Abstract
Objective
This study aimed to compare treatment outcomes and factors associated with mortality in HIV-1-positive and HIV-1-negative individuals.
Methods
We conducted a cohort study between July 2008 and December 2016. Logistic regression was used to determine factors associated with outcomes and death after tuberculosis (TB) treatment.
Results
A total of 996 individuals with TB, 228 (22.9%) with HIV-1 co-infection and 770 (77.1%) who were HIV-1 negative were reviewed. The overall treatment success rate was 74.3%. The HIV-1-negative individuals with TB had significantly higher treatment success rates (77.2% vs 64.5%, P < 0.001). Using logistic regression analysis, age >50 years (adjusted odds ratio [aOR] 3.89, 95% confidence interval [CI] 2.24–6.76; P < 0.001), body weight ≤45 kg (aOR 2.19, 95% CI 1.14–4.19; P = 0.02) and HIV-1-positive status (aOR 3.31, 95% CI 1.84–5.91; P < 0.001) were independently associated with death during TB treatment. Among HIV-1-positive individuals, not undergoing antiretroviral therapy (ART), having diabetes and a CD4 T cell count of <50 cells/mm3 were significantly associated with death.
Conclusion
Individuals who had both TB and HIV-1 in Thailand had lower TB treatment success and higher mortality rates compared with individuals with TB without HIV-1. Strategies to improve ART uptake and to reduce risk of developing active TB among individuals with advanced HIV-1 infection should be scaled up.
Keywords: treatment outcome, risk factors, TB/HIV, antiretroviral therapy
Introduction
Tuberculosis (TB) remains a major global health problem and one of the top 10 causes of death globally. In 2016, the World Health Organization (WHO) estimated that the incidence of TB was at 10.4 million cases worldwide, including 1 million (10%) among people living with HIV (PLHIV). In addition, there were an estimated 1.3 million TB deaths, 374,000 of which were among PLHIV [1].
With successful antiretroviral therapy (ART), PLHIV life expectancy has dramatically increased [2]. Nevertheless, HIV-1 co-infection is the most important risk factor for developing active TB. High HIV-1 prevalence rates are significantly correlated with high TB incidence rates [3]. The WHO recommends that all individuals with TB co-infected with HIV (TB/HIV) should be started on ART, irrespective of CD4 count [4] because treatment initiation during TB therapy significantly improves survival in these individuals [5–8].
In 2016, Thailand was classified by the WHO as one of the 14 countries with the highest TB burden, multidrug resistant tuberculosis (MDR-TB) and TB/HIV co-infection [9]. The universal coverage system of the National Health Security Office now provides both anti-TB drugs and ART free of charge, and Thai guidelines recommend initiating ART at any CD4 T cell count. A 2014 survey conducted in public hospitals and government-owned TB treatment centres found that 13% of the new TB cases occurred in PLHIV, but that only 69% had positive TB treatment outcomes compared with 80% in HIV-1-negative individuals, both results being below the WHO target for treatment success at 85% [10]. However, there are no data regarding TB treatment outcomes in tertiary care hospitals. We therefore aimed to evaluate TB treatment outcomes and factors associated with mortality among individuals with TB/HIV co-infection who underwent treatment in this setting, where ART use is implemented nationwide.
Methods
Study populations
We conducted a combination of prospective and retrospective cohort studies in three large tertiary care hospitals with 300–1500 beds: the King Chulalongkorn Memorial Hospital, Rajavithi Hospital and the Bamrasnaradura Infectious Diseases Institute, which together represent the ‘Thailand Big City TB Research Network’ clinical cohort. The retrospective study included individuals treated between July 2008 and December 2016, and the prospective study included those treated from August 2011 to December 2016.
The study design and diagnostic efficacy, but not the clinical outcomes, of the prospective cohort were described previously [11]. Briefly, the main inclusion criteria for the prospective cohort study were adult individuals suspected of active pulmonary TB and whose HIV-1 status was known. All provided written informed consent for the study.
The retrospective cohort study was conducted among adult individuals who were diagnosed with active pulmonary or extrapulmonary TB and were treated at the King Chulalongkorn Memorial Hospital. Data were extracted from individual medical records. Criteria for inclusion were individuals with known HIV-1 status diagnosed with active TB using clinical symptoms, positive results for either acid-fast staining or PCR testing, or positive culture for Mycobacterium tuberculosis who underwent TB therapy.
The primary outcome of the current study was to investigate TB treatment outcomes among individuals with TB with and without HIV-1 co-infection. Treatment outcome definitions were modified from the WHO 2013 ‘Definitions and Reporting Framework for Tuberculosis’ as follows [12]: (1) cure = individuals with bacteriologically confirmed TB at the beginning of treatment who became sputum smear- or culture-negative at the last month of treatment, (2) treatment completed = individuals who completed treatment without evidence of failure and bacteriological confirmation at the last month of treatment, (3) treatment success = the sum of cured and treatment-completed individuals, (4) treatment failed = individuals with sputum smear- or culture-positive results at month 5, (5) died = individuals who died for any reason during the course of TB treatment and (6) lost to follow-up = individuals who did not return to clinic to complete 6 months of treatment. Outcomes of cases who had transferred from the treatment centre were defined as ‘not evaluated’ and excluded from the analysis.
The secondary objectives were to determine factors associated with mortality in both cohorts.
Extrapulmonary TB was defined as TB diagnosed in the lymph nodes, the pleura, the gastrointestinal tract or the central nervous system. Medical records of individuals who had died in each of the hospitals were reviewed by the same physician. Cause of death was defined as the last event responsible for the individuals’ death, whatever the underlying condition may have been.
Data management and analysis
Medical data were transferred in electronic format. Mean (SD), median (interquartile range [IQR]) and frequencies (%) were used to describe individuals’ characteristics in each study. Group χ2 and Mann–Whitney U tests were used to formally compare categorical and continuous variables between the two groups, respectively.
Multivariable logistic regression was used to determine factors associated with death after TB treatment and treatment success. Covariates assessed included demographics, sex, age, low body weight (body weight ≤45 kg classified by IQR), as well as clinical variables such as HIV-1 status, history of previous TB diagnosis, comorbidities including diabetes and chronic kidney disease (defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2), sputum smear result and susceptibility of M. tuberculosis to TB drugs. Covariates significant at P < 0.1 in univariable models were adjusted for in a multivariable model. A separate model was developed for HIV-1-positive individuals that included the CD4 T cell count at TB treatment initiation. Statistical analyses were performed using Stata 15.1 (StataCorp, College Station, TX, USA). The study was reviewed and approved by each institution's review board. The study was carried out in compliance with the relevant laws and guidelines, in accordance with the ethical standards of the Declaration of Helsinki.
Results
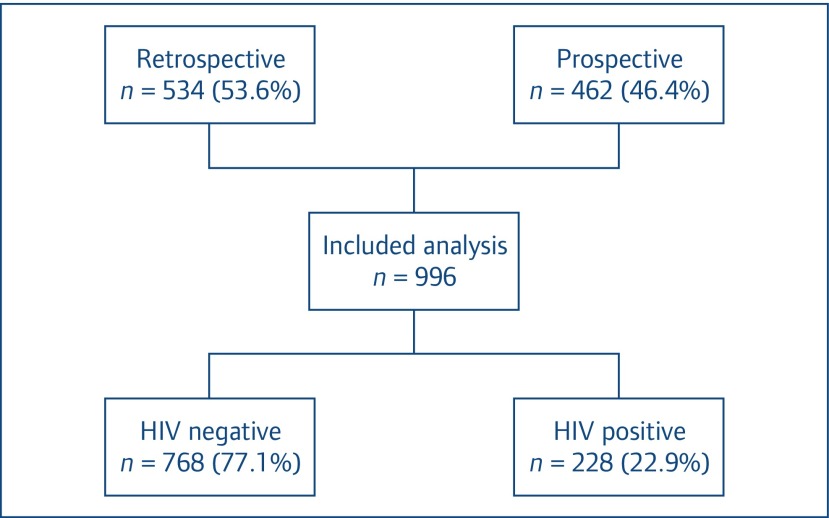
A total of 1222 individuals with TB were enrolled in the study; 198 individuals were excluded since their HIV-1 status could not be confirmed, 28 individuals were excluded due to transfer out to other hospitals and 996 individuals fulfilled the inclusion criteria and were analysed (Figure 1). Individuals’ characteristics are shown in Table 1. Two hundred twenty-eight (22.9%) were HIV-1 positive and 770 (77.1%) HIV-1 negative. The majority of participants were male (60.1%), with a median age of 41 (IQR 30–55) years. The HIV-1-positive participants had a lower body weight than the HIV-1-negative ones (P = 0.009) and had a higher rate of previous TB diagnosis (P < 0.001). Among HIV-1 infected participants, the baseline median (IQR) CD4 T cell count was 74 (IQR 27–199) cells/mm3. Most of the study participants were diagnosed with pulmonary TB (75%). Forty per cent of individuals had a positive acid-fast bacilli (AFB) smear. The HIV-negative individuals with TB had higher rates of positive AFB smear than those with HIV-1 co-infection (41% vs 33%, P = 0.05). Forty-one per cent of all TB cases were found to be culture positive. Drug susceptibility testing (DST) was performed in 75% (308 of 409) of culture-positive samples with 72.7%, 16.2%, 4.9% and 6.2% displaying no resistance, monoresistance, polyresistance and multidrug resistance, respectively. There was no difference in culture positivity and DST results according to HIV-1 status.
Figure 1.
Overview of cases included in the analysis
Table 1.
Individuals’ characteristics
| Variables | Total (N = 996) | HIV negative (n = 768) | HIV positive (n = 228) | P-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 41.3 (30.4–54.8) | 43.7 (30.3–58.5) | 35.1 (30.4–42.6) | <0.001 |
| Sex: male (n)* | 593 (60.1) | 430 (56.7) | 163 (71.5) | <0.001 |
| Weight (kg), median (IQR) | 51.2 (45.8–58.9) | 52 (46.0–59.3) | 50 (43.7–57.2) | 0.009 |
| Type of TB | 0.36 | |||
| Pulmonary | 744 (74.7) | 579 (75.4) | 165 (72.4) | |
| Extrapulmonary | 252 (25.3) | 189 (24.6) | 63 (27.6) | |
| Prior TB diagnosis | 189 (18.9) | 121 (15.6) | 68 (29.8) | <0.001 |
| Culture results | 0.12 | |||
| Not done | 449 (45.1) | 350 (45.6) | 99 (43.4) | |
| Negative | 138 (13.8) | 97 (12.6) | 41 (18) | |
| Positive | 409 (41.1) | 323 (41.8) | 88 (38.6) | |
| Smear results | 0.05 | |||
| Not done | 112 (11.2) | 88 (11.5) | 24 (10.5) | |
| Negative | 490 (49.2) | 362 (47.1) | 128 (56.1) | |
| Positive | 394 (39.6) | 320 (41.4) | 76 (33.3) | |
| Drug resistance profile | 0.66 | |||
| No resistance | 224 (72.7) | 167 (72.3) | 57 (74) | |
| Mono resistance | 50 (16.2) | 38 (16.5) | 12 (15.6) | |
| Polyresistance | 15 (4.9) | 13 (5.6) | 2 (2.6) | |
| Multidrug resistant | 19 (6.2) | 13 (5.6) | 6 (7.8) | |
| Diabetes | 81 (8.1) | 77 (10) | 4 (1.8) | <0.001 |
| Chronic kidney disease | 19 (1.9) | 16 (2.1) | 3 (1.3) | 0.46 |
| CD4 cell count (cells/mm3), median (IQR) | 74 (27–199) |
IQR: interquartile range; TB: tuberculosis.
Data are n (%) unless otherwise specified.
Tuberculosis treatment outcome
The overall successful TB treatment outcome (defined as either ‘cured’ or ‘treatment completed’) was 74.3%. The HIV-1-negative individuals with TB had significantly higher treatment success rates than those with HIV-1 co-infection (77.2% vs 64.5%, P < 0.001; Table 2). Among individuals with HIV-1/TB, the cure rate was significantly lower and the death rate was significantly higher than among individuals who were HIV-1 negative (8.8% vs 20.3%, P < 0.001, and 12.7% vs 7.7%, P = 0.02, respectively). We also found that individuals with HIV co-infection with TB had higher lost-to-follow-up rates (22.4% vs 14.2%, P = 0.004).
Table 2.
Treatment outcomes by HIV status
| Total
n (%) |
HIV negative
n (%) |
HIV positive
n (%) |
P-value | |
|---|---|---|---|---|
| Cured | 176 (17.7) | 156 (20.3) | 20 (8.8) | <0.001 |
| Treatment completed | 564 (56.6) | 437 (56.9) | 127 (55.7) | 0.75 |
| Treatment success (cured or treatment completed) | 740 (74.3) | 595 (77.2) | 147 (64.5) | <0.001 |
| Treatment failed | 8 (0.8) | 7 (0.9) | 1 (0.4) | 0.69 |
| Lost to follow-up | 160 (16.1) | 109 (14.2) | 51 (22.4) | 0.004 |
| Died | 88 (8.8) | 59 (7.7) | 29 (12.7) | 0.02 |
| TB related | 44 (4.4) | 30 (3.9) | 14 (6.1) | |
| Unrelated to TB | 44 (4.4) | 29 (3.8) | 15 (6.6) |
TB: tuberculosis.
Results from univariate logistic regression analysis showed that there were more deaths among individuals aged >50 years, those with body weight ≤45 kg, those with underlying diabetes mellitus (DM), and those who were HIV-1 positive. In multivariable logistic regression analysis, age >50 years (adjusted odds ratio [aOR] 3.89, 95% confidence interval [CI] 2.24–6.76; P < 0.001), body weight ≤45 kg (aOR 2.19, 95% CI 1.14–4.19; P = 0.02) and HIV-1 positivity (aOR 3.31, 95% CI 1.84–5.91; P < 0.001) were independently associated with death during TB treatment (Table 3). In a multivariate model with treatment success as the outcome, only the site of TB infection (pulmonary vs extrapulmonary, aOR 5.82, 95% CI 2.67–12.67; P < 0.001) was associated with successful treatment outcome (Table 4).
Table 3.
Risk factors for death during TB treatment
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Adjusted odds ratio (95% CI) | P-value | |
| All individuals | ||||
| Age >50 vs ≤50 years | 3.19 (2.04–4.99) | <0.001 | 3.89 (2.24–6.76) | <0.001 |
| Male | 1.47 (0.92–2.35) | 0.11 | ||
| Weight ≤45 kg vs >45 kg | 2.65 (1.42–4.94) | 0.002 | 2.19 (1.14–4.19) | 0.02 |
| Pulmonary vs extrapulmonary TB | 0.74 (0.43–1.27) | 0.28 | ||
| Prior TB diagnosis | 1.38 (0.82–2.31) | 0.22 | ||
| TB/HIV co-infection vs TB mono-infection | 1.75 (1.09–2.81) | 0.02 | 3.31 (1.84–5.91) | <0.001 |
| Diabetes | 2.14 (1.13–4.06) | 0.02 | 1.71 (0.84–3.47) | 0.14 |
| CKD* | 2.84 (0.92–8.75) | 0.10 | ||
| Multidrug resistance | 1.12 (0.24–5.10) | 0.89 | ||
| AFB smear positive | 0.86 (0.55–1.37) | 0.54 | ||
| Individuals with TB/HIV co-infection only | ||||
| Age >50 vs ≤50 years | 4.46 (1.51–13.19) | 0.007 | 1.75 (0.30–10.04) | 0.53 |
| Male | 1.62 (0.63–4.17) | 0.32 | ||
| Weight ≤45 kg vs >45 kg | 1.83 (0.65–5.22) | 0.25 | ||
| Pulmonary vs extrapulmonary TB | 0.81 (0.33–2.0) | 0.65 | ||
| Prior TB diagnosis | 1.80 (0.81–4.0) | 0.15 | ||
| Diabetes | 7.3 (0.99–53.9) | 0.05 | 29.8 (1.55–574.2) | 0.02 |
| CD4 cell count <50 vs ≥50 cells/mm3 | 3.25 (1.22–8.62) | 0.02 | 4.27 (1.48–12.3) | 0.007 |
| Not undergoing ART | 2.4 (1.10–5.33) | 0.03 | 3.59 (1.30–9.92) | 0.01 |
| Multidrug resistance | 2.94 (0.47–18.5) | 0.25 | ||
| AFB smear positive | 0.64 (0.27–1.52) | 0.31 | ||
AFB: acid-fast bacilli; ART: antiretroviral therapy; CI: confidence interval; CKD: chronic kidney disease; TB: tuberculosis.
CKD was not included in the multivariate model because three of four diabetic individuals also had CKD, leading to collinearity problems in the model.
Table 4.
Factors associated with treatment success
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Adjusted odds ratio (95% CI) | P-value | |
| All individuals | ||||
| Age >50 vs ≤50 years | 1.6 (1.05–2.43) | 0.03 | 1.5 (0.95–2.37) | 0.08 |
| Male | 0.99 (0.68–1.44) | 0.96 | ||
| Weight ≤45 kg vs >45 kg | 0.77 (0.55–1.1) | 0.15 | ||
| Pulmonary vs extrapulmonary TB | 5.58 (2.58–12.07) | <0.001 | 5.82 (2.67–12.67) | <0.001 |
| Prior TB diagnosis | 0.73 (0.47–1.14) | 0.16 | ||
| TB/HIV co-infection vs TB mono-infection | 1.25 (0.8–1.96) | 0.32 | ||
| Diabetes | 0.57 (0.29–1.1) | 0.09 | 0.67 (0.33–1.38) | 0.28 |
| Chronic kidney disease | 0.77 (0.19–3.1) | 0.71 | ||
CI: confidence interval; TB: tuberculosis.
In the subgroup analysis of 228 HIV-1-positive participants, 83 (36.4%) were not on any ART until the end of their follow-up period. Eighty-five (37.3%) participants were ART naive and underwent ART after TB treatment. Sixty (26.3%) participants were on ART before starting TB treatment. The median baseline CD4 T cell count among participants who underwent ART before TB treatment, after TB treatment and did not undergo ART were 106, 51.5 and 76 cells/mm3 (P=0.06), respectively.
Multivariable logistic regression analysis among individuals with TB/HIV co-infection demonstrated that DM (aOR 29.8, 95% CI 1.55–574.2; P = 0.02), a CD4 T cell count of <50 cells/mm3 (aOR 4.27, 95% CI 1.48–12.3; P = 0.007) and the absence of ART at baseline (aOR 3.59, 95% CI 1.30–9.92; P = 0.01) were associated with death.
The overall occurrence of adverse events, such as rash, hepatotoxicity, nausea and fever, was significantly higher in individuals with HIV-1 co-infection than in HIV-1-negative ones (17.5% vs 10.6%, P = 0.005). Participants with a history of previous TB had a higher lost-to-follow-up rate than those who were first diagnosed with TB (aOR 1.53, 95% CI 1.02–2.30; P = 0.04).
Discussion
This study evaluated TB treatment outcomes and factors associated with mortality between individuals with TB/HIV-1 co-infection and those without HIV-1 co-infection from three tertiary care hospitals in Bangkok, Thailand. The overall treatment success rate was 74.3%, which was higher than that found in a previous study that was also conducted in Bangkok and in the Nonthaburi province in Thailand [13]. Our cohort outcome may have improved because of its prospective component, which may have resulted in a better follow-up rate. Nevertheless, our success rate remains lower than the WHO-recommended target of 85% [10]. Successful treatment outcomes were lower among similar individuals with TB/HIV-1 co-infection as compared with HIV-1-negative ones, as found in previous studies [14,15]. In addition, the mortality rate was higher among individuals with TB/HIV-1 co-infection [16,17]. Notably, individuals with TB/HIV co-infection in our study were in the advanced stages of HIV-1 infection with a median CD4 T cell count of 74 cells/mm3.
It is an alarming fact that many individuals with HIV-1 co-infection with TB did not undergo ART during TB treatment. Thailand has implemented ART universal access and we would have expected that all individuals with TB/HIV-1 co-infection would have been on ART; however, this was not the case in our study. Approximately one-third of participants with TB/HIV-1 co-infection were not on ART during their entire TB treatment time, a finding similar to the data from the Thailand National TB and HIV programme, where only 69% underwent ART [10]. There is strong evidence that individuals with TB/HIV have a higher mortality rate if HIV is not treated [7,8,16,18–20]. These data were confirmed by multivariable logistic regression analysis among individuals with TB/HIV-1 co-infection in our study, which demonstrated that a CD4 T cell count of <50 cells/mm3 and the absence of ART during TB treatment were associated with death. Interestingly, we found that TB/HIV co-infection with DM was associated with death, whereas DM was not associated with death in the entire cohort. This can be explained by a good control of blood sugar among HIV-negative participants with TB (median fasting blood sugar and HbA1c during TB treatment were 132 mg/dL, IQR 114–176 mg/dL and 7.7%, IQR 6.35%–9.0%, respectively).
DM is also associated with immunosuppression and increased risk of acquiring TB [21]. Several previous studies have suggested that it is an important risk factor for treatment failure and death among individuals with TB [22–24]. The recent global burden of TB study suggests that efforts to prevent DM can have a substantial collateral impact on TB burden because many low- and middle-income countries have increasing DM prevalence rates due to demographic and epidemiological transition, with, as a result, increased burden of both TB and DM [25].
Besides HIV-1 co-infection, a higher rate of mortality was also observed for individuals >50 years of age or with a body weight <45 kg. It is known that TB is difficult to treat in older people due to the presence of non-specific symptoms or atypical presentations [26], high lost-to-follow-up rates and adverse drug events [27]. Previous studies have shown that mortality rate is higher in individuals who are older with TB than in younger populations [28]. However, correctly identifying the cause of death in older individuals with TB remains challenging because of the presence of other comorbidities and the absence of autopsies.
Another factor affecting mortality in our TB cohort was low body weight. Previous studies have shown that it is associated with an increased risk of death during TB treatment [29,30]. Malnutrition can suppress cell-mediated immunity, which represents the principal host defence against TB. Therefore, high mortality rates among underweight individuals with TB may be due to impaired immunity and, as a result, a more severe infection in this population [31].
MDR-TB is a major clinical challenge, particularly in individuals with HIV-1 co-infection. Previous studies have reported high mortality rates in MDR-TB/HIV-1 co-infection [32,33], which was not present in our study. This may be explained by the small number of MDR-TB cases (7%) and the fact that many were on ART during TB treatment, a finding consistent with a recent study showing that mortality rates did not differ by HIV-1 status [34].
There are some limitations to our study, the main one being that part of it was retrospective. Therefore, some factors that may have affected TB treatment outcomes are unavailable, such as socioeconomic status, alcohol consumption, smoking or ART regimen for participants with HIV-1 co-infection. Secondly, time from diagnosis to starting TB treatment was not recorded in that part of the study. We cannot demonstrate either the change in diagnosis, treatment or mortality rates of TB after the advent of Xpert MTB/RIF use. Third, since there were a limited number of TB/HIV cases with DM (n = 4) in our cohort, this finding would need to be confirmed with a larger sample size. Fourth, we could not contact lost-to-follow-up individuals. Since individuals with TB/HIV in our study had very advanced HIV-1 disease, those lost to follow-up may have died if they could not access ART. Lastly, our study population was only from Bangkok and Nonthaburi province and may not be representative of other hospitals in Thailand.
In conclusion, the individuals with TB/HIV co-infection at tertiary hospitals in the big Thai cities had a lower ART uptake (63.6%) and success rate of TB treatment (63%), as well as a higher mortality rate than the TB/HIV-1-negative population. These findings have significant implications for Thailand, aiming at ending the TB epidemic by 2030. Therefore, strategies to improve ART uptake should be massively scaled up. Additionally, the presence of DM in individuals with TB needs to be assessed with fasting glucose.
Acknowledgements
The authors thank the Tuberculosis Research Unit, Faculty of Medicine, Chulalongkorn University, and King Chulalongkorn Memorial Hospital, the Thai Red Cross Society, Rajavithi Hospital and Bamrasnaradura Infectious Diseases Institute for their support, as well as the supervisors, data collectors and study participants.
Conflicts of interest
AA participated in a company-sponsored speaker's bureau from Janssen-Cilag, Gilead and Bristol-Myers Squibb. KR received the Senior Research Scholar from Thailand Research Fund and honoraria/consultation fees from Merck, Roche, Janssen-Cilag, Tibotec, Mylan and Government Pharmaceutical Organization (GPO, Thailand). KR also participated in a company-sponsored speaker's bureau for Abbott, Gilead, Bristol-Myers Squibb, Merck, Roche, Janssen-Cilag, GlaxoSmithKline and GPO. The other authors declared no conflict of interest.
Funding
This study was funded by the TB National Research University (grant no. HR1161 A(1)), Chulalongkorn University Ratchaphisek Somphot Endowment Fund and TB Research Unit (grant no. GRU 6104030007-1) and HIV-NAT.
References
- 1. WHO Global tuberculosis report 2018. Available at: www.who.int/tb/publications/global_report/en/ ( accessed May 2019).
- 2. Teeraananchai S, Chaivooth S, Kerr SJ et al. Life expectancy after initiation of combination antiretroviral therapy in Thailand. Antivir Ther 2017; 22: 393– 402. [DOI] [PubMed] [Google Scholar]
- 3. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24: 351– 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second edition 2016. Available at: www.who.int/hiv/pub/arv/arv-2016/en/ ( accessed May 2019). [PubMed]
- 5. WHO Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach: 2010 revision. Available at: www.who.int/hiv/pub/arv/adult2010/en/ ( accessed May 2019). [PubMed]
- 6. Abdool Karim SS, Naidoo K, Grobler A et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362: 697– 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanc FX, Sok T, Laureillard D et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365: 1471– 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Havlir DV, Kendall MA, Ive P et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365: 1482– 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO Global tuberculosis report. 2016. Available at: apps.who.int/medicinedocs/en/d/Js23098en/ ( accessed May 2019).
- 10. SEARO Bending the curve – ending TB: annual report 2017. 2017. Available at: www.who.int/iris/handle/10665/254762 ( accessed May 2019).
- 11. Kawkitinarong K, Suwanpimolkul G, Kateruttanakul P et al. Real-life clinical practice of using the Xpert MTB/RIF assay in Thailand. Clin Infect Dis 2017; 64: S171– S178. [DOI] [PubMed] [Google Scholar]
- 12. WHO Definitions and reporting framework for tuberculosis. 2013 revision, updated December 2014 Available at: www.who.int/tb/publications/definitions/en/ ( accessed May 2019).
- 13. Manosuthi W, Kawkitinarong K, Suwanpimolkul G et al. Clinical characteristics and treatment outcomes among patients with tuberculosis in Bangkok and Nonthaburi, Thailand. Southeast Asian J Trop Med Public Health 2012; 43: 1426– 1436. [PubMed] [Google Scholar]
- 14. Ali SA, Mavundla TR, Fantu R, Awoke T. Outcomes of TB treatment in HIV co-infected TB patients in Ethiopia: a cross-sectional analytic study. BMC Infect Dis 2016; 16: 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tweya H, Feldacker C, Phiri S et al. Comparison of treatment outcomes of new smear-positive pulmonary tuberculosis patients by HIV and antiretroviral status in a TB/HIV clinic, Malawi. PLoS One 2013; 8: e56248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagu TJ, Aboud S, Mwiru R et al. Tuberculosis associated mortality in a prospective cohort in sub Saharan Africa: association with HIV and antiretroviral therapy. Int J Infect Dis 2017; 56: 39– 44. [DOI] [PubMed] [Google Scholar]
- 17. Field N, Lim MS, Murray J et al. Timing, rates, and causes of death in a large South African tuberculosis programme. BMC Infect Dis 2014; 14: 3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manosuthi W, Chottanapand S, Thongyen S et al. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 2006; 43: 42– 46. [DOI] [PubMed] [Google Scholar]
- 19. Abdool Karim SS, Naidoo K, Grobler A et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365: 1492– 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta A, Nadkarni G, Yang WT et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 2011; 6: e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dooley KE, Tang T, Golub JE et al. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80: 634– 639. [PMC free article] [PubMed] [Google Scholar]
- 23. Oursler KK, Moore RD, Bishai WR et al. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis 2002; 34: 752– 759. [DOI] [PubMed] [Google Scholar]
- 24. Wang CS, Yang CJ, Chen HC et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect 2009; 137: 203– 210. [DOI] [PubMed] [Google Scholar]
- 25. Kyu HH, Maddison ER, Henry NJ et al. The global burden of tuberculosis: results from the Global Burden of Disease Study. Lancet Infect Dis 2015; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajagopalan S, Yoshikawa TT. Tuberculosis in the elderly. Z Gerontol Geriatr 2000; 33: 374– 380. [DOI] [PubMed] [Google Scholar]
- 27. Velayutham BR, Nair D, Chandrasekaran V et al. Profile and response to anti-tuberculosis treatment among elderly tuberculosis patients treated under the TB Control programme in South India. PLoS One 2014; 9: e88045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagu T, Ray R, Munseri P et al. Tuberculosis among the elderly in Tanzania: disease presentation and initial response to treatment. Int J Tuberc Lung Dis 2017; 21: 1251– 1257. [DOI] [PubMed] [Google Scholar]
- 29. Yen YF, Tung FI, Ho BL et al. Underweight increases the risk of early death in tuberculosis patients. Br J Nutr 2017; 118: 1052– 1060. [DOI] [PubMed] [Google Scholar]
- 30. Lai HH, Lai YJ, Yen YF. Association of body mass index with timing of death during tuberculosis treatment. PLoS One 2017; 12: e0170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8: 286– 298. [PubMed] [Google Scholar]
- 32. Farley JE, Ram M, Pan W et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One 2011; 6: e20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gandhi NR, Shah NS, Andrews JR et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010; 181: 80– 86. [DOI] [PubMed] [Google Scholar]
- 34. Brust JCM, Shah NS, Mlisana K et al. Improved survival and cure rates with concurrent treatment for MDR-TB/HIV co-infection in South Africa. Clin Infect Dis 2017; 66: 1246– 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]