Abstract
Background:
Abnormal expression of ABCC transporter genes has been associated with treatment failure in pediatric patients with acute lymphoblastic leukemia (ALL). The aim of this study was to evaluate the expression pattern of ABCC1-6 and ABCC10 genes in Iranian pediatric patients with ALL relapse and determine the potential predictive value of determining ALL relapse from ABCC expression.
Methods:
Patients with ALL were divided into two separate groups, either the case group with relapsed ALL or the control group in which ALL patients have been in progression-free survival for at least 3 years A total of thirty-nine participants (23 with relapsed ALL; 16 controls) were enrolled over 26 months. To determine the levels of ABCC1-6 and ABCC10 transporter gene expression RT-PCR was used. Cumulative doses of the chemotherapy drugs, VCR, DNR and L-ASP, were calculated for each patient.
Results:
Our findings showed elevated expression of ABCC2-6 and decreased expression of ABCC1 and ABCC10 to be associated with an increased risk of ALL relapse. The mean-fold expression of ABCC2 was significantly increased in the ALL relapse group. Additionally, the expression pattern of the ABCC transporter genes was associated with high doses of three chemotherapy drugs, VCR, DNR and L-ASP.
Conclusion:
Evaluating the expression pattern of ABCC transporter genes may be a potential biomarker for predicting the occurrence of ALL relapse in Iranian pediatric patients and improve cancer prognosis.
Key Words: ABC transporters, Childhood, Gene expression, Pattern, Recurrence
Introduction
The Iranian Cancer Registry has previously reported that in 2007, the incidence of pediatric cancer was 176 cases per 1 million children in Tehran, Iran (1). In patients with ALL, the most common factor involved in treatment failure is the occurrence of cancer relapse, often leading to a poor prognosis (3) The chemotherapy treatment is often rendered ineffective due to the development of multidrug resistance (MDR). This can be a result of abnormal ATP-binding cassette (ABC) transporter gene expression (4-6). The ABCC transporter subfamily of the ABC transporters is also known as multidrug resistance proteins (MRPs). These membrane proteins are able to render the chemotherapeutic agents ineffective due to their ability to transport anticancer agents across membranes and excrete substances out of the cell (7). Due to their ability to interfere with treatments, MRPs are an attractive target for improving the treatment of ALL and preventing the development of cancer cell resistance to chemotherapy (8). Important genes of this subfamily which relate to multidrug resistance in patients with malignancy are ABCC1-6 and ABCC10 (9).
Previous reports have examined the relationship between ABCC transporter gene expression and the prognosis of pediatric patients with ALL. Some research has found no significant relationship between the gene expression of ABCC1 or ABCC3 and ALL prognosis in pediatric patients (10-12). However, it has been found that one of the main functions of ABCC1-6 is the transportation of chemotherapeutic agents, such as Adriamycin (ADR), Vincristine (VCR), Etoposide (VP16), 6-Mercaptopurine (6-MP), and Methotrexate (MTX) (13-16).
The aim of this study was to examine the gene expression profile of ABCC1-6 and ABCC10 in Iranian pediatric patients with ALL and determine if this expression pattern can predict ALL relapse. Additionally, we examined the relationship between chemotherapy dose and ABCC gene expression.
Materials and Methods
Ethics approval
Permission to conduct this study and ethical approval was obtained from the Ethics Committee of the Shahid Beheshti University of Medical Science. The ethics approval code was IR.SBMU.RETECH.REC.1396.533.
Patient samples and demographic
In this prospective case-control study, we evaluated the gene expression of ABCC1-6 and ABCC10 in pediatric ALL patients. The participants in this study were children referred to the MAHAL’s Pediatric Cancer Treatment and Research Center in Tehran, Iran, with either a previous or current diagnosis of ALL. Patients included in this study were under the age of 15, had an immunophenotyping report of ALL, and had previously completed their chemotherapy treatment. The case group included patients who have had ALL relapse immediately following or during their chemotherapy regimen. The control group consisted of patients who successfully completed their chemotherapy treatment and had at least 3 years of progression-free survival.
Informed consent was obtained from each patient’s parent prior to enrollment in the study. Consent was also given to collect 3 mL of peripheral blood (PB) in EDTA-treated tubes before beginning the chemotherapy cycle.
For each participant a questionnaire about their clinical, pathological, and demographic characteristics was completed by a physician. These characteristics included age at the time of diagnosis, sex, French-American-British (FAB) classification and immunophenotype of ALL, date chemotherapy regimen was initiated, date of first complete remission, and the total doses of each chemotherapy agent used during the treatment, prognostic factor and National Cancer Institute (NCI) risk group (17). In addition, the date, site, and number of recurrences were reported for the case group.
Cumulative dose of chemotherapy agents
The chemotherapy regimen was based on the ALL-BFM (Berlin-Frankfurt-Munster) protocol 2009. Cumulative doses of the chemotherapy drugs administered during the induction phase were calculated. The cumulative dose of VCR was categorized as low (<20 mg/m2), intermediate (20-40 mg/m2), or high (>40 mg/m2) (18, 19). According to the standard dose of Daunorubicin (DNR) (20), the cumulative dose was categorized as standard (<100 mg/m2) or high (≥100 mg/m2). The cumulative dose of L-Asparginase (L-ASP) was categorized as standard (<60,000 u/m2) or high (≥60,000 u/m2).
Total RNA isolation
Each PB sample was transferred to the laboratory immediately after collection. White blood cells were separated by red blood cell lysis buffer and suspended in PBS (21). Total RNA was isolated by YTzol Pure RNA reagent according to the manufacturer’s instructions (Cat No: YT9063, Yekta Tajhiz Azma, Iran). The concentration of total RNA was determined by Thermo Scientific NanoDrop (Thermo Fisher Scientific, USA), and the purity was examined via OD260/OD280 absorption. Total RNA samples were stored in -70 °C until cDNA synthesis.
cDNA synthesis
The cDNA was prepared using standard total RNA. A cDNA synthesis kit (Cat No: YT4500, Yekta Tajhiz Azma, Iran) was used according to the manufacturer’s instructions. The template RNA (based on 1 μg) was mixed with 1 μL random hexamer primer (184.84 μl) and finalized with DEPC treated water to the volume of 13.4 μL. After incubation at 70 °C for 5 minutes, each product was mixed with 4 μL 5× first-strand buffer (200 μl), 1 μL dNTP (50 μl), 0.5 μL RNasin (25 μl), and 1 μL MMLV (50 μl). The mixture was incubated for 1 hour at 37 °C and was terminated by heating at 70 °C for 5 minutes. The PCR products were stored at –70 °C prior to RT-PCR analysis (9).
Real-Time PCR
The primers used for ABCC1-6 and ABCC10 genes are shown in Table 1 and designed as previously described (22) . The GAPDH gene was used to normalize the results through RT-PCR with the forward primer (5'–3'): GAAGGTGAAGGTCGGAGTC and reverse primer (5'–3'): GAAGATGGTGATGGGATTTC. All primers were obtained from Pishgam Biotech Company, Iran. Primers had been purified using the Macrogen Oligonucleotide Purification Cartridge™ purification method. Prior to beginning RT-PCR, products of cDNA synthesis were diluted at a ratio of 1:3. The mixture for RT-PCR consisted of 0.5 μL forward primer (0.2 μM) + 0.5 μL reverse primer (0.2 μM) + cDNA template according to 1 μg/μL + 7.5 μL Syber Green QPCR master mix 2× (cat no: YT2551, Yekta Tajhiz Azma, Iran;) and nuclease-free water to achieve a final volume of 20 μL. The RT-PCR program was set up as follows: one cycle of pre-incubation (95 °C = 300 s), 38 cycles of 3-step amplification (95 °C = 60 s, 60 °C = 60 s, 72 °C = 30 s), and one cycle of melting (95 °C = 10 s, 65 °C = 60 s, 97 °C = 1 s). Quantifications were determined by Light Cycler 96 Real-Time PCR Cycler (Hoffmann-La Roche AG, Basel, Switzerland). For checking the fluorescent signals of specific bands, all RT-PCR products were subjected to 1.5% agarose gel electrophoresis.
Table 1.
Forward and reverse primers of ABCC1-6 and ABCC10
| Gene | Forward primer, 5'–3' | Reverse primer, 5'–3' | Gene bank accession no | Position (bp) |
|---|---|---|---|---|
| ABCC1 | GAAGGCCATCGGACTCTTCA | CAGCGCGGACACATGGT | L05628 | 3097–3166 |
| ABCC2 | TGCAGCCTCCATAACCATGAG | GATGCCTGCCATTGGACCTA | U63970 | 2728–2807 |
| ABCC3 | CACACGGATCTGACAGACAATGA | ACAGGGCACTCAGCTGTCTCA | AB_010887 | 2670–2745 |
| ABCC4 | AAGTGAACAACCTCCAGTTCCAG | GGCTCTCCAGAGCACCATCT | AF_071202 | 2026–2144 |
| ABCC5 | TGAAAGCCATTCGAGGAGTTG | CGGAAAAGCTCGTCATGCA | AF_146074 | 2979–3054 |
| ABCC6 | AGACACGGTTGACGTGGACAT | GCTGACCTCCAGGAGTCCAA | AF_168791 | 3156–3231 |
| ABCC10 | GCGGGTTAAGCTTGTGACAGA | CCCACCCGCAGAACTTGA | XM_052745 | 1585–1646 |
Statistical analysis
The raw data was analyzed by light cycler software. Relative-expression analysis was done by using REST 2009 software. Statistical calculations were performed using SPSS (version 22) software. The mean fold expression and ROC curve analysis was determined using GraphPad PRISM version 8.1.2 software. The relative changes in gene expression were analyzed by the Livak method (23). A P-value of ≤0.05 indicates statistical significance.
Results
Patients
A total of 39 pediatric patients with ALL were enrolled in this study over a period of 26 months. Table 2 shows the demographic data of our patient cohort. The case group included 23 patients, and the control group included 16 patients. In this study 18 children were from Tehran (the capital city of Iran) and others had been referred from different provinces of Iran.
Table 2.
Patient characteristics and cumulative doses of chemotherapy agents
| Sex (n) & Age | Immunophenotypes type of ALL (n) | BMA (%) at the end of induction | Prognostic group (n) | NCI risk groups (n) | Vincristine dose (%) | Daunorubicin dose (%) | L-asparginase dose (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M/F | MA.DX | PB | EPB | TC | POB | CR | Hy | PR | FV | LFV | LR | HR | VHR | L | I | H | SD | H | SD | H | |
| Case group | 17 | 6 | 2.8 | 5.1 ± 0.9 | 13 | 6 | 2 | 2 | 50 | 35.7 | 14.3 | 9 | 6 | 9 | 5 | 1 | 57.1 | 14.3 | 28.6 | 75 | 25 | 84.6 | 15.4 |
| Control group | 5 | 11 | 0.04 | 7.3 ± 1.0 | 8 | 7 | 1 | - | 76.9 | 23.1 | - | 6 | 1 | 12 | 4 | - | 13.3 | 26.7 | 60 | 90 | 10 | 15.4 | 73.3 |
Abbreviations: n, number; BMA, Bone Marrow Aspiration; NCI, National Cancer Institute; M, Male; F, Female; M/F, Male to Female; MA.DX, Mean Age at Diagnosis; PB, Pre-B; EPB, Early Pre-B; TC, T-Cell; POB, Pro-B; CR, Complete Remission; Hy, Hypocellular; PR, Partial Remission; FV, Favorable; LFV, Less Favorable; LR, Low Risk; HR, High Risk; VHR, Very High Risk; L, Low; I, Intermediate; H, High; SD, Standard
Our cohort included a total of 22 boys and 17 girls. The mean age at diagnosis was 6.1 ± 4.1 years (range 7 months–14 years). The immunophenotypes of patients were determined as follows: Pre-B ALL (n=21); early Pre-B ALL (n=13); T-cell ALL (n=3); and Pro-B ALL (n=2). The BMA (Bone Marrow Aspiration) at the end of induction showed that 63% were in complete remission, 29.6% were hypocellular, and 7.4% were in partial remission.
The sites of relapse in the case group were as follows: central nervous system (CNS): 55%, bone marrow: 25%, testis: 13%, and one patient experienced relapse in the bone marrow and CNS. The median time to relapse from diagnosis was 23 months (range, 65 days–5 years).
Nine patients (8 from the case group and 1 from the control group) did not fall into a prognostic group. Additionally, eight participants in the case group did not have an NCI risk evaluation. Of the 31 patients who had NCI risk evaluations, 21 had low-risk ALL, nine had high-risk ALL, and one had very high–risk ALL.
Patient Follow-up
At the time of preparing this manuscript, nine patients from the case group had died of relapsed ALL. The median time for the follow-up was 4 years (ranging from 14 months–10 years) for all patients. The median time of follow-up for patients in the case group was 36 months, and for the control group was 61 months. The 3-year overall survival and 5-year overall survival, according to Kaplan-Meier analysis was 82.3% ± 0.06% and 75.4% ± 0.07%, respectively.
Cumulative Doses of Chemotherapy Agents
The maximum and mean (±SD) cumulative dose of VCR was 90.9 mg/m2 and 38.5 ± 22 mg/m2, respectively (34.5% low dose, 20.7% intermediate dose, and 44.8% high dose). The maximum and mean (±SD) cumulative dose of DNR was 185 mg/m2 and 67.3 ± 43.5 mg/m2, respectively (standard dose: 81.8%, high dose: 18.2%). Results showed that the maximum and mean (±SD) cumulative dose of L-ASP were 92,500 u/m2 and 43,894.26 ± 22,717.9 u/m2, respectively (standard dose: 78.6%, high dose: 21.4%). Table 2 shows the dose categorizations of chemotherapy agents used during the induction phase for the two groups.
Expression of ABCC1-6 and ABCC10 by RT-PCR
Using RT-PCR, the reaction efficiency for ABCC transporter genes was 0.96, and 0.98 for GAPDH. Patients with ALL relapse had significantly (P-Value: 0.0161) higher expression of ABCC2 in comparison to the patients with at least 3 years of progression free survival (figure 1). The expression level of ABCC3, ABCC4, ABCC5 and ABCC6 was higher in patients with relapse ALL, while the expression levels of ABCC1 and ABCC10 was lower in patients with ALL relapse (Fig. 1). Table 3 shows the mean fold expression in the both the control and case groups of our ALL patients.
Fig. 1.
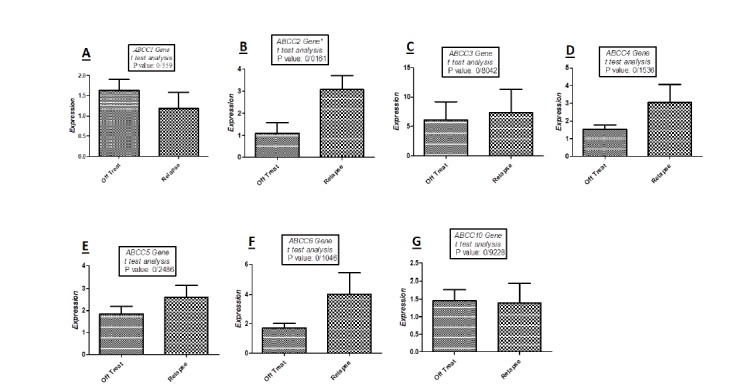
Quantitative real-time PCR analysis with t-test for the mRNAs expression levels of ABCC transporter genes in two groups of relapse and off treatment (3 years progression free survival) patients:
A: low expression of ABCC1 in relapse group in comparison to off treatment patients (P-Value: 0.359);
B: high expression of ABCC2 in relapse group in comparison to off treatment patients (P-Value: 0.0161);
C: high expression of ABCC3 in relapse group in comparison to off treatment patients (P-Value: 0.8042);
D: high expression of ABCC4 in relapse group in comparison to off treatment patients (P-Value: 0.1536);
E: high expression of ABCC5 in relapse group in comparison to off treatment patients (P-Value: 0.2486);
F: high expression of ABCC6 in relapse group in comparison to off treatment patients (P-Value: 0.1046);
G: low expression of ABCC10 in relapse group in comparison to off treatment patients (P-Value: 0.9228)
Table 3.
Mean fold of expressions in considered patients
| MF±SEM | P-Value | MF±SEM | P-Value | MF±SEM | P-Value | MF±SEM | P-Value | MF±SEM | P-Value | MF±SEM | P-Value | MF±SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case group | 1.18 ± 0.4 | 0.3592 | 3.06 ± 0.63 | 0.0161 | 7.34 ± 3.93 | 0.8042 | 3.04 ± 1.01 | 0.1536 | 2.59 ± 0.54 | 0.2486 | 4 ± 1.4 | 0.1046 | 1.39 ± 0.56 |
| Control group | 1.63 ± 0.27 | 1.09 ± 0.48 | 6.11 ± 3.06 | 1.52 ± 0.25 | 1.84 ± 0.36 | 1.71 ± 0.33 | 1.45 ± 0.31 |
ROC Curve analysis for the expressed ABCC genes revealed that ABCC1 and ABCC2 had significantly increased sensitivity of expression in relapsed patients (Fig. 2 and Table 4).
Fig. 2.
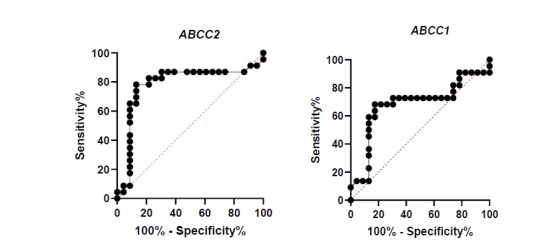
ROC curves of ABCC1 and ABCC2 expression for evaluation of sensitivity and specificity in patients:
A: high sensitivity between low expression of ABCC1 and occurrence of relapse in considered patients;
B: high sensitivity between high expression of ABCC2 and occurrence of relapse in considered patients
Table 4.
ROC analysis for ABCC1 and ABCC2
| Area under the ROC curve | ABCC1 | ABCC2 |
|---|---|---|
| Area | 0.6808 | 0.7845 |
| Std. Error | 0.08592 | 0.07786 |
| 95% confidence interval | 0.5124-0.8492 | 0.6319-0.9371 |
| P-Value | 0.0377 | 0.0009 |
The one-way ANOVA test showed a significant relationship between high doses of administered chemotherapeutic agents (VCR, DNR, L-Asp) and types of expression (high expression of ABCC2-6 and low expression of ABCC1, ABCC10) in relapsed ALL patients (P-Value< 0.0001).
Discussion
The abnormal expression of ABC transporters is a major factor leading to the development of multidrug resistant cancer cells. Their role in the regulation of chemotherapeutic agents across the plasma membrane can enable them to render chemotherapy treatments ineffective. This function has made ABC transporters an attractive target for improving cancer therapy and preventing the development of multidrug resistance (8).
Sensitivity to chemotherapeutic agents is regulated by the expression of ABC transporter genes, mainly the ABCC subfamily of transporters (6,9,24). ABCC1-6 are responsible for transporting drug agents like ADR, VP-16, VCR, and 6-MP (25).
Studies examining the role of ABC transporter gene expression on the outcome of treatment response (e.g., poor prognosis, early relapse, reduced progression-free survival) have shown contracting findings.
Despite the improved survival of pediatric patients with ALL who receive modern treatment regimens, relapse remains to be the most common cause for treatment failure (26). Most studies have examined the effect of MDR1 (P-gp) (27) and MRP1 (ABCC1) in ALL (28).
A previous study evaluated the expression of MDR1 and MRP1 in 167 patients with ALL at different stages (new cases, complete remission, and relapses) by RT-PCR analysis. Their results showed that the level of MRP1 expression was higher in patients with ALL relapse, and those individuals who expressed more MRP1 did not experience complete remission. However, further research into the functional characteristics of MRP1 through drug transporters is required to better understand the role of MRP1 in ALL relapse (29).
A study by Gurbuxani et al. examined 32 adult and pediatric cases of untreated de novo ALL, observing a low correlation between MDR1 and MRP1 gene expression and a poor response to chemotherapy. Their results showed that there was no relation between gene expression and treatment outcomes or the occurrence of ALL relapse (30).
The prognostic value of MDR1 and ABCC1 in patients with acute leukemia is well known (31-34). A study of 34 pediatric patients with ALL revealed that high expression of ABCC1 may affect complete remission and lead to decreased 2-year overall survival (35). Additional reports have demonstrated that there is no relationship between the expression of ABCC1 or MDR1 and poor treatment responses in adult or pediatric ALL patients (36). A separate controversial study of 140 pediatric patients with ALL showed that low expression of ABCC1 and ABCC3 is related to a high risk of death caused by treatment toxicity (37).
Plasschaert et al. examined 56 pediatric patients with newly diagnosed ALL to evaluate the effect of ABCC1-6 expression on prognosis and treatment response. Their findings revealed that high expression of ABCC1, ABCC3, and ABCC5 was related to poor prognosis. They suggested that characterizing the expression profile for the upregulated ABCC transporter genes may help identify early relapse in pediatric patients with ALL (24).
Few studies have explored the role of gene expression of ABC transporters in relation to the prognosis of Iranian pediatric patients with ALL. In a study by Mahjoubi et al. Iranian pediatric patients with relapsed ALL were observed to have high levels of ABCC1 gene expression. (21).
In accordance with the Plasschaert et al. study, our cohort of Iranian children with relapsed ALL expressed higher levels of ABCC2-6 than those who had finished their treatment regimens 3 years earlier without any disease recurrence. The expression of ABCC1 and ABCC10 was lower in patients with relapsed ALL than in those in the control group of our study. Our results also showed that high expression of ABCC2-6 and low expression of ABCC1 and ABCC10 were significantly associated (P <0.05) with high doses of the chemotherapeutic agents, VCR, DNR, and L-ASP. These results suggest that ABCC gene profiling should be done for pediatric patients with ALL to identify those who have a high probability of ALL recurrence.
The most common cause of treatment failure in children with ALL is relapse. Since the expression of ABCC transporter genes has been associated with the clinical outcomes of pediatric ALL patients, characterizing the expression pattern of ABCC genes could help predict the probability of treatment failure and improve patient prognosis. The results of this study show that characterizing the gene expression profile of ABCC1-6 and ABCC10 may offer a means of predicting the incidence ALL relapse in Iranian pediatric patients. Additionally, our study has revealed a relationship between high doses of chemotherapeutic agents (VCR, DNR, L-Asp) and ABCC transporter gene expression in ALL relapsed patients. As this study only recruited patients from a single center, a major drawback for this study was the small sample size. Future studies should examine patients across multiple different centers in order to better evaluate the role ABCC transporter gene expression has in ALL relapse and the role of high dose chemotherapeutic agents in ABCC gene expression.
Acknowledgements
We would like to thank the pediatric hematologist-oncologists at the MAHAK’s Pediatric Cancer Treatment and Research Center for managing and caring of considered patients of this study and the professors at the Hematology Library at the Iran University of Medical Sciences for their support of making equipment’s availability for conducting the study. The authors would also like to acknowledge and thank Dr. Angela J. McArthur for editing this manuscript.
References
- 1.Mosavi-Jarrahi A, Moini M, Mohagheghi M-A, Alebouyeh M, Yazdizadeh B, Shahabian A, et al. Clustering of childhood cancer in the inner city of Tehran metropolitan area: a GIS-based analysis. International journal of hygiene and environmental health. 2007;210(2):113–9. doi: 10.1016/j.ijheh.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Mehrvar A, Faranoush M, Asl AAH, Tashvighi M, Fazeli MA, Mehrvar N, et al. Epidemiological features of childhood acute leukemia at MAHAK’s Pediatric Cancer Treatment and Research Center (MPCTRC), Tehran, Iran. Basic & Clinical Cancer Research. 2015;7(1):9–15. [Google Scholar]
- 3.Oskarsson T, Söderhäll S, Arvidson J, Forestier E, Montgomery S, Bottai M, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. haematologica. 2015 doi: 10.3324/haematol.2015.131680. haematol. 2015.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calcagno AM, Ambudkar SV. Analysis of expression of drug resistance-linked ABC transporters in cancer cells by quantitative RT-PCR. Membrane Transporters in Drug Discovery and Development: Springer; 2010:121–32. doi: 10.1007/978-1-60761-700-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nature Reviews Cancer. 2002;2(1):48. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 6.Roundhill E, Fletcher J, Haber M, Norris M. Clinical relevance of ultidrug-resistance-proteins (MRPs) for anticancer drug resistance and prognosis. Resistance to targeted ABC transporters in cancer: Springer. 2015:27–52. [Google Scholar]
- 7.Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cellular and molecular life sciences. 2008;65(20):3145. doi: 10.1007/s00018-008-8111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Human genomics. 2009;3(3):281. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrvar N, Akbari ME, Rezvany MR, Abolghasemi H, Saberynejad J, Mehrvar A, et al. ATP-Binding Cassette transporters' gene expression in pediatric patients with acute leukemia; a comprehensive analysis of published reports through PubMed search engine. Cellular and molecular biology (Noisy-le-Grand, France). 2019;65(2):7–13. [PubMed] [Google Scholar]
- 10.Plasschaert SL, Vellenga E, de Bont ES, van der Kolk DM, Veerman AJ, Sluiter WJ, et al. High functional P-glycoprotein activity is more often present in T-cell acute lymphoblastic leukaemic cells in adults than in children. Leukemia & lymphoma. 2003;44(1):85–95. doi: 10.1080/1042819021000040288. [DOI] [PubMed] [Google Scholar]
- 11.Sauerbrey A, Voigt A, Wittig S, Häfer R, Zintl F. Messenger RNA analysis of the multidrug resistance related protein (MRP1) and the lung resistance protein (LRP) in de novo and relapsed childhood acute lymphoblastic leukemia. Leukemia & lymphoma. 2002;43(4):875–9. doi: 10.1080/10428190290017024. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, et al. The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood. 2003;102(13):4493–8. doi: 10.1182/blood-2002-11-3461. [DOI] [PubMed] [Google Scholar]
- 13.Belinsky MG, Chen Z-S, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer research. 2002;62(21):6172–7. [PubMed] [Google Scholar]
- 14.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. Journal of Biological Chemistry. 2000;275(39):30069–74. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 15.Kool M, Van Der Linden M, de Haas M, Scheffer GL, De Vree JML, Smith AJ, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proceedings of the National Academy of Sciences. 1999;96(12):6914–9. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proceedings of the National Academy of Sciences. 2000;97(13):7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health NCIatNIo. Risk Groups for Childhood Acute Lymphoblastic Leukemia.
- 18.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in motor performance in children with cancer is independent of the cumulative dose of vincristine. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2006;106(6):1395–401. doi: 10.1002/cncr.21706. [DOI] [PubMed] [Google Scholar]
- 19.van de Velde ME, Kaspers GL, Abbink FC, Wilhelm AJ, Ket JC, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Critical reviews in oncology/hematology. 2017;114:114–30. doi: 10.1016/j.critrevonc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Rammeloo L, Postma A, Sobotka-Plojhar M, Bink-Boelkens MTE, Berg AvDv, Veerman A, et al. Low-dose daunorubicin in induction treatment of childhood acute lymphoblastic leukemia: No long-term cardiac damage in a randomized study of the Dutch Childhood Leukemia Study Group. Medical and Pediatric Oncology: The Official Journal of SIOP—International Society of Pediatric Oncology (Societé Internationale d'Oncologie Pédiatrique. 2000;35(1):13–9. doi: 10.1002/1096-911x(200007)35:1<13::aid-mpo3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Mahjoubi F, Akbari S. Multidrug resistance-associated protein 1 predicts relapse in Iranian childhood acute lymphoblastic leukemia. Asian Pacific Journal of Cancer Prevention. 2012;13(5):2285–9. doi: 10.7314/apjcp.2012.13.5.2285. [DOI] [PubMed] [Google Scholar]
- 22.Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clinical chemistry. 2003;49(2):230–8. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Plasschaert SL, de Bont ES, Boezen M, vander Kolk DM, Daenen SM, Faber KN, et al. Expression of multidrug resistance–associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clinical Cancer Research. 2005;11(24):8661–8. doi: 10.1158/1078-0432.CCR-05-1096. [DOI] [PubMed] [Google Scholar]
- 25.Safa AR. Identification and characterization of the binding sites of P-glycoprotein for multidrug resistance-related drugs and modulators. Current Medicinal Chemistry-Anti-Cancer Agents. 2004;4(1):1–17. doi: 10.2174/1568011043482142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oskarsson T, Söderhäll S, Arvidson J, Forestier E, Montgomery S, Bottai M, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. haematologica. 2016;101(1):68–76. doi: 10.3324/haematol.2015.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaszubiak A, Kupstat A, Müller U, Hausmann R, Holm PS, Lage H, et al. Regulation of MDR1 gene expression in multidrug-resistant cancer cells is independent from YB-1. Biochemical and biophysical research communications. 2007;357(1):295–301. doi: 10.1016/j.bbrc.2007.03.145. [DOI] [PubMed] [Google Scholar]
- 28.Nies AT, Magdy T, Schwab M, Zanger UM. Role of ABC transporters in fluoropyrimidine-based chemotherapy response. Advances in cancer research. 2015;125:217–43. doi: 10.1016/bs.acr.2014.10.007. Elsevier. [DOI] [PubMed] [Google Scholar]
- 29.Gurbuxani S, Arya LS, Raina V, Sazawal S, Khattar A, Magrath I, et al. Significance of MDR1, MRP1, GSTπ and GSTμ mRNA expression in acute lymphoblastic leukemia in Indian patients. Cancer letters. 2001;167(1):73–83. doi: 10.1016/s0304-3835(00)00684-4. [DOI] [PubMed] [Google Scholar]
- 30.Gurbuxani S, Zhou D, Simonin G, Raina V, Arya L, Sazawal S, et al. Expression of genes implicated in multidrug resistance in acute lymphoblastic leukemia in India. Annals of hematology. 1998;76(5):195–200. doi: 10.1007/s002770050388. [DOI] [PubMed] [Google Scholar]
- 31.Huh HJ, Park C-J, Jang S, Seo E-J, Chi H-S, Lee J-H, et al. Prognostic significance of multidrug resistance gene 1 (MDR1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) mRNA expression in acute leukemia. Journal of Korean medical science. 2006;21(2):253–8. doi: 10.3346/jkms.2006.21.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamidah N. Assessment of P-gp and MRP1 activities using MultiDrugQuant™ Assay Kit: a preliminary study of correlation between protein expressions and its functional activities in newly diagnosed acute leukaemia patients. Malays J Pathol. 2008;30:87–93. [PubMed] [Google Scholar]
- 33.Valera ET, Scrideli CA, Queiroz RGdP, Mori BMO, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Medical Journal. 2004;122(4):166–71. doi: 10.1590/S1516-31802004000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kourti M, Vavatsi N, Gombakis N, Sidi V, Tzimagiorgis G, Papageorgiou T, et al. Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. International journal of hematology. 2007;86(2):166–73. doi: 10.1532/IJH97.E0624. [DOI] [PubMed] [Google Scholar]
- 35.El-Sharnouby JA, Abou El-Enein AM, El Ghannam DM, El-Shanshory MR, Hagag AA, Yahia S, et al. Expression of lung resistance protein and multidrug resistance-related protein (MRP1) in pediatric acute lymphoblastic leukemia. Journal of Oncology Pharmacy Practice. 2010;16(3):179, 88. doi: 10.1177/1078155209351329. [DOI] [PubMed] [Google Scholar]
- 36.Wuchter C, Leonid K, Ruppert V, Schrappe M, Buchner T, Schoch C, et al. Clinical significance of P-glycoprotein expression and function for response to induction chemotherapy, relapse rate and overall survival in acute leukemia. Haematologica. 2000;85(7):711–21. [PubMed] [Google Scholar]
- 37.Cortez MA, Scrideli CA, Yunes JA, Valera ET, Toledo SR, Pavoni-Ferreira PC, et al. mRNA expression profile of multidrug resistance genes in childhood acute lymphoblastic leukemia. Low expression levels associated with a higher risk of toxic death. Pediatric blood & cancer. 2009;53(6):996–1004. doi: 10.1002/pbc.22220. [DOI] [PubMed] [Google Scholar]


