ABSTRACT
Blepharospasm (BPS) is one of the most frequent types of facial dystonia and, at the same time, one of the most disabling, being able to trigger functional blindness if not treated. Our aim with this work was to evaluate the efficacy and safety of long-term onabotulinum A toxin (BAT) treatment in a cohort of patients with BPS.
The retrospective study was conducted on consecutive patients with BPS treated with subcutaneous BAT. The selection of muscles and dose was made based on each patient’s needs. The clinical and demographic characteristics, number of sessions, dose, duration and effectiveness of treatment, and adverse events were analysed.
130 patients were included in the study. The median (95% confidence interval) length of follow-up was 14 (13–15.6) years with an average of 20.5 sessions (range from 10 to 57). Regarding the efficacy of the treatment, 114 (87.7%) experienced satisfactory results with functional and aesthetics recovery. Patient evaluation of global response suggested a clear improvement without adverse events in 72 (55.4%) patients. Adverse events developed at least once during the treatment in 39% of patients, with transient ptosis and haematoma the most common reported both by physician and patient.
The results of our study suggest that botulin toxin A is a safe and effective long-term treatment for blepharospasm with mild, transient and well-tolerated side effects when they appear.
KEYWORDS: Botulinum toxin, blepharospasm, efficacy, safety, long-term
Introduction
As mentioned above, blepharospasm (BPS) is one of the most frequent types of facial dystonia and, at the same time, one of the most disabling. Clinical findings of the disease include excessive blinking, photophobia, and forceful involuntary eyelid closure due to involuntary contractions of the orbicularis oculi and surrounding muscles1, leading in some cases to functional blindness.
The prevalence is estimated around 12–133 cases per million.2 It usually appears between the fifth to the seventh decades of life and affects predominately women.3,4
The primary form is often called benign essential blepharospasm (BEB). However, BPS can also occur in the setting of neurodegenerative diseases, such as parkinsonism, hereditary ataxias and generalised dystonias. Although the aetiology of the disease is not well known, several lines of indirect evidence, including electrophysiological studies5, structural6,7 and functional brain imaging8, and autopsy findings9, suggest a central origin with a dysfunction of the thalamus, basal ganglia, or brainstem.
Concerning the genetic aspects of BPS, it seems that there is a higher frequency of focal dystonia in relatives, but with a considerable phenotypic variability.10
Since the BPS was classified, different therapeutic options have been proposed. These include anticholinergics, dopamine agonists, presynaptic monoamine-depleting agents, botulinum toxin injections, and, in refractory cases, myomectomy of the orbicularis oculi muscle.4,11 The administration of Botulinum A toxin therapy (BAT) into the eyelids and eyebrows, is now generally considered as the treatment of choice.12 BAT is a potent biological neurotoxin that blocks the release of acetylcholine at the neuromuscular junction leading to a state of muscle paralysis that may last for months.
The association of BPS and dystonia of lower facial, jaw, and neck muscles has been classified as a form of segmental dystonia called cranial dystonia, or ‘‘Meige syndrome”.11 Its symptoms usually begin in the fifth or sixth decade of life with a twofold higher incidence in women. Similarly to BEB, the aetiology is unknown and the first line treatment is botulinum toxin injections.
Many different studies have shown both efficacy and safety of treatment with BAT.13–17 However, it has been suggested that long-term botulinum toxin treatment might result in a decreasing of the effectiveness of treatment over time.18,19
The longest reported follow-up period to assess the efficacy and safety of botulinum toxin therapy was published by Czyz et al in. 201311 This study included 37 patients, 26 with BPS, 7 with hemifacial spams, and 4 with Meige syndrome, with average treatment duration of 19.4 years.
Our study aimed to evaluate the efficacy and safety of long-term BAT treatment in a cohort of patients with BPS.
Methods
A retrospective study was conducted on consecutive patients with BPS treated with Onabotulinumtoxin A (BOTOX® Allergan, Inc., Irvine, CA) at the Movement Disorder Unit of the Neurology Service (La Paz Hospital, Autonomous University of Madrid) between 1991 and 2011. During the follow-up, clinical data were recorded using a standard clinical protocol.
The study was approved by the local ethics committee. All patients were fully informed about the details of the protocol and patients provided written informed consent. The ethical principles outlined in the Declaration of Helsinki and Good Clinical Practice were followed.
All participants were required to meet the following inclusion criteria: age equal or greater than 18 years; clinical diagnosis of BPS or Meige syndrome; request for BAT treatment; minimum of 1 BAT injection per year; and 10 or more years of consecutive botulinum toxin treatments. Patients with clinical diagnosis other than BPS or Meige syndrome; discontinuation of the treatment longer than 1 year; any condition considered to be a contraindication to botulinum toxin treatment (allergic reaction, diseases of the neuromuscular junction, peripheral neuropathic diseases, etc…); and pregnancy or lactation were exclusion criteria for the study.
The criteria for diagnosis of BPS included observation of involuntary bilateral increased blinking with intermittent dystonic eyelid and eyebrow contractions. On the other hand, Meige syndrome was diagnosed when clinical criteria for BPS combined with dystonia of the lower facial and/or cervical muscles were fulfilled.20
Injections of BAT were administered subcutaneously selecting the muscles and dose based on the effects of the treatment and patient needs, following the preseptal technique. Initial dose was 2.5 units per muscle treated, ranging the total administered dose of BAT from 15 to 80 units per session. In the following visits doses were calculated according to therapeutic response and tolerance. In cases of partial response to preseptal technique additionally injections in the proximity of the pretarsal portion of the orbicularis muscle were applied.
We evaluated the clinical and demographic characteristics of the sample (with special interest in the impact of the sex), number of sessions, dose of botulin toxin administered, duration and effect of the treatment, impact of the technique, and patient satisfaction. We also analysed these parameters comparing patients treated with the preseptal technique and those treated with the pretarsal technique.
The effect of the treatment was graded according to the following scale: 0 = no effect; 1 = unsatisfactory results (the adverse effects are more relevant than the functional benefit); 2 = no completely satisfactory (appropriate functional benefit with some minor adverse events); 3 = satisfactory results with clear functional benefits; 4 = satisfactory results with functional and aesthetics recovery. Treatment response was gauged based on the relief of symptoms between first and last injections.
In order to evaluate the efficacy of the treatment, from the patient`s perspective, we used the following scale: 1 = no improvement with serious adverse events, 2 = some improvement with moderate to serious adverse events; 3 = some improvement with mild to moderate adverse events; 4 = moderate improvement with none to mild adverse events; and 5 = clear improvement without adverse events.
During the course of follow-up both physician-observed and patient-related adverse events were recorded.
Statistical analysis
Statistical analysis was performed using MedCalc 12.2.1.0 (MedCalc Software, Mariakerke, Belgium).
Descriptive statistics (mean [standard deviation]) and 95% Confidence intervals (95% CIs) were used for demographic and clinical characteristics.
Data were tested for normal distribution using a D’Agostino-Pearson test.21 If data were normally distributed, a two-tailed independent-samples Student’s t-test was used to compare means between quantitative variables. When a normal distribution was not expected (time of follow-up, number of sessions, etc.) the Mann-Whitney test was used. Categorical variables were compared using a Chi-square test and a Fisher`s exact test, as needed.
Because of the large number of tests, simultaneous inference using the Bonferroni correction was used to correct the P-value (α/4). Statistical significance was accepted for P < 0.0125.
Results
Of the 161 patients who were screened, 130 fulfilled the respective demands of the inclusion and exclusion criteria. 13 (10%) patients met criteria for Meige syndrome and 12 subjects (9.2) had cervical dystonia.
This study included 85 (65.4%) women and 45 (34.6) men with a mean (standard deviation) age of 51.4 (13.0) years at initial treatment and 65.8 (12.3) years at last treatment. The median (95% CI) length of follow-up was 14 (13–15.6) years, ranging from 10 to 21 years, with an average of 20.5 sessions (range from 10 to 57). The mean dose applied over the course of follow-up per application was 32.9 (13.8) units with a range from 15 to 80 (Table 1). Additional pretarsal injections were required in 20 (15.4%) patients.
Table 1.
Demographic and clinical characteristics of study population.
| Mean (SD) | 95% CI | Range | |
|---|---|---|---|
| Age at initial treatment (years) | 51.4 (13.0) | 49.2 to 53.7 | 18–78 |
| Age at last treatment (years) | 65.8 (12.3) | 63.6 to 67.9 | 34–90 |
| Follow-up (years) | 14.5 (3.3) | 13.9 to 15.1 | 10–21 |
| Number of BAT treatments | 20.5 (13.7) | 18.2 to 22.9 | 10–57 |
| Min D1S (units) | 27.7 (10.0) | 23.9 to 31.5 | 15–45 |
| Max D1S (units) | 43.4 (12.5) | 38.7 to 48.2 | 20–80 |
| Mean D1S (units) | 32.9 (13.8) | 23.9 to 48.2 | 15–80 |
Notes. SD = Standard deviation; 95% CI = 95% Confidence interval; BAT = botulinum A toxin; Min D1S = Minimum dose in one session; Max D1S = Maximum dose in one session; Mean D1S = Mean dose in one session.
Age at the initial treatment was significantly greater in women than in men, 53.9 (13.5) vs. 46.8 (10.6) years, respectively, p = 0.0025. Additionally, the length of follow-up was significantly lower in women as compared with that in men, p = 0.007 (Table 3). However, there were no significant differences regarding BAT doses between women and men (Table 2).
Table 3.
Comparison of age at initial treatment, length of follow-up, number of BAT treatments, and minimum and maximum dose in one session between patients treated with preseptal injections as compared with those treated with pretarsal location.
| |
Preseptal (110) |
Pretarsal (20) |
|
||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | p Value 1 | |
| Age at initial treatment (years) | 50.9 (13.1) | 48.4 to 53.3 | 54.5 (12.1) | 48.8 to 60.1 | 0.2578 |
| Age at last treatment (years) | 65.1 (12.2) | 62.8 to 67.4 | 69.4(12.1) | 63.8 to 75.0 | 0.1520 |
| Follow-up (years) | 14.4 (3.2) | 13.8 to 15.0 | 15.0 (4.0) | 13.1 to 15.0 | 0.5913 * |
| Number of BAT treatments | 17.6 (11.6) | 15.4 to 19.7 | 36.9 (13.0) | 30.8 to 43.0 | < 0.0001* |
| Min D1S (units) | 22.2 (4.4) | 18.8 to 25.6 | 30.1 (11.0) | 25.0 to 35.3 | 0.0101 |
| Max D1S (units) | 33.3 (10.9) | 25.0 to 41.7 | 48.0 (10.6) | 43.1 to 52.9 | 0.0020 |
| Adverse Events None Mild Severe |
69 (62.7%) 34 (30.9%) 7 (6.4%) |
10 (50%) 9 (45%) 1 (5%) |
0.6860+ 0.4798+ 0.9061+ |
||
Notes. SD = Standard deviation; 95% CI = 95% Confidence interval; BAT = botulinum A toxin; Min D1S = Minimum dose in one session; Max D1S = Maximum dose in one session; 1 = independent samples t- test, * = Mann-Whitney test; + = Fisher’s exact test; p Values were considered statistically significant if lower than 0.0125.
Table 2.
Comparison of age at initial treatment, length of follow-up, number of BAT treatments, and minimum and maximum dose in one session between women and men.
| Women (85) |
Men (45) |
||||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | p Value 1 | |
| Age at initial treatment (years) | 53.9 (13.5) | 51.0 to 56.8 | 46.8 (10.6) | 43.6 to 50.0 | 0.0025 |
| Age at last treatment (years) | 67.7 (12.4) | 65.1 to 70.4 | 62.1 (11.2) | 58.7 to 65.5 | 0.0121 |
| Follow-up (years) | 13.9 (3.2) | 13.3 to 14.6 | 15.6 (3.3) | 14.6 to 16.6 | 0.007* |
| Number of BAT treatments | 19.5 (14.0) | 16.5 to 22.5 | 22.5 (13.0) | 18.6 to 26.4 | 0.1417* |
| Min D1S (units) | 27.5 (10.7) | 22.3 to 32.7 | 28.0 (9.2) | 21.4 to 34.6 | 0.9013 |
| Max D1S (units) | 45.5 (12.29 | 39.6 to 51.4 | 39.5 (12.8) | 30.3 to 48.7 | 0.2251 |
Notes. SD = Standard deviation; 95% CI = 95% Confidence interval; BAT = botulinum A toxin; Min D1S = Minimum dose in one session; Max D1S = Maximum dose in one session; 1 = independent samples t- test, * = Mann-Whitney test; p Values were considered statistically significant if lower than 0.0125.
The mean (95% CI) number of sessions differed significantly among those patients treated with the preseptal technique [17.6 (15.4–19.7)] as compared with those treated with the pretarsal one [36.9 (30.8–43.0)], p < 0.0001. The minimum dose of BAT administered in one session was significantly higher in those patients requiring pretarsal injections, 30.1 (11.0) units, as compared with those treated with preseptal locations, 22.2 (4.4) units, p = 0.013. Similarly, the maximum dose administered in one session was significantly higher in the pretarsal administration as compared with the preseptal location, 48 (10.6) vs. 33.3 (10.9) units, respectively, p = 0.002 (Table 3).
Regarding the efficacy of the treatment, 114 (87.7%) experienced satisfactory results with functional and aesthetics recovery. However, 13 (10%) patients needed the administration of concomitant medication, such as pimozide, baclofen, clonazepam, and trihexyphenidyl, and 3 (2.3%) needed surgery (myomectomy of the orbicularis oculi).
The efficacy of those 20 patients treated with pretarsal injections, 2 (10%) patients did not show any positive effect, 8 (40%) obtained satisfactory results with clear functional benefits, and 10 (50%) experienced satisfactory results with functional and aesthetics recovery.
Patient evaluation of global response suggested some improvement with none to mild adverse events in 39 (30%) and a clear improvement without adverse events in 72 (55.4%) patients (Figure 1).
Figure 1.
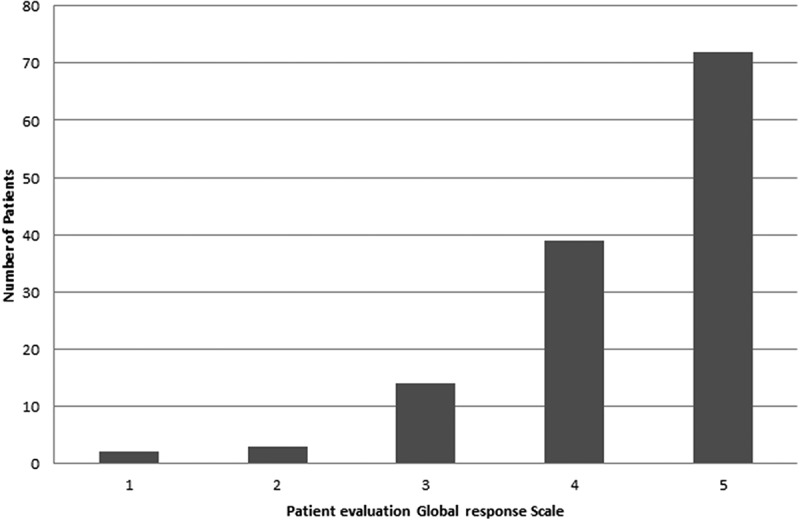
Patient Evaluation of Global Response Scale. The following scale was used for this purpose: 1 = no improvement with serious adverse events; 2 = some improvement with moderate to serious adverse events; 3 = some improvement with mild to moderate adverse events; 4 = moderate improvement with none to mild adverse events; and 5 = clear improvement without adverse events.
Adverse events developed at least once during the treatment in 39% of patients with transient ptosis and haematoma being the most common reported by physician and patient. Among serious adverse events the most commonly observed was diplopia affecting 5% of patients (Table 4). Our results suggest that there are no differences in the incidence of either mild or severe adverse events between subjects treated with preseptal injections and those treated with the pretarsal location (Table 3).
Table 4.
Adverse events associated with the botulin A toxin administration over the duration of the study.
| Adverse Event (AE) | Number (%) |
|---|---|
| Subjects with at least 1 AE | 51 (39.2) |
| Ptosis | 32 (24.6) |
| Haematoma | 12 (9.2) |
| Dry Eye | 2 (1.5) |
| Dysgeusia | 1 (0.8) |
| Diplopia | 7 (5.3) |
| Corneal Ulcer | 1 (0.8) |
| Dysphagia * | 4 (3.1) |
Note. * Dysphagia is related to the need for cervical approach in some patients.
Despite the occurrence of adverse events they were transient and well tolerated for all patients and the withdrawal of the treatment was not necessary in any case. The treatment was neither retired for another reason.
Discussion
The results of our study suggest that the BAT therapy offered very good response rate, 88% of patients, with sustained effects over the long-term.
Our results are in agreement with those previously published by Czyz et al in 201317 This study evaluated 37 patients with BPS followed for at least 15 years (average duration of the treatment 19.4 years) who underwent treatment with BAT. This study reported that the mean duration of the effect and botulinum toxin dose required for treatment remained unchanged during the follow-up of the study.17 Although the mean length of follow-up of our study was slightly lower (14.5 years) as compared with that in the Czyz et al study17 (19.4), the sample of our study was significantly greater, 130 vs. 37 patients.
Additionally, our study partially agree with that published by Gil-Polo et al.22 who reported that repeated injections of BTA are efficacious and well tolerated in the long-term treatment of BPS. Although the follow-up and the sample of our study were greater, we observed fewer incidences of pretarsal injections (15.4%) than in the Gil-Polo study (41.2%).22
Additionally, the results of our study are consistent with many of those previously published with shorter follow-up periods22–25 regarding not only the efficacy of the BTA but also the epidemiological findings (age at onset and female predominance).
Because of the small number of patients with Meige syndrome (13) or cervical dystonia (12), we did not think that a separate statistical analysis can provide relevant findings.
Curiously, our study found some significant differences between women and men in both age at initial treatment and length of follow-up. But, due to the design, our study is not capable to give an explanation to these findings.
As regards the effectiveness of the different infiltration techniques, it has been suggested that pretarsal injections have better outcomes than the preseptal injections.26 Cakmur et al.26 performed a retrospective study in 25 patients with BPS and 28 patients with hemifacial spasm that compared pretarsal with preseptal injections. The results of this study suggested that pretarsal injections produced significantly higher response rate and longer duration of maximum response in both patient groups.26 Conversely, our study showed that patients treated with the pretarsal technique received higher doses of BAT and more sessions of treatment, but this technique did not seem to be more effective.
The incidence of adverse events in our study (39%) is slightly higher than that published in different studies. Kim et al27 reported a very low rate of adverse events in a sample of 1819 patients treated with BTA for different s purposes. This study found an incidence of adverse events of the 8.3% in patients with BPS. In line with these results, Aquino et al25 reported an incidence of adverse events of the 14% in a cohort of 113 patients treated with BTA. Nevertheless, the incidence of adverse events in our study is similar to that previously reported by other studies16,24,27 and significantly lower than that published in the Gil-Polo et al’s study.22 As in prior studies16,22,24,25,28 ptosis was the most common side effect (25%) following by haematoma (9%). Nevertheless, side effects were well tolerated in our series and were not related to treatment withdrawal. Furthermore, it is essential to keep in mind that this is the total adverse events over the course of the 20 years of follow-up, so the annual rate of adverse events was much lower.
Our study has some limitations. The most important one is its retrospective design. Selection and confounding bias are all inherent limitations of retrospective studies. Nevertheless, the strict inclusion/exclusion criteria applied in our study was such to minimise this potential bias. The second limitation of our study is that it is a single centre study. Nevertheless, the fact of including a large number of patients with a long follow-up minimises the impact of such limitation.
Despite these limitations, we found that botulin toxin A was a safe and effective long-term treatment for BPS and most of the side effects were well tolerated.
Funding Statement
This work has not received any funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Nicoletti AGB, Aoki L, Nahas TR, Matayoshi S.. Blefaroespasmo essencial: revisão da literatura. Arq Bras Oftalmol. 2010;73:469–473. [DOI] [PubMed] [Google Scholar]
- 2.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF international workshop. Neurology. 2008;71:1275–1282. doi: 10.1212/01.wnl.0000327601.46315.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic J, Orman J. Blepharospasm: demographic and clinical survey of 250 patients. Ann Ophthalmol. 1984;16(4):371–376. [PubMed] [Google Scholar]
- 4.Grandas F, Elston J, Quinn N, Marsden CD. Blepharospasm: a review of 264 patients. J Neurol Neurosurg Psychiatry. 1988;51(6):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller J, Rinnerthaler M, Poewe W, Kofler M. Auditory startle reaction in primary blepharospasm. Mov Disord. 2007;22:268–272. doi: 10.1002/mds.21270. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J, Patel S. Blepharospasm associated with brain stem lesions. Neurology. 1983;33:1237–1240. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J. Blepharospasm with basal ganglia lesions. Arch Neurol. 1986;43:866–868. [DOI] [PubMed] [Google Scholar]
- 8.Esmaeli-Gutstein B, Nahmias C, Thompson M, Kazdan M, Harvey J. Positron emission tomography in patients with benign essential blepharospasm. Ophthal Plast Reconstr Surg. 1999;15:23–27. [DOI] [PubMed] [Google Scholar]
- 9.Kulisevsky J, Marti MJ, Ferrer I, Tolosa E. Meige syndrome: neuropathology of a case. Mov Disord. 1988;3:170–175. doi: 10.1002/mds.870030209. [DOI] [PubMed] [Google Scholar]
- 10.Defazio G, Martino D, Aniello MS, Masi G, Abbruzzese G, Lamberti S, Valente EM, Brancati F, Livrea P, Berardelli A. A family study on primary blepharospasm. J Neurol Neurosurg Psychiatry. 2006;77:252–254. doi: 10.1136/jnnp.2005.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft SP, Lang AE. Cranial dystonia, blepharospasm and hemifacial spasm: clinical features and treatment, including the use of botulinum toxin. CMAJ. 1988;139:837–844. [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson DM, Blitzer A, Brashear A, Comella C, Dubinsky R, Hallett M, Jankovic J, Karp B, Ludlow CL, Miyasaki JM, Naumann M, So Y; Therapeutics and technology assessment subcommittee of the American Academy of Neurology. Practice parameter: botulinum neurotoxin for the treatment of movement disorders and spasticity: an evidence-based report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37:616–623. [DOI] [PubMed] [Google Scholar]
- 14.Truong DD, Jost WH. Botulinum toxin: clinical use. Parkinsonism Relat Disord. 2006;12(6):331–355. doi: 10.1016/j.parkreldis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Truong D, Comella C, Fernandez HH, Ondo WG. Dysport benign essential blepharospasm study group. Efficacy and safety of purified botulinum toxin type A (Dysport) for the treatment of benign essential blepharospasm: a randomized, placebo-controlled, phase II trial. Parkinsonism Relat Disord. 2008;14:407–414. doi: 10.1016/j.parkreldis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, Gasser T, Krauss JK, Nardocci N, Newton A, Valls-Solé J. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18(1):5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 17.Czyz CN, Burns JA, Petrie TP, Watkins JR, Cahill KV, Foster JA. Long-term botulinum toxin treatment of benign essential blepharospasm, hemifacial spasm, and meige syndrome. Am J Ophthalmol. 2013;156(1):173–177. doi: 10.1016/j.ajo.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Haussermann P, Marczoch S, Klinger C, Landgrebe M, Conrad B, Ceballos-Baumann A. Long-term follow-up of cervical dystonia patients treated with botulinum toxin A. Mov Disord. 2004;19(3):303–308. doi: 10.1002/(ISSN)1531-8257. [DOI] [PubMed] [Google Scholar]
- 19.Brin MF1, Comella CL, Jankovic J, Lai F, Naumann M. CD-017 BoNTA study group. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23(10):1353–1360. doi: 10.1002/mds.v23:10. [DOI] [PubMed] [Google Scholar]
- 20.Tolosa E, Marti MJ. Blepharospasm-oromandibular dystonia syndrome (Meige’s syndrome): clinical aspects. Adv Neurol. 1988;49:73–84. [PubMed] [Google Scholar]
- 21.Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 5th ed. Boca Raton: Chapman & Hall /CRC; 2011. [Google Scholar]
- 22.Gil Polo C, Rodríguez Sanz MF, Berrocal Izquierdo N, Castrillo Sanz A, Gutiérrez Ríos R, Zamora García MI, Mendoza Rodríguez A, Duarte García-Luis J. Blepharospasm and hemifacial spasm: long-term treatment with botulinum toxin. Neurologia. 2013;28(3):131–136. doi: 10.1016/j.nrl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Snir M, Weinberger D, Bourla D, Kristal-Shalit O, Dotan G, Axer- Siegel R. Quantitative changes in botulinum toxin a treatment over time in patients with essential blepharospasm and idiopathic hemifacial spasm. Am J Ophthalmol. 2003;136:99—105. [DOI] [PubMed] [Google Scholar]
- 24.Silveira-Moriyama L, Gonçalves LR, Chien HF, Barbosa ER. Botulinum toxin a in the treatment of blepharospasm: a 10-year experience. Arq Neuropsiquiatr. 2005;63:221–224. [DOI] [PubMed] [Google Scholar]
- 25.Aquino CC, Felício AC, Castro PC, Oliveira RA, Silva SM, Borges V, Ferraz HB. Clinical features and treatment with botulinum toxin in blepharospasm: a 17-year experience. Arq Neuropsiquiatr. 2012;70:662–666. [DOI] [PubMed] [Google Scholar]
- 26.Cakmur R, Ozturk V, Uzunel F, Donmez B, Idiman F. Comparison of preseptal and pretarsal injections of botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neurol. 2002;249:64—8. [DOI] [PubMed] [Google Scholar]
- 27.Kim BW, Park GH, Yun WJ, Rho NK, Jang KA, Won CH, Chang SE, Chung SJ, Lee MW. Adverse events associated with botulinum toxin injection: A multidepartment, retrospective study of 5310 treatments administered to 1819 patients. J Dermatolog Treat. 2014;25(4):331–336. doi: 10.3109/09546634.2013.789473. [DOI] [PubMed] [Google Scholar]
- 28.Ainsworth JR, Kraft SP. Long-term changes in duration of relief with botulinum toxin treatment of essential blepharospasm and hemifacial spasm. Ophthalmology. 1995;102(12):2036–2040. [DOI] [PubMed] [Google Scholar]


