Abstract
Background:
Breast cancer is the leading cause of cancer related death in women worldwide. The development of metastatic cancer is the main factor contributing to mortality. The molecular mechanisms underlying the metastatic process have yet to be clearly elucidated. However, the interplay between the tumor microenvironment and the cancer cells hold a critical role in influencing the progression of cancer metastasis. Within the microenvironment of solid tumors, the lack of sufficient vasculature leads to the development of nutrient deprived conditions. This study aimed to examine how nutrient deprivation influences factors involved in cancer progression and metastasis. Specifically, we examined how nutrient stress changes cancer cell migration, the gene expression, and cytokine production of metastasis-related factors in a human breast cancer cell line.
Methods:
MCF7 breast cancer cells were cultured in serum-free media for 24, 48, and 72 h. Cell migration was evaluated using a transwell migration assay. The transcriptional expression of metastatic related genes was examined via real-time PCR. Cytokine production was examined via enzyme-linked immunosorbent assay.
Results:
Nutrient deprivation of the MCF7 cells significantly reduced cell migration after 24 h. However, following 72 h of nutrient deprivation, there was significant increase in cell migration compared to the 24 h group. Transcriptional expression of markers involved in migration including, β-catenin, twist, vimentin, fibronectin, ICAM1, VCAM1, and VEGF were up regulated after 72 h of nutrient deprivation. The cytokines TGFβ1, IL-8, and MCP1 were differentially secreted.
Conclusion:
Nutrient deprivation is an environmental stress factor that can influence the behavior of cancer cells. Current treatments implement nutrient deprivation as a potential cancer treatment. Under short periods of nutrient deprivation, cancer cell migration is inhibited. However, our findings show that following extended lengths of nutrient deprivation, cancer cells are capable of adapting themselves to the environmental condition and restoring their migratory abilities. This, in part, may be a result of increased expression of metastasis-related genes. Further research is required to accurately identify how the expression of metastasis-related genes is modulated and controlled in response to nutrient deprivation and environmental stress.
Key Words: Cancer cell migration, Epithelial mesenchymal transition, Nutrient deprivation
Introduction
Breast cancer is the most common invasive cancer in women, and the second leading cause of death due to cancer in women worldwide (1). The main cause of mortality from breast cancer is due to the progression of metastasis. However, many of the treatment strategies currently in practice, such as a lumpectomy, mastectomy, and radiation therapy, are developed to target the primary tumor. However, metastasis commonly occurs in breast cancer patients, and thus treatment of the primary tumor is not sufficient to cure most individuals (2). More recently, the focus of cancer research has shifted towards finding ways to combat tumor metastasis and identifying the molecular pathways involved in its development and progression.
Nutrient deprivation is one of the hallmark conditions of the tumor microenvironment. The rapid growth of the tumor leads to the development of a hypoxic and nutrient deprived microenvironment within the core of the tumor mass due to an insufficient blood supply (3). Previous research has revealed that nutrient deprivation can modulate the behavior of cancer cells (4). In this study, we investigated the effect of nutrient deprivation on cancer cell migration. As the epithelial to mesenchymal transition (EMT) is involved in tumor invasion and metastasis (5), the second objective of this study was to examine whether nutrient deprivation modulates the expression of key factors involved in EMT including, β-catenin, twist, vimentin, and fibronectin in MCF7 breast cancer cells. Components of the extracellular matrix (ECM) have also been known to hold critical roles in the development of metastasis such as, intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and matrix metallopeptidase 9 (MMP9). Therefore, we examined how the nutrient deprivation impacts the expression of these genes in the MCF7 cell line (6, 7). The expression of vascular endothelial growth factor (VEGF) was also examined due to its role as an inducer of angiogenesis and tumor progression (7). We also aimed to elucidate whether nutrient deprivation contributes to any changes in the secretion of cytokines integral to the development of tumor metastasis. These factors include MMP9, transforming growth factor beta 1 (TGFβ1), interleukin 8 (IL-8), and monocyte chemoattractant protein 1 (MCP1). Additionally, as a marker of oxidative stress, the levels of nitric oxide (NO) production were examined.
In understanding how cancer cells respond to nutrient deprivation provides new insight into how cancer cells adapt in response to environmental stress and offer a potential underlying cause contributing to the initiation of cancer cell migration and metastasis.
Materials and Methods
Cell culture
MCF7 human breast cancer cells were purchased from the Pasteur Institute (Tehran, Iran). Cells were cultured in Dulbecco modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), and antibiotics (Penicillin 100 IU/ml, Streptomycin 100 μg/ml). Cells were incubated at 37 °C in a controlled atmosphere composed of 95% air and 5% CO2.
In vitro nutrient deprivation
To investigate the effects of nutrient deprivation on cell migration, MCF7 cells were exposed to nutrient deprived environmental conditionals, specifically media without FBS supplementation. Cells were cultured in the nutrient deprived condition for 24, 48 and 72 h.
Transwell migration assay
The transwell migration assay was performed as previously described (8). Briefly, nutrient deprived MCF7 cells were plated on the upper side of a transwell membrane in serum-free media. The transwells were placed into 24-well plates containing 800 μl of media containing 10% FBS in the lower chamber. After 24 h, membranes were fixed and stained with 4',6-diamidino-2- phenylindole (DAPI). Four random fields of cells migrating through the membrane were counted on an Olympus IX71 fluorescence microscope.
RNA extraction and real-time PCR
Total RNA was isolated from the MCF7 cells following nutrient deprivation using TRIZOL reagent (Roche, Germany). cDNA was synthesized by the RevertAid First Strand cDNA Synthesis kit (Fermentas), followed by real time PCR. Reactions were carried out using SYBR Green PCR Master Mix (Takara, Japan) in a Real-Time PCR System (Bio-Rad) by denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s (9). Primer sequences used in this study are shown in Table 1. The housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was used to normalize target gene expression. Relative quantification of gene expression was performed according to the 2-ΔΔCT method. Melting curves were used to determine non-specific amplification.
Table 1.
Real-time PCR primer sequences.
| Primers | Sequence | Product size |
|---|---|---|
| β-catenin | F: AAAATGGCAGTGCGTTTAG | 100 bp |
| R: TTTGAAGGCAGTCTGTCGTA | ||
| Twist | F: TGCGGAAGATCATCCCCACG | 137 bp |
| R: GCTGCAGCTTGCCATCTTGGA | ||
| Vimentin | F: ACCCGCACCAACGAGAAGGT | 90 bp |
| R: ATTCTGCTGCTCCAGGAAGCG | ||
| Fibronectin | F: TCCTTGCTGGTATCATGGCAG | 74 bp |
| R: AGACCCAGGCTTCTCATACTTGA | ||
| ICAM1 | F: AGGCCACCCCAGAGGACAAC | 406 bp |
| R: CCCATTATGACTGCGGCTGCTA | ||
| VCAM1 | F: CGTCTTGGTCAGCCCTTCCT | 460 bp |
| R: ACATTCATATACTCCCGCATCCTTC | ||
| MMP9 | F: GCACGACGTCTTCCAGTACC | 124 bp |
| R: CAGGATGTCATAGGTCACGTAGC | ||
| VEGF | F: CCTTGCTGCTCTACCTCCAC | 280 bp |
| R: ATCTGCATGGTGATGTTGGA | ||
| GAPDH | F: AAGGTGAAGGTCGGAGTCAAC | 102 bp |
| R: GGGGTCATTGATGGCAACAATA |
Enzyme-Linked Immunosorbent Assay (ELISA)
MCF7 cells were seeded in 96-well plates in the nutrient deprived media for 24, 48, and 72 hrs. The supernatant was collected and the levels of secreted MMP9, TGFβ1, IL-8, and MCP1 were determined using immunoassay kits (IBL, Germany) according to the manufacturer’s instructions. The concentration of secreted NO in the medium was also determined using an ELISA kit (Eastbiopharm, China) in accordance with the manufacturer’s instructions. The optical density (OD) of each well was measured using a microplate reader (Stat Fax 2100, Palm City, FL) at 540 nm.
Statistical analysis
The data was analyzed using GraphPad Prism version 6.0. The significant difference between groups were assessed using a one-way ANOVA followed by Dunnett’s test. The data is presented as mean ± standard deviation (SD). A p value of, P < 0.05 and P< 0.01, was considered to be statistically significant.
Results
Nutrient deprivation modulates the migration ability of MCF7 cells
The cell migration assay was performed using a two-chamber transwell system. MCF7 cells were cultured under nutrient deprived conditions for 24, 48, and 72 h. Cells that had traversed the membrane to the bottom side of the well were fixed and stained (Fig. 1A). The results showed that cell migration significantly decreased after 24 h of nutrient deprivation compared to the control group (P< 0.01). However, the migration ability of cells increased after 48 and 72 h compared to the 24 h group and this increase was significant at 72 h (P< 0.01) (Fig. 1B).
Fig. 1.
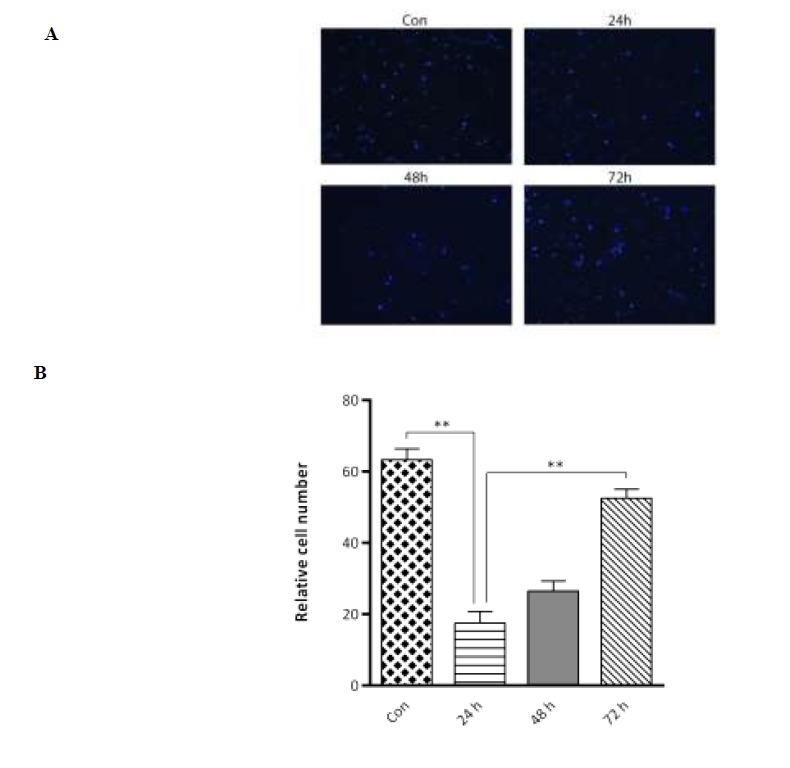
Nutrient deprivation modulates the migration of MCF7 cells. (A) Cells on the transwell filter that migrated to the lower surface of the filter were stained and photographed. (B) The results presented are an average of the number of migrated cells on the underside of the filter. Statistical significance was determined at **P< 0.01.
Nutrient deprivation modulates the expression of metastasis-associated genes
Exposure of MCF7 cells to nutrient deprivation for 72 h showed a significant increase in the expression of β-catenin, twist, vimentin, fibronectin, ICAM1, VCAM1, and VEGF (Fig. 2). Among the different genes examined, MMP9 was downregulated
Fig. 2.
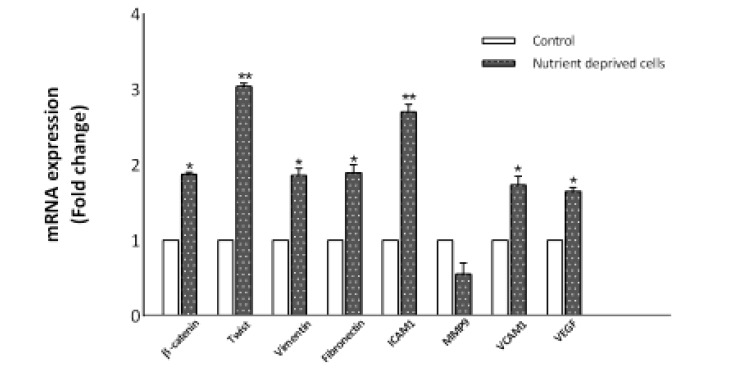
RT-PCR analysis indicates that nutrient deprivation modulates gene expression in MCF7 cells. Expression of β-catenin, twist, vimentin, fibronectin, ICAM1, VCAM1, and VEGF were found to be significantly upregulated after 72 h nutrient deprivation. The levels of MMP9 expression were downregulated. However, this was not significant. The data represent the means ± SD (*P< 0.05, **P< 0.01).
Nutrient deprivation influences cytokine secretion by MCF7 cells
The concentrations of MMP9, TGFβ1, IL-8, MCP1, and NO within the supernatant of MCF7 cells after 24, 48, and 72 h nutrient deprivation were examined via ELISA (Fig. 3). The secretion of MMP9 was below the detection limit in the control and nutrient deprived cells at all examined time points. The mean concentrations of TGFβ1 in the supernatant was 23.13 ± 4, and 15.33 ± 2.51 pg/ml, after 24 and 48 h of nutrient deprivation, respectively. The secretion of TGFβ1 was below the detection limit in the serum deprived cells after 72 h and was significantly decreased compared to the concentration of TGFβ1 in the supernatant of control cells (32.63 ± 5 pg/ml; p<0.05, and p<0.01).
Fig. 3.
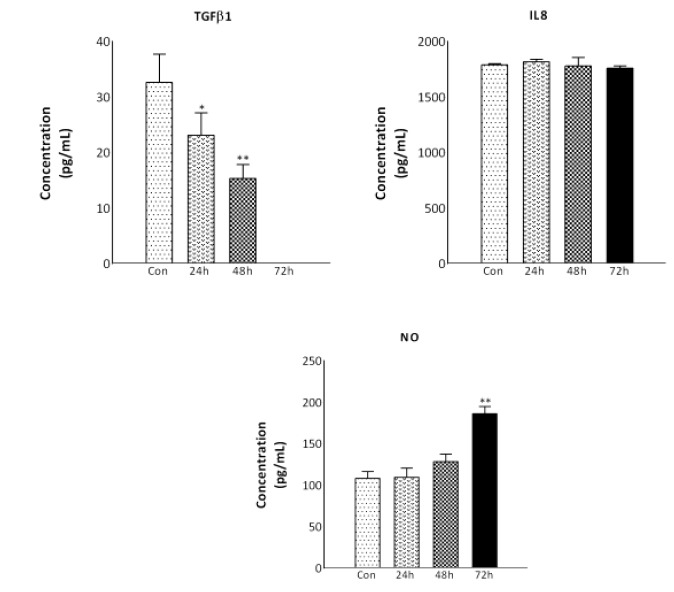
Secretion of TGFβ1, IL-8, and NO was assessed by ELISA. MCF7 cells were cultured for 24, 48, and 72 h under nutrient deprived conditions. The levels of TGFβ1, IL-8, and NO in the medium following nutrient deprivation were measured by ELISA. The results were expressed as mean ± standard deviation (SD). (*P< 0.05, **P< 0.01)
The concentration of IL-8 did not change over time in the nutrient deprived condition. The secretion of MCP1 was below the detection limit in nutrient deprived cells at all the studied time points compared to the control cells (data not shown). Additionally, nutrient deprivation induced a significant increase in NO production in the MCF7 cells (186.23± 8.51 pg/ml; p<0.05) after 72 h compared to the control cells (108.53 ± 8.53 pg/ml).
Discussion
Previous research has shown that cancer cells resistant to nutrient deprivation are associated with a poor prognosis in colorectal cancer. This suggests that the adaptations undergone by cancer cells in response to nutritional stress strengthens the resilience of cancer cells to treatment (10). It has been demonstrated that nutrient deprivation elicits the activation of different signaling pathways modifying the characteristics and behavior of tumor cells (11, 12). How nutrient deprivation influences cancer cells has been mostly studied with respect to radiotherapy (13, 14), cell cycle arrest (15, 16), and autophagy (17, 18). In the present study, we investigated the influence of nutritional stress on the cellular migration of the human breast cancer cell line, MCF7. Additionally, the expression of metastasis-related genes was examined in response to nutrient deprivation. We observed that serum deprivation inhibits the migratory capacity of the MCF7 human breast cancer cells after 24 h. However, there was a gradual increase in cell migration following 48, and 72 h of nutrient deprivation compared to 24 h. Moreover, our findings showed that 72 h nutrient deprivation in serum free media resulted in the increased expression of genes involved in the epithelial to mesenchymal transition (EMT) including, β-catenin, twist, vimentin, fibronectin, ICAM1, and VCAM1. The EMT is a physiological process by which epithelial cells lose their adherent junctions and their apical–basal cell polarity to form spindle shaped cells that contribute to their ability to migrate as single cells. Research from Zangh et al. revealed that the expression of β-catenin was associated with the metastasis of spontaneous breast cancer in mice (19). Similarly, De et al. showed that tumor cells acquire metastasis-associated phenotypes following activation of Wnt-beta-catenin pathway (20). In the absence of a Wnt signal, the cytoplasmic β-catenin is linked to a destruction complex (Axin, APC, CK1, GSK-3 beta), which facilitates β-catenin phosphorylation and degradation by proteasome. In the presence of a Wnt signal, the destruction complex is inactivated, which leads to stabilization and accumulation of β-catenin in the cytoplasm. Consequently, β-catenin translocates to the nucleus and binds to T-cell factor/lymphoid-enhancer factor, resulting in the activation of transcription of various target genes, such as CXCR4, CXCL12, MMP7, Fibronectin, and Hyaluronan synthase-2 (HAS2) which are involved in invasion and metastasis (21, 22). Moreover, it has been found that twist, the suppressor of the E-cadherin adhesion molecule, plays an essential role in cancer metastasis (23). Additionally, it was demonstrated that ICAM1 and VCAM1 expression levels positively correlates with the metastatic potential of breast cancer cell lines (24, 25). Our data suggests that increased expression of the EMT/metastasis related genes may be partially involved in the increased observed in cell migration following serum deprivation after 72 h compared to 24 h. Nutrient deprivation has been widely used as a cancer treatment in a variety of approaches, such as the administration of anti-angiogenic agents, restricting blood supply via surgical ligation, and embolization (3). In this study, the increased expression of EMT/metastasis related markers under nutrient deprivation suggests a potential detrimental consequence for nutrient deprivation in cancer treatment.
Various members of the matrix metalloproteinase (MMP) family, such as MMP9, also hold a role in cancer cell invasion (26). However, our findings show no increase expression of MMP9. One of the other adaptive responses of tumor cells in serum deprived conditions is to increase the secretion of VEGF (27). Capillaries consist of a single layer of endothelial cells that express the VEGF receptor. In response to VEGF, the endothelium sprouts resulting in the formation of new blood vessels (28). Our findings showed that following 72 h of nutrient deprivation, there was an increase in the expression of VEGF. This increased expression of VEGF may be a prerequisite for enhanced cancer progression and metastasis in vivo.
To better understand the possible mechanisms involved in modifying the cellular migration of cancer cells under nutrient deprived conditions, the secretion of the cytokines, TGFβ1, MCP1, and IL-8 were measured. These cytokines have been shown to hold an important role in cancer metastasis (29, 30). Our findings demonstrate that the incubation of MCF7 cancer cells in serum free media leads to a decrease in the secretion of TGFβ1, MCP1, and no changes in the secretion of IL-8 for all time points. These findings do not correlate with our findings of a resurgence in cell migration after 72 h of nutrient deprivation, compared to 24 h. Additionally, increased expression of NO in our study suggests a role for nutrient deprivation as an oxidative stress on the MCF7 cells. Accordingly, it seems that the cellular behavior induced by nutrient deprivation may be attributed to an interplay between different simultaneous synergistic or antagonistic inputs.
This study is one of the first reports to demonstrate that nutrient deprivation increases the expression of EMT/metastasis related genes. As nutrient deprivation can lead to increased expression of genes involved in cellular migration, special care should be taken when nutrient deprivation is used as a cancer treatment.
Acknowledgements
The authors acknowledge the financial support provided by Cancer Prevention Research Center, Shahroud University, project No 94108.
The corresponding author (NA) has completed and submitted the International Committee of Medical Journal Editors (ICMJE) Uniform Disclosure Form for Potential Conflicts of Interest on behalf of all authors. None of the authors disclose any conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bertozzi S, Londero AP, Cedolini C, Uzzau A, Seriau L, Bernardi S, et al. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. SpringerPlus. 2015;4:688. doi: 10.1186/s40064-015-1449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin TW, Chen MT, Lin LT, Huang PI, Lo WL, Yang YP, et al. TDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastoma. Oncotarget. 2017;8(34):56612–25. doi: 10.18632/oncotarget.17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wek RC, Staschke KA. How do tumours adapt to nutrient stress? The EMBO journal. 2010;29(12):1946–7. doi: 10.1038/emboj.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes & development. 2013;27(20):2192, 206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedicto A, Romayor I, Arteta B. Role of liver ICAM-1 in metastasis. Oncology letters. 2017;14(4):3883–92. doi: 10.3892/ol.2017.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)--an increasing insight into its role in tumorigenicity and metastasis. International journal of cancer. 2015;136(11):2594–14. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 8.Mirahmadi M, Ahmadiankia N, Naderi-Meshkin H, Heirani-Tabasi A, Bidkhori HR, Afsharian P, et al. Hypoxia and laser enhance expression of SDF-1 in muscles cells. Cellular and molecular biology (Noisy-le-Grand, France). 2016;62(5):31–7. [PubMed] [Google Scholar]
- 9.Ahmadiankia N, Moghaddam HK, Mishan MA, Bahrami AR, Naderi-Meshkin H, Bidkhori HR, et al. Berberine suppresses migration of MCF-7 breast cancer cells through down-regulation of chemokine receptors. Iranian journal of basic medical sciences. 2016;19(2):125–31. [PMC free article] [PubMed] [Google Scholar]
- 10.Miyo M, Konno M, Nishida N, Sueda T, Noguchi K, Matsui H, et al. Metabolic Adaptation to Nutritional Stress in Human Colorectal Cancer. Scientific reports. 2016;6:38415. doi: 10.1038/srep38415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efeyan A, Comb WC, Sabatini DM. Nutrientsensing mechanisms and pathways. Nature. 2015;517(7534):302–10. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastasiou D. Tumour microenvironment factors shaping the cancer metabolism landscape. British journal of cancer. 2017;116(3):277–86. doi: 10.1038/bjc.2016.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champ CE, Baserga R, Mishra MV, Jin L, Sotgia F, Lisanti MP, et al. Nutrient restriction and radiation therapy for cancer treatment: when less is more. The oncologist. 2013;18(1):97–103. doi: 10.1634/theoncologist.2012-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell cycle (Georgetown, Tex). 2013;12(12):1955–63. doi: 10.4161/cc.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao YJ, Chang WH, Ting HC, Chao WT, Hsu YH. Cell cycle arrest and cell survival induce reverse trends of cardiolipin remodeling. PloS one. 2014;9(11):e113680. doi: 10.1371/journal.pone.0113680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda F, Ishii M, Mori A, Uehara L, Yanagida M, Takeda K, et al. Glucose restriction induces transient G2 cell cycle arrest extending cellular chronological lifespan. Scientific reports. 2016;6:19629. doi: 10.1038/srep19629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Guo X, Xie X, Zhao X, Li D, Deng W, et al. Autophagy in hypoxia protects cancer cells against apoptosis induced by nutrient deprivation through a Beclin1-dependent way in hepatocellular carcinoma. Journal of cellular biochemistry. 2011;112(11):3406–20. doi: 10.1002/jcb.23274. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Xia X, Pan H. Active autophagy in the tumor microenvironment: A novel mechanism for cancer metastasis. Oncology letters. 2013;5(2):411–6. doi: 10.3892/ol.2012.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Fei F, Li S, Zhao Y, Yang Z, Qu J, et al. The role of beta-catenin in the initiation and metastasis of TA2 mice spontaneous breast cancer. Journal of Cancer. 2017;8(11):2114–23. doi: 10.7150/jca.19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De P, Carlson JH, Wu H, Marcus A, Leyland-Jones B, Dey N. Wnt-beta-catenin pathway signals metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget. 2016;7(28):43124–49. doi: 10.18632/oncotarget.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Park SY, Jun Y, Kim JY, Nam JS. Roles of Wnt Target Genes in the Journey of Cancer Stem Cells. International journal of molecular sciences. 2017;18(8) doi: 10.3390/ijms18081604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao C, Xiao G, Hu J. Regulation of Wnt/betacatenin signaling by posttranslational modifications. Cell & bioscience. 2014;4(1):13. doi: 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell research. 2011;21(2):275–89. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, et al. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26(5):943–50. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Zhang J, Li H, Lu Z, Shan W, Mercado-Uribe I, et al. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. American journal of translational research. 2013;5(3):336–46. [PMC free article] [PubMed] [Google Scholar]
- 26.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. The Journal of biological chemistry. 2005;280(11):9963–72. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- 28.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4(4):241–6. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer metastasis reviews. 2006;25(3):435–57. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 30.Dutta P, Sarkissyan M, Paico K, Wu Y, Vadgama JV. MCP-1 is overexpressed in triplenegative breast cancers and drives cancer invasiveness and metastasis. Breast cancer research and treatment. 2018;170(3):477–86. doi: 10.1007/s10549-018-4760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


