Abstract
Ventilator-associated lung injury (VALI) remains a significant medical problem in intensive care units. The present study aimed to investigate the role of sphingosine kinase 1 (SPHK1) in VALI using a two-hit model and explore the potential underlying molecular mechanism. Mice were divided into five groups: i) Non-ventilated group; ii) non-ventilated + lipopolysaccharide (LPS) group; iii) ventilated group; iv) ventilated + LPS group; and v) ventilated + LPS + SPHK1 inhibitor group. Mice were administered LPS (1 mg/kg) via an intraperitoneal injection. After 12 h, the mice were anesthetized and connected to a ventilator (10 ml/kg at 150 breaths/min) for 4 h. SPHK1 inhibitor (50 mg/kg) was injected intraperitoneally 1 h prior to ventilation. Mouse lung vascular endothelial cells were treated with LPS and SPHK1 inhibitor, and then subjected to cyclic stretch for 4 h. The present results suggested that the expression of SPHK1 and sphingosine 1 phosphate was upregulated in the two-hit model of VALI; SPHK1 inhibitor could attenuate VALI in the two-hit model as observed by hematoxylin and eosin staining, and affected the cell count and the protein content levels in the bronchoalveolar lavage fluid. In addition, treatment with SPHK1 inhibitor reduced the wet-to-dry ratio of the lungs and suppressed Evans blue dye leakage into the lung tissue. Furthermore, SPHK1 inhibitor exhibited protective effects on the two-hit model of VALI by inhibiting the Ras homolog family member a-mediated phosphorylation of myosin phosphatase target subunit 1 (MYPT-1) and endothelial hyperpermeability. Additionally, mice were divided into five additional groups: i) Non-ventilated group; ii) non-ventilated + LPS group; iii) ventilated group; iv) ventilated + LPS group; and v) ventilated + LPS + Rho-associated coiled-coil forming protein kinase (ROCK)1 inhibitor group. ROCK1 inhibitor (10 mg/kg) was injected intraperitoneally 1 h prior to ventilation. The present results suggested that ROCK1 inhibitor could attenuate mechanical stretch-induced lung endothelial injury and the phosphorylation of MYPT-1 in vivo and in vitro. Collectively, the present findings indicated that upregulation of SPHK1 may contribute to VALI in a two-hit model.
Keywords: mechanical stretch, acute lung injury, sphingosine kinase 1, ras homolog family member a, endothelial permeability
Introduction
Mechanical ventilation is a life-saving therapy for patients with acute lung injury or acute respiratory distress syndrome (ARDS), which are characterized by intractable hypoxemia and bilateral lung infiltration (1). Moderate tidal volume does not cause lung injury itself; however, several studies have demonstrated that it may initiate or promote pre-existing lung injuries, causing further damage to the endothelial barrier with subsequent inflammation and pulmonary edema (2-4). This syndrome has been termed as ventilator-associated lung injury (VALI). Due to the high morbidity and mortality of patients with ARDS, VALI remains a significant medical problem in intensive care units (5). Therefore, further investigation is required to develop effective strategies to mitigate VALI.
Sphingosine 1-phosphate (S1P) is a pleiotropic bioactive mediator that is involved in a variety of key biological processes, including cellular proliferation, apoptosis, migration and survival (6). The formation of S1P is catalyzed by the phosphorylation of sphingosine by sphingosine kinase (SPHK). Recent evidence has suggested a key role for SPHK in several lung pathologies, including lung fibrosis (7), pulmonary artery hypertension (8), asthma (9) and ischemia reperfusion injury (10). Nishiuma et al (11) reported that the inhalation of SPHK1 inhibitor could attenuate airway inflammation in a mouse model of asthma. A recent study revealed the expression of SPHK to be dysregulated in VALI; however, whether this contributes to the pathogenesis of VALI remains unclear.
The increased permeability of endothelial cells has been shown to be the prominent feature of VALI (12,13). The disruption of the endothelial barrier induces the transmigration of inflammatory cells, such as neutrophils, and the formation of edema. Our previous study showed that the activation of Ras homolog family member a (RhoA), and subsequent phosphorylation of myosin light chain and contraction of endothelial cells, may be involved in VALI (14). Furthermore, high tidal volume ventilation-induced lung inflammation was found to be associated with the upregulation of RhoA; treatment with RhoA inhibitor suppressed the expression of Rho-associated coiled-coil forming protein kinase (ROCK) and alleviated lung inflammation (15). A previous study showed that, in a partial urethral obstruction model, upregulation of SPHK1 was accompanied with the induction of RhoA expression, suggesting an association between SPHK and RhoA in regulating endothelial barrier function (16).
Through the comprehensive analysis of mouse VALI genomics data, the present study found that the mRNA expression levels of SPHK1, rather than SPHK2, were significantly upregulated in mouse lung tissues following ventilation. Therefore, the present results suggested that upregulation of SPHK1 may contribute to endothelial hyperpermeability during the development of a two-hit model of VALI by activating the RhoA signaling pathway. It is well-known that bacteremia and/or circulating bacterial products, such as lipopolysaccharide (LPS), are present in the circulation of critically ill individuals (17,18). Therefore, in the present study, a two-hit mouse model was established by systemic LPS (1 mg/kg) followed by ventilation with a low tidal volume of 10 ml/kg. The improvements identified in the present study were similar to the reported clinical mechanical ventilation strategies (2,3). Therefore, the present findings may have potential clinical applications. In addition, in the present study, an in vitro mechanical stretch system was used on primary cultured mouse lung microvascular endothelial cells to evaluate the role of SPHK1 in the mechanical stretch-induced activation of the RhoA signaling pathway and endothelial hyperpermeability.
Materials and methods
Microarray data collection
The present study used the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) to retrieve expression profile datasets. The search term used was: 'Ventilator lung'.
Animal preparation
In total, 280 male ICR mice (aged 8-10 weeks, weighting 25-30 g) were purchased from the Animal Experimentation Center of the Second Military Medical University. All mice had free access to water and food and were housed at room temperature (20-22°C) with 30-70% humidity under a 12-h light/dark cycle. All experimental protocols were approved by The Shanghai Jiaotong University School of Medicine and the methods were conducted in accordance with the institutional guidelines (ethical approval nos. XHEC-C-2017-058 and XHEC-F-NSFC-2018-057).
Mechanical ventilation and drug treatment
Mice underwent ventilation and were intraperitoneally administered with 1 mg/kg LPS (LPS + ventilation group) or saline (ventilation group) (2). After 12 h, the mice were anesthetized with ketamine (70 mg/kg) and xylazine (10 mg/kg) (19), and connected to a ventilator (Inspira, Harvard Apparatus Ltd.) following tracheotomy (10 ml/kg at 150 breaths/min) for 4 h. SPHK1 inhibitor SKI II (50 mg/kg) or ROCK1 inhibitor RKI-1447 (10 mg/kg) were injected intraperitoneally 1 h prior to ventilation. All animals were sacrificed following ventilation, and bronchoalveolar lavage (BAL) fluid or lung tissues were collected. The blood samples were centrifuged at 956 × g for 10 min at 4°C. Lung samples were fixed in 4% paraformaldehyde for 1 day at room temperature, employed to determine the lung wet-to-dry (W/D) ratio or stored at −80°C until use.
Experimental groups
For the first set of experiments, the present study aimed to investigate the effects of SPHK1 inhibition on VALI. The mice were randomly assigned to five groups (n=7 per group): i) Saline administration followed by spontaneous breathing (non-ventilated group); ii) LPS administration followed by spontaneous breathing (non-ventilated + LPS group); iii) saline followed by ventilation (ventilated group); iv) LPS administration followed by ventilation (ventilated + LPS group); and v) LPS and subsequent SPHK1 inhibitor administration with ventilation (ventilated + LPS + SPHK1 inhibitor group).
For the second set of experiments, the present study aimed to investigate the effects of ROCK1 inhibition on VALI. The mice were randomly allocated to the following five groups: i) Saline administration followed by spontaneous breathing (non-ventilated group); ii) LPS administration followed by spontaneous breathing (non-ventilated + LPS group); iii) saline administration followed by ventilation (ventilated group); iv) LPS administration followed by ventilation (ventilated + LPS group); and v) LPS and subsequent ROCK1 inhibitor administration with ventilation (ventilated + LPS + ROCK1 inhibitor group).
BAL
BAL fluid collection was performed three times with 1 ml of saline as previously described (12). The total cell counts were determined as previously described (20) and the protein concentration of BAL fluid was analyzed using a commercially available bicinchoninic protein assay kit.
Lung W/D weight ratio
The index of pulmonary edema formation was calculated by obtaining the lung W/D weight ratio. The lung weights prior to and following drying were used to calculate the lung W/D ratio as previously described (13).
Evans blue dye extravasation assay
Evans blue dye was injected intravenously at 30 mg/kg 1 h prior to the termination of ventilation to assess vascular leak, according to a previously described protocol (13).
Examination of lung histopathology
The left lung tissues were fixed in 4% paraformaldehyde at room temperature for 1 day, and then stained with hematoxylin for 5-10 min at room temperature and eosin (Beyotime Institute of Biotechnology) for 1-2 min at room temperature. The pathogenesis of lung samples was graded by two pathologists in a blinded manner as previously reported: i) 0=Normal tissue; ii) 1=minor inflammatory alterations; iii) 2=mild to moderate inflammatory alterations without marked damage in the lung architecture; iv) 3=moderate inflammatory injury with thickening of the alveolar septa; v) 4=moderate to severe inflammatory injury with the formation of nodules or areas of pneumonitis; and vi) 5=severe inflammatory injury with total obliteration of the field. The mean score was reported per section.
Assay for S1P levels
The content of S1P in the serum was determined via ELISA using a commercial kit, according to the manufacturer's instructions (cat. no. abx585002; Abbexa Ltd.).
Assay for ROCK1 activity
The activities of ROCK1 in the lung tissue and in mouse lung vascular endothelial cells (MLVECs) were analyzed via ELISA using a commercial kit, according to the manufacturer's instructions (cat. no. STA-416; Cell Biolabs, Inc.).
Isolation of MLVECs and cyclic stretch
MLVECs isolation and culture were performed using a modified method as previously described (9). Briefly, the anesthetized mice were perfused with DMEM (Gibco; Thermo Fisher Scientific, Inc.) from the right ventricle to remove blood from the lungs. Then, the subpleural pulmonary tissue was cut into pieces and cultured in DMEM with 20% FBS under 5% CO2 at 37°C for 60 h. The tissue was removed and the adherent cells at passages three and four were used. For cyclic stretch, MLVECs (1×106 cells/well) were seeded onto collagen I-coated Bioflex® 6-well culture plates (Flexcell International Corporation) and grown to 80% confluence. MLVECs were then exposed to cyclic stretch (8% linear elongation, sinusoidal wave, 30 cycles/min) for 4 h, as previously described (21), by using the Flexcell® FX-5000 Tension system (FX5K; Flexcell International Corporation). The cells that did not receive cyclic stretch were placed in the same incubator for 4 h at 37°C.
Analysis of MLVEC permeability
Endothelial cell permeability was analyzed according to a previously published protocol (22). The assay was based on the high affinity binding of an avidin-conjugated FITC-labeled tracer to biotinylated extracellular matrix proteins immobilized on the bottom of culture dishes covered with an MLVEC monolayer. The BioFlex plates (Flexcell International Corporation) were coated with biotinylated gelatin (Sigma-Aldrich; Merck KGaA), and MLVECs were seeded in the wells (1×106 cells/well). After cyclic stretch, cells were fixed with 3.7% formaldehyde at room temperature for 10 min, and FITC-avidin (25 µg/ml) was added to the culture medium for 3 min at room temperature. After washing, elastic bottoms of plates were excised with a scalpel and transferred onto a microslide. The fluorescence was then analyzed under a microscope (magnification, ×20; Olympus Corporation).
Western blot analysis
For western blotting, the lung tissues and MLVECs were homogenized in cold RIPA lysis buffer (Beyotime Institute of Biotechnology) containing proteinase and phosphatase inhibitor cocktail (Roche Diagnostics). Equal amounts of protein extract (30-40 µg) were separated by 8-12% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). After blocking in 5% non-fat dry milk for 2 h at room temperature, the membranes were probed overnight at 4°C with anti-SPHK1 (cat. no. ab71700; 1:500; Abcam), anti-phosphorylated (p)-myosin phosphatase target subunit 1 (p-MYPT1; cat. no. 5163S; 1:1,000; Cell Signaling Technology, Inc.), anti-MYPT1 (cat. no. 2634S; 1:1,000; Cell Signaling Technology, Inc.), anti-RhoA (cat. no. ab187027; 1:3,000; Abcam) and anti-β-actin (cat. no. sc-47778; 1:500; Santa Cruz Biotechnology, Inc.) antibodies. After washing, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (cat. nos. ab6721 and ab6728; 1:5,000; Abcam). The antibody-reactive bands were visualized via an enhanced chemiluminescence western blotting detection system (EMD Millipore). The membranes were visualized using the UVP Bio-Imaging system. Densitometric analysis was performed using Quantity One software (version 4.6; Bio-Rad Laboratories, Inc.).
Analysis of RhoA via pull-down assay
RhoA activity was measured via a pull-down assay using glutathione S-transferase (GST) fusion of Rho-binding domain (RBD) (Cell Signaling Technology, Inc.) according to the manufacturer's instructions. Following treatment, lung tissues and MLVECs were collected and lysed in cold RIPA lysis buffer. Supernatants were incubated for 2 h at 4°C with GST-RBD coupled beads. Centrifuge the samples at 3,824 × g for 10-30 sec and precipitates were washed three times with RIPA lysis buffer and suspended in 2X SDS sample buffer (Cell Signaling Technology, Inc.). The activated RhoA bound to the beads or total RhoA in cell extracts was detected using western blot analysis with a RhoA antibody following the aforementioned protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA isolated from mouse lungs tissue using TRIzol® reagent (Thermo Fisher Scientific, Inc.) was used for RT. Subsequently, qPCR was performed using SYBR® Premix Ex Taq (2X; Takara Bio, Inc.) with a StepOne Plus system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The amplification conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec, with a final extension at 72°C for 5 min. Mouse β-actin served as an internal control, using the 2−ΔΔCq method (23). The sequences of the primers used are listed in Table I.
Table I.
Primers used for qPCR.
| Gene symbol | Primer sequence (5′-3′) |
|---|---|
| SPHK1 | F: GAAGACCTGCTCATCAACTGC |
| R: GGTGCCCACTGTGAAACG | |
| β-actin | F: CTGTATGCCTCTGGTCGTAC |
| R: TGATGTCACGCACGATTTCC |
SPHK1, sphingosine kinase 1; F, forward; R, reverse.
Statistical analysis
Data are presented as the mean ± SEM. Normal distribution was assessed by Shapiro-Wilk test. Statistical significance was measured according to sample distribution and homogeneity of variance. Statistical comparison between two groups were determined by Student's t-test for normally distributed data and by Mann-Whitney U test for non-normally distributed data. One-way ANOVA with Bonferroni's post hoc test was performed to compare multiple groups using SPSS 19 (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Pulmonary levels of SPHK1 and S1P are upregulated in the two-hit model of VALI
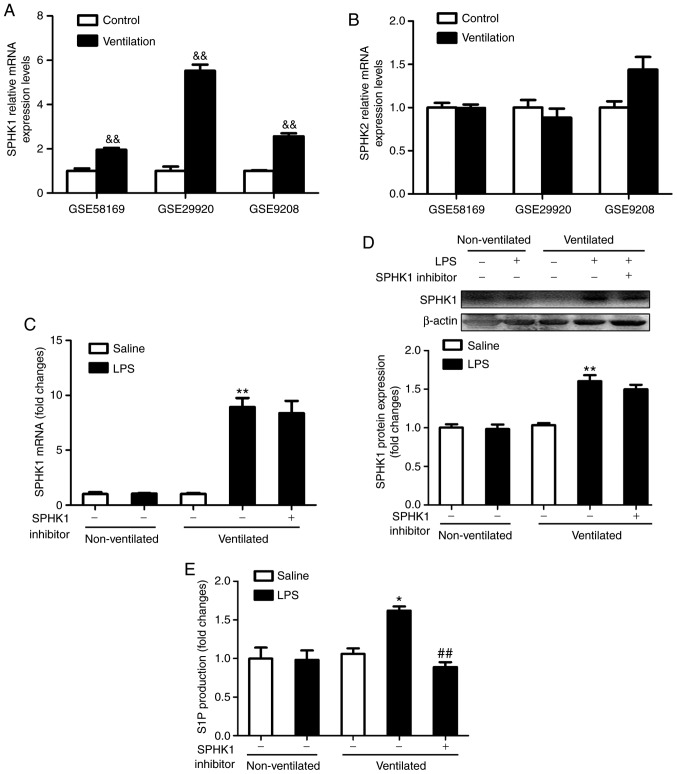
To determine the expression levels of SPHK in ventilated mouse lung tissues, the present study analyzed the gene expression profiles of the GEO mouse VALI datasets GSE58169 (24), GSE29920 and GSE9208 (25). Collectively, the analysis of these data revealed that the mRNA expression of SPHK1, rather than SPHK2, was significantly upregulated in ventilated mouse lung tissues (Fig. 1A and B). To verify these findings, the expression of SPHK1 was investigated by RT-qPCR and western blotting. As shown in Fig. 1C and D, there were no significant differences in mRNA and protein concentration of SPHK1 in LPS alone or ventilated alone group. However, in the ventilated + LPS group, the expression of SPHK1 was significantly increased compared with that in the non-ventilated group. SPHK1 inhibitor had no effects on the mRNA and protein expression of SPHK1. Furthermore, consistent with the increase in SPHK1 expression, the levels of pulmonary S1P were significantly increased in the ventilated + LPS group, but decreased following treatment with SPHK1 inhibitor (Fig. 1E).
Figure 1.
Pulmonary levels of SPHK1 and S1P are upregulated in the two-hit model of ventilator-associated lung injury. mRNA expression levels of (A) SPHK1 and (B) SPHK2 in ventilated mouse lung tissues. Data were obtained from the following Gene Expression Omnibus datasets: GSE58169, GSE29920 and GSE9208. Data are presented as the mean ± SEM. (C) mRNA expression levels of SPHK1 were detected by reverse transcription-quantitative PCR. Protein expression of SPHK1 was normalized to β-actin. (D) Representative protein bands are shown. (E) At the end of the experiment, blood was collected from the heart, and the supernatant was removed after centrifugation. Alterations in the levels of S1P were detected by ELISA. Data are presented as the mean ± SEM (n=7). &&P<0.01 vs. Control group. *P<0.05, **P<0.01 vs. LPS or ventilated groups. ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; S1P, sphingosine 1 phosphate; SPHK, sphingosine kinase.
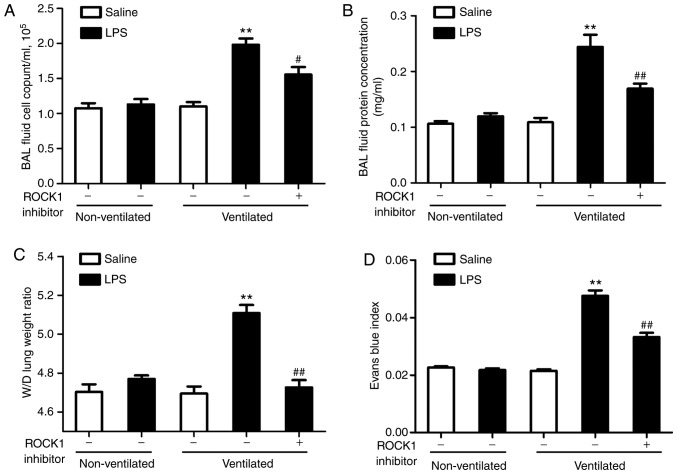
SPHK1 inhibitor attenuates lung vascular hyperpermeability in the two-hit model of VALI
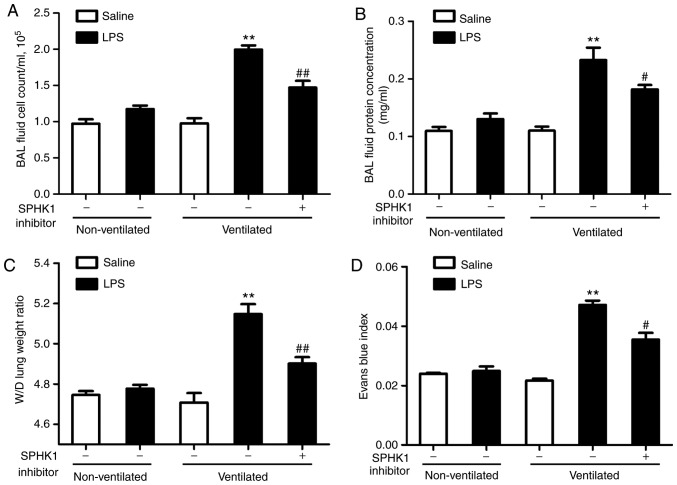
In the present study, it was investigated whether SPHK1 inhibitor could exhibit a protective effect on the two-hit of model of VALI. As shown in Fig. 2A and B, LPS or ventilation alone induced minor non-significant lung injury compared with the non-ventilated group. In line with our previous study (13), low tidal volume ventilation did not exert significant lung injury. However, when combined with LPS, ventilation significantly induced lung injury, indicating a synergistic effect of these two stimuli. In addition, the degree of lung injury in the two-hit VALI models was significantly attenuated following treatment with SPHK1 inhibitor. Similar results were observed in the lung W/D weight ratio and the Evans blue assay (Fig. 2C and D). Ventilation + LPS led to increased lung W/D ratio and Evans blue leakage from the vascular space into the lung parenchyma, which were significantly alleviated following treatment with SPHK1 inhibitor.
Figure 2.
SPHK1 inhibitor attenuates lung vascular hyperpermeability in the two-hit model of ventilator-associated lung injury. (A) Cell count and (B) measurements of protein concentration in BAL fluid. (C) Lung W/D was calculated as an index of pulmonary edema. (D) Pulmonary vascular permeability was determined via the analysis of Evans blue-labeled albumin extravasation into the lung tissue. Data are presented as the mean ± SEM (n=7). **P<0.01 vs. LPS or ventilated groups; #P<0.05, ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; BAL, bronchoalveolar lavage; W/D wet-to-dry weight ratio; SPHK, sphingosine kinase.
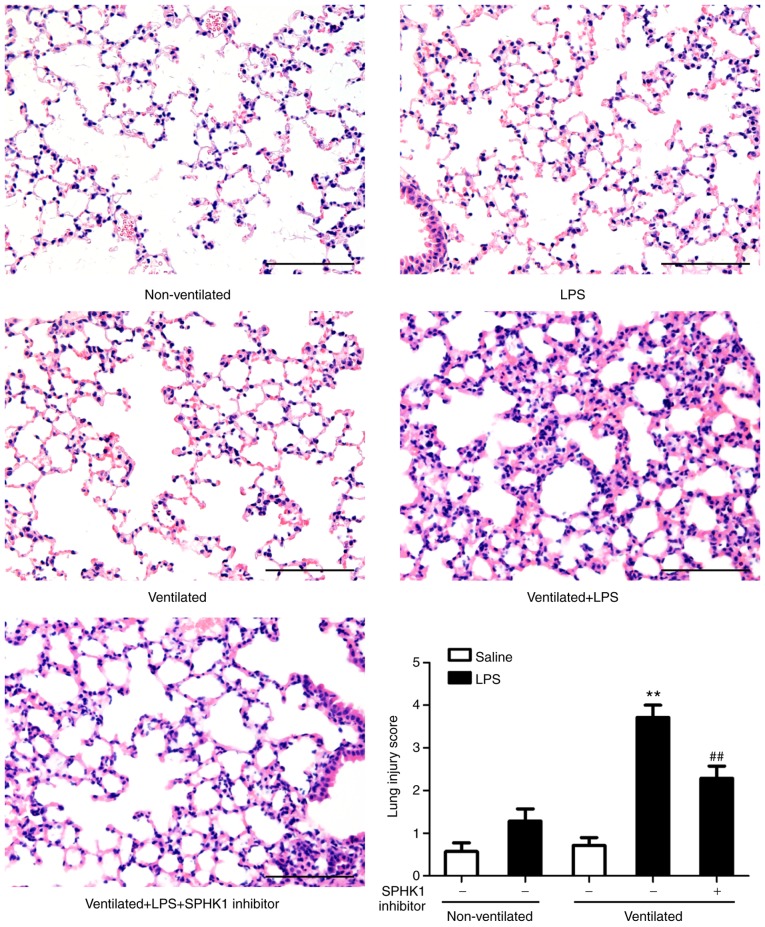
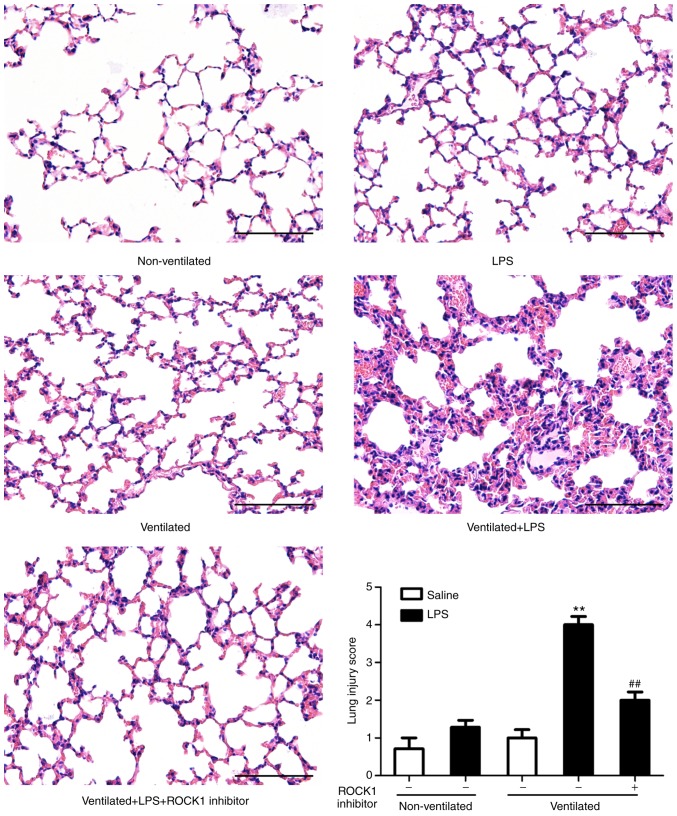
H&E staining was then performed to evaluate the histology of tissues (Fig. 3). Histopathological analysis revealed that mice in the two-hit VALI group (ventilated + LPS) exhibited diffuse interstitial edema and infiltration around the pulmonary vessels; alveolar and interstitial edema were also observed compared with the LPS and ventilated groups. Quantitative analysis demonstrated a significant increase in the lung injury score in the ventilated + LPS group. Following treatment with SPHK1 inhibitor, lung injury induced by ventilation + LPS was significantly attenuated.
Figure 3.
SPHK1 inhibitor suppresses lung histopathological alterations in the two-hit model of ventilator-associated lung injury. Representative hematoxylin and eosin staining of lung tissues. The severity of lung injury was scored to quantify the degree of lung pathology. Original magnification, ×200. Scale bar, 100 µm. Data are presented as the mean ± SEM (n=7). **P<0.01 vs. LPS or ventilated groups; ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; SPHK, sphingosine kinase.
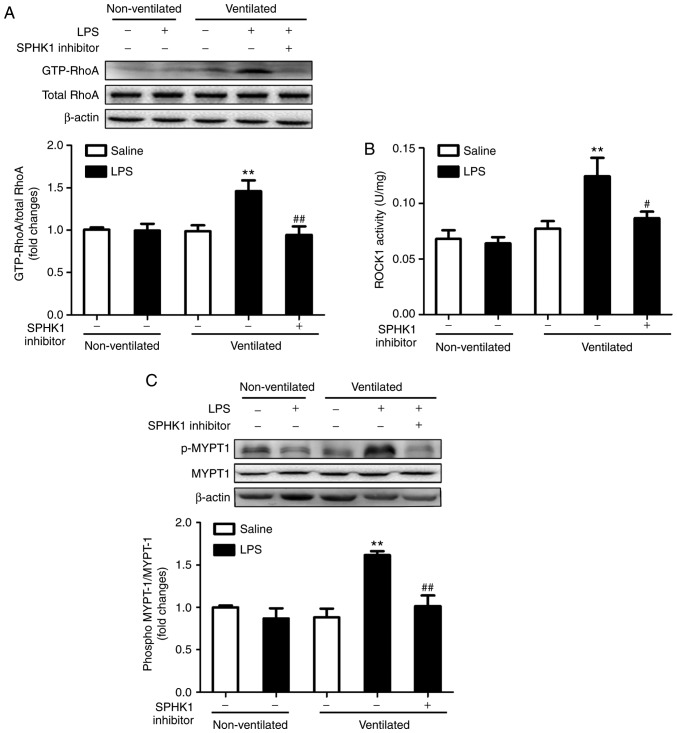
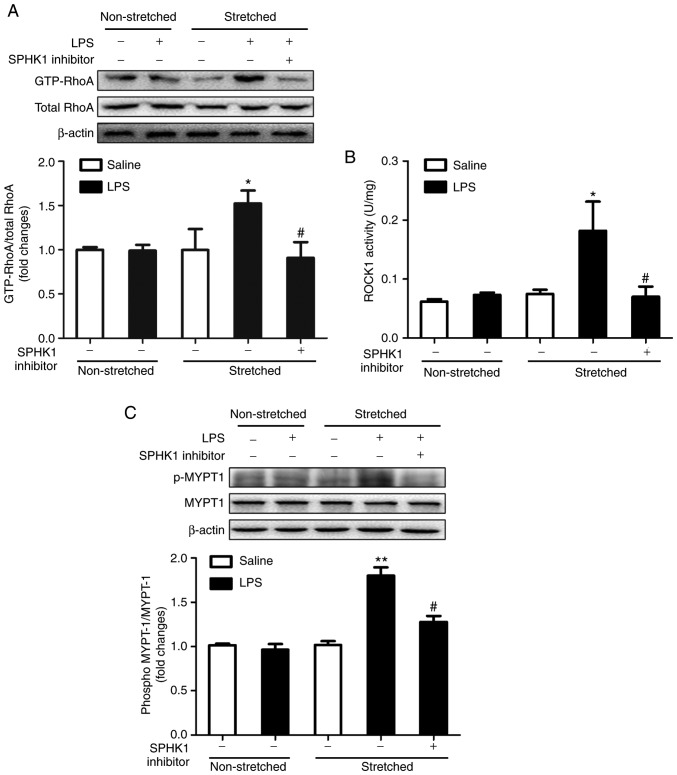
Effects of SPHK1 inhibitor on the RhoA/ROCK pathway in a two-hit model of VALI
RhoA and its target protein, ROCK, are involved in the calcium-sensitizing signaling pathway associated with the cytoskeletal contractile response via the activity of myosin ATPase (26). ROCK mediates the phosphorylation of myosin light chain, ultimately resulting in the reorganization of actin myosin, tension fiber formation and cell contraction (27). MYPT-1 is the regulatory subunit of myosin light chain phosphatase, which functions as a downstream target of ROCK (28). The present study investigated the activation of RhoA and ROCK1, and the phosphorylation of MYPT-1 in the two-hit model of VALI. As presented in Fig. 4, LPS or ventilation alone had no significant effect on RhoA and ROCK1 activity, and on the phosphorylation levels of MYPT1. By contrast, mice in the two-hit VALI group (ventilated + LPS) exhibited an increase in RhoA and ROCK1 activity, and MYPT1 phosphorylation compared with the non-ventilated + LPS or ventilated groups. Additionally, SPHK1 inhibitor treatment significantly attenuated the increases in RhoA and ROCK1 activity, and the phosphorylation levels of MYPT1 in the lung tissues from mice in the ventilated + LPS group. The present results suggested that SPHK1 inhibitor suppressed the activity of the RhoA/ROCK signaling pathway in animals with VALI.
Figure 4.
Effect of SPHK1 inhibitor on the RhoA/ROCK pathway in the two-hit model of ventilator-associated lung injury. Levels of (A) GTP-RhoA, (B) ROCK1 and (C) p-MYTP1 were determined by western blot analysis. GTP-RhoA levels were normalized to total RhoA expression, and the p-MYTP1/total-MYTP1 ratio was evaluated. The representative protein bands were shown. Data are presented as the mean ± SEM (n=7). **P<0.01 vs. LPS or ventilated groups; #P<0.05, ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; MYTP1, myosin phosphatase target subunit 1; p, phosphorylated; ROCK, Rho-associated coiled-coil forming protein kinase; RhoA, Ras homolog family member A; SPHK, sphingosine kinase.
Effects of SPHK1 inhibitor on the RhoA/ROCK pathway in MLVECs
As lung endothelial cell hyperpermeability is caused by endothelial overdistension during ventilation (1,29), the effects of cyclic stretch and LPS were examined, and the effect of SPHK1 inhibition on the activation of the RhoA/ROCK pathway was investigated in primary cultured MLVECs. In the present study, MLVECs were subjected to LPS treatment for 12 h and then exposed to cyclic stretch (8% elongation) for 4 h. Consistent with the findings in vivo, LPS or stretch alone led to minor changes in the activation of RhoA and ROCK1, and the phosphorylation of MYPT1. On the contrary, MLVECs subjected to the combination of stretch and LPS treatment exhibited significant increases in the activity of RhoA and ROCK1, and in the phosphorylation levels of MYPT1. Treatment with SPHK1 inhibitor significantly inhibited the activation of RhoA and ROCK1, and the phosphorylation of MYPT1 induced by cyclic stretch + LPS (Fig. 5).
Figure 5.
Effects of SPHK1 inhibitor on the RhoA/ROCK pathway in mouse lung vascular endothelial cells. Cells were collected to analyze (A) the protein expression levels of GTP-RhoA, (B) ROCK1 and (C) p-MYTP1 expression. GTP-RhoA levels were normalized to total RhoA expression and the p-MYTP1/total-MYTP1 ratio was evaluated. The representative protein bands were shown. Data are presented as the mean ± SEM (n=4). *P<0.05, **P<0.01 vs. LPS or stretched groups; #P<0.05, ##P<0.01 vs. Stretched + LPS group. LPS, lipopolysaccharide; MYTP1, myosin phosphatase target subunit 1; p, phosphory-lated; ROCK, Rho-associated coiled-coil forming protein kinase; RhoA, Ras homolog family member A; SPHK, sphingosine kinase.
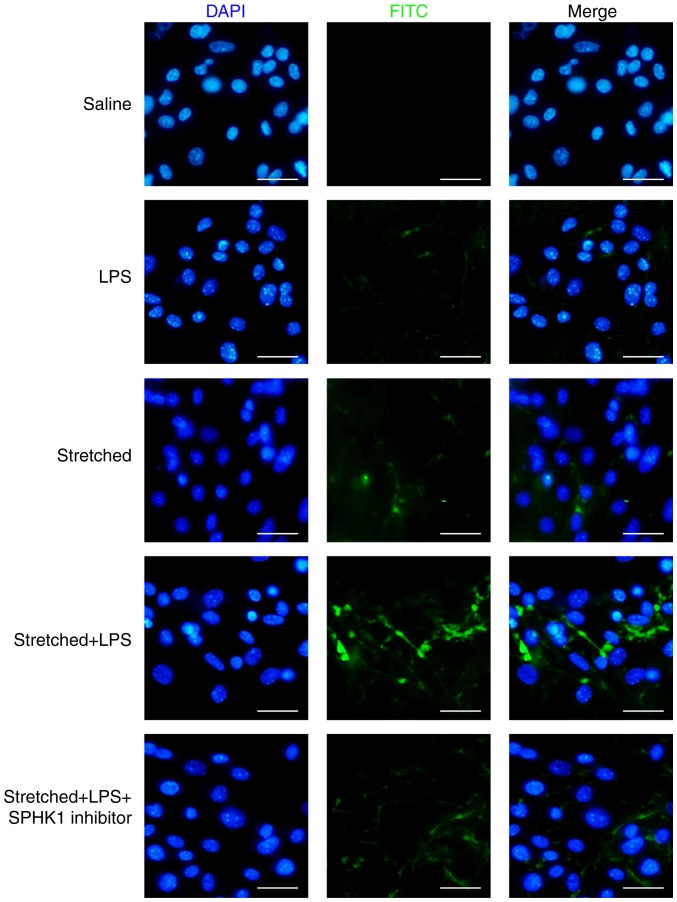
Effects of SPHK1 inhibitor on the permeability of MLVECs
The protective effects of SPHK1 inhibitor against stretch-associated endothelial hyperpermeability were further assessed by a permeability assay. As presented in Fig. 6, LPS or mechanical stretch alone had limited effects on endothelial permeability. By contrast, analysis of the permeability sites in the LPS + stretch-challenged endothelial monolayers revealed the presence of FITC-labeled avidin, which entered cells via weakened cell junctions and stretch-induced paracellular gaps; the combination of stretch and LPS treatment markedly increased endothelial monolayer permeability to FITC-labeled avidin. In addition, SPHK1 inhibitor treatment markedly decreased the cellular entry of the fluorescent probe via intercellular junctions, indicating that inhibition of SPHK1 attenuated the disruptive effects of LPS + cyclic stretch on the permeability function of endothelial cells in vitro.
Figure 6.
Effects of SPHK1 inhibitor on the permeability of mouse lung vascular endothelial cells. Cells were collected for the analysis of membrane permeability. FITC was analyzed via fluorescence microscopy. Original magnification, ×200. Scale bar, 20 µm. SPHK, sphingosine kinase; LPS, lipopolysaccharide.
ROCK1 inhibitor attenuates lung vascular hyperpermeability in the two-hit model of VALI
To assess the role of the RhoA/ROCK pathway in the two-hit model of VALI, mice were treated with ROCK1 inhibitor RKI-1447 (10 mg/kg) prior to ventilation. As presented in Fig. 7A and B, ROCK1 inhibitor exerted a significant protective effect on the two-hit model of VALI as assessed by measuring the cell count and the protein levels in the BAL fluid. The results of the lung W/D ratio and Evans blue index analyses also supported these findings (Fig. 7C and D). In addition, H&E staining was performed to evaluate the effects of ROCK1 inhibitor on lung histology (Fig. 8). The degree of lung injury induced by LPS and ventilation was significantly attenuated by ROCK1 inhibitor treatment.
Figure 7.
ROCK1 inhibitor attenuates lung vascular hyperpermeability in the two-hit model of ventilator-associated lung injury. (A) Cell count and (B) protein concentration in BAL fluid. (C) Lung W/D was calculated as an index of pulmonary edema. (D) Pulmonary vascular permeability was determined via the analysis of Evans blue-labeled albumin extravasation into the lung tissue. Data are presented as the mean ± SEM (n=7). **P<0.01 vs. LPS or ventilated groups; #P<0.05, ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; ROCK, Rho-associated coiled-coil forming protein kinase; W/D wet-to-dry weight ratio; BAL, bronchoalveolar lavage.
Figure 8.
ROCK1 inhibitor suppresses lung histopathological changes in the two-hit model of ventilator-associated lung injury. Representative hematoxylin and eosin staining of lung tissues. The severity of lung injury was scored to quantify the degree of lung pathology. Original magnification, ×200. Scale bar, 100 µm. Data are presented as the mean ± SEM (n=7). **P<0.01 vs. LPS or ventilated groups; ##P<0.01 vs. Ventilated + LPS group. LPS, lipopolysaccharide; ROCK, Rho-associated coiled-coil forming protein kinase.
Additionally, the present study investigated the effects of ROCK1 inhibitor on the ROCK1/MYPT1 pathway in vivo. As presented in Fig. S1, ROCK activity was suppressed in response to ROCK1 inhibitor, and the levels of p-MYPT1 were also downregulated.
Effects of ROCK1 inhibitor on the permeability of MLVECs
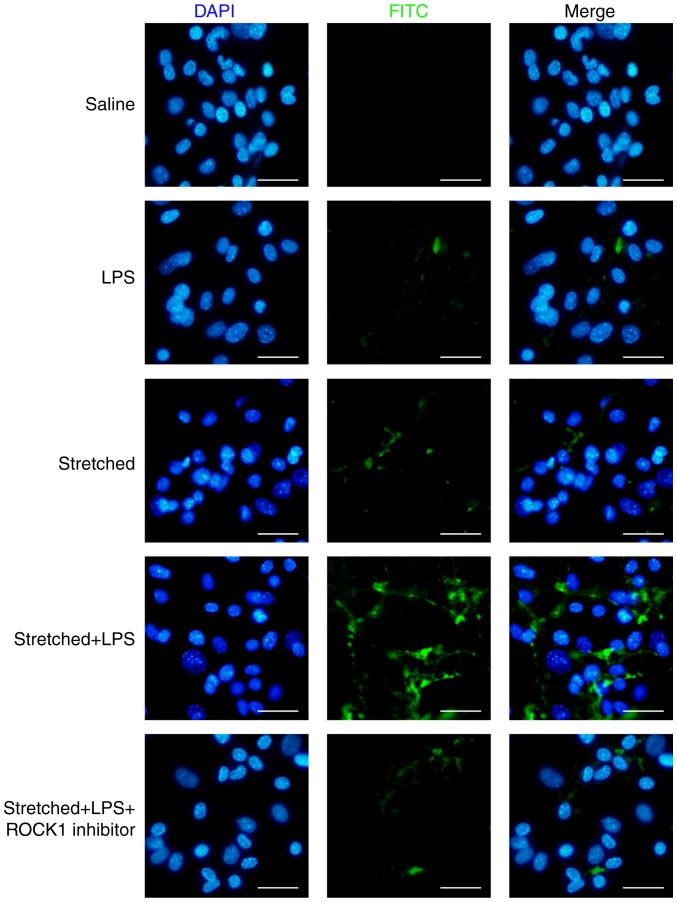
The present study investigated the effects of ROCK1 inhibitor on endothelial permeability in vitro. As presented in Fig. 9, ROCK1 inhibition by RKI-1447 (1 µM) could inhibit the entry of the fluorescent probe in cells in the stretch + LPS group. The present results suggested that inhibition of ROCK1 attenuated the barrier-disruptive effects of LPS treatment and mechanical stretch in vitro. In addition, in MLVECs, the activity of ROCK1 and the phosphorylation levels of MYPT1 were reversed by treatment with ROCK1 inhibitor (Fig. S2).
Figure 9.
Effects of ROCK1 inhibitor on the permeability of mouse lung vascular endothelial cells. Cells were collected for the analysis of membrane permeability. Fluorescein isothiocyanate fluorescence was analyzed via fluorescence microscopy. Original magnification, ×200. Scale bar, 20 µm. ROCK, Rho-associated coiled-coil forming protein kinase; LPS, lipopolysaccharide.
Discussion
In the present study, it was reported that SPHK1/S1P signaling was induced in a two-hit model of VALI, and the present results suggested that inhibition of SPHK1 activity may be a potential therapeutic approach for treating VALI.
Previous studies have shown that positive-pressure ventilation via moderate ventilation does not generally cause extensive lung injury in animals with healthy lungs (3,13); however, whether ventilation promotes injury in pre-injured lungs requires further investigation. Investigating the effects of ventilation is clinically important as patients receiving ventilation therapy in intensive care with lung injury can exhibit complications, such as sepsis (17,30,31). Brégeon et al (3) revealed that positive-pressure ventilation using a tidal volume of 10 ml/kg was harmful under conditions of endotoxemia, and may increase the susceptibility to lung injury in the presence of this complication. In the present study, a tidal volume of 10 ml/kg was selected and LPS (1 mg/kg) was administered to mimic the ventilation strategy commonly used in clinic. In addition, the concentration of LPS used reflected the concentration range of circulating LPS associated with pre-injured lungs (2,3). When establishing the two-hit model, the present study intraperitoneally administrated 1 mg/kg LPS, according to the study of O'Mahony et al (2); and no notable lung injury was observed at this dose. Similarly, the present study reported that ventilation or LPS alone could result in mild changes, not meeting the criteria for ALI or ARDS (5,29). By contrast, in the present study, the combined treatment of LPS and ventilation resulted in molecular and histological alterations associated with lung injury. These findings may support future clinical application of the present findings. Furthermore, Wadgaonkar et al (32) revealed that the mRNA expression levels of SPHK1 increased ~5-fold following the intratracheally administration of 2 mg/kg LPS; in addition, significant upregulation of SPHK1 protein expression was reported. The present study showed that intraperitoneal administration of LPS at 1 mg/kg had no significant effect on pulmonary SPHK1 expression. The discrepancy between our findings and those of Wadgaonkar et al (32) may be due to an increased dose and a different administration route of LPS.
Mammals have two isoforms of SPHK, including SPHK1 and SPHK2 (33). The expression of these two isoenzymes has been identified in endothelial cells and the lungs, and these enzymes were found to generate S1P following activation (34). In a previous study, SPHK1 rather than SPHK2, was found to be the major isoenzyme mediating endothelial barrier function (35). Billich et al (36) reported that SPHK1 activity was 3-20 times increased compared with SPHK2 in mouse tissues. In addition, the expression of SPHK1 was found to increase following ventilation at 30 ml/kg for 4 h (24,25). During the development of pulmonary arterial hypertension, unlike Sphk2−/− mice, Sphk1−/− mice exhibit protection against hypoxia-mediated pulmonary hypertension (8). In the present study, the gene expression profiles from datasets generated in mice with VALI were analyzed. In the present study, the mRNA expression levels of SPHK1, rather than SPHK2, were significantly upregulated in ventilated mouse lung tissues. Using an in vivo two-hit model of VALI, it was observed that the mRNA and protein expression levels of SPHK1 were increased following the combined treatment of LPS and ventilation. In addition, the present study identified that SPHK1 inhibitor treatment improved LPS + ventilation-induced lung injury, suggesting that upregulation of SPHK1 may contribute to LPS + ventilation-induced lung injury.
Accumulating evidence demonstrated that mechanical stretch disrupts the function of endothelial adherens junctions and promotes vascular endothelial hyperpermeability (1,37). However, conflicting findings regarding the effects of SPHK1 on the regulation of endothelial function have been reported (38,39). For example, the activation of SPHK1 was determined to mediate endothelial damage under high glucose conditions or in streptozotocin-induced diabetic rats, and inhibition of SPHK1 markedly protected endothelial cells and suppressed the formation of vascular lesions (38). These previous findings were consistent with the present study, as mechanical stretch resulted in the upregulation of SPHK1, and SPHK1 inhibitor attenuated mechanical stretch-induced lung edema and endothelial hyperpermeability. In addition, SPHK1 has been reported to induce endothelial cell proliferation, thus promoting hemangiogenesis and lymphangiogenesis in breast cancer, both of which could be suppressed by SPHK1 inhibitor (39). Collectively, these previous and the present findings indicated that SPHK1 may modulate endothelial function in a context-dependent manner.
The increased production of ROS (1), and the disorganized structure of the actin and tubulin cytoskeleton (37), endothelial apoptosis (40) and disruptions in endothelial interactions (13) are the main causes of endothelial hyperpermeability in VALI. The present study and several previous reports revealed that RhoA/ROCK1-mediated phosphorylation of myosin light chain led to the contraction of endothelial cells and breakdown of the pulmonary endothelial barrier (14,27). In the present study, it was identified that SPHK inhibitor attenuated the activity of RhoA and ROCK1, and the phosphorylation of MYPT1 in vivo and in vitro; consequently, the function of the pulmonary vascular endothelial barrier was found to be improved following SPHK inhibition. ROCK1 inhibitor could also attenuate lung injury and the phosphorylation of MYPT1 in vivo and in vitro. In addition, inhibition of SPHK1 was observed to notably protect against high glucose-induced endothelial cell injury in a protein kinase C (PKC)- and ERK1/2-dependent manner (38). SPHK1 has been reported as a modulator of hypoxia inducible factor (HIF)-1α, which contributes to hypoxia-induced endothelial dysfunction (41). Whether the PKC, ERK1/2 or HIF-1α signaling pathways are involved in mediating the protective effects of SPHK1 inhibitor against mechanical stretch-induced endothelial hyperpermeability requires further investigation.
In conclusion, by using an LPS + ventilation model, the present study suggested that upregulation of SPHK1 served an essential role in the two-hit model of VALI. Additionally, SPHK1 inhibitor could exert protective effects via the suppression of RhoA-mediated phosphorylation of MYPT1 and endothelial hyperpermeability. Furthermore, lung injury induced by the combined treatment of LPS and ventilation could be reversed by ROCK1 inhibitor.
However, the present study presents certain limitations; the present findings were not confirmed using an SPHK1 activator or SPHK1 knockout mice, which may provide further insight into the mechanisms underlying the protective effects of SPHK1 inhibitor in the two-hit VALI animal model. Nevertheless, although the exact role of the SPHK1/S1P pathway in VALI remains to be elucidated, the present findings suggested that SPHK1 inhibitor could be a novel therapeutic agent for treating VALI.
Supplementary Data
Acknowledgments
The authors would like to thank Dr Yan-fei Mao for excellent technical support. (Department of Anesthesiology and Surgical Intensive Care Unit, Xinhua Hospital, Shanghai Jiaotong University School of Medicine).
Funding
The present study was supported by grants from The Shanghai Municipal Commission of Health and Family Planning to Dr Jiang (grant no. 2017BR062), Shanghai Science and Technology Commission to Dr Wang (grant no. 18YF1415500), and from The National Natural Science Foundation of China to Dr Jiang (grant nos. 81772117 and 81571929) and Dr Liu (grant no. 81672266).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW analyzed the data and drafted the manuscript. TTG performed the experiments and designed the study. DFX performed the experiments and collected the data. XYZ performed the experiments and drafted the manuscript. WWD performed the experiments. ZL performed the experiments and analyzed the data. YJL performed the experiments and supervised the study. LJ designed and supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols were approved by The Shanghai Jiaotong University School of Medicine and the methods were conducted in accordance with the institutional guidelines (ethical approval nos. XHEC-C-2017-058 and XHEC-F-NSFC-2018-057).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lv Z, Wang Y, Liu YJ, Mao YF, Dong WW, Ding ZN, Meng GX, Jiang L, Zhu XY. NLRP3 inflammasome activation contributes to mechanical stretch-induced endothelial-mesenchymal transition and pulmonary fibrosis. Crit Care Med. 2018;46:e49–e58. doi: 10.1097/CCM.0000000000002799. [DOI] [PubMed] [Google Scholar]
- 2.O'Mahony DS, Liles WC, Altemeier WA, Dhanireddy S, Frevert CW, Liggitt D, Martin TR, Matute-Bello G. Mechanical ventilation interacts with endotoxemia to induce extrapulmonary organ dysfunction. Crit Care. 2006;10:R136. doi: 10.1186/cc5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brégeon F, Delpierre S, Chetaille B, Kajikawa O, Martin TR, Autillo-Touati A, Jammes Y, Pugin J. Mechanical ventilation affects lung function and cytokine production in an experimental model of endotoxemia. Anesthesiology. 2005;102:331–339. doi: 10.1097/00000542-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JG, Letsiou E, Dudek SM. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1294–L1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 7.Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, Ma W, Noth I, Ma SF, Pendyala S, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. 2013;27:1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, Conrad D, Ryan JJ, Milstien S, Spiegel S. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J Allergy Clin Immunol. 2013;131:501–511.e1. doi: 10.1016/j.jaci.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Chen F, Pan Y, Lin L, Xiong X. Effects of FTY720 on lung injury induced by hindlimb ischemia reperfusion in rats. Mediators Inflamm. 2017;2017:5301312. doi: 10.1155/2017/5301312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li SY, Zhu XY, Jiang L. Salidroside attenuates ventilation induced lung injury via sirt1-dependent inhibition of NLRP3 inflammasome. Cell Physiol Biochem. 2017;42:34–43. doi: 10.1159/000477112. [DOI] [PubMed] [Google Scholar]
- 13.Dong WW, Liu YJ, Lv Z, Mao YF, Wang YW, Zhu XY, Jiang L. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Radic Biol Med. 2015;88:404–416. doi: 10.1016/j.freeradbiomed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Liu K, Mao YF, Zheng J, Peng ZY, Liu WW, Liu Y, Xu WG, Sun XJ, Jiang CL, Jiang L. SC5b9-induced pulmonary microvascular endothelial hyperpermeability participates in ventilator-induced lung injury. Cell Biochem Biophys. 2013;67:1421–1431. doi: 10.1007/s12013-013-9675-8. [DOI] [PubMed] [Google Scholar]
- 15.Dai H, Zhang S, Du X, Zhang W, Jing R, Wang X, Pan L. RhoA inhibitor suppresses the production of microvesicles and rescues high ventilation induced lung injury. Int Immunopharmacol. 2019;72:74–81. doi: 10.1016/j.intimp.2019.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Aydin M, Downing K, Villegas G, Zhang X, Chua R, Melman A, Disanto ME. The sphingosine-1-phosphate pathway is upregulated in response to partial urethral obstruction in male rats and activates RhoA/Rho-kinase signalling. BJU Int. 2010;106:562–571. doi: 10.1111/j.1464-410X.2009.09156.x. [DOI] [PubMed] [Google Scholar]
- 17.Parsons PE, Worthen GS, Moore EE, Tate RM, Henson PM. The association of circulating endotoxin with the development of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:294–301. doi: 10.1164/ajrccm/140.2.294. [DOI] [PubMed] [Google Scholar]
- 18.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155:937–944. doi: 10.1164/ajrccm.155.3.9117029. [DOI] [PubMed] [Google Scholar]
- 19.Iwai Y, Honda S, Ozeki H, Hashimoto M, Hirase H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci Res. 2011;70:124–127. doi: 10.1016/j.neures.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L924–L935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol. 2013;190:3590–3599. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest. 2013;93:254–263. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Spassov S, Pfeifer D, Strosing K, Ryter S, Hummel M, Faller S, Hoetzel A. Genetic targets of hydrogen sulfide in ventilator-induced lung injury-a microarray study. PLoS One. 2014;9:e102401. doi: 10.1371/journal.pone.0102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaiahgari S, Yerrapureddy A, Reddy SR, Reddy NM, Dodd OJ, Crow MT, Grigoryev DN, Barnes K, Tuder RM, Yamamoto M, et al. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am J Respir Crit Care Med. 2007;176:1222–1235. doi: 10.1164/rccm.200701-060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sari-Hassoun M, Clement MJ, Hamdi I, Bollot G, Bauvais C, Joshi V, Toma F, Burgo A, Cailleret M, Rosales-Hernández MC, et al. Cucurbitacin I elicits the formation of actin/phosphomyosin II co-aggregates by stimulation of the RhoA/ROCK pathway and inhibition of LIM-kinase. Biochem Pharmacol. 2016;102:45–63. doi: 10.1016/j.bcp.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Dong M, Liu W, Wang L, Luo Y, Li Z, Jin F. 1α,25-dihydroxyvitamin D3 ameliorates seawater aspiration-induced acute lung injury via NF-κB and RhoA/Rho kinase pathways. PLoS One. 2014;9:e104507. doi: 10.1371/journal.pone.0104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKay CE, Shaifta Y, Snetkov VV, Francois AA, Ward JPT, Knock GA. ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1. Free Radic Biol Med. 2017;110:316–331. doi: 10.1016/j.freeradbiomed.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu CF, Liu YJ, Wang Y, Mao YF, Xu DF, Dong WW, Zhu XY, Jiang L. Downregulation of R-Spondin1 contributes to mechanical stretch-induced lung injury. Crit Care Med. 2019;47:e587–e596. doi: 10.1097/CCM.0000000000003767. [DOI] [PubMed] [Google Scholar]
- 30.Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 31.Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, et al. Diagnostic and prognostic implications of endotoxemia in critical illness: Results of the MEDIC study. J Infect Dis. 2004;190:527–534. doi: 10.1086/422254. [DOI] [PubMed] [Google Scholar]
- 32.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JG, Natarajan V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296:L603–L613. doi: 10.1152/ajplung.90357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: Role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 37.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837–L848. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Xing XP, Holmes A, Wadham C, Gamble JR, Vadas MA, Xia P. Activation of the sphingosine kinase-signaling pathway by high glucose mediates the proinflammatory phenotype of endothelial cells. Circ Res. 2005;97:891–899. doi: 10.1161/01.RES.0000187469.82595.15. [DOI] [PubMed] [Google Scholar]
- 39.Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angio-genesis and lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raaz U, Kuhn H, Wirtz H, Hammerschmidt S. Rapamycin reduces high-amplitude, mechanical stretch-induced apoptosis in pulmonary microvascular endothelial cells. Microvasc Res. 2009;77:297–303. doi: 10.1016/j.mvr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Lee SO, Kim JS, Lee MS, Lee HJ. Anti-cancer effect of pristimerin by inhibition of HIF-1α involves the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer. 2016;16:701. doi: 10.1186/s12885-016-2730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.