Abstract
Objectives
The aim of this study was to investigate associations between baseline characteristics and CD4 cell count response on first-line antiretroviral therapy and risk of virological failure (VF) with or without drug resistance.
Methods
We conducted an analysis of UK Collaborative HIV Cohort data linked to the UK HIV Drug Resistance Database. Inclusion criteria were viral sequence showing no resistance prior to initiation of first-line efavirenz + tenofovir disoproxil fumarate + emtricitabine and virological suppression within 6 months. Outcomes of VF (≥200 copies/mL) with or without drug resistance were assessed using a competing risks approach fitted jointly with a model for CD4 cell count recovery. Hazard ratios for each VF outcome were estimated for baseline CD4 cell count and viral load and characteristics of CD4 cell count response using latent variables on a standard normal scale.
Results
A total of 3640 people were included with 338 VF events; corresponding viral sequences were available in 134 with ≥1 resistance mutation in 36. VF with resistance was associated with lower baseline CD4 (0.30, 0.09–0.62), lower CD4 recovery (0.04, 0.00–0.17) and higher CD4 variability (4.40, 1.22–12.68). A different pattern of associations was observed for VF without resistance, but the strength of these results was less consistent across sensitivity analyses. Cumulative incidence of VF with resistance was estimated to be <2% at 3 years for baseline CD4 ≥350 cells/μL.
Conclusion
Lower baseline CD4 cell count and suboptimal CD4 recovery are associated with VF with drug resistance. People with low CD4 cell count before ART or with suboptimal CD4 recovery on treatment should be a priority for regimens with high genetic barrier to resistance.
Keywords: antiretroviral therapy, ART, drug resistance, HIV, NNRTI, NRTI, viral failure, viral suppression
Introduction
Amongst people diagnosed with HIV in whom there is initial viral suppression, subsequent virological failure (VF) rates on modern ART regimens are low [1]. However, there remains interest in identifying those people living with HIV (PLHIV) at highest risk of VF, particularly that with emergence of drug resistance. The incidence of acquired drug resistance has been falling in resource-rich countries [2,3], but preservation of future treatment options in PLHIV is important, given that people are likely to need antiretroviral therapy (ART) for life. Although it has been argued that routine monitoring of CD4 cell count observations could be reduced for PLHIV on suppressive ART [4,5], the CD4 cell count remains an important marker of immunological status [6], and so there is a motivation to further investigate associations between baseline and post-treatment CD4 cell counts and risk of VF and acquired drug resistance.
It has been previously found that low baseline CD4 cell count (<200 cells/μL) and high baseline viral load (VL) (≥100,000 copies/mL) are risk factors for acquired drug resistance following ART initiation [3,7,8]. The expected level of CD4 cell count recovery on virally suppressive ART is strongly dependent on the baseline observation at ART initiation [9–12], and so CD4 cell count recovery itself needs to be evaluated relative to that expected, given the baseline value. One option for evaluation of CD4 cell count response is to track centile values with reference to population charts [13], and another approach is to model CD4 cell count recovery conditional on baseline using mixed-effects models [14,15]. Using the latter approach, it can be shown that there is considerable between-person variation in CD4 cell count recovery that is not explained by baseline characteristics and also differences in the variability of observations over time [15].
There is some evidence from randomised controlled trials that clinical and CD4 cell count monitoring of response to ART is not inferior to VL monitoring with respect to clinical endpoints within a few years of treatment initiation [16,17]. It is also known that CD4 cell count recovery provides important prognostic information for the outcomes of mortality and AIDS progression amongst PLHIV on ART with viral suppression [18], but the available literature suggests only a limited association with risk of VF. Badri et al. found that slope of increase and absolute change in CD4 cell count from baseline were not predictive of VF [19], and immunological criteria have shown poor performance as a direct proxy for VF on ART [20]. However, it is possible that the association between CD4 cell count recovery and VF could differ according to whether this is coincident with the appearance of acquired drug resistance.
In this paper, we develop a joint competing risks model for CD4 cell count measurements on ART and occurrence of VF with or without emergence of drug resistance mutations. This approach allows estimation of associations between the events of interest and baseline CD4 cell count and VL, CD4 cell count response relative to that expected, given the baseline level, and CD4 cell count variability over time, as well as other individual characteristics. We investigate PLHIV on first-line efavirenz + tenofovir disoproxil fumarate + emtricitabine (EFV + TDF + FTC), an established regimen with widespread global use.
Methods
We conducted an analysis of pseudo-anonymised clinical records of PLHIV included in the UK Collaborative HIV Cohort [21] (CHIC) linked to viral sequences collected by the UK HIV Drug Resistance Database [22]. Data from the UK Register of Seroconverters [23] (UKR) cohort were also used to calibrate parameters of statistical models developed.
The primary analyses included PLHIV in UK CHIC with viral sequence showing the absence of reverse transcriptase major drug resistance mutations (following the International Antiviral Society-USA list [24]) prior to the initiation of first-line ART. PLHIV were included if they started ART on EFV + TDF + FTC in the period 2004–2014 and were observed to achieve virological suppression (defined as single VL measurement <50 copies/mL) within 6 months of initiation. All CD4 cell counts and VF data were censored at change to or interruption (≥14 days) of ART regimen, and CD4 cell counts observed on or after the date of an observed VF (≥200 copies/mL) were also excluded. Only a very small number of people in the UKR and UK CHIC cohorts with nonsexual acquisition of HIV met the inclusion criteria for each stage of the analysis, and so they were excluded.
The statistical methodology was developed using a ‘calibration’ dataset comprising 339 seroconverters (with well-estimated dates of seroconversion) from the UKR cohort who started ART on EFV + TDF + FTC. The purpose of the additional calibration step was to provide information regarding model parameters for variance components and those linking CD4 cell count and VL at treatment initiation to the expected trajectory of CD4 cell count recovery, for use in the primary analyses; fitting of the primary analysis models without this was found to be unstable.
Statistical methods
All models were developed within a Bayesian framework using the Stan probabilistic programming language [25] run on a cluster computer. Full technical details of the statistical models developed are given in Supplementary Appendix S1A, with files to simulate data and run the models also provided. We conducted modelling using the square-root scale for CD4 cell counts and log10 scale for VL.
Calibration analysis
Models were fitted to pre- and post-treatment CD4 cell counts in the UKR ‘calibration dataset’ for the specified ART regimen based on those previously developed by Stirrup et al. [14,15,26]. Briefly, the methodology constitutes an extension of the nonlinear mixed-effects framework in which characteristics of CD4 cell count recovery are modelled conditional on latent variables representing ‘true’ baseline CD4 cell count and VL at treatment initiation. Following previous work [14,27], the pretreatment model comprised a ‘random intercepts and slopes model’ with fractional Brownian motion stochastic processes included in the variance structure alongside the measurement error term, and a simple asymptotic curve is used for the CD4 cell count recovery submodel [14].
Primary analyses
The median post-treatment CD4 cell count recovery for seroprevalent PLHIV was also modelled using a simple asymptotic curve. The true baseline CD4 cell count (square-root scale) and VL (log10 scale) were assumed to follow a bivariate normal distribution. The closest CD4 cell count and VL observation within 6 months prior to (or on the day of) treatment initiation were used as baseline observations, and PLHIV without these were excluded. The baseline CD4 cell count measurement was included as the t = 0 observation for the asymptotic recovery model, whilst the baseline VL observation was modelled as following a normal distribution conditional on the ‘true baseline VL’ latent variable [28]. Informative priors were used based on the posterior of the calibration model for residual variance parameters and for those linking the shape of the median CD4 cell count recovery to true baseline CD4 cell count and VL.
The main outcome for the primary analysis was observation of VF, defined as a single VL measurement of ≥200 copies/mL with or without observation of any new major resistance mutation at resistance test using a blood sample obtained within 6 months. These two outcomes were assessed using Weibull survival models, defined relative to initial viral suppression, following a competing risks approach. For cases in which a viral sequence was recorded in the database after ART initiation but prior to any VL observations ≥200 copies/mL, the VF date was set at the date of blood sampling for resistance testing (unless later than the last possible UK CHIC follow-up for most sites: 31 December 2014). VF events with no viral sequence within 6 months were included in the analyses by treating the event as having a masked/missing cause of failure [29,30].
The VF model was fitted jointly with the nonlinear mixed-effects model for CD4 cell count recovery. The hazard ratios (HRs) for each VF outcome were estimated for the level of CD4 cell count recovery relative to that expected, given the baseline value, the degree of CD4 cell count variability over time (in comparison to a smooth curve) and the true baseline CD4 cell count and VL; each of these predictive variables is not directly observed but rather represented by a latent variable in the mixed-effects model (illustrated in Figure 1). The analysis therefore constitutes a joint modelling approach to longitudinal measurements and competing risks failure time data [31], with the novel feature that one of the latent variables included relates to differences in variability over time.
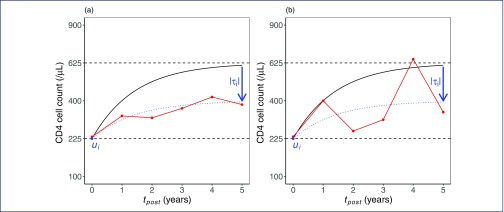
Figure 1.
Plots of hypothetical individual-level data and model fit illustrating latent variables included in the post-treatment CD4 cell count submodel. In each plot, the ‘true’ baseline CD4 cell count (ui) is 225 cells/μL, and the long-term median CD4 cell count following the expected trajectory (solid black line) is 625 cells/μL, but recovery in the observed individual (dotted line) is below average conditional on their baseline (τi is negative with magnitude indicated by the blue arrow). Plots (a) and (b) show people living with HIV with low and high CD4 cell count variability, respectively, with observed CD4 counts shown in red
Baseline true CD4 cell count and VL follow a bivariate normal distribution as described, shift in long-term median CD4 cell count relative to that expected (‘CD4 cell count recovery’) is normally distributed and CD4 cell count variability takes the form of a gamma-distributed variable. Effects on hazard of VF were estimated for each latent variable transformed to a standard normal scale for ease of comparison, that is, effect estimates are reported for a difference of 1 SD from the mean in the studied population (on square-root scale for CD4 cell count and log10 scale for VL). The fitted models were used to generate cumulative incidence functions for VF with or without resistance for specific baseline CD4 cell count values.
Models were fitted including parameters linking the following individual characteristics to the asymptotic long-term median post-ART CD4 cell count and hazard functions for VF with and without resistance: age at treatment initiation (linear effect centred at 38 years), men who have sex with women, women who have sex with men, black African ethnicity, black Caribbean ethnicity, any other nonwhite or unknown ethnicity, and viral subtype.
Results
There were 3640 PLHIV on first-line EFV + TDF + FTC included in the primary analysis, with VF observed in 338 of these at a median of 1.2 (interquartile range (IQR) 0.6–2.6) years. The overall median follow-up time for the analysis was 2.4 (IQR 0.9–4.6) years. A summary of the study population is given in Table 1. A viral resistance test was available in 134 (40%) of PLHIV with VF, and in 36 (27%) of these, there was at least one resistance mutation observed (mutations listed in Supplementary Appendix S1B). Viral subtype was available in all but three PLHIV, and so the potential associations between individual characteristics other than CD4/VL and VF outcomes were evaluated on a complete case basis.
Table 1.
Characteristics of people living with HIV starting ART on efavirenz + tenofovir disoproxil fumarate + emtricitabine included in the primary analysis (n = 3640)
| n (%) or median (IQR) | |
|---|---|
| Sex/mode of infection group | |
| MSM | 2666 (73.2) |
| MSW | 469 (12.9) |
| WSM | 505 (13.9) |
| Ethnicity | |
| Black African | 636 (17.5) |
| Black Caribbean | 113 (3.1) |
| Other/unknown | 525 (14.4) |
| White | 2366 (65.0) |
| Viral subtype | |
| A | 147 (4.0) |
| B | 2458 (67.6) |
| C | 448 (12.3) |
| CRF | 444 (12.2) |
| Other | 136 (3.7) |
| Unknown | 3 (0.1) |
| Age at ART initiation (years) | 38.1 (31.7–44.3) |
| CD4 cell count at baseline (cells/μL) | 280 (192–367) |
| RNA at baseline (copies/mL) | 51 000 (13 000–147 000) |
| Year of ART initiation | |
| 2004 | 72 (2.0) |
| 2005 | 183 (5.0) |
| 2006 | 160 (4.4) |
| 2007 | 213 (5.9) |
| 2008 | 583 (16.0) |
| 2009 | 576 (15.8) |
| 2010 | 541 (14.9) |
| 2011 | 443 (12.2) |
| 2012 | 407 (11.2) |
| 2013 | 342 (9.4) |
| 2014 | 120 (3.3) |
ART, antiretroviral therapy; CRF, circulating recombinant form; MSM, men who have sex with men; MSW, men who have sex with women; WSM, women who have sex with men.
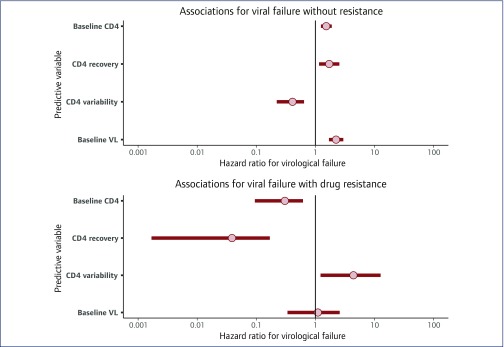
A plot of estimated associations between VL and CD4 cell count characteristics and the hazard of VF without adjustment for demographic variables is presented in Figure 2. The event of VF without any resistance mutations was associated with higher baseline CD4 cell count (HR 1.52, 1.24–1.89), CD4 cell count recovery (1.71, 1.15–2.54) and baseline VL (2.23, 1.70–2.97) and lower CD4 cell count variability (0.41, 0.22–0.64). Conversely, VF with resistance was associated with lower baseline CD4 cell count (0.30, 0.09–0.62), lower CD4 cell count recovery (0.04, 0.00–0.17) and higher CD4 cell count variability (4.40, 1.22–12.68). Further details of the fitted model are given in Supplementary Appendix S1C.
Figure 2.
Plot of hazard ratios linking VL and CD4 cell count characteristics to risk of virological failure with and without the appearance of resistance mutations. Results are for people living with HIV on first-line efavirenz + tenofovir disoproxil fumarate + emtricitabine regimen from the fitted model without adjustment for demographic variables. Results are shown as posterior mean with 95% credibility interval. All predictive variables in this plot relate to modelled latent variables transformed to a standard normal scale, with the effect estimate reported for a difference of 1 SD from the mean (on square-root scale for CD4 cell count and log10 scale for VL). VL, viral load
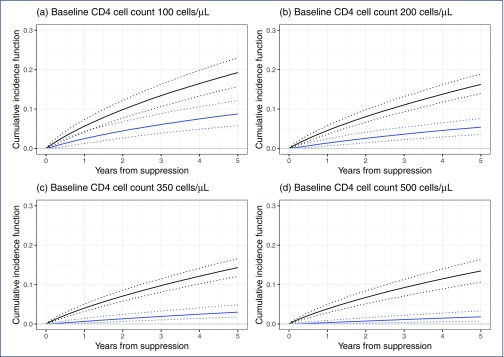
Plots of the estimated cumulative incidence of PLHIV experiencing VF with or without emergence of resistance according to the true baseline CD4 cell count level are shown in Figure 3. The number of PLHIV expected to have a VF event without resistance by 3 years from initial suppression is fairly stable across baseline CD4 cell count levels at 7%–8%. However, the proportion of PLHIV expected to experience VF with the emergence of resistance by 3 years is lower for higher baseline CD4 cell count levels, falling from 6% for a baseline of 100 cells/μL to 1% for a baseline of 500 cells/μL.
Figure 3.
Estimated cumulative incidence functions for virological failure with or without resistance (black line) and virological failure with resistance (blue line), derived within a competing risks framework. Ninety-five per cent credibility intervals are shown (dotted lines). Plots are shown for ‘true’ CD4 cell count at baseline set to (a) 100 cells/μL, (b) 200 cells/μL, (c) 350 cells/μL and (d) 500 cells/μL. The estimates presented are averaged over the expected distribution of individual-level CD4 cell count recovery and baseline viral load characteristics, with the distribution for baseline viral load adjusted conditional on the specified CD4 cell count level
The addition of predictive variables relating to age at ART, sex/mode of infection, ethnicity and viral subtype did not have a substantial impact on the estimated associations between VL and CD4 cell count and VF events (Table 2). None of the additional factors analysed showed a definitive association with the events of VF with or without resistance, but credibility intervals for individual factors were wide, and so the fitted model is not inconsistent with strong associations being present.
Table 2.
Estimates of associations between CD4 cell count and VL baseline and response variables and the events of VF with or without the appearance of resistance mutations, unadjusted and with adjustment for demographic and viral characteristics
| VF without resistance | VF with resistance | |
|---|---|---|
| Unadjusted model | ||
| Baseline CD4 cell count* | 1.52 (1.24–1.89) | 0.30 (0.09–0.62) |
| CD4 cell count recovery* | 1.71 (1.15–2.54) | 0.04 (0.00–0.17) |
| CD4 cell count variability* | 0.41 (0.22–0.64) | 4.40 (1.22–12.68) |
| Baseline VL* | 2.23 (1.7–2.97) | 1.10 (0.34–2.58) |
| Adjusted model | ||
| Baseline CD4 cell count* | 1.59 (1.29–1.97) | 0.27 (0.06–0.64) |
| CD4 cell count recovery* | 1.63 (1.11–2.52) | 0.01 (0–0.03) |
| CD4 cell count variability* | 0.41 (0.2–0.63) | 8.18 (1.47–28.22) |
| Baseline VL* | 2.4 (1.79–3.18) | 1.49 (0.3–4.87) |
| Age at ART initiation (years)† | 0.99 (0.97–1.01) | 0.96 (0.87–1.05) |
| Mode of transmission | ||
| MSM | Reference | Reference |
| MSW | 0.95 (0.49–1.63) | 3.29 (0.21–13.42) |
| WSM | 0.95 (0.46–1.77) | 6.28 (0.63–30.58) |
| Ethnicity | ||
| White | Reference | Reference |
| Black Caribbean | 1.52 (0.48–3.13) | 14.34 (0.67–69.23) |
| Black African | 1.57 (0.84–2.67) | 2.13 (0.19–8.41) |
| Other/unknown | 1.06 (0.65–1.56) | 1.17 (0.12–4.99) |
| Viral subtype | ||
| A | 1.34 (0.48–2.69) | 4.82 (0.22–25.43) |
| B | Reference | Reference |
| C | 0.85 (0.39–1.54) | 4.9 (0.42–23.39) |
| CRF | 1.27 (0.69–2.04) | 1.75 (0.08–8.26) |
| Other | 1 (0.4–2.16) | 0.69 (0.02–3.48) |
CRF, circulating recombinant form; MSM, men who have sex with men; MSW, men who have sex with women; VF, virological failure; VL, viral load; WSM, women who have sex with men.
Results reported as posterior expectation of hazard ratio (95% credibility interval).
*Modelled latent variable on standard normal scale, effect estimate reported for a difference of 1 SD from the mean (on square-root scale for CD4 cell count and log10 scale for VL).
†Centred at 38 years.
Some additional details and sensitivity analyses are presented in a supplementary file: the diagnostic performance of our model to predict resistance status at VF (Supplementary Appendix S1D), VL values at VF (Supplementary Appendix S1E), an extended model with quadratic associations between CD4 cell count and VL characteristics and risk of VF (Supplementary Appendix S1F), and results from alternative simplified models (Supplementary Appendix S1G). The relative importance of predictive factors for VF without drug resistance varied under the sensitivity analyses (Supplementary Appendix S1G); the positive association with CD4 cell count recovery found in the primary analysis was less strong if cases of VF with unknown resistance status were excluded, censored or assumed to have no resistance, and the association with baseline VL was weaker and that with baseline CD4 cell count absent using a competing risks approach considering only observed baseline measurements. However, baseline CD4 cell count showed a strong negative relationship with the hazard of VF with drug resistance for all sensitivity analyses, and the level of CD4 recovery also showed a strong negative association with VF with drug resistance for all analyses that included post-treatment CD4 cell counts.
Discussion
We have found that for PLHIV who achieve initial viral suppression on EFV + TDF + FTC, the characteristics of CD4 cell count response to first-line ART are strongly associated with the risk of VF with emergence of drug resistance. Our results also suggest that, in this cohort, there was a different set of factors associated with the event of VF without drug resistance. We used the model developed to investigate the cumulative incidence of VF according to baseline CD4 cell count level and found the incidence of VF with resistance to be substantially lower for baseline CD4 cell count levels of 350 cells/μL or above.
The finding that low baseline CD4 cell count is a risk factor for acquired drug resistance on first-line EFV + TDF + FTC is consistent with previous research on this particular regimen [32] and that on combination ART more generally both in the UK [7,8,33] and elsewhere [3]. Previous studies have also found an association between high baseline VL and risk of acquired drug resistance [3,7,8,34], which we did not observe. However, we considered only resistance tests within 6 months of the first observed VF unlike some previous studies [3,7,8], and we did find high baseline VL to be associated with VF without resistance in our primary analysis. We considered only the first VF event in each person and did not assess whether people went on to develop resistance mutations at a later date; the rationale for this is that adherence interventions or treatment changes would be possible once VF is detected, but it would be useful to be able to identify high-risk individuals before VF occurs. Furthermore, we only included PLHIV with initial viral suppression, differing from Fogel et al. [34], who observed most cases of acquired drug resistance in PLHIV who never achieved suppression.
The model developed predicts a cumulative incidence of VF with emergence of resistance below 2% at 3 years from initial suppression for a baseline CD4 cell count level of ≥350 cells/μL compared with around 6% for a baseline of 100 cells/μL, despite a fairly consistent cumulative incidence of VF without resistance of 7%–8% across CD4 cell count levels. Our modelling framework has the advantage that it takes the occurrence of VF events with unknown resistance status into account, and so our estimate of the cumulative incidence of VF with resistance includes cases that may not be identified within 6 months of a detectable VL.
For the estimated associations between CD4 cell count response on ART and the risks of VF with or without resistance, interpretation is made difficult by a lack of information on drug adherence for the studied PLHIV. Poor drug adherence is known to predict both VF overall [35] and the emergence of drug resistance [3,36]. It is also known that low or inconsistent ART adherence is associated with reduced CD4 cell count recovery [37,38], and so the association that we have identified between lower than expected CD4 cell count response and the emergence of drug resistance may be due to consistent poor adherence in the affected PLHIV. However, new drug resistance mutations can nonetheless occur in PLHIV with perfect self-reported adherence [36].
The level of CD4 cell count recovery was not found to be negatively associated with the risk of VF without resistance, which could indicate that these events were primarily caused by short-term lapses in adherence in our study population. The fact that we identified distinct sets of associations for the events of VF with and without resistance suggests that they are linked to different combinations of biological and behavioural factors, but we do not know whether differences in adherence fully explain the findings in our studied cohort or whether there could also be a physiological basis for the link between low CD4 cell count and the emergence of drug resistance.
The variability in CD4 cell counts over time was also found to be associated with the risk of VF with emergence of resistance in the primary analysis; it may seem that erratic CD4 cell count trajectories are likely due to inconsistent treatment adherence, but similar differences between PLHIV in the level of CD4 cell count variability can also be observed in pretreatment data [14,27], indicating that this could reflect a biological characteristic of immune response.
A limitation of this study is that it made use of classical Sanger consensus sequences, both for the inclusion criterion of confirmed lack of resistance mutations at baseline and for the classification of VF events. There is evidence that the presence of minority variants with resistance mutations at baseline is a risk factor for subsequent VF [39,40]. A study of men who have sex with men in the UK with probable recent HIV infection in 2011–2013 using next-generation sequencing (NGS) found minority variant (2%–20% thresholds) transmitted drug resistance in 2.3% of PLHIV for nucleoside reverse transcriptase inhibitor and in 1.4% for non-nucleoside reverse-transcriptase inhibitor mutations [41]. It is therefore possible that new drug resistance mutations observed at VF in the studied cohort could have been present as an undetected minority variant prior to the initiation of ART. Although we cannot therefore be sure whether suboptimal CD4 cell count recovery represents a cause of new drug resistance or an effect of pre-existing minority drug resistance mutations in each individual, our results would still be of clinical relevance if the latter were true in the absence of routine NGS screening; in this scenario, suboptimal CD4 cell count recovery would be predictive of VF caused by pre-existing but undetected drug resistance.
The EFV + TDF + FTC regimen investigated in this study is no longer recommended as first-line ART in the UK [42]. Dolutegravir, in particular, is now being used as the basis for first-line treatment in both low-/middle- and high-income countries [42,43]. It will be several years before enough follow-up data would be available for an equivalent analysis to be conducted for PLHIV starting first-line dolutegravir in the UK, and this drug is known to have a higher genetic barrier to resistance [44] than does EFV, meaning that there are likely to be fewer cases of acquired resistance. Our results suggest that a high genetic barrier to resistance should be a particular priority when deciding on treatment for people with low baseline CD4 cell count. PLHIV with viral suppression but lower than expected CD4 cell count recovery might also benefit from a switch to such a regimen, whatever the underlying cause of their suboptimal recovery and particularly if adherence issues can be ruled out.
This study included PLHIV starting ART in the years 2004–2014 and during this time, the guidelines for ART initiation in the UK shifted from starting when PLHIV have a CD4 cell count that has dropped below 350 cells/μL [45] to starting at any CD4 cell count following diagnosis [42]. Public Health England has also recently reported that improved uptake of regular testing has led to an increase in the proportion of PLHIV diagnosed with high baseline CD4 cell count in some centres [46]. We would therefore expect a substantially higher average baseline CD4 cell count for PLHIV now starting first-line ART in the UK. In our analyses, we did not account for the possibility that the risk of VF might differ for PLHIV in whom ART is initiated close to the date of infection, as this would have been a rare event in the cohort under investigation, and this warrants further investigation. However, in the UK and worldwide, there will continue to be large numbers of PLHIV in whom HIV is not diagnosed early and who will initiate ART at a level comparable to the cohort in this analysis.
There is limited information available in the literature regarding the link between CD4 cell response to treatment and the risk of VF and acquired drug resistance. This is due in part to the fact that it is difficult to appropriately quantify the level of an individual's CD4 cell response, as the expected recovery is dependent on their baseline characteristics and individual trajectories can be highly erratic. The modelling framework that we have developed has identified an association between lower than expected CD4 cell count response and the risk of VF with emergence of drug resistance for PLHIV on first-line EFV + TDF + FTC. Our results suggest that policies to ensure that people who acquire HIV are diagnosed early and initiate ART at as high a CD4 cell count level as possible have the potential to substantially further reduce the incidence of acquired drug resistance, and that people with a low CD4 cell count at ART initiation or with suboptimal CD4 cell count recovery on treatment might benefit from the use of an ART regimen with higher genetic barrier to resistance.
Acknowledgements
The authors acknowledge the use of the UCL Legion High Performance Computing Facility (Legion@UCL) and associated support services in the completion of this work.
This work was presented at the 22nd International Workshop on HIV and Hepatitis Observational Databases (IWHOD), Fuengirola, Spain, 2018 (no record is published online for this conference).
Conflicts of interest
CAS has received funding from Gilead Sciences, ViiV Healthcare and Janssen-Cilag for participation in data safety and monitoring boards, advisory boards, speaker panels and for the preparation of educational materials. DC has received travel grants and payment for speaking or attending advisory boards from Gilead, MSD and Janssen. All other authors report no potential conflicts.
Source of funding
This work was funded by the UK Medical Research Council (Award Number 164587).
UK HIV Drug Resistance Database
Steering committee: David Asboe, Anton Pozniak (Chelsea & Westminster Hospital, London); Patricia Cane (Public Health England, Porton Down); David Chadwick (South Tees Hospitals NHS Trust, Middlesbrough); Duncan Churchill (Brighton and Sussex University Hospitals NHS Trust); Duncan Clark (Barts Health NHS Trust, London); Simon Collins (HIV i-Base, London); Valerie Delpech (National Infection Service, Public Health England); Samuel Douthwaite (Guy's and St. Thomas’ NHS Foundation Trust, London); David Dunn, Esther Fearnhill, Kholoud Porter, Anna Tostevin, Oliver Stirrup (Institute for Global Health, UCL); Christophe Fraser (University of Oxford); Anna Maria Geretti (Institute of Infection and Global Health, University of Liverpool); Rory Gunson (Gartnavel General Hospital, Glasgow); Antony Hale (Leeds Teaching Hospitals NHS Trust); Stéphane Hué (London School of Hygiene and Tropical Medicine); Linda Lazarus (Expert Advisory Group on AIDS Secretariat, Public Health England); Andrew Leigh-Brown (University of Edinburgh); Tamyo Mbisa (National Infection Service, Public Health England); Nicola Mackie (Imperial NHS Trust, London); Chloe Orkin (Barts Health NHS Trust, London); Eleni Nastouli, Deenan Pillay, Andrew Phillips, Caroline Sabin (University College London, London); Erasmus Smit (Public Health England, Birmingham Heartlands Hospital); Kate Templeton (Royal Infirmary of Edinburgh); Peter Tilston (Manchester Royal Infirmary); Erik Volz (Imperial College London, London); Ian Williams (Mortimer Market Centre, London); Hongyi Zhang (Addenbrooke's Hospital, Cambridge).
Coordinating centre: Institute for Global Health, UCL (David Dunn, Keith Fairbrother, Esther Fearnhill, Kholoud Porter, Anna Tostevin and Oliver Stirrup).
Centres contributing data: Clinical Microbiology and Public Health Laboratory, Addenbrooke's Hospital, Cambridge (Justine Dawkins); Guy's and St Thomas’ NHS Foundation Trust, London (Siobhan O’Shea and Jane Mullen); PHE – Public Health Laboratory, Birmingham Heartlands Hospital, Birmingham (Erasmus Smit); Antiviral Unit, National Infection Service, Public Health England, London (Tamyo Mbisa); Imperial College Health NHS Trust, London (Alison Cox); King's College Hospital, London (Richard Tandy); Medical Microbiology Laboratory, Leeds Teaching Hospitals NHS Trust (Tracy Fawcett); Specialist Virology Centre, Liverpool (Mark Hopkins); Department of Clinical Virology, Manchester Royal Infirmary, Manchester (Peter Tilston); Department of Virology, Royal Free Hospital, London (Clare Booth and Ana Garcia-Diaz); Edinburgh Specialist Virology Centre, Royal Infirmary of Edinburgh (Lynne Renwick); Department of Infection & Tropical Medicine, Royal Victoria Infirmary, Newcastle (Matthias L Schmid and Brendan Payne); South Tees Hospitals NHS Trust, Middlesbrough (David Chadwick); Department of Virology, Barts Health NHS Trust, London (Jonathan Hubb); Molecular Diagnostic Unit, Imperial College, London (Simon Dustan); University College London Hospitals (Stuart Kirk); West of Scotland Specialist Virology Laboratory, Gartnavel, Glasgow (Rory Gunson and Amanda Bradley-Stewart).
UK Collaborative HIV Cohort
Steering committee: Jonathan Ainsworth, Sris Allan, Jane Anderson, Abdel Babiker (MRC Clinical Trials Unit, UCL), David Chadwick, Duncan Churchill, Valerie Delpech, David Dunn, Brian Gazzard, Richard Gilson, Mark Gompels, Phillip Hay, Teresa Hill, Margaret Johnson, Sophie Jose, Stephen Kegg, Clifford Leen, Fabiola Martin, Dushyant Mital, Mark Nelson, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Frank Post, Jillian Pritchard, Caroline Sabin, Achim Schwenk, Anjum Tariq, Roy Trevelion, Andy Ustianowski, John Walsh.
Central coordination: University College London (Teresa Hill, Sophie Jose, Andrew Phillips, Caroline Sabin, Alicia Thornton and Susie Huntington); Medical Research Council Clinical Trials Unit at UCL, London (David Dunn, Adam Glabay and Shaadi Shidfar).
Participating centres: Barts Health NHS Trust, London (Chloe Orkin, Janet Lynch, James Hand and Carl de Souza); Brighton and Sussex University Hospitals NHS Trust (Nicky Perry, Stuart Tilbury, Elaney Youssef and Duncan Churchill); Chelsea and Westminster Hospital NHS Foundation Trust, London (Brian Gazzard, Mark Nelson, Tracey Mabika, David Asboe and Sundhiya Mandalia); Homerton University Hospital NHS Trust, London (Jane Anderson and Sajid Munshi); King's College Hospital NHS Foundation Trust, London (Frank Post, Ade Adefisan, Chris Taylor, Zachary Gleisner, Fowzia Ibrahim and Lucy Campbell); Middlesbrough, South Tees Hospitals NHS Foundation Trust (David Chadwick and Kirsty Baillie); Mortimer Market Centre, Central and North West London NHS Foundation Trust/University College London (Richard Gilson, Nataliya Brima and Ian Williams); North Middlesex University Hospital NHS Trust, London (Jonathan Ainsworth, Achim Schwenk, Sheila Miller and Chris Wood); Royal Free NHS Foundation Trust/University College London (Margaret Johnson, Mike Youle, Fiona Lampe, Colette Smith, Rob Tsintas, Clinton Chaloner, Samantha Hutchinson, Caroline Sabin, Andrew Phillips, Teresa Hill, Sophie Jose, Susie Huntington and Alicia Thornton); Imperial College Healthcare NHS Trust, London (John Walsh, Nicky Mackie, Alan Winston, Jonathan Weber, Farhan Ramzan and Mark Carder); The Lothian University Hospitals NHS Trust, Edinburgh (Clifford Leen, Alan Wilson and Sheila Morris); North Bristol NHS Trust (Mark Gompels and Sue Allan); Leicester, University Hospitals of Leicester NHS Trust (Adrian Palfreeman and Adam Lewszuk); Woolwich, Lewisham and Greenwich NHS Trust (Stephen Kegg, Akin Faleye, Victoria Ogunbiyi and Sue Mitchell), St. George's Healthcare NHS Trust (Phillip Hay and Christian Kemble); York Teaching Hospital NHS Foundation Trust (Fabiola Martin, Sarah Russell-Sharpe and Janet Gravely); Coventry, University Hospitals Coventry and Warwickshire NHS Trust (Sris Allan and Andrew Harte); Wolverhampton, The Royal Wolverhampton Hospitals NHS Trust (Anjum Tariq, Hazel Spencer and Ron Jones); Chertsey, Ashford and St. Peter's Hospitals NHS Foundation Trust (Jillian Pritchard, Shirley Cumming and Claire Atkinson); Milton Keynes Hospital NHS Foundation Trust (Dushyant Mital, Veronica Edgell and Julie Allen); The Pennine Acute Hospitals NHS Trust (Andy Ustianowski, Cynthia Murphy and Ilise Gunder); Public Health England, London (Valerie Delpech); i-Base (Roy Trevelion).
Supplementary files
Appendix containing further details of statistical methodology and fitted models is available at the following website link: https://doi.org/10.6084/m9.figshare.9884627.v1
Contributor Information
UK HIV Drug Resistance Database and the UK Collaborative HIV Cohort:
David Asboe, Anton Pozniak, Patricia Cane, David Chadwick, Duncan Churchill, Duncan Clark, Simon Collins, Valerie Delpech, Samuel Douthwaite, David Dunn, Esther Fearnhill, Kholoud Porter, Anna Tostevin, Oliver Stirrup, Christophe Fraser, Anna Maria Geretti, Rory Gunson, Antony Hale, Stéphane Hué, Linda Lazarus, Andrew Leigh-Brown, Tamyo Mbisa, Nicola Mackie, Chloe Orkin, Eleni Nastouli, Deenan Pillay, Andrew Phillips, Caroline Sabin, Erasmus Smit, Kate Templeton, Peter Tilston, Erik Volz, Ian Williams, Hongyi Zhang, Keith Fairbrother, Justine Dawkins, Siobhan O’Shea, Jane Mullen, Alison Cox, Richard Tandy, Tracy Fawcett, Mark Hopkins, Clare Booth, Lynne Renwick, Lynne Renwick, Matthias L. Schmid, Brendan Payne, Jonathan Hubb, Simon Dustan, Stuart Kirk, Amanda Bradley-Stewart, Abdel Babiker, Teresa Hill, Sophie Jose, Alicia Thornton, Susie Huntington, Adam Glabay, Shaadi Shidfar, Janet Lynch, James Hand, Carl de Souza, Nicky Perry, Stuart Tilbury, Elaney Youssef, Brian Gazzard, Mark Nelson, Tracey Mabika, Sundhiya Mandalia, Jane Anderson, Sajid Munshi, Frank Post, Ade Adefisan, Chris Taylor, Zachary Gleisner, Fowzia Ibrahim, Lucy Campbell, Kirsty Baillie, Richard Gilson, Nataliya Brima, Jonathan Ainsworth, Achim Schwenk, Sheila Miller, Chris Wood, Margaret Johnson, Mike Youle, Fiona Lampe, Colette Smith, Rob Tsintas, Clinton Chaloner, Samantha Hutchinson, John Walsh, Nicky Mackie, Alan Winston, Jonathan Weber, Farhan Ramzan, Mark Carder, Clifford Leen, Alan Wilson, Sheila Morris, Mark Gompels, Sue Allan, Adrian Palfreeman, Adam Lewszuk, Stephen Kegg, Akin Faleye, Victoria Ogunbiyi, Sue Mitchell, Phillip Hay, Christian Kemble, Fabiola Martin, Sarah Russell-Sharpe, Janet Gravely, Sris Allan, Andrew Harte, Anjum Tariq, Hazel Spencer, Ron Jones, Jillian Pritchard, Shirley Cumming, Claire Atkinson, Dushyant Mital, Veronica Edgell, Julie Allen, Andy Ustianowski, Cynthia Murphy, Ilise Gunder, and Roy Trevelion
References
- 1. O'Connor J, Smith C, Lampe FC et al. Durability of viral suppression with first-line antiretroviral therapy in patients with HIV in the UK: an observational cohort study. Lancet HIV 2017; 4: e295– e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scherrer A, Wyl V, Yang W-L et al. Emergence of acquired HIV-1 drug resistance almost stopped in Switzerland: a 15-year prospective cohort analysis. Clin Infect Dis 2016; 62: 1310– 1317. [DOI] [PubMed] [Google Scholar]
- 3. Rocheleau G, Brumme C, Shoveller J et al. Longitudinal trends of HIV drug resistance in a large Canadian cohort, 1996-2016. Clin Microbiol Infect 2017. [DOI] [PubMed] [Google Scholar]
- 4. Caniglia E, Sabin C, Robins J et al. When to monitor CD4 cell count and HIV RNA to reduce mortality and AIDS-defining illness in virologically suppressed HIV-positive persons on antiretroviral therapy in high-income countries: a prospective observational study. J Acquir Immune Defic Syndr (1999) 2016; 72: 214– 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ford N, Meintjes G, Pozniak A et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015; 15: 241– 247. [DOI] [PubMed] [Google Scholar]
- 6. Peeling R, Ford N. Reprising the role of CD4 cell count in HIV programmes. Lancet HIV 2017; 4: e377– e378. [DOI] [PubMed] [Google Scholar]
- 7. Phillips A, Dunn D, Sabin C et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS 2005; 19: 487– 494. [DOI] [PubMed] [Google Scholar]
- 8. Cozzi-Lepri A, Dunn D, Pillay D et al. Long-term probability of detecting drug-resistant HIV in treatment-naive patients initiating combination antiretroviral therapy. Clin Infect Dis 2010; 50: 1275– 1285. [DOI] [PubMed] [Google Scholar]
- 9. Kaufmann G, Perrin L, Pantaleo G et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med 2003; 163: 2187– 2195. [DOI] [PubMed] [Google Scholar]
- 10. Gras L, Kesselring A, Griffin J et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr 2007; 45: 183– 192. [DOI] [PubMed] [Google Scholar]
- 11. Hughes R, Sterne J, Walsh J et al. Long-term trends in CD4 cell counts and impact of viral failure in individuals starting antiretroviral therapy: UK Collaborative HIV Cohort (CHIC) study. HIV Med 2011; 12: 583– 593. [DOI] [PubMed] [Google Scholar]
- 12. Mocroft A, Phillips AN, Gatell J et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet 2007; 370: 407– 413. [DOI] [PubMed] [Google Scholar]
- 13. Bouteloup V, Sabin C, Mocroft A et al. Reference curves for CD4 T-cell count response to combination antiretroviral therapy in HIV-1-infected treatment-naïve patients. HIV Med 2017; 18: 33– 44. [DOI] [PubMed] [Google Scholar]
- 14. Stirrup O, Babiker A, Copas A. Combined models for pre- and post-treatment longitudinal biomarker data: an application to CD4 counts in HIV-patients. BMC Med Res Methodol 2016; 16: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stirrup O, Copas A, Phillips A et al. Predictors of CD4 cell recovery following initiation of antiretroviral therapy among HIV-1 positive patients with well-estimated dates of seroconversion. HIV Med 2018; 19: 184– 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mermin J, Ekwaru J, Were W et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ 2011; 343: d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jourdain G, Cœur SL, Ngo-Giang-Huong N et al. Switching HIV treatment in adults based on CD4 count versus viral load monitoring: a randomized, non-inferiority trial in Thailand. PLoS Med 2013; 10: 1– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Opportunistic Infections Project Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012; 9: e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badri M, Lawn S, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis 2008; 8: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutherford G, Anglemyer A, Easterbrook P et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS 2014; 28: S161– S169. [DOI] [PubMed] [Google Scholar]
- 21. UK Collaborative HIV Cohort Steering Committee The creation of a large UK-based multicentre cohort of HIV-infected individuals: The UK Collaborative HIV Cohort (UK CHIC) Study. HIV Med 2004; 5: 115– 124. [DOI] [PubMed] [Google Scholar]
- 22. UK Group on Transmitted HIV Drug Resistance Time trends in primary resistance to HIV drugs in the United Kingdom: multicentre observational study. BMJ 2005; 331: 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. UK Register of HIV Seroconverters Steering Committee The UK Register of HIV Seroconverters: methods and analytical issues. Epidemiol Infect 1996; 117: 305– 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wensing A, Calvez V, Günthard H et al. 2017 Update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24: 132– 133. [PMC free article] [PubMed] [Google Scholar]
- 25. Carpenter B, Lee D, Brubaker M et al. Stan: a probabilistic programming language. J Stat Softw 2017; 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stirrup OT, Dunn DT. Estimation of delay to diagnosis and incidence in HIV using indirect evidence of infection dates. BMC Med Res Methodol 2018; 18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stirrup O, Babiker A, Carpenter J et al. Fractional Brownian motion and multivariate-t models for longitudinal biomedical data, with application to CD4 counts in HIV-patients. Stat Med 2016; 35: 1514– 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thiébaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed 2004; 74: 255– 260. [DOI] [PubMed] [Google Scholar]
- 29. Guess F, Usher J, Hodgson T. Estimating system and component reliabilities under partial information on cause of failure. J Stat Plan Inference 1991; 29: 75– 85. [Google Scholar]
- 30. Basu S, Basu A, Mukhopadhyay C. Bayesian analysis for masked system failure data using non-identical Weibull models. J Stat Plan Inference 1999; 78: 255– 275. [Google Scholar]
- 31. Elashoff R, Li G, Li N. An approach to joint analysis of longitudinal measurements and competing risks failure time data. Stat Med 2007; 26: 2813– 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blanco J, Montaner J, Marconi V et al. Lower prevalence of drug resistance mutations at first-line virological failure to first-line therapy with atripla vs. tenofovir + emtricitabine/lamivudine + efavirenz administered on a multiple tablet therapy. AIDS 2014; 28: 2531– 2539. [DOI] [PubMed] [Google Scholar]
- 33. Jose S, Quinn K, Dunn D et al. Virological failure and development of new resistance mutations according to CD4 count at combination antiretroviral therapy initiation. HIV Med 2016; 17: 368– 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fogel J, Hudelson S, Ou S-S et al. HIV drug resistance in adults failing early antiretroviral treatment: results from the HIV prevention trials network 052 trial. J Acquir Immune Defic Syndr 2016; 72: 304– 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bezabhe W, Chalmers L, Bereznicki L et al. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wyl V, Klimkait T, Yerly S et al. Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV cohort study. PLoS ONE 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mannheimer S, Friedland G, Matts J et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34: 1115– 1121. [DOI] [PubMed] [Google Scholar]
- 38. Boussari O, Subtil F, Genolini C et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Paredes R, Ribaudo H et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA 2011; 305: 1327– 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cozzi-Lepri A, Noguera-Julian M, Giallonardo FD et al. Low-frequency drug-resistant HIV-1 and risk of virological failure to first-line NNRTI-based ART: a multicohort European case-control study using centralized ultrasensitive 454 pyrosequencing. J Antimicrob Chemother 2015; 70: 930– 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunningham E, Chan Y-T, Aghaizu A et al. Enhanced surveillance of HIV-1 drug resistance in recently infected MSM in the UK. J Antimicrob Chemother 2017; 72: 227– 234. [DOI] [PubMed] [Google Scholar]
- 42. Waters L, Ahmed N, Angus B et al. BHIVA guidelines for the treatment of HIV-1- positive adults with antiretroviral therapy 2015 (2016 interim update). British HIV Association, 2016. Available at: www.bhiva.org/HIV-1-treatment-guidelines ( accessed September 2019). [DOI] [PubMed]
- 43. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach – Second edition. WHO ( 2016). [PubMed]
- 44. Llibre JM, Pulido F, García F et al. Genetic barrier to resistance for dolutegravir. AIDS Rev 2015; 17: 56– 64. [PubMed] [Google Scholar]
- 45. BHIVA Writing Committee. Gazzard B, on behalf of the British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2005). HIV Med 2005; 6: 1– 61. [DOI] [PubMed] [Google Scholar]
- 46. Brown A, Mohammed H, Ogaz D et al. Fall in new HIV diagnoses among men who have sex with men (MSM) at selected London sexual health clinics since early 2015: testing or treatment or pre-exposure prophylaxis (PrEP)? Eurosurveillance 2017; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix containing further details of statistical methodology and fitted models is available at the following website link: https://doi.org/10.6084/m9.figshare.9884627.v1