ABSTRACT
DBI/ACBP (diazepam binding protein, acyl-CoA binding protein) participates in the regulation of fatty acid metabolism when it is localized within cells, whereas outside of cells it acts as a diazepam-binding protein. Recent results indicate that many different mammalian cell types release DBI/ACBP upon in vitro or in vivo starvation in a macroautophagy/autophagy-dependent fashion. The autophagy-associated release of DBI/ACBP elicits feedback inhibition of autophagy through 3 independent mechanisms. First, the depletion of DBI/ACBP from cells limits autophagy in a cell-autonomous fashion. Second, extracellular DBI/ACBP acts in a paracrine fashion to inhibit autophagy. Third, DBI/ACBP increasing in the systemic circulation acts as an activator of lipo-anabolism and feeding behavior, thus removing the cause of autophagy induction (starvation) and suppressing the phenomenon. DBI/ACBP expression is upregulated at the mRNA and protein levels in obese mice and humans, and its extracellular neutralization by antibodies controls food intake and increases lipo-catabolism. Current data support the contention that DBI/ACBP is an important pro-obesity factor.
Abbreviations: DBI: diazepam binding protein, acyl-CoA binding protein; GABR: gamma-aminobutyric acid type A receptor; TSPO: translocator protein
KEYWORDS: Autophagy, lipids, metabolism, obesity, unconventional protein secretion
DBI/ACBP is a protein that participates in the buffering of potentially toxic medium and long-acyl CoA molecules as well as in their transport between organelles. One particularity of this phylogenetically conserved protein is that it is released from cells through an unconventional (Golgi-independent) pathway, in an autophagy-dependent fashion, as was first demonstrated for several fungal species. Outside of cells, this protein is best known as a diazepam binding inhibitor because it displaces diazepam (a benzodiazepine) from its binding site at the GABR (gamma-aminobutyric acid type A receptor). This receptor, a ligand-gated ion channel, is activated in response to the chief inhibitory neurotransmitter, gamma-aminobutyric acid, and is mostly known for its role in the central nervous system (CNS), although it is also expressed on most peripheral cell types, outside of the CNS.
We recently found that multiple different human and mouse cell types release DBI/ACBP upon induction of autophagy by starvation or rapamycin [1]. This effect is blocked by bafilomycin A1, chloroquine, or leupeptin, as well as by genetic inhibition of autophagy (such as knockout of Atg4b in mice). In response to starvation, the intracellular levels of DBI/ACBP decline and DBI/ACBP accumulates in the extracellular space (in vitro) or in the plasma (in vivo). Intrigued by these findings, we characterized the effects of DBI/ACBP on autophagy, reasoning that this factor might engage in regulatory circuits that amplify or inhibit its own release. Indeed, we found that the translocation of DBI/ACBP from the intracellular to the extracellular compartment causes feedback inhibition of autophagy at 3 different levels.
At the first level, reduction of the intracellular pool of DBI/ACBP by RNA interference, inhibits starvation-induced autophagy, whereas its transgene-enforced overexpression enhances autophagy in fed cells and further increases autophagy in response to nutrient depletion. Of note, the knockdown of TSPO (translocator protein), which is best known as the “peripheral benzodiazepine receptor”, phenocopies the knockdown of DBI/ACBP with respect to autophagy inhibition, suggesting that the interaction between DBI/ACBP and TSPO might be functionally relevant for this effect (Figure 1A).
Figure 1.
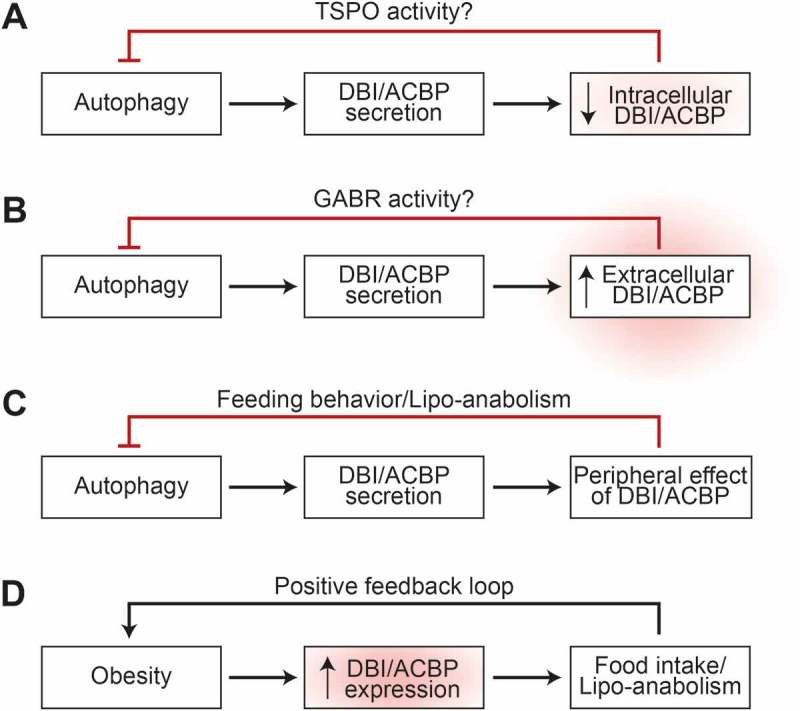
Implication of DBI/ACBP in feedback circuits regulating autophagy at the cell-autonomous (A), paracrine (B) and neuroendocrine (C) levels. Moreover, the role of DBI/ACBP in obesity is depicted (D).
At the second level, addition of recombinant DBI/ACBP protein (recDBI) to cultured cells or injection of recDBI into mice inhibits starvation-induced autophagy. Conversely, addition of neutralizing mono- or polyclonal antibodies specific for DBI/ACBP to cell cultures (or injection of such antibodies into the peritoneal cavity of mice) stimulates baseline autophagy and enhances starvation-induced autophagy. The autophagy-stimulatory effect of anti-ACBP can be prevented by bicuculline methiodide, an antagonist of GABR, indicating that it is possibly mediated by an action of DBI/ACBP on GABR. Autophagy inhibition induced by recDBI/ACBP is accompanied by the activation of the AKT-MTORC1 pathway, as well as inhibition of AMPK, supporting the contention that extracellular DBI/ACBP can inhibit autophagy through a conventional pathway (Figure 1B).
At the third level, DBI/ACBP has widespread systemic metabolic effects in vivo, in mice. Most importantly, measures designed to elevate extracellular DBI/ACBP levels cause hyperphagy. Thus, intravenous injection of recDBI causes a rapid incorporation of glucose into the white adipose tissue for fatty acid synthesis, causing a transient reduction of blood glucose. Concomitant with this reduction of glycemia, mice rapidly activate orexigenic neurons in the hypothalamus and increase their food intake. When glucose levels are artificially maintained at a high level by concomitant intraperitoneal injection of glucose together with intravenous recDBI, this orexigenic reaction is inhibited, supporting the idea that DBI/ACBP stimulates central appetite centers via a peripheral action. Conversely, when mice are starved for 24 h (which causes a ~ 10% reduction in body weight), their plasma DBI/ACBP levels ramp up. When confronted with food, these mice logically manifest a hyperphagic behavior. However, when extracellular DBI/ACBP is neutralized by injection of a specific monoclonal antibody, this post-starvation overeating behavior is inhibited and, instead of orexigenic neurons, anorexigenic centers are activated in the hypothalamus. These results can be tentatively incorporated into a scheme (Figure 1C) in which fasting causes an autophagy-dependent increase in extracellular DBI/ACBP that then acts as a factor to stimulate appetite and to suppress the primary cause of autophagy, which is the scarcity of nutrients.
Of note, it appears that the aforementioned homeostatic feedback system can be geared to affect body mass and body composition. Thus, transgenic overexpression of DBI/ACBP in the liver causes a systemic increase in circulating DBI/ACBP levels that finally results in enhanced feeding, weight gain, and an increase in adiposity. These changes phenocopy human obesity in that obese patients contain higher levels of circulating DBI/ACBP in their plasma than lean subjects. In mice, DBI/ACBP can be chronically neutralized by breaking self-tolerance against DBI, resulting in the generation of anti-DBI autoantibodies. This manipulation reduces weight gain induced by high-fat diet or by LEP (leptin) deficiency and also reduces the co-morbidities of obesity such as hepatosteatosis and diabetes, establishing causality between the DBI/ACBP elevation observed in obese subjects and their phenotype. Indeed, DBI/ACBP not only stimulates lipo-anabolic reactions (such as conversion of glucose into lipids), but also acts to inhibit lipo-catabolic reactions (such as fatty acid oxidation, lipolysis in white adipose tissue, browning of adipose tissue and lipo-autophagy) and to stimulate appetite. In other words, DBI/ACBP may well be the elusive “obesity factor” (Figure 1D). This contention is based on the observations that, in humans, circulating DBI/ACBP concentrations strongly correlate with body mass and that, in mice, artificial elevations of DBI/ACBP induce weight gain, while DBI/ACBP neutralization counteracts obesity.
Funding Statement
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”, Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China [GDW20171100085], Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology [ANR-18-IDEX-0001]; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Disclosure statement
GK is a scientific co-founder of Samsara Therapeutics.
Reference
- [1].Bravo-San Pedro JM, Sica V, Martins I, et al. Acyl-CoA-binding protein is a lipogenic factor that triggers food intake and obesity. [Google Scholar]


