Abstract
Objective:
Using an integrative view of psychology, neuroscience, immunology and psychophysiology, the present review of literature curates the findings that have had an impact on the field of bereavement research, and shaped its development.
Methods:
Beginning with Lindemann’s systematic descriptions of medical and psychological responses to the death of a loved one, specific studies that investigate medical outcomes following loss, their psychological predictors, and biopsychosocial mechanisms are discussed. This selective review culminates in recommendations for the field for future research, including greater integration of these disparate fields of inquiry.
Results:
Morbidity and mortality following the death of a loved one has long been a topic of research. Early researchers characterized somatic and psychological symptoms and studied immune cell changes in bereaved samples. More recent research has repeatedly demonstrated increased rates of morbidity and mortality in bereaved samples, as compared to married controls, in large epidemiological studies. Recent developments also include the development of criteria for prolonged grief disorder (also termed complicated grief). Newer methods, including neuroimaging, have observed that the greatest impact of the death of a loved one is in those who have the most severe psychological grief reactions. Mechanisms tying bereavement to medical outcomes are scarce, but differences in rumination, in inflammation and in cortisol dysregulation between those who adapt well and those who do not, have been offered with some evidence.
Conclusions:
Recommendations to propel the field forward include longitudinal studies to understand differences between acute reactions and later adaptation, comparing samples with grief disorders from those with more typical responses, and integrating responses in brain, mind and body.
Keywords: bereavement, grief, default mode, widow, morbidity, prolonged grief disorder
Psychosomatic medicine has a long and storied history of studying the health effects of bereavement. The death of a loved one has been recognized as the greatest life stressor that we face as humans, heading the list of stressful life events compiled by Holmes and Rahe(1). These researchers were attempting to quantify the relationship between life events that require an ongoing adjustment (e.g., chronic stress) and the timing of illness onset. The earliest accounts of the symptomatology and management of acute grief harken back to the beginning of the field of psychosomatic medicine. In 1944, Erich Lindemann published data collected from bereaved family members following the Cocoanut Grove Fire that killed 492 people, the deadliest nightclub fire in history. This was the first-ever systematic study of the somatic and psychological aspects of bereavement(2), which continue to interest the field today.
The present paper will cover topics that are not frequently combined in a review, despite the fact that they all include the empirical investigation of grief. In the first section I will discuss some important historical and contemporary developments in the field of bereavement research, including theoretical models that can be used to understand the experience and process of grieving. These historical events in the field affected the way that subsequent grief research has been conducted. The next section will cover adaptation to grief in the body, chronicling investigations of medical outcomes during bereavement, followed by acute and chronic changes seen in the biomarkers of diverse physiological systems. Although not often studied in a combined research design, these biomarkers are the presumed mechanisms linking the loss event with medical morbidity and mortality. The next section covers advancements in what is known about how the mind adapts during grief, preferentially covering those mental processes that are amenable to psychological intervention. The following section reviews findings in neuroscience that speak to how the brain adapts following the death of a loved one. Last, a summary section makes recommendations for future research and integration of these disparate subfields. As a final note, the present review is not a systematic or comprehensive one, but rather highlights particular studies in the field that I believe may point us to a greater understanding of the role of grief in illness.
Important historical developments in bereavement research
Descriptions and theories of what happens in grief have largely come from psychiatry and psychology. From these domains, current grief research relies heavily on attachment theory and cognitive stress theory to understand the process of adapting after the death of a loved one, rather than the outdated and inaccurate five-stage model of grief(3). Acute grief, or the period immediately following a death, is often characterized by a loss of regulation. This can be observed as increased intensity and frequency of sadness, anger and/or anxiety, and also emotional numbness and difficulty concentrating, in addition to dysregulation in sleep and appetite.
There are wide individual differences in the adaptation process, but George Bonanno has demonstrated a small number of trajectories, using prospective data to examine adaptation after a death(4,5). One insight from this work, which disrupted the field of bereavement research, was that the vast majority of individuals are very resilient (approximately 60%). By six months, the resilient group shows no elevation in depressive symptoms or functional impairment. This does not mean that resilient people do not experience the intense short-term pangs of grief, but these emotional waves do not cause functional impairment. The realization that previous theories of grief were largely based on a treatment-seeking population forced the field to reconsider some of its assumptions. Consequently, a very influential model of grief, the dual process model of coping, was adopted to reflect the oscillation that occurs in typical grief(6). In day-to-day life during bereavement, healthy people oscillate between focusing on loss-related stressors (e.g., the pain of living without the person) and restoration-related stressors (e.g., engaging in new roles and identities due to the loss), and at other times are simply engaged in everyday life experience.
Importantly, Bonanno’s research demonstrated that the functioning of a person prior to the death event is also an important aspect of their trajectory of adaptation. Those who are depressed prior to bereavement may need different interventions from those who develop depression only after the event. Depression and grief can be distinguished clinically, statistically(7), and even pharmacologically, as antidepressants do not ameliorate grief symptoms(8). It is notable that there is very little application of these trajectories of adaptation to physiological systems (thus far). Sporadic work has compared acute grief (from immediately following the death event to six months after the loss) to later grief (from six months to a lifetime exposure to deaths), but it is difficult to compare across these studies, and they have rarely taken advantage of sophisticated longitudinal statistical analyses that are now available.
The most recent insight that has changed the field of grief research is the development of criteria characterizing disordered grief. Although for decades psychiatry and psychology have described the fact that some people experience grieving of greater intensity and functional impairment in comparison to others, criteria were developed in the 1990’s to define what is now most often termed “complicated grief disorder” or “prolonged grief disorder”. Symptoms are divided into separation distress, including persistent yearning and pre-occupation with the loss, and traumatic distress. These may include difficulties accepting the death, feeling one has lost a part of one’s self, anger about the loss, guilt, or difficulty in engaging with social or other activities. These disorders now appear in the Diagnostic and Statistical Manual-5 (DSM-5; as an area for continued study)(9) and in the International Classification of Diseases-11 (ICD-11). The advent of a discrete disorder required a name for “non-complicated grief,” (i.e., those who are bereaved, but resilient in integrating the experience). This term is based on the label used for “non-depressed”. However, additional work is needed to validate the diagnostic criteria, especially across cultures, and to compare diagnostic criteria sets that have been developed(10). Although the criteria sets share the hallmark symptoms of intense yearning and preoccupation with the deceased, additional criteria requirements for diagnosis vary in type and number. As with all mental disorders, the rates of complicated grief are very low (approximately 10 percent of bereaved individuals(11)) and likely form a continuous phenomenon of grief severity, with a chosen cut-off point for diagnosis.
This historical inflection point of diagnostic criteria means that it is now difficult to compare studies done prior to the advent of the grief disorder category to those done after, because earlier studies looked at health effects of grief across the full range of severity. The samples from these earlier studies include people with complicated grief and bereaved people who do not. Later studies often specifically model complicated grief or grief severity as a predictor of health outcomes. Because of the recency of these diagnostic criteria, the vast majority of studies reviewed in the present paper investigate bereavement as a category, and not grief severity or disordered grief.
Absent grief, or a lack of overt expression of grief through denial or suppression, was described originally with psychoanalytic theories. As a construct, absent grief has been clarified through contributions of psychology research (although more research is needed in this area). The difficulty in distinguishing resilience (which appears as a lack of overt grief expression) and suppression (which also appears as a lack of overt grief expression, but masks intense emotional experience) has made this area difficult to study. Elegant laboratory work has distinguished these two phenomena under conditions of cognitive load(12), but clinicians rarely have laboratory tasks to rely on with individual patients. It has been demonstrated that delayed increased medical consequences are not commonly seen in those who do not express overt grief. However, there is still the open question as to whether discriminating true resilience from suppression (the latter being employed by a much smaller group) would reveal mechanisms of poor physical health outcomes in those who suppress grief emotions.
Adaptation of the body during grief
In 1961 in Psychosomatic Medicine, George Engel wrote an article entitle, “Is Grief a Disease? A Challenge for Medical Research(13).” Engel is often misquoted by relying on the title of the article, and although he did not state that grief was a disease, he did suggest that grief was a legitimate topic for medical research(14). Nonetheless, he pointed in the direction that the field has followed ever since:
“Until—and not until—much more is known about the biochemical, physiological, and psychological consequences of such losses, no one is justified in passing judgment as to how important this factor is in the genesis of the disease states that seem so often to follow close upon an episode of grief.” (p. 21)
The study of these “biochemical, physiological” mechanisms can be traced back to the earliest publication of immune correlates of bereavement, published by Roger Bartrop and colleagues in 1977(15). In the past forty years, the field of psychosomatic medicine has investigated biomarkers that may help to explain the relationship between bereavement and medical outcomes, including mechanisms in autonomic (particularly cardiovascular), endocrine, and immune systems. Additionally, the neural correlates of bereavement have been investigated, specifically attempting to determine the role of the brain in the relationship between the death event and subsequent medical illness. Notably, Engel also presciently considered this: “…whatever the consequences of object loss and grief may be, whether manifest ultimately in biochemical, physiological, psychological or social terms, they must first be initiated in the central nervous system (emphasis added).” In the past fifteen years or so, studies of the physiological concomitants of grief have included functional magnetic resonance imaging (fMRI), a method Engel would no doubt have found exciting.
Perhaps the most compelling evidence that there is a connection between bereavement and medical consequences is documentation of the “broken-heart phenomenon”, or the increased risk of mortality for bereaved people in the first six months after the loss event compared to their married counterparts. Evidence of his phenomenon was first published in 1963 in the Lancet(16) and in the British Medical Journal(17). Unfortunately, the term “broken-heart” has become associated with a specific medical condition in the literature. First reported in 1990 by Sato and colleagues, Takotsubo cardiomyopathy is acute stress-induced cardiomyopathy involving left ventricular apical ballooning that mimics acute myocardial infarction(18). Because the stressful event leading to Takotsubo cardiomyopathy is sometimes (though not always) the death of a loved one, the condition has become synonymous with the “broken heart”. For this reason, the increased risk of all-cause morbidity and mortality in the bereaved has alternatively been called “the widowhood effect”. However, this term is also somewhat unsatisfying, as the stressful event does not have to be the death of a spouse, but can be the death of any attachment figure.
In the past twenty years, multiple epidemiological studies have verified the excess morbidity and mortality following the death of a loved one. In a study of 1.5 million Finns, risk of chronic ischemic heart disease was 2.08-fold higher in men in the six months after the death of their wife, compared to the continuously married cohort(19). In the Health and Retirement Study (N=12,316), mortality risk for widowed men was 1.87 adjusting for demographics, behavioral risk factors and co-morbidities(20). The relative risk of death is 22% higher for both widows and widowers compared to married individuals, adjusting for age and other relevant covariates(21), although the effect may also be moderated by sex(22). The increased risk is for all-cause mortality, including cardiovascular disease, acute health events (e.g. infections), chronic diseases (e.g. diabetes), and cancer(23). This increased risk from bereavement is higher than well-established cardiovascular risk factors, such as smoking(24).
All-cause morbidity is also increased following the death of a loved one, including cardiovascular events(25), vascular disease(23), incidence of cancer(26,27) and self-reported hypertension(28). In a case crossover study, increase in the incidence of a non-fatal myocardial infarction was 21 times higher in the 24 hours after the death of a loved one when compared to an a priori control period in the previous 6 months of the patient’s life(25). The risk in the first day was almost 28 times higher when the patient reported that the death was moderately or extremely meaningful, pointing to the psychological aspect of grief contributing to the medical outcome. Although the death of a loved one is a rare event in the life of an individual, it is a nearly universal experience across the population. This means that in absolute terms, there is one excess heart attack per 1394 people at low cardiovascular risk, and one excess heart attack per 320 people at high cardiovascular risk(25). These numbers demonstrate that the effect of bereavement on medical outcomes is a significant public health concern.
Changes in biomarkers during grief
Although the links between bereavement, morbidity, and mortality highlight the importance of bereavement as a public health concern, measuring changes in biomarkers following the death of a loved one can help us to understand the mechanisms that may lead to these medical endpoints. As mentioned above, autonomic, cardiovascular, endocrine and immune biomarkers are likely candidates. In particular, endocrine and immune biomarkers have a widespread effect on end organs and systems of the body, making them likely mechanisms, given the all-cause nature of bereavement-related morbidity and mortality.
Cardiovascular biomarkers have shown consistent changes in bereavement when comparing acute (e.g., <6 weeks) and chronic grief within bereaved individuals, and also between bereaved and nonbereaved groups. The shift is seen in tonic activity, although there are some indications that reactivity measures (i.e., phasic activity) may also differ(29,30). These biomarkers include increased heart rate (resting and 24-hour), heart rate variability, systolic and diastolic blood pressure, von Willebrand factor, and platelet/granulocyte aggregates(31–34). Higher levels of cortisol(35–37) and dysregulated HPA axis activity are also seen consistently in bereavement(38,39). The mechanisms linking biomarkers to medical outcome may have mediators or moderators as well. For example, the psychological reactions to the death (such as grief severity or numbness) influence cortisol levels following the event. Men who experience high levels of numbness following the death have high levels of cortisol at 18 months(40). Two studies have demonstrated that those with complicated grief drove the cortisol effect compared to other bereaved adults without the disorder(41,42).
Immune changes following bereavement are also documented, although not ubiquitously, as shown in a recent systematic review(43). Pro-inflammatory markers IL-6 and IL-1 are higher in bereaved adults(44–46). One of these studies found that the elevated IL-6 levels were moderated by a pro-inflammatory variant of the IL-6 −174 single-nucleotide polymorphism (SNP)(47). However, another inflammatory marker, C-reactive protein, is not higher in bereaved compared to non-bereaved adults, even with reasonably large sample sizes(32,46). Therefore, inflammatory responses in the wake of bereavement may be specific, and these inflammatory changes may be due to cellular immune changes that are also observed. In vitro lymphocyte proliferative response to mitogens, natural killer cell activity, and neutrophil function are decreased in bereavement and this impairment occurs independent of changes in absolute numbers and percentages of lymphocytes and lymphocyte subpopulations(15,32,36,48,49). Finally, bereavement is associated with decreased antibody response to vaccination(50). In summary, early studies indicate changes in the physiological systems of the bereaved that could be investigated as a link between the bereavement event and the survivor’s morbidity or mortality.
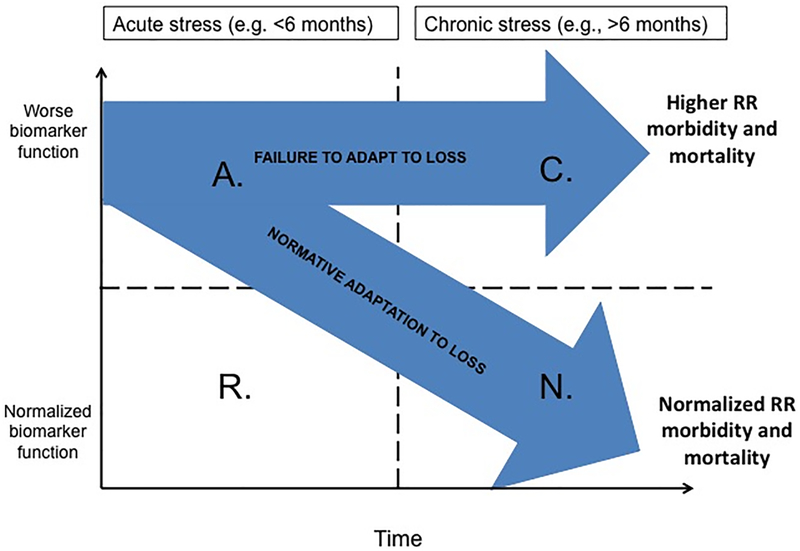
Figure 1 illustrates a model of the potential trajectories that biomarkers might take, forming a link between bereavement and medical outcomes (reproduced with permission from Knowles, Ruiz, O’Connor, in press). This model has the advantage of pointing out the importance of time in the normalization of biomarkers during grieving. Time since loss is on the x-axis and biomarkers (e.g., inflammation, heart rate, blood pressure) are on the y-axis. The y-axis could be replaced with any specific parameter under investigation. With a process model, we can easily see where previous studies already provide information about biomarkers. For example, IL-6 is increased in bereaved people compared to married controls at one year and two years (45,46), but we know nothing about this pro-inflammatory marker during acute grief. We may hypothesize that increased cardiovascular events during acute grief are related to inflammation, but a process model is required to determine the mechanistic links.
Figure 1. (adapted with permission from Knowles, Ruiz, O’Connor, in press).
Broad Model of Acute and Chronic Alterations in Biomarkers Following Bereavement. Quadrants: A = Acute dysregulation, R = Resilient to changes, C = Chronic dysregulation, N = Normalized function. The vertical transition line refers to the time point where most individuals show normalization in parameters. The horizontal clinical cutoff line refers to the level at which parameters affect pathophysiology of disease. RR = relative risk.
In addition, the model highlights fact that not everyone will react in the same way following the death of a loved one. The model creates the opportunity to show multiple trajectories. Quadrant A refers to people with Acute biomarker changes following bereavement and quadrant R refers to those in acute grief who are Resilient to the disruption. Quadrant C refers to those who show Chronic dysregulation over time and quadrant N shows those whose dysregulation Normalizes over time. Most markers normalize over time for most people (path A to N). However, a subset of bereaved people show dysregulation in biomarkers that persists over time and the putative outcomes of increased morbidity and mortality (path A to C). The vertical line in the figure can be used to delineate the point in time at which the majority of people have normalized function, providing useful comparative information for clinicians. The horizontal line can be used to indicate the clinical cut-off point for biomarkers that have known medical consequences or clinical guidelines (e.g., 140 for systolic blood pressure).
In the face of clear epidemiological evidence of increased morbidity and mortality during bereavement, the field would benefit from moving beyond documenting evidence of the widowhood effect, and focusing efforts on how the effect occurs. Longitudinal studies could investigate individual differences in the trajectories of physiological adaptation, as we have seen done for psychological adaptation. The medical effects during the first weeks post-loss may be distinct from those occurring later in adaptation. Discovering whether the physiological mechanisms operating during these two periods are independent or causally related would advance the field enormously. No longer should studies lump acute grief and chronic grief together, nor combine individuals with a resilient trajectory with those diagnosed with complicated grief.
Adaptation of the mind during grief
Unfortunately, in the field of bereavement research, scientists who study the effects of grief in the body and those who study the effects of grief in the mind do not very often interact, attend the same conferences, or read the same journals. Although this split can be seen in many subfields (and psychosomatic medicine often attempts to bring subfields together), this lack of communication seems particularly problematic for comprehending the effects of bereavement; therefore it has been my goal to attempt to bridge these research areas in my own work and to introduce the methods, topics, and research advancements to each respective community.
A number of factors are associated with greater grief and depressive symptoms following bereavement, including avoidant attachment, neuroticism, unexpectedness of the loss, adequacy of financial situation and low social support(51). However, these factors would not be easily changed by clinical intervention. Thus, the field may benefit by focusing on the processes (cognitive, emotional, and behavioral) that are more amenable to intervention and processes that mediate the adaptation trajectory in bereavement.
Processes that mediate the relationship between risk factors and mental health outcomes include (among others) rumination(52), deliberate grief avoidance(53), emotional expression(54), cognitive appraisals(55), and meaning-making(56). In elegant work comparing theoretically important mediators, rumination was found to mediate the relationship between several risk factors and greater grief and depressive symptoms(51). These risk factors included gender, attachment avoidance, neuroticism, social support and expectedness of the loss. Thus, those who experience an unexpected death are more likely to ruminate, which causes them to have higher levels of grief and depressive symptoms, as shown through mediation analyses. Although rumination has been studied in the context of some medical outcomes(57), this has not been closely investigated in bereavement research.
Avoidance is a natural and adaptive response during grieving in small doses; however, high levels of deliberate avoidance of grief-related emotions may lead to prolonged activation of the suppressed thoughts and physiological arousal, poorer concentration and functioning on tasks in the moment, and prolonged likelihood of recurrent intrusive thoughts in the future. Surprisingly, rumination can also be a form of avoidance. Maarten Eisma, Maggie Stroebe, and Henk Schut have showed this in a series of elegant studies. Grief rumination includes repetitive thinking focused on the causes and consequences of the loss and loss-related emotions. The specific content of grief-related rumination has been studied, and maladaptive grief rumination includes counterfactuals (e.g., could I have done something to prevent the death?) and self-focused perseveration on the injustice of the death (e.g., why did this happen to me and not someone else?). Maladaptive rumination predicts depressive and complicated grief symptoms. As shown through eye tracking, those high in rumination avoid looking at reminders of the death compared to those bereaved individuals lower in rumination(58), and using a reaction time task, high ruminators are faster to push reminders away from themselves than low ruminators(59). To summarize, high levels of avoidance of grief (even while simultaneously ruminating about other aspects of the death) appear to be detrimental to long-term adaptation.
Adaptation of the brain during grief
Neuroscience provides us with another lens through which to view grief and the process of adaptation (or lack thereof). After all, it is the perception of the death event through seeing or hearing about the death, followed by the comprehension of that information and its consequences, which leads to the psychobiological reaction. The neurobiology of grief is still in its infancy, but several seminal pieces of research have been conducted thus far. These have included functional neuroimaging, structural neuroimaging and even an animal model of bereavement (i.e., between monogamous, pair-bonded voles)(60,61).
In the first functional neuroimaging study of grief ever conducted, we chose to have participants view a photo of their deceased loved one captioned with a grief-related words contrasted with viewing a matched photo of a stranger, captioned with neutral words in order to elicit grief. This original study was descriptive, and we realized that grief is a complex emotional state, incorporating many mental functions. Resulting activated brain regions are involved in emotional processing, mentalizing, episodic memory retrieval, processing of familiar faces, visual imagery, autonomic regulation, and modulation or coordination of these functions(62). Regions activated by personally relevant grief-related words compared to neutral words, including posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC), are now considered to be the core regions in the default network. Regions activated by the photo of the deceased compared to a stranger, including dorsal anterior cingulate cortex (dACC) and insula, are now considered to be hubs in the salience network. The default network and salience network have become critical in understanding social neuroscience in the decade and a half since this first study was conducted (63) and the relationship between them is now considered a critical aspect of mood disorders (64).
Following the descriptive study on neural activation during grief, we moved to looking at what distinguished complicated grief from non-complicated grief during the same grief elicitation task (65). Although replication of areas from the first study was seen across the whole sample of participants, results of this second study demonstrated a single area that was more active in the complicated grief group than a group of bereaved participants adapting well: part of the basal ganglia called the nucleus accumbens. Nucleus accumbens activation positively correlated with self-reported yearning across all participants. In contrast, there was no correlation between accumbens activation and time since loss, or self-reported positive or negative affect, suggesting specificity of the association between yearning and regional activation.
Interpreting the increased nucleus accumbens activation in those with complicated grief necessitated relying on prior studies. Imaging studies of romantic love (partner vs. stranger) and parental love (one’s own child vs. another child) of living attachment figures also shows activity in this region(66,67). Because nucleus accumbens activity is high in response to living loved ones, and is high in those with complicated grief, one speculative possibility is that activation in this region in response to reminders of the deceased decreases over time in non-complicated grief, as the reminder of the attachment figure no longer generates an intense yearning response. In contrast, accumbens activation appears to remain high in complicated grief, associated with the continued yearning for the deceased loved one. However, longitudinal fMRI studies are needed to determine if changes in nucleus accumbens activation over time remain elevated in complicated grief. Yearning is likely a part of the “wanting” portion of reward, known to activate nucleus accumbens, although it could also be the “liking” part of reward (68). An animal model of bereavement lends support to this idea that nucleus accumbens activation is a critical aspect of attachment to loved ones. Nucleus accumbens activation is critical for pair bonding in the monogamous vole and oxytocin receptor signaling in this region decreases following partner loss(60).
Because of interest in how bereaved people regulate experiences of strong emotions, such as yearning and pangs of grief, several researchers have investigated regions in the executive network. Three studies have used an emotional Stroop task during neuroimaging in bereavement. The emotional Stroop measures reaction time to deceased-related words compared to matched neutral words, indexing the capacity to disengage from emotionally salient stimuli in order to respond to the task at hand. In the first study, attentional bias to grief-related stimuli correlated with amygdala, insula, and dorsolateral prefrontal cortex (DLPFC) activation(69). In addition, a continuous measure of self-reported intrusiveness of grief-related thoughts correlated with ventral amygdala and rostral anterior cingulate activation, while avoidance correlated with deactivation of dorsal amygdala and DLPFC. In the second study, participants with non-complicated grief exhibited activity in the rostral anterior cingulate/orbitofrontal cortex to grief-related vs. matched neutral words, and this region was not observed in the non-bereaved control group(70). This rostral area is important for emotion regulation in other fMRI emotional Stroop studies, and would be expected in a bereaved group facing greater emotional distress. However, the complicated grief group displayed no rostral anterior cingulate activation, even when examining this circumscribed area. This could be interpreted as a relative inability of individuals with complicated grief to recruit the regions needed to down-regulate emotional responses in order to successfully complete the task. In the third study using the emotional Stroop and bereaved participants across the spectrum of grief severity(71), bereaved individuals did not show differential brain activation to words related to the deceased versus living attachment figures, even at a lenient statistical threshold. Notably, this was despite their finding of a behavioral attentional bias towards the deceased, with greater attentional bias associated with higher levels of complicated grief symptoms.
Looking across these three fMRI studies of the emotional Stroop task, we do not see a clear picture of the neural foundations of this task in grief or complicated grief. This may be due to the very wide heterogeneity between these three studies (e.g., type of loss, time since death, participant age). However, as a follow up to the last study, a multivoxel pattern analysis was used to identify a pattern of brain activity associated with intrusive deceased-related thoughts. The authors focused on interacting connectivity between the salience network, and the ventral attention and default networks (72). This interaction was different among those high and low in deliberate avoidance as a coping strategy. Those high in avoidance appeared to maintain continuous application of the attentional network during a mind-wandering task, and this monitoring was associated with a lower likelihood of reporting conscious thoughts of the loss. It may be that deliberate avoidance, also predictive in behavioral and clinical studies, is a neural signature in those who are not adapting well during grieving. Avoiding the situations and reminders of loss may prolong the time it takes to learn how to adapt to a world without the attachment figure.
One possibility when considering neurobiology of grief is that cognitive impairment may help to explain differences between those who are adapting well, and those who have prolonged grief severity. In the largest comprehensive study of neuropsychological testing in a bereaved sample (n’s = 150 with complicated grief, 615 with non-complicated grief and 4731 non-bereaved), group differences emerged(73). Neuropsychological testing demonstrated that participants with complicated grief performed poorly in cognitive tests compared to those with non-complicated grief and the non-bereaved, although effect sizes were small. Those with complicated grief also had a smaller total brain volume, for both white matter and gray matter. Longitudinally, participants with complicated grief showed greater cognitive decline than matched, non-bereaved participants during seven years of follow-up in a very large sample(74). Those with non-complicated grief did not show cognitive decline over this period. This suggests that complicated grief is a risk factor for cognitive decline, and as with physical health, effects seem to be driven by those with the most severe grief reactions. Therefore, future research seeking a mechanistic understanding should assess grief severity, and not lump those with complicated and non-complicated grief together.
In conclusion, at least three possible explanations should be considered for the lack of decisive, replicated findings so far in neuroimaging studies of bereavement (and the author’s knowledge of some unpublished null findings). First, the tasks used thus far (i.e., passive viewing of deceased-related cues; the emotional Stroop) may not be ideal for discriminating neural differences between bereaved and non-bereaved, or complicated and non-complicated grief. As Schneck and colleagues point out(71), there may be a great deal of similarity in the way that deceased and living loved ones are encoded in the brain, and therefore the typical analytic imaging method of subtracting activation in one condition from another may lead to minimal (or potentially less replicable) activations. New, validated tasks that index the cognitive and affective mechanisms of grief and complicated grief are needed (possibly related to grief rumination or avoidance), and behavioral tasks that also show discrimination between complicated and non-complicated groups would be preferable.
Second, with the eventual progress toward more reliable diagnostic criteria for complicated (or prolonged) grief disorder (which would capture a smaller and more severely affected portion of the population), studies that compare disordered grief to controls may reveal more reliable differences in neural processing. Studies to date have used a range of diagnostic criterion sets, and occasionally phenomena that co-occur with complicated grief, such as intrusive thoughts or poor coping. Hopefully, better validity and reliability in the most critical psychological aspects of grief will lead to greater understanding of the neurobiology.
Third, the sample sizes of imaging studies of grief have been quite small, although as with all areas of neuroimaging research, this is changing. Brains have considerable structural as well as functional heterogeneity, which only increases with age, and when we add the heterogeneity of the mental aspects of grief, larger samples would increase the chances of finding convergent and reliable results. As grief research becomes more common, likely we will see more established research programs with the grant funding, infrastructure and collaborations needed to recruit larger samples. Taken together, researchers need more signal (e.g., better tasks and diagnostic criteria) and less noise (e.g., less heterogeneity through larger samples) in order to make progress in the neurobiology of grief.
Future directions
I hope that adaptation by the mind, brain, and body during bereavement will not be studied apart indefinitely, and that future research will reflect a greater integration of the depth of knowledge developed in each area. Better assessment of grief severity can be applied to future study of the medical consequences of bereavement. Early indications suggest that grief severity (including meeting complicated grief criteria or major depression) as a reaction to bereavement may drive the observed morbidity. Additional basic psychological science discriminating resilience from suppression or avoidance would further clarify the mechanisms that may lead to poor health following this stressful life event.
Finally, as researchers with interest in translational applications, clinical trials should examine how intervention during acute and chronic grief could improve health. In acute grief, we have published a very small feasibility trial of low-dose aspirin as a potential primary prevention strategy(29). As a risk factor, bereavement is often predictable and the increased risk is temporary. Low-dose aspirin targets some of the main cardiovascular biomarkers affected during acute grief, is inexpensive, is widely available, does not require a prescription, and is feasible in other short-term interventions. Effective psychotherapeutic interventions for complicated grief have been developed and empirically tested(75,76). These manualized treatments are based on the dual-process model and cognitive behavioral principles, and have demonstrated efficacy even in those who have had complicated grief for many years. Future research should assess whether remission of complicated grief co-occurs with improvement in biomarkers, and ultimately, in health.
The field of psychoneuroimmunology has proposed that mind, brain, and body interact, especially under stressful circumstances; for example, circulating inflammation may be related to cognitive, emotional and physical dysregulation. Combining the neuroimaging method with the assessment of immune activation, O’Connor and colleagues(77) looked at the correlation between regional activation during the photo/word grief elicitation task described above and circulating inflammatory markers in a bereaved sample. The subgenual anterior cingulate cortical activation was correlated with circulating interleukin (IL)-1β, suggesting that those with the highest level of inflammatory activity following bereavement stress are also processing deceased-related stimuli differently. This cingulate region is active in many mental functions, but also reliably shows high levels of activation in other mood disorders. Given the known interplay between physical health and mood disorders (which may include complicated grief disorder), further investigation of this area may be a fruitful area for future research linking bereavement with medical outcomes through neural and immune processes. Future research could integrate whether the neural signatures of plausible mental processes (avoidance, rumination) are mechanisms that mediate the relationship between psychological experiences (yearning, grief severity) and medical outcomes (biomarker changes, morbidity and mortality).
Overall, progress has been made in the field of grief research, investigating how body, mind, and brain adapt. This progress has led to the awareness that nuances of the bereavement experience must be captured in order to explain medical outcomes, despite the universality of this experience. More integration between the subfields studying this unique stressful life event is needed. The historical study of grief in psychosomatic medicine has a bright and growing future.
Support received:
NIA K01 AG028404, The DANA Foundation, UCLA Cousins Center for Psychoneuroimmunology, NIMH T32-MH19925, and the California Breast Cancer Research Program 10IB-0048.
List of abbreviations:
- DSM-5
Diagnostic and Statistical Manual-5
- DLPFC
dorsolateral prefrontal cortex
- fMRI
functional magnetic resonance imaging
- IL
interleukin
- ICD-11
International Classification of Diseases
- SNP
single-nucleotide polymorphism
Footnotes
The author has no conflicts of interest to report.
References
- 1.Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967; [DOI] [PubMed] [Google Scholar]
- 2.Lindemann E. Symptomatology and management of acute grief. American Journal of Psychiatry J Psychiatry. 1944;101:141–48. [DOI] [PubMed] [Google Scholar]
- 3.Stroebe M, Schut H, Boerner K. Cautioning Health-Care Professionals. Omega [Internet]. 2017. [cited 2019 Feb 6];74:455–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28355991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galatzer-Levy IR, Bonanno G a. Beyond normality in the study of bereavement: heterogeneity in depression outcomes following loss in older adults. Social science & medicine (1982) [Internet]. 2012;74:1987–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22472274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maccallum F, Galatzer-Levy IR, Bonanno GA. Trajectories of depression following spousal and child bereavement: A comparison of the heterogeneity in outcomes. Journal of Psychiatric Research [Internet]. 2015;69:72–79. Available from: 10.1016/j.jpsychires.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 6.Stroebe MS, Schut H. The dual process model of coping with bereavement: a decade on. Omega. 2010;61:273–89. [DOI] [PubMed] [Google Scholar]
- 7.Boelen PA, Reijntjes A, J. Djelantik AAAM, Smid GE. Prolonged grief and depression after unnatural loss: Latent class analyses and cognitive correlates. Psychiatry Research [Internet]. 2016;240:358–63. Available from: 10.1016/j.psychres.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 8.Shear MK, Reynolds CF, Simon NM, Zisook S, Wang Y, Mauro C, Duan N, Lebowitz B, Skritskaya N. Optimizing treatment of complicated grief a randomized clinical trial. JAMA Psychiatry. 2016;73:685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington. 2013. [Google Scholar]
- 10.Killikelly C, Maercker A. Prolonged grief disorder for ICD-11: the primacy of clinical utility and international applicability. European Journal of Psychotraumatology [Internet]. 2017;8:1476441 Available from: https://www.tandfonline.com/doi/full/10.1080/20008198.2018.1476441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundorff M, Holmgren H, Zachariae R, Farver-Vestergaard I, O’Connor M. Prevalence of prolonged grief disorder in adult bereavement: A systematic review and meta-analysis. Vol. 212, Journal of Affective Disorders. 2017. p. 138–49. [DOI] [PubMed] [Google Scholar]
- 12.Mikulincer M, Dolev T, Shaver PR. Attachment-related strategies during thought suppression: Ironic rebounds and vulnerable self-representations. Journal of Personality and Social Psychology. 2004;87:940–56. [DOI] [PubMed] [Google Scholar]
- 13.Engel GL. Is Grief a Disease? Psychosomatic Medicine. 1961; [Google Scholar]
- 14.Stroebe M. “Is Grief a Disease ?” Why Engel Posed the Question. 2015;1–8. [Google Scholar]
- 15.Bartrop RW, Lazarus L, Luckhurst E, Kiloh LG, Penny R. Depressed lymphocyte function after bereavement. The Lancet [Internet]. 1977;309:834–36. Available from: http://www.sciencedirect.com/science/article/pii/S0140673677927805 [DOI] [PubMed] [Google Scholar]
- 16.Young M, Benjamin B, Wallis C. The Mortality of Widowers. The Lancet. 1963;282:454–57. [DOI] [PubMed] [Google Scholar]
- 17.Rees WD, Lutkins SG. Mortality of bereavement. British Medical Journal. 1967;4:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai RI, Morii I. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. Journal of the American College of Cardiology. 2001;38:11–18. [DOI] [PubMed] [Google Scholar]
- 19.Martikainen P, Valkonen T. Mortality after the death of a spouse: Rates and causes of death in a large Finnish cohort. American Journal of Public Health. 1996; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon JR, Glymour MM, Vable AM, Liu SY, Subramanian SV. Short- and long-term associations between widowhood and mortality in the United States: longitudinal analyses. Journal of Public Health [Internet]. 2014;36:382–89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24167198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shor E, Roelfs DJ, Curreli M, Clemow L, Burg MM, Schwartz JE. Widowhood and Mortality: A Meta-Analysis and Meta-Regression. Demography. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahl ST, Arnold AM, Chen J, Anderson S, Schulz R. Mortality After Bereavement: The Role of Cardiovascular Disease and Depression. 2016; [DOI] [PMC free article] [PubMed]
- 23.Elwert F, Christakis NA. The effect of widowhood on mortality by the causes of death of both spouses. American Journal of Public Health. 2008;98:2092–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman M a. Risk of acute myocardial infarction after the death of a significant person in one’s life: the Determinants of Myocardial Infarction Onset Study. Circulation [Internet]. 2012;125:491–96. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3397171&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JH, Bierhals AJ, Prigerson HG, Kas l S. V., Mazure CM, & Jacobs S. Gender diff erences in the effects of bereavement-related psychological distress in health outcomes. Psychol Medicine Journal. 1999;29:376–80. [DOI] [PubMed] [Google Scholar]
- 27.Stroebe M, Stroebe W, Schut H, Boerner K. Grief is not a disease but bereavement merits medical awareness. The Lancet. 2017;389:347–49. [DOI] [PubMed] [Google Scholar]
- 28.Prigerson HG, Bierhals AJ, Kasl SV., Reynolds CF, Shear MK, Day N, Beery LC, Newsom JT, Jacobs S. Traumatic grief as a risk factor for mental and physical morbidity. American Journal of Psychiatry. 1997; [DOI] [PubMed] [Google Scholar]
- 29.Karl S, Fallon M, Palitsky R, Martinez JA, Gündel H, O’Connor M-F. Low-Dose Aspirin for Prevention of Cardiovascular Risk in Bereavement: Results from a Feasibility Study. Psychotherapy and Psychosomatics. 2018; [DOI] [PubMed] [Google Scholar]
- 30.Fallon MA, Careaga JS, Sbarra DA, Connor MO, O’Connor M-F. Utility of a virtual Trier Social Stress Test: Initial findings and benchmarking comparisons. Psychosomatic Medicine [Internet]. 2016;78:835–40. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=psyc13&NEWS=N&AN=2016-44062-008 [DOI] [PubMed] [Google Scholar]
- 31.Buckley T, Stannard A, Bartrop R, McKinley S, Ward C, Mihailidou AS, Morel-Kopp M-C, Spinaze M, Tofler G. Effect of Early Bereavement on Heart Rate and Heart Rate Variability. The American journal of cardiology [Internet]. 2012; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22853984 [DOI] [PubMed] [Google Scholar]
- 32.Buckley T, Morel-Kopp M-C, Ward C, Bartrop R, McKinley S, Mihailidou AS, Spinaze M, Chen W, Tofler G. Inflammatory and thrombotic changes in early bereavement: a prospective evaluation. European Journal of Preventive Cardiology [Internet]. 2012;19:1145–52. Available from: http://journals.sagepub.com/doi/10.1177/1741826711421686 [DOI] [PubMed] [Google Scholar]
- 33.Buckley T, Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp M-C, Spinaze M, Tofler GH. Haemodynamic changes during early bereavement: potential contribution to increased cardiovascular risk. Heart, lung & circulation [Internet]. 2011;20:91–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21147029 [DOI] [PubMed] [Google Scholar]
- 34.O’Connor M-F, Allen JJB, Kaszniak AW. Autonomic and emotion regulation in bereavement and depression. Journal of Psychosomatic Research. 2002;52. [DOI] [PubMed] [Google Scholar]
- 35.Buckley T, Bartrop R, McKinley S, Ward C, Bramwell M, Roche D, Mihailidou AS, Morel-Kopp MC, Spinaze M, Hocking B, Goldston K, Tennant C, Tofler G. Prospective study of early bereavement on psychological and behavioural cardiac risk factors. Internal Medicine Journal. 2009;39:370–78. [DOI] [PubMed] [Google Scholar]
- 36.Gerra G, Monti D, Panerai AE, Sacerdote P, Anderlini R, Avanzini P, Zaimovic A, Brambilla F, Franceschi C. Long-term immune-endocrine effects of bereavement: Relationships with anxiety levels and mood. Psychiatry Research. 2003; [DOI] [PubMed] [Google Scholar]
- 37.Irwin M, Daniels M, Rish SC, Bloom E, Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biol Psychiatry. 1988;24:173–78. [DOI] [PubMed] [Google Scholar]
- 38.Ong AD, Fuller-Rowell TE, Bonanno GA, Almeida DM. Spousal Loss Predicts Alterations in Diurnal Cortisol Activity Through Prospective Changes in Positive Emotion. Health Psychology. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fallon MA, Careaga JS, Sbarra DA, O’Connor M-F. Utility of a Virtual Trier Social Stress Test: Initial Findings and Benchmarking Comparisons. Psychosomatic Medicine. 2016;78. [DOI] [PubMed] [Google Scholar]
- 40.Richardson VE, Bennett KM, Carr D, Gallagher S, Kim J, Fields N. How Does Bereavement Get Under the Skin? The Effects of Late-Life Spousal Loss on Cortisol Levels. The journals of gerontology. Series B, Psychological sciences and social sciences [Internet]. 2013;1–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24259378 [DOI] [PubMed] [Google Scholar]
- 41.Pérez HCS, Direk N, Milic J, Ikram MA, Hofman A, Tiemeier H. The impact of complicated grief on diurnal cortisol levels two years after loss: A population-based study. Psychosomatic Medicine. 2017; [DOI] [PubMed] [Google Scholar]
- 42.O’Connor MF, Wellisch DK, Stanton AL, Olmstead R, Irwin MR. Diurnal cortisol in Complicated and Non-Complicated Grief: Slope differences across the day. Psychoneuroendocrinology. 2012;37:725–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowles Lindsey M., Ruiz John M., O’Connor M-F. A Systematic Review of the Association Between Bereavement and Biomarkers of Immune Function. [DOI] [PubMed]
- 44.Cankaya B, Chapman BP, Talbot NL, Moynihan J, Duberstein PR. History of sudden unexpected loss is associated with elevated interleukin-6 and decreased insulin-like growth factor-1 in women in an urban primary care setting. Psychosomatic medicine. 2009;71:914–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultze-Florey CR, Martínez-Maza O, Magpantay L, Breen EC, Irwin MR, Gündel H, O’Connor MF. When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity [Internet]. 2012;26:1066–71. Available from: 10.1016/j.bbi.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen M, Granger S, Fuller-Thomson E. The association between bereavement and biomarkers of inflammation. Behavioral Medicine. 2015;41:49–59. [DOI] [PubMed] [Google Scholar]
- 47.Schultze-Florey CR, Martínez-Maza O, Magpantay L, Breen EC, Irwin MR, Gündel H, O’Connor M-F. When grief makes you sick: Bereavement induced systemic inflammation is a question of genotype. Brain, Behavior, and Immunity. 2012;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khanfer R, Lord JM, Phillips AC. Neutrophil function and cortisol:DHEAS ratio in bereaved older adults. Brain, Behavior, and Immunity. 2011;25:1182–86. [DOI] [PubMed] [Google Scholar]
- 49.Irwin M, Daniels M, Craig Risch S, Bloom E, Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biological Psychiatry. 1988; [DOI] [PubMed] [Google Scholar]
- 50.Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain, Behavior, and Immunity. 2006;20:279–89. [DOI] [PubMed] [Google Scholar]
- 51.van der Houwen K, Stroebe M, Schut H, Stroebe W, van den Bout J. Mediating processes in bereavement: the role of rumination, threatening grief interpretations, and deliberate grief avoidance. Social science & medicine (1982) [Internet]. 2010;71:1669–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20832924 [DOI] [PubMed] [Google Scholar]
- 52.Nolen-Hoeksema S. Ruminative coping and adjustment to bereavement In: Stroebe M, Hansson R, Stroebe W, Schut H, editors. Handbook of bereavement research: consequences, coping, and care. Washington, DC, DC: American Psychological Association; 2001. [Google Scholar]
- 53.Shear K, Monk T, Houck P, Melhem N, Frank E, Reynolds C, Sillowash R. An attachment-based model of complicated grief including the role of avoidance. Vol. 257, European Archives of Psychiatry and Clinical Neuroscience. 2007. p. 453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroebe M, Stroebe W, Schut H, Zech E, Van Den Bout J. Does disclosure of emotions facilitate recovery from bereavement? Evidence from two prospective studies. Journal of Consulting and Clinical Psychology. 2002;70:169–78. [PubMed] [Google Scholar]
- 55.Boelen PA, van den Bout J, van den Hout MA. Negative cognitions and avoidance in emotional problems after bereavement: A prospective study. Behaviour Research and Therapy. 2006;44:1657–72. [DOI] [PubMed] [Google Scholar]
- 56.Davis CG, Nolen-Hoeksema S, Larson J. Making Sense of Loss and Benefiting from the Experience: Two Construals of Meaning. Journal of Personality and Social Psychology. 1998;75:561–74. [DOI] [PubMed] [Google Scholar]
- 57.Busch LY, Pössel P, Valentine JC. Meta-analyses of cardiovascular reactivity to rumination: A possible mechanism linking depression and hostility to cardiovascular disease. Psychological Bulletin. 2017;143:1378–94. [DOI] [PubMed] [Google Scholar]
- 58.Eisma MC, Rinck M, Stroebe MS, Schut H a W, Boelen P a, Stroebe W, van den Bout J. Rumination and implicit avoidance following bereavement: an approach avoidance task investigation. Journal of behavior therapy and experimental psychiatry [Internet]. 2015;47:84–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25499772 [DOI] [PubMed] [Google Scholar]
- 59.Eisma MC, Schut H a W, Stroebe MS, van den Bout J, Stroebe W, Boelen P a. Is rumination after bereavement linked with loss avoidance? Evidence from eye-tracking. PloS one [Internet]. 2014;9:e104980–e104980. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4139328&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosch OJ, Dabrowska J, Modi ME, Johnson ZV., Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osako Y, Nobuhara R, Arai YCP, Tanaka K, Young LJ, Nishihara M, Mitsui S, Yuri K. Partner Loss in Monogamous Rodents: Modulation of Pain and Emotional Behavior in Male Prairie Voles. Psychosomatic Medicine. 2018;80:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gündel H, O’Connor M-F, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: an FMRI study. The American journal of psychiatry [Internet]. 2003;160:1946–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14594740 [DOI] [PubMed] [Google Scholar]
- 63.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA psychiatry [Internet]. 2015;72:603–11. Available from: http://archpsyc.jamanetwork.com/article.aspx?articleid=2203837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. NeuroImage. 2008;42:969–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004; [DOI] [PubMed] [Google Scholar]
- 67.Wittfoth-Schardt D, Gründing J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gündel H, Waller C. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knutson B, Fong CAGW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Imaging. 2001;12:3683–87. [DOI] [PubMed] [Google Scholar]
- 69.Freed PJ, Yanagihara TK, Hirsch J, Mann JJ. Neural Mechanisms of Grief Regulation. Biological Psychiatry [Internet]. 2009;66:33–40. Available from: 10.1016/j.biopsych.2009.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arizmendi B, Kaszniak AW, O’Connor MF. Disrupted prefrontal activity during emotion processing in complicated grief: An fMRI investigation. NeuroImage [Internet]. 2016;124:968–76. Available from: 10.1016/j.neuroimage.2015.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schneck N, Tu T, Michel CA, Bonanno GA, Sajda P, Mann JJ. Attentional Bias to Reminders of the Deceased as Compared With a Living Attachment in Grieving. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneck N, Tu T, Haufe S, Bonanno GA, GalfaIvy H, Ochsner KN, Mann JJ, Sajda P. Ongoing monitoring of mindwandering in avoidant grief through cortico-basal-ganglia interactions. Social Cognitive and Affective Neuroscience. 2018;14:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saavedra Pérez HC, Ikram M a, Direk N, Prigerson HG, Freak-Poli R, Verhaaren BFJ, Hofman a, Vernooij M, Tiemeier H. Cognition, structural brain changes and complicated grief. A population-based study. Psychological medicine [Internet]. 2015;45:1389–99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25363662 [DOI] [PubMed] [Google Scholar]
- 74.Pérez HCS, Ikram MA, Direk N, Tiemeier H. Prolonged Grief and Cognitive Decline: A Prospective Population-Based Study in Middle-Aged and Older Persons. American Journal of Geriatric Psychiatry [Internet]. 2018; Available from: 10.1016/j.jagp.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 75.Shear K, Wang Y, Skritskaya N, Duan N, Mauro C, Ghesquiere A. Treatment of complicated grief in elderly persons: a randomized clinical trial. JAMA psychiatry [Internet]. 2014;71:1287–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25250737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boelen PA, de Keijser J, Van den Hout MA, Van den Bout J. Treatment of complicated grief: A comparison between cognitive-behavioral therapy and supportive counseling. Journal of Consulting and Clinical Psychology. 2007;75:277–84. [DOI] [PubMed] [Google Scholar]
- 77.O’Connor M-F, Irwin MR, Wellisch DK. When grief heats up: Pro-inflammatory cytokines predict regional brain activation. NeuroImage. 2009;47. [DOI] [PMC free article] [PubMed] [Google Scholar]