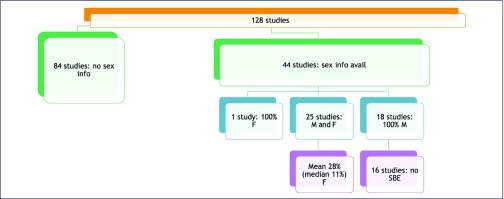
Although the survey asked respondents to provide demographic breakdowns when available, not all respondents were able to provide this information (whether it was not yet available, not yet sufficient to be meaningful or for other reasons). Approximately half of the respondents (n = 39) provided sex information about participants. Thirty-one respondents provided some degree of descriptive racial or ethnic information. Some studies that have been completed made this information available in clinicaltrials.gov, and published studies included demographic information on participants [16–18]. Combining these three sources of demographic data (clinicaltrials.gov, survey responses and publications), we identified race or ethnicity breakdowns for 34 studies, sex breakdowns for 44 of 128 studies and limited age information for 35 of 128 studies.
Data related to racial and ethnic diversity in trial participants were insufficient to conduct analysis for non-US or multinational studies. In US-only studies, enrolment (or enrolment to date) is as follows: 39% black or African American, 52% white and 16% Hispanic. There does not appear to be any correlation between a study's category and its demographic diversity.
Reported percentages of female participants ranged from 100% to 0%, with a mean across categories of 17% female and 83% male. Five respondents gave descriptive information (i.e. ‘mostly male’ or ‘most are males’) as opposed to estimated percentages. These descriptive entries were coded as 51% male and 49% female, so the actual percentage of males enrolled may be higher than is reflected in this descriptive analysis.
Of the 44 studies where participant sex was reported, 40% (n = 18) enrolled 100% male participants (Figure 2). One of these studies was limited to male participants, and another recruited from a cohort that was predominantly male. The remaining 16 studies that enrolled only male participants did not have sex-based exclusion criteria. In other words, there was no scientific or study-based rationale for enrolling only males, but only males were enrolled.
Figure 2.
Participant sex information. F, female; M, male; SBE, sex-based exclusion
Twenty-five of the 44 studies that provided participant sex information enrolled both male and female participants. The mean female enrolment in these studies is 28% (median 11%). When studies enrolling newborns and infants were excluded, the mean female enrolment dropped to 16%. Available data indicated that studies involving ATIs had enrolled 89% male participants.
Women's under-representation varied across curative strategy (Table 3), with five categories of curative strategy reporting no female participants enrolled to date. There was no apparent correlation between higher rates of female enrolment and study sponsor, study location or category of trial. Studies that enrolled infants and preadolescent children reported approximately equal sex distributions.
Table 3.
Sex distribution (when reported/available)
| Category | Male (%) | Female (%) | Number of studies in category with sex data available (of total number of studies in category) |
|---|---|---|---|
| Anti-inflammatory | 100 | 0 | 2 (of 3) |
| Antiproliferative | 100 | 0 | 1 (of 1) |
| Antibodies | 73 | 27 | 7 (of 13) |
| Antifibrotic | 100 | 0 | 1 (of 2) |
| Combinations | 79 | 21 | 6 (of 17) |
| Gene therapies | 77 | 23 | 1 (of 8) |
| Immune checkpoint inhibitors | 73 | 27 | 3 (of 4) |
| Mammalian target of rapamycin inhibitors | 100 | 0 | 1 (of 2) |
| Observational | 86 | 14 | 7 (of 29) |
| Proteasome inhibitors | 100 | 0 | 1 (of 1) |
| Stem cell transplantation | 75 | 25 | 2 (of 4) |
| Therapeutic vaccines | 96 | 4 | 5 (of 8) |
| Treatment intensification/early tx | 73 | 27 | 7 (of 10) |
| Total | 82.73 | 17.27 |
Discussion
The current landscape of cure-related clinical research includes a diverse array of interventional and observational studies. In the majority of cases, trials are early stage (phase I or II) and sample sizes are small. Results are expected to become available over the next 2–4 years and will likely inform future curative research.
Potential obstacles to the implementation of study protocols that were identified in our survey included local regulatory review, which may suggest a need to enhance the knowledge of institutional review board members regarding HIV cure research. Securing funding was also cited as an obstacle to trial development, emphasising the importance of increasing financial investment in the field. The field of HIV research – biomedical prevention as well as treatment and cure – is quite complex and requires increased engagement and understanding from all invested parties: review boards [19], communities [20–23] and referring providers.
Factors impacting enrolment
The reported enrolment obstacles – and the high reports of no obstacles to enrolment – merit further discussion and collaboration between community and researchers, particularly in light of evolving conversations around the acceptability of ATIs and other invasive procedures in HIV cure research [24,25]. Because these small early-phase studies are not required to enrol diverse participants, it will be important for study teams to balance the need to enrol quickly and to meet stringent inclusion/exclusion criteria with the potential ramifications of not including any female participants.
Survey responses indicated that, even with a high number of highly invasive procedures, many cure trials are able to enrol quickly. These data suggest that despite the lack of benefit for trial participants and the intense nature of sampling procedures, HIV cure-related trials that require invasive procedures and/or ATIs are able to enrol at a pace similar to those without such requirements.
Participant demographics
In agreement with prior analyses [26,27], our findings identified an under-representation of women in current studies. Our findings, including that women's under-representation is more pronounced in some categories of cure research, are in line with earlier findings on women's under-representation in cure research [26]. The demographic information provided in surveys is incomplete – respondents were asked to provide information ‘to date’ – meaning participant demographics may change as trials fully accrue. Trends should be interpreted with caution; however, we are still able to identify areas where increased participant diversity is needed, including the five categories of cure trials that reported zero female enrolment.
Importantly, several studies were able to enrol higher numbers of adult female participants, including one study that was open only to female participants. The enrolment strategies these studies reported did not differ significantly from studies that exclusively or primarily enrolled male participants. It may be worthwhile to ask representatives of these studies to provide additional qualitative information about their recruitment practices, including the use of financial incentives. A planned follow-up survey in 2019 will seek to further explore these issues.
Given that the vast majority of studies in this analysis (113 of 128) are phase I or II, they are not required to enrol adequate numbers of women or minorities under the National Institutes of Health Revitalization Act, and studies taking place outside of the NIH's purview are not bound by this mandate. However, because the current landscape of cure trials is still in its early stages, now is a critical moment to incorporate measures to increase participant diversity (geographic, racial/ethnic, sex and gender). Previously identified sex differences in HIV reservoirs and persistence [28–31] underscore the importance of increasing female enrolment in HIV cure studies.
Lack of diversity, both in terms of participants and the geographic location of the research, could significantly affect the generalisability of anticipated results. Enrolling diverse participants not only may allow researchers to identify potentially important safety signals or relevant endpoints but also will ensure that mechanisms are in place for curative strategies to reach all populations impacted by HIV and perhaps avoid implementation challenges akin to those seen with pre-exposure prophylaxis rollout [20]. Since HIV cure research is increasingly asking participants to give more in terms of number and intensity of procedures while receiving little or no personal benefit, every effort should be made to ensure that results can be as broadly applicable as possible.
Ethical considerations
This analysis highlights a need to try and diversify participation, while recognising that the absence of potential benefit raises difficult questions regarding how to ethically manage risk and places an onus on altruism as a motivation to join studies. Facilitating the informed participation of a broader spectrum of people living with HIV is likely to require increasing the availability and accessibility of information on cure research. While simply enrolling larger numbers of women or minorities is an insufficient solution to the structural health disparities that decrease their participation in the first place [32,33], a body of research has demonstrated that historically under-represented populations are willing to participate in research when they are asked [34,35], and at least one study of screen-out rates in a cohort of AIDS Clinical Trials Group trials found that women's screen-out rates do not differ significantly from men's [36].
Community enthusiasm for HIV cure research remains high, as does momentum to diversify the participant base of HIV cure research [37,38]. Many researchers have recognised the importance of early and meaningful community engagement from trial development through implementation [39]. We are heartened that over half of survey respondents reported community involvement during trial development and encourage investigators to continue seeking input from diverse communities. Further characterising community involvement and engagement – how this is understood and operationalised in different settings and by different stakeholders – will be an important area of future research.
Limitations
There are a number of potentially important limitations to our analysis. The primary means of identifying cure-related clinical trials and observational studies was the clinicaltrials.gov registry, which relies on those conducting the research to supply information and does not mandate entry of phase I trials.
The response rate to our survey was 57%; however, we had hoped for higher overall responses and representation from all categories of curative strategy. Not all respondents provided elaboration or comments for different survey questions, including describing community engagement, participant demographics or enrolment challenges, which limits our ability to make comparisons across trials. Notably, detailed breakdowns on the sex and ethnicity of participants were only available for a subset of studies. This is understandable since survey respondents were often providing real-time estimates for studies that are still enrolling, and we hope that more information will be available during the planned follow-up.
Responses were not anonymous. We disclosed to respondents in survey instructions that the survey had been requested by The Bill and Melinda Gates Foundation, an organisation that is known to fund global HIV research. These two factors may have produced possible response bias in our results.
Acknowledgements
The authors thank Mike McCune and Emily Turner of the Bill & Melinda Gates Foundation for guidance on the project and editorial assistance with the manuscript, and Karine Dubé, DrPH, MPhil (Oxon), of the University of North Carolina for her comments on the manuscript. The authors would also like to thank all the respondents who took time to complete the survey and made this analysis possible.
Source of Funding
This study was supported by the Bill & Melinda Gates Foundation.
Conflicts of interest
All authors declare that they have no competing interests.
Authors’ contributions
RJ initiated the TAG Research Toward an HIV Cure online listing. LB designed and conducted the survey and performed the data collection and analyses. Both authors collaborated on writing the manuscript.
Appendix
Survey instrument
-
1.
What is the clinicaltrials.gov NCT number for this study?
-
2.
If there is no NCT number, please provide the study's name.
-
3.
What is your role in the study?
Study development
-
4.
When did your trial/study open to enrolment? (List anticipated date if not yet enrolling.)
-
5.
Approximately how long was the development process for this study?
-
6.
If any community advisory bodies/representatives provided input during study development, please comment on that process (e.g. Were they enthusiastic about the study? Did they express concerns? How involved were they in study planning?)
-
7.Did you encounter any of the following challenges during study development?
-
○Local regulatory obstacles (IRB)
-
○Regulatory obstacles from funder
-
○Study team concerns over intensity of participation (e.g. a specific procedure and number of procedures)
-
○Community concerns over an aspect of study (please specify)
-
○Securing funding
-
○Securing study products and/or required infrastructure
-
○Other
-
○
-
8.
Any additional comments on study development?
Study design
-
9.
How many participants are you planning to enrol?
-
10.
What are the study's demographic targets for enrolment (e.g. percentages of total in terms of age, sex, gender and ethnicity)? If there are no targets, please write ‘none’.
-
11.
If enrolment targets exist, how were they determined and what plans are in place to meet them?
-
12.Is the study taking place at a single site or multiple sites?
-
○Single site
-
○Multiple sites
-
○
-
13.In what countries is this study enrolling? Select all that apply:
-
○Argentina
-
○Australia
-
○Belgium
-
○Botswana
-
○Brazil
-
○Canada
-
○China
-
○Denmark
-
○France
-
○Germany
-
○Haiti
-
○India
-
○Italy
-
○Kenya
-
○Malawi
-
○Netherlands
-
○South Africa
-
○Spain
-
○Sweden
-
○Switzerland
-
○Tanzania
-
○Thailand
-
○Uganda
-
○UK
-
○USA
-
○Zimbabwe
-
○Other
-
○
-
14.Why were these locations chosen? Select all that apply
-
○Location of PI's institution
-
○Sites’ infrastructure/capacity
-
○Demographic considerations
-
○Funding considerations
-
○Regulatory considerations
-
○To draw on existing connections in local community
-
○To draw on existing connections at sites
-
○Other
-
○
-
15.
Any additional comments on site selection
Study recruitment and enrolment
-
16.What recruitment strategies are being employed? Select all that apply:
-
○Printed flyers and posters
-
○Social media campaigns
-
○Word of mouth
-
○Physician/clinic referral
-
○Media outreach (press releases and interviews)
-
○Professional marketing/communications effort
-
○Other
-
○
-
17.
Have you consulted with community advisory bodies or representatives regarding recruitment and/or recruitment strategies? If yes, please describe.
-
18.What is the study's current enrolment status?
-
○Less than 25% enrolled
-
○25%–49% enrolled
-
○50%–74% enrolled
-
○More than 75% enrolled
-
○
-
19.
If possible, please provide an approximate demographic breakdown of current enrollees (e.g. age, ethnicity and sex).
-
20.How does this breakdown compare to your enrolment targets (if applicable)?
-
○There were no demographic targets.
-
○We are on track to meet some demographic targets (specify which ones below).
-
○We are on track to meet all demographic targets.
-
○We will not meet any demographic targets.
-
○It is too early to tell.
-
○Other
-
○
-
21.Which demographic targets will you meet?
-
○Age targets
-
○Ethnicity targets
-
○Gender targets
-
○Sex targets
-
○Other
-
○
-
22.
How long do you estimate enrolment will take from start to finish?
-
23.What is the basis for this estimate? Select all that apply.
-
○Current rate of enrolment
-
○Historical enrolment at these sites
-
○Other
-
○
-
24.What obstacles to enrolment have you encountered? Select all that apply:
-
○Participants are hesitant to undergo study procedures.
-
○Participants are nervous about jeopardising current health.
-
○Providers are unwilling to refer participants to the study.
-
○Study has no benefit for participants.
-
○Challenges developing effective recruitment materials.
-
○Difficulties meeting demographic enrolment targets.
-
○Other
-
○
-
25.
Please elaborate on enrolment obstacles, if desired.
Study completion
-
26.
What is the current projected study completion date?
-
27.Do you have an anticipated timeline for presentation/publication of results?
-
○Yes
-
○No
-
○Other
-
○
-
28.
If you answered yes above, please describe your timeline and plans for disseminating study results (e.g. publication, presentation and/or reporting back to participants).
-
29.
Is there anything you wish to add regarding any aspect of this study?
References
- 1. Deeks SG, Lewin SR, Ross AL et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22: 839– 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allers K, Hütter G, Hofmann J et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 2011; 117: 2791– 2799. [DOI] [PubMed] [Google Scholar]
- 3. Henrich TJ, Hanhauser E, Marty FM et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 2014; 161: 319– 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luzuriaga K, Gay H, Ziemniak C et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372: 786– 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frange P, Faye A, Avettand-Fenoël V et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3: e49– e54. [DOI] [PubMed] [Google Scholar]
- 6. Henrich TJ, Hatano H, Bacon O et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med 2017; 14: e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sáez-Cirión A, Bacchus C, Hocqueloux L et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin GE, Frater J. Post-treatment and spontaneous HIV control. Curr Opin HIV AIDS. 2018; 13: 402– 407. [DOI] [PubMed] [Google Scholar]
- 9. Walensky RP, Office of AIDS Research Advisory Council (OARAC) HIV/AIDS Research Portfolio Review Working Group. Auerbach JD. Focusing National Institutes of Health HIV/AIDS research for maximum population impact. Clin Infect Dis 2015; 60: 937– 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins FS. Statement on NIH efforts to focus research to end the AIDS pandemic. National Institutes of Health, August 11, 2015 Available at: www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-efforts-focus-research-end-aids-pandemic ( accessed July 2019).
- 11. AVAC , Global investment in HIV cure research and development in 2017. (July 2018). Available at: www.avac.org/resource/global-investment-hiv-cure-research-and-development-2017 ( accessed July 2019).
- 12. Treatment Action Group Research toward a cure trials. Available at: www. treatmentactiongroup.org/cure/trials ( accessed July 2019).
- 13. Clinical Trials Registration and Results Information Submission 42 C.F.R. § 11. [PubMed]
- 14. UK Clinical Trials Gateway Available at: www.ukctg.nihr.ac.uk/ ( accessed July 2019).
- 15. The EPIC4 Study Website Available at: epic4.ca/ ( accessed July 2019).
- 16. Sneller MC, Justement JS, Gittens KR et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chintanaphol M, Sacdalan C, Chottanapund S et al. Brief Report: Safety and Tolerability of Inguinal Lymph Node Biopsy in Individuals With Acute HIV Infection in Thailand. J Acquir Immune Defic Syndr 2018; 79: 244– 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tebas P, Stein D, Binder-Scholl G et al. Antiviral effects of autologous CD4 T cells genetically modified with a conditionally replicating lentiviral vector expressing long antisense to HIV. Blood 2013; 121: 1524– 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slack C, Wilkinson A, Salzwedel J et al. Strengthening stakeholder engagement through ethics review in biomedical HIV prevention trials: opportunities and complexities. J Int AIDS Soc. 2018; 21 ( Suppl 7): e25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo YR, Chu C, Ananworanich J et al. Stakeholder engagement in HIV cure research: lessons learned from other HIV interventions and the way forward. AIDS Patient Care STDS 2015; 29: 389– 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathews A, Farley S, Blumberg M et al. HIV cure research community engagement in North Carolina: a mixed-methods evaluation of a crowdsourcing contest. J Virus Erad. 2017; 3: 223– 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacQueen KM, Auerbach JD. It is not just about “the trial”: the critical role of effective engagement and participatory practices for moving the HIV research field forward. J Int AIDS Soc. 2018; 21 ( S7): e25179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newman PA, Rubincam C.. Advancing community stakeholder engagement in biomedical HIV prevention trials: principles, practices and evidence. Expert Rev Vaccines 2014; 13: 1553– 1562. [DOI] [PubMed] [Google Scholar]
- 24. Julg B, Dee L, Ananworanich J et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials – report of a consensus meeting. Lancet HIV 2019; [ Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubé K, Henderson GE, Margolis DM. Framing expectations in early HIV cure research. Trends Microbiol 2014; 22: 547– 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnston RE, Heitzeg MM. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum Retroviruses 2015; 31: 85– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curno MJ, Rossi S, Hodges-Mameletzis I et al. Systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr 2016 ; 71: 181– 188. [DOI] [PubMed] [Google Scholar]
- 28. Scully EP, Gandhi M, Johnston R et al. Sex-based differences in HIV-1 reservoir activity and residual immune activation. J Infect Dis 2018; [ Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das B, Dobrowolski C, Luttge B et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci USA 2018; 115: E7795– E7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep. 2018; 15: 136– 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gianella S, Tsibris A, Barr L et al. Barriers to a cure for HIV in women. J Int AIDS Soc. 2016; 19: 20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oh SS, Galanter J, Thakur N et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med 2015; 12: e1001918 Published 2015 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erves JC, Mayo-Gamble TL, Malin-Fair A et al. Needs, priorities, and recommendations for engaging underrepresented populations in clinical research: a community perspective. J Community Health 2017; 42: 472– 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garber M, Hanusa BH, Switzer GE et al. HIV-infected African Americans are willing to participate in HIV treatment trials. J Gen Intern Med 2007; 22: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolak C, Bass SB, Tedaldi E et al. Minority HIV patients’ perceptions of barriers and facilitators to participation in clinical research. Curr HIV Res 2012; 10: 348– 355. [DOI] [PubMed] [Google Scholar]
- 36. Smeaton L, Mykhalchenko K, Koletar SL et al. Screening and enrollment by sex in HIV clinical trials – a cross-protocol analysis. 37th Society for Clinical Trials Annual Meeting, 15–18 May, 2016, Montreal, Quebec, Canada.
- 37. Dubé K, Evans D, Sylla L et al. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J Virus Erad. 2017; 3: 40– 50, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Women's Research Initiative on HIV/AIDS Advocating for, discovering and delivering a cure. 2017. Available at: www.thewellproject.org/sites/default/files/WRI%20BRIEF%20FINAL.pdf ( accessed July 2019).
- 39. Grossman CI, Ross AL, Auerbach JD et al. Towards multidisciplinary HIV-cure research: integrating social science with biomedical research. Trends Microbiol 2016; 24: 5– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]