Abstract
Huntington's disease (HD) is a lethal neurodegenerative disorder for which no cure is available yet. It is caused by abnormal expansion of a CAG triplet in the gene encoding the huntingtin protein (Htt), with consequent expansion of a polyglutamine repeat in mutated Htt (mHtt). This makes mHtt highly unstable and aggregation prone. Soluble mHtt is linked to cytotoxicity and neurotoxicity, whereas mHtt aggregates are thought to be neuroprotective. While Htt and mHtt are ubiquitously expressed throughout the brain and peripheral tissues, HD is characterized by selective degradation of the corpus striatum, without notable alterations in peripheral tissues. Screening for mRNAs preferentially expressed in rodent striatum led to the discovery of a GTP binding protein homologous to Ras family members. Due to these features, the newly discovered protein was termed Ras Homolog Enriched in Striatum (RHES). The aetiological role of RHES in HD has been ascribed to its small ubiquitin-like modifier (SUMO)-E3 ligase function. RHES sumoylates mHtt with higher efficiency than wild-type Htt, thereby protecting mHtt from degradation and increasing the amounts of the soluble form. Although RHES is an attractive target for HD treatment, essential information about protein structure and function are still missing. With the aim of investigating RHES 3D structure and function, bioinformatic analyses and molecular modelling have been performed in the present study, based on which, RHES regions predicted to be involved in the interaction with mHtt or the SUMO-E2 ligase Ubc9 have been identified. These regions have been used to design peptides aimed at inhibiting RHES interactions and, therefore, mHtt sumoylation; in turn, these peptides will be used to develop small molecule inhibitors by both rational design and virtual screening of large compound libraries. Once identified, RHES sumoylation inhibitors may open the road to the development of therapeutic agents against the severe, and currently untreatable, HD.
Keywords: Huntington's disease, Ras homologue enriched in the striatum, huntingtin, ubiquitin carrier protein 9, homology modelling, molecular docking, peptide design
Introduction
Huntington's disease (HD) (1) is an autosomal dominant lethal neurodegenerative disorder that causes a wide range of symptoms including: Involuntary movements, clumsiness, lack of concentration, memory lapses, mood swings and depression. It is caused by an abnormal expansion (>35 copies) of a CAG triplet located in exon 1 of the gene encoding the huntingtin protein (Htt), with consequent expansion of a polyglutamine repeat in the protein (2). The expansion of the CAG-rich region makes mutant Htt (mHtt) very unstable and able to aggregate with itself and/or other proteins, forming clumps. However, cytotoxicity and neurodegeneration has been shown to correlate with the amount of soluble, monomeric mHtt levels but not with mHtt aggregates and oligomers, which are thought to be neuroprotective, at least at some stages (3-6). Although several symptomatic treatments are available, no cure for this serious neurological disease is available (1). Furthermore, neither Htt function has been entirely understood, nor have the molecular mechanisms underlying mHtt cytotoxicity been clarified yet (3,7).
While Htt and mHtt are ubiquitously expressed throughout the brain and peripheral tissues, HD is characterized by highly selective degradation of the corpus striatum (8), with no notable alterations in peripheral tissues. The corpus striatum is a brain region that regulates the tuning and planning of movements, emotion, and higher brain function (9). Therefore, the cardinal symptoms of HD include a choreiform involuntary movement disorder, clumsiness, concentration deficiency, memory lapses, mood swings and depression (10).
Screening for mRNAs preferentially expressed in rodent striatum led to the discovery of a gene encoding for a GTP binding protein with similarity to Ras family members (11). Due to these features, i.e., enrichment in the striatum and homology with Ras proteins, the newly discovered protein was termed Ras homolog enriched in striatum (RHES). The striatal high expression levels suggest that RHES is likely to play an important role in striatal physiology and pathology, and in particular in HD. Indeed, RHES has been shown to physiologically bind mHtt to a larger extent that wild-type Htt, and greatly increase mHtt sumoylation in a time- and concentration-dependent manner, while decreasing ubiquitination. This results in a decrease of mHtt degradation and increase in the levels of the cytotoxic, soluble mHtt form (12). While RHES can sumoylate other substrates, like Ran guanosine triphosphatase (GTPase)-activating protein, mHtt sumoylation is highly specific with respect to both wild-type Htt (that RHES does not sumoylate) and ataxin, another protein containing a poly-Q stretch (that RHES does not bind) (12). RHES plays several roles in the sumoylation process: It acts as a SUMO-E3 ligase, significantly enhancing the ability of the ubiquitin carrier protein 9 (Ubc9), the only known SUMO-E2 ligase, to sumoylate mHtt (preferentially on lysine residues at positions 6, 9, 15 and 91). Additionally, it enhances cross-sumoylation between SUMO-activating enzyme subunits 1 (SAE1) and 2 (SAE2), the only known SUMO-E1 ligase, and Ubc9, in a bidirectional fashion (13). As expected on the basis of these RHES activities, RHES deletion by RNA interference was neuro-protective in HD cellular models (5,14); additionally, motor symptoms were either delayed or reduced in two different RHES knock-out HD mouse models (15,16). Intriguingly, a conflicting result was also reported according to which RHES silencing by inhibitory RNA did not improve motor function in HD mouse models and even increased anxiety and striatal atrophy (17). A possible explanation for this result lies in the fact that RHES has other activities, in addition to SUMO-E3 ligase, which are protective towards HD symptoms. In fact, in addition to the SUMO-E3 ligase activity, RHES exerts a dual role on autophagy by acting on two different pathways. On the one hand, it reduces autophagy by activating the mammalian target of rapamycin (mTOR) autophagy inhibitor (18,19). On the other, it enhances autophagy independent of mTOR, by directly interacting with autophagy activator Beclin-1 and freeing it from its interaction with B-cell lymphoma 2 protein (20). Autophagy is a lysosomal degradation process implicated in both aging and neurodegeneration, which has been demonstrated to have a protective role in both cellular and animal HD models. The result of RHES dual activity is autophagy activation (20), which explains both the delayed onset of symptoms in HD and the reported worsening of HD symptoms upon RHES knocking-out in mouse models (17).
The SUMO-E3 ligase activity on mHtt, bidirectional regulation of autophagy induction and specific expression in the corpus striatum, make RHES an intriguing regulator of cell survival and metabolism through different pathways, as well as an attractive target for HD treatment. In spite of these intriguing features, essential information about RHES structure and function are still missing. As an example, determination of 3D structure or identification of interactors in human cells have not been performed so far.
With the aim of shedding light on RHES structural features, assessing the potential role of RHES as a target for HD therapy and setting the basis for the development of new treatments against HD, a 3D model of the RHES region homologous to the Ras domain was built by homology modelling and analysed. The model was accurately compared with the experimentally determined 3D structures of representative members of the Ras family to identify conserved and variable regions, which correspond to those of higher and lower reliability in the model, respectively. Since structures of RHES homologs in complex with molecular partners required to carry out the SUMO-E3 ligase activity are not available, models of RHES in complex with either the Htt target or the SUMO-E2 ligase Ubc9 were built by molecular docking. Analysis of the interfaces involved in these complexes allowed identification of RHES regions that can be targeted by either peptides mapping on the RHES surface or small molecules identified by in silico screenings of virtual compound libraries.
The identification of compounds able to interfere with RHES interactions required for mHtt sumoylation and able to revert the HD phenotype in available cellular and animal HD models (7,21-23), would represent an important step towards the development of novel therapeutic strategies against HD.
Materials and methods
The amino acid sequences and 3D structures (Table I) of proteins analysed in this study were downloaded from the UniProt (http://www.uniprot.org/) (24) and Protein Data Bank (PDB; http://www.rcsb.org/pdb/home/home.do) (25) databases, respectively. The UniProt and PDB Identifiers (IDs) of the proteins analysed in the present study are listed in Table I.
Table I.
Sequence and 3D structure identifiers of the proteins analysed in this work. RHES UniProt ID is: RHES_HUMAN (Q96D21).
| Protein name | UniProt ID | PDB ID | Chain | GTP/GDP ligand | Resolution (Å) |
|---|---|---|---|---|---|
| DiRas1 | DIRA1_HUMAN (O95057) | 2GF0 | B | GDP | 1.9 |
| DiRas2 | DIRA2_HUMAN (Q96HU8) | 2ERX | A | GDP | 1.65 |
| K-Ras | RASK_HUMAN (P01116) | 5F2E | A | GDP | 1.4 |
| H-Ras | RASH_HUMAN (P01112) | 2CE2 | A | GDP | 1.0 |
| H-Ras | RASH_HUMAN (P01112) | 2CL7 | A | GTP | 1.25 |
| Rap1A | RAP1A_HUMAN (P62834) | 1C1Y | A | GTP | 1.9 |
| Htt | HD_HUMAN (P42858) | 6EZ8 | A | - | 4.0 |
| Ubc9 | UBC9_HUMAN (P63279) | 5F6E | A | - | 1.12 |
Htt, huntingtin; RHES, Ras Homolog Enriched in Striatum; Ubc9, ubiquitin carrier protein 9; DiRas1 and DiRas2, distinct subgroup of the Ras family member 1 and 2; GTP, guanosine triphosphate; GDP, guanosine diphosphatase.
The BLASTp algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (26) was used to search the NCBI databases for homologous protein sequences. Multiple Sequence Alignments (MSAs) of homologous protein sequences were obtained using ClustalO (http://www.clustal.org) (27).
Visual inspections and structure analyses were performed using several programs: SwissPDBViewer (http://spdbv.vital-it.ch/) (28), PyMol (Schrödinger LLC; http://www.pymol.org/), UCSF Chimera package (29) and InsightII (Accelrys Inc.).
Pairwise structural comparisons were performed using PDBeFOLD (30), as well as tools provided by the aforementioned programs. The initial superimpositions provided by these automated methods were manually refined by including the highest possible number of residues whose Cα-Cα distance was ≤3.0 Å and discarding those whose Cα-Cα distance was >3.0 Å, to produce optimal Structure-Based MSAs (SB-MSAs).
Complete lists of Ras family members and proteins homologous to the Ubc9 E2-ligase whose 3D structure has been experimentally determined, were obtained from the Pfam (http://pfam.xfam.org) (31) and Superfamily 2 (32) databases.
Homology modelling of RHES in the GTP- or GDP-bound conformation was performed using the following programs: I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) (33), HHPred (https://toolkit.tuebingen.mpg.de/hhpred) (34), Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) (35) and Swiss-Model (https://swissmodel.expasy.org/) (28).
Secondary structure predictions were provided by the JPRED (36) and PSIPRED (37) servers. Short Linear Motifs were annotated by the ELM resource (38). Signal peptide predictions were obtained from several servers: SignalP 4.1 (39), Signal-CF (40), Signal-BLAST (41) and Signal-3L 2.0 (42).
Overall, polar and non-polar solvent accessible surface areas were calculated using the program Naccess (http://wolf.bms.umist.ac.uk/naccess/).
Docking simulations of the interaction between Htt (Table I) and RHES, and between RHES and Ubc9 (Table I) were performed using the protein-protein docking program ZDOCK (http://zdock.umassmed.edu/; version 3.0.2) (43). ZDOCK implements a Fast Fourier Transform algorithm and a scoring system based on a combination of shape complementarity, electrostatics and statistical potential terms. The 2000 complexes generated by ZDOCK were re-ranked using ZRANK (44), which uses a more detailed potential including electrostatics, van der Waals and desolvation terms. In both cases, the best ranking complexes obtained by ZRANK are described and discussed in the following sections. RHES residues were defined to be at the interface if they have at least one atom within 4.0 Å from any atom of the Htt or Ubc9 interaction partner in the respective complexes.
Sumoylation sites on Htt sequence were predicted by Jassa (http://www.jassa.fr/), a tool that uses a scoring system based on a Position Frequency Matrix derived from the alignment of experimental sumoylation sites or SUMO-interacting motifs (SIMs) (45).
All software and databases used in this study are publicly available. For all software, default parameters and thresholds were used. Databases were last queried on January 15th, 2019.
Images were generated using either PyMol (http://www.pymol.org/) or the UCSF Chimera package (29).
Results
RHES homology models building and expected accuracy
The results of a BLASTp search in the NCBI database of sequences of known 3D structures (pdb) (46) using RHES sequence as input, indicate that the two closest homologues of known structure are the distinct subgroup of the Ras family member 1 and 2 (DiRas1 and DiRas2) human proteins (Table I), both of which were determined in the inactive GDP bound state. These proteins match RHES regions comprised between residues 18-191 and 13-189 with E-values of 5x10-36 and 5x10-42, respectively. The closest RHES homologue determined in the GTP-bound conformation is Ras-related protein 1A (Rap1A; Table I), which matches RHES region 20-186 with an E-value of 8x10-32.
Conversely, neither the N-terminal (residues 1-12) nor the C-terminal (residues 192-253) region matches any protein of known structure with significant E-values (i.e., below 10-2), either when the whole RHES sequence or the sequence of these regions alone is given as input to BLASTp.
Several automated methods were exploited to build 3D models of RHES in the active or 'on' GTP-bound (RHES-GTP) and inactive or 'off' GDP-bound (RHES-GDP) conformations (see Materials and methods). These programs were chosen because of their ability to consistently provide accurate protein structure predictions in blind tests, i.e., in the absence of information about the experimental structure, in several rounds of the Critical Assessment of Structure Prediction experiment (http://predictioncenter.org/) (47).
Automated structure comparisons indicated that in the Ras domain region, the best RHES models provided by the different structure prediction methods for each conformation were highly similar to one another and to the main templates used in the modelling procedure. Based on their highest structural similarity with the main templates, the best models provided by Swiss-Model were selected for further studies.
To obtain models of complexes with GTP and GDP ligands, RHES-GTP and RHES-GDP models were optimally superimposed to the experimentally determined structures of the closest homologues determined in each conformation, i.e., Rap1A and DiRas2, respectively. Once optimal model-to-structure superimpositions had been obtained (Fig. 1), GTP and GDP ligands were docked to the RHES-GTP and RHES-GDP models, respectively, by importing their coordinates from the structure of Rap1A-GTP and DiRas2-GDP complexes and performing small manual adjustments to relieve unfavourable van der Waals contacts.
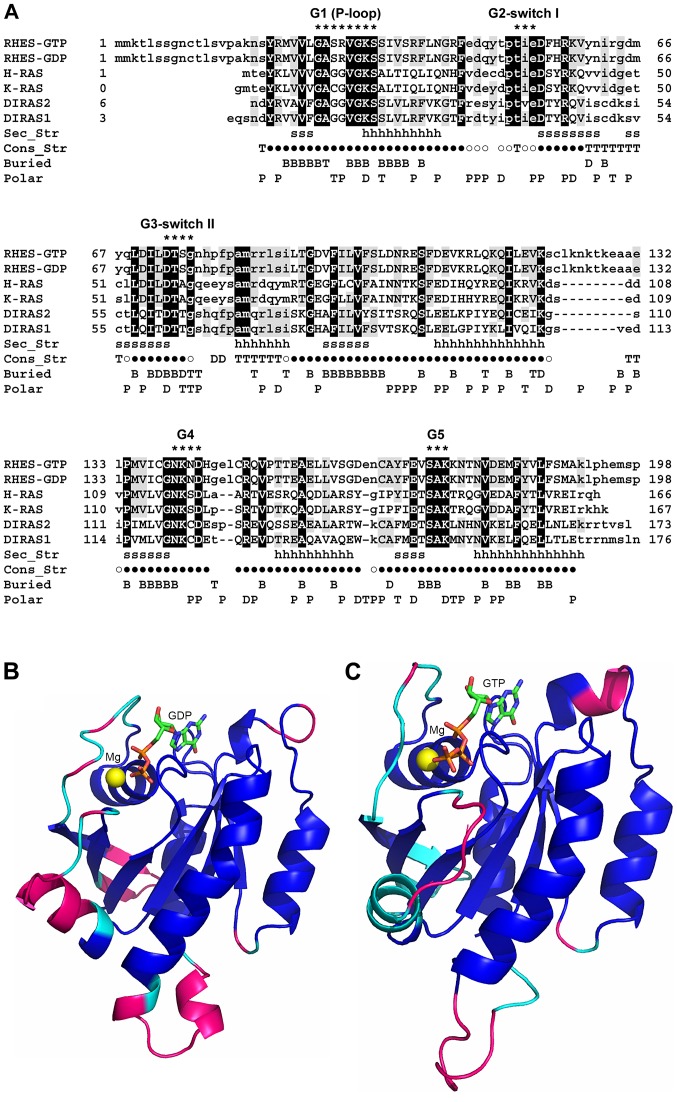
Figure 1.
Sequence and structure comparison between RHES and homologous proteins belonging to the Ras family. (A) SB-MSA of RHES 3D models with proteins of known structure bound to either GTP or GDP. Both RHES-GTP and RHES-GDP models were included in the SB-MSA. RHES sequence is truncated at residue 198, since the remaining C-terminal region (199-253) does not have homologs of known structure. Other sequences comprise only residues that are visible in the experimentally determined 3D structures (Table I). Upper- and lower-case letters indicate residues that are and are not structurally aligned, respectively. Black and grey background shows RHES residues whose identity is conserved in all or at least one template, respectively. Sec_Str: Secondary structure elements, i.e., β strands and α-helices, which are present in both GTP and GDP-binding H-Ras structures, are marked with 's' and 'h' letters, respectively. Con_Str: Residues that are structurally conserved between all the experimentally determined structures are indicated by '●' symbols; 'T', 'D' and '○' symbols indicate residues that are structurally conserved between GTP-bound H-Ras and Rap1A, GDP-bound H-Ras and DiRas2, and both the aforementioned pairs of structures, respectively. Buried: Residues whose SASA is ≤20 Å in RHES-GTP model, RHES-GDP model and both models are indicated with 'T', 'D' and 'B', respectively. Polar: Residues whose SASA in RHES-GTP model, RHES-GDP model and both models are predominantly polar, are indicated with 'P', 'T' and 'D', respectively. Residues belonging to conserved RAS family motifs G1-G5 are indicated by '*'. The consensus sequences of these motifs are: G1 (P-loop) = GXXXXGK(S/T); G2 switch I = XTX; G3 switch II = DXXG; G4 = (N/T)(K/Q)XD; G5 = (T/G/C)(C/S)A. (B) Molecular model of RHES-GDP. The model is represented as a ribbon and colour coded as follows. Blue: Residues that are structurally conserved among GTP- and GDP-bound structures, indicated by a '●' symbol in panel (A). Cyan: Additional residues that are structurally conserved between GDP-bound structures, indicated by '○' and 'D' symbols in panel (A). Magenta: Residues that are not structurally conserved in either of the aforementioned groups of structures. GDP is shown as sticks and coloured by atom type (C, green; N, blue; O, red; P, orange). The magnesium ion is shown as a sphere and coloured yellow. (C) Molecular model of RHES-GTP. The model is represented as a ribbon and colour coded as follows. Blue: Same as in panel (B). Cyan: Additional residues that are structurally conserved between GTP-bound structures, indicated by '○' and 'T' symbols in panel (A). Magenta: Residues that are not structurally conserved in either of the aforementioned groups of structures. GTP is shown as sticks and coloured by atom type (C, green; N, blue; O, red; P, orange). The magnesium ion is shown as a sphere and coloured yellow. RHES, Ras Homolog Enriched in Striatum; SB-MSA, Structure-Based-Multiple Sequence Alignments; GTP, guanosine triphosphate; GDP, guanosine diphosphatase.
To provide an estimate of the accuracy of RHES models, hand-curated SB-MSAs were produced comprising: i) GTP-bound RHES, Rap1A and H-Ras; ii) GDP-bound RHES, DiRas2 and H-Ras; iii) both GTP-bound and GDP-bound RHES models and experimental structures (Fig. 1).
The structurally conserved regions (SCRs) among the experimentally determined structures of GTP- and GDP-bound Ras family members comprise 122 residues (Fig. 1A). In agreement with previous assignment of Ras family members to different subfamilies (48), the values of percentage sequence identity (%_ID) and root mean square deviation (RMSD) value in the SCRs reported in Table II indicate that GTP- and GDP-bound H-Ras and GDP-bound K-Ras (all of which were assigned to the Ras subfamily) have higher sequence identity (%_ID=93%) and structure similarity (RMSD=0.62-0.80 Å) with one another than each of them with either DiRas1 or DiRas2 (both of which were assigned to the Ras-extended subfamily). Similarly, in the SCRs, DiRas1 and DiRas2 have higher sequence identity (83%) and structure similarity (RMSD: 0.67 Å) with each other than each of them with either GTP- or GDP-bound H-Ras, or K-Ras. Comparison of structures from different subfamilies, namely: GTP-bound or GDP-bound H-Ras, or K-Ras vs. DiRas1 or DiRas2 yields %_ID in the range 40-43% and RMSD values in the range 1.06-1.17 Å. Based on the established relationship between the percentage of sequence identity and structure similarity in the core regions of homologous proteins (49), it is possible to infer the accuracy that RHES models are expected to have in the SCRs. The %_ID between RHES and experimentally determined GTP-bound or GDP-bound structures is 42% (with H-Ras-GTP) and 48% (with DiRas2), respectively, which is comparable to or better than the %_IDs calculated between members of the different (i.e., Ras and Ras-extended) subfamilies. As a consequence, the main-chain co-ordinates of both RHES-GTP and RHES-GDP models in the SCRs are expected to have RMSD values with the real structures ≤1.2 Å, which is the highest value calculated after optimal pair-wise superimpositions between members of different subfamilies.
Table II.
Sequence and structure similarity of Ras family members in the SCRs indicated in Fig. 1A. Each Ras family member is indicated by the name of the protein (see Table I); in the case of H-Ras, the bound ligand is also shown in parenthesis. The lower and upper part of the matrix contain the percentage of sequence identity and the RMSD values (Å) calculated after optimal pair-wise structure superimposition of the main-chain atoms in the SCRs, respectively.
| H-RAS (GTP) | H-RAS (GDP) | K-RAS | DIRAS1 | DIRAS2 | |
|---|---|---|---|---|---|
| H-RAS (GTP) | - | 0.80 | 0.78 | 1.12 | 1.17 |
| H-RAS (GDP) | 100 | - | 0.62 | 1.08 | 1.06 |
| K-RAS | 93 | 93 | - | 1.13 | 1.11 |
| DIRAS1 | 40 | 40 | 42 | - | 0.67 |
| DIRAS2 | 42 | 42 | 43 | 83 | - |
SCRs, structurally conserved regions; DiRas1 and DiRas2, distinct subgroup of the Ras family member 1 and 2; GTP, guanosine triphosphate; GDP, guanosine diphosphatase.
The SCRs described above are conserved in both GTP- and GDP-bound RHES conformations. However, the regions where RHES-GTP and RHES-GDP models are reliable are more extended than the SCRs (Fig. 1). i) The optimal structure alignment between the GDP-bound structures of H-Ras and DiRas2 comprises 138 residues (SCRs-GDP) whose %_ID and RMSD value of the main chain atoms are 53% and 1.06 Å, respectively. The %_ID between RHES and the two structures in the SCRs-GDP is 40 and 45%, respectively. Optimal structure superimposition between the GTP-bound structures of H-Ras and Rap1A comprise 154 residues whose %_ID and RMSD value of the main chain atoms after optimal structure superimposition are 57% and 0.96 Å, respectively. The %_ID between RHES and each of the two structures in the SCRs-GTP is 40%. Based on sequence-structure relationships previously defined for pairs of homologous proteins with different folds (49) and on those reported above for members of the Ras family, RHES-GDP and RHES-GTP models are expected to have RMSD values not higher than 1.2 Å in the SCR-GDP and SCR-GTP, respectively.
Analysis of RHES sequence-structure-function relationships
Based on sequence and structure analyses described in the previous section, RHES comprises three distinct regions, namely an N-terminal sequence, RAS domain and C-terminal region, which are predicted to encompass residues 1-18, 19-190 and 191-266, respectively.
The Ras domain is homologous to members of the globular Ras-like small G protein family and has highest similarity to the DiRas members of the Ras-extended subfamily members (48). The Ras domain comprises the signatures of the conserved GTP-binding motifs G1-G5, all of which are fully conserved in RHES (Fig. 1). The G1 motif or 'P-loop' (residues 26-33) interacts with the oxygen atoms of β and γ phosphate groups and is crucial for nucleotide binding. The G2 motif (residues 50-52) comprises a threonine residue (T35 in H-Ras), which is responsible for GTP γ phosphate sensing and is part of the 'Switch I' region (residues 48-52) that undergoes a conformational change after GTP hydrolysis. The G3 motif (residues 73-76) partially overlaps with the 'Switch II' site (residues 75-83 in H-Ras) and comprises a conserved glycine residue (G76), which is involved in the interaction with effector molecules and plays a role in guanine nucleotide exchange factors-catalysed nucleotide exchange and GTPase activating proteins-mediated GTP hydrolysis. The G4 and G5 motifs encompass RHES residues 140-143 and 172-174, respectively. Based on the conservation of G1-G5 motifs, as well as of the SCRs-GTP and SCRs-GDP (see above), the mode of GTP- and GDP-binding by RHES is expected to be conserved with respect to homologous GTP- and GDP-bound structures. As a consequence, these structures have been used as templates to dock the co-ordinates of GTP and GDP to RHES-GTP and RHES-GDP models, respectively. According to the ELM resource, the Ras domain comprises a LIG_SUMO_SIM_par_1 motif for non-covalent SUMO binding in the 115-121 region (Table SI).
The N-terminal sequence is predicted by several methods not to be a signal peptide, nor to assume a regular secondary structure. Interestingly, the ELM resource identifies in the 16-20 region the DOC_USP7_MATH_1 binding motif for USP7, a deubiquitinating enzyme that cleaves ubiquitin moieties from its substrates (Table SI).
The C-terminal tail (residues 193-266) has been termed the 'cationic region' due to its enrichment in positively charged residues (50). A C-terminal tail of the same length or longer is present in RHES proteins from other species, whereas it is not conserved in other Ras homologs. The C-terminal CAAX motif comprises one cysteine residue that undergoes farnesylation, a post-translational modification that allows G-proteins to be localized at the level of plasma and intracellular membranes. It is predicted to contain one α-helix in the 240-250 region. A slightly longer C-terminal region of RHES has been shown to be both required and sufficient for Beclin-1 binding (20), and is therefore responsible for RHES autophagy activating activity.
Modelling of RHES complexes with Htt and Ubc9 by molecular docking
To identify RHES regions required for mHtt sumoylation, molecular models of RHES complexes were built with the Htt target and with the E2-ligase Ubc9.
Extensive searches in sequence and structure databases could not detect any 3D structure of complexes between proteins homologous to RHES and to either Htt or Ubc9, to be used as templates for modelling RHES-Htt or RHES-Ubc9 interaction by homology, respectively. Therefore, a model of each of these complexes was built by molecular docking, using: i) The 3D model of RHES-GTP, which is in the GTP-bound, active ('on') conformation; and ii) the 3D structure of Htt or Ubc9 (Table I), which have been experimentally determined by cryoelectron microscopy and X-ray crystallography, respectively.
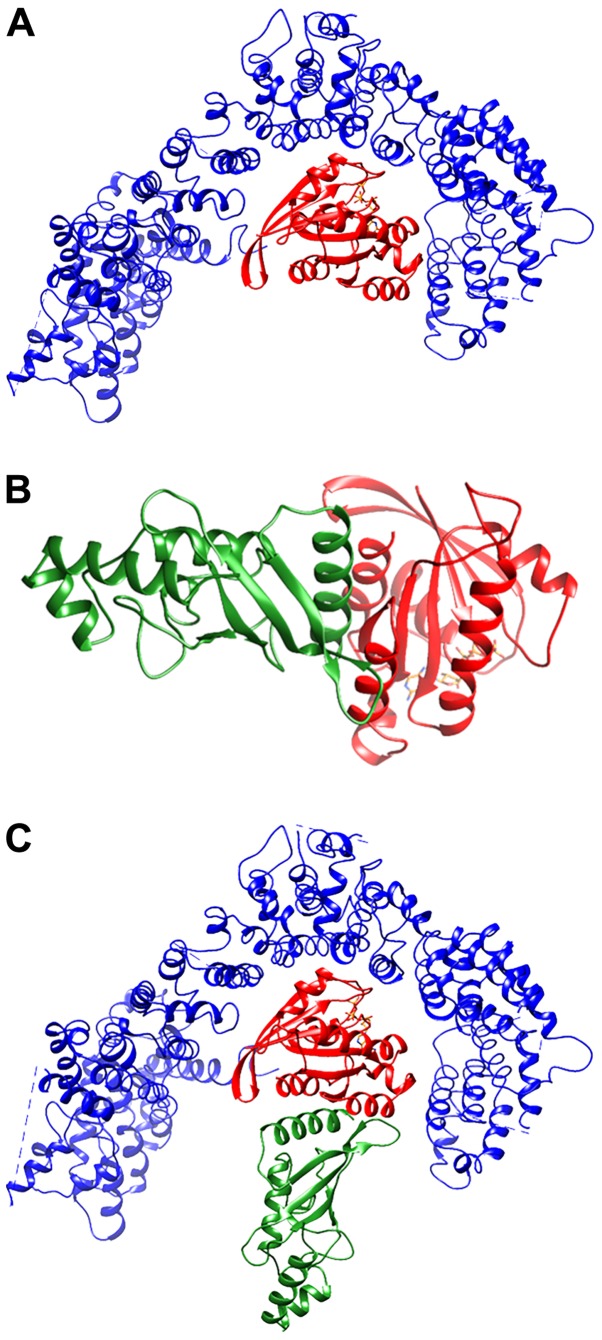
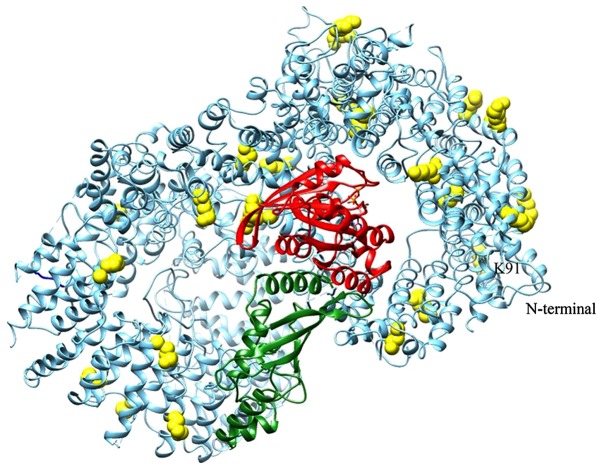
The putative Htt domain responsible for the interaction with GTP-binding proteins has been previously identified (51). For this reason, molecular docking simulations have been focused only on this domain, corresponding to Htt residues 1223-2324. Accordingly, in the best-ranked complex (Fig. 2A; Table III) RHES interacts with Htt in the same region in which other GTP-binding proteins have been predicted to bind (51). Fig. 2B shows the best-ranked RHES-Ubc9 complex. The superimposition between Htt-RHES and RHES-Ubc9 predicted complexes (Fig. 2C) is compatible with formation of a ternary complex. In Fig. 3 the ternary complex is shown in the context of the whole Htt protein, together with the predicted sumoylation sites. Several sumoylation sites of Htt are located in regions spatially close to Ubc9 and/or at the interface with RHES. However, since the only available experimental structure of Htt has been determined in complex with Htt-associated protein 40, the Htt conformation present in the complex with RHES is representative of this particular bimolecular complex. It is well known that Htt assumes different conformations depending on its intra- and inter-molecular interactions (52); therefore, other Htt sumoylation sites, including the ones located in the 1-90 N-terminal region, which is not present in the experimentally determined structure, may be located within reach of Ubc9 in the ternary Htt-RHES-Ubc9 complex.
Figure 2.
Models of RHES complexes with Htt and Ubc9. (A) Best-ranked ZDOCK complex between RHES and Htt. RHES and Htt are shown as a ribbon and coloured red and blue, respectively. RHES-bound GTP is orange. Only the Htt domain predicted to be involved in the interaction with GTP-binding proteins (51) is shown. (B) Best-ranked ZDOCK complex between RHES and Ubc9. RHES and Ubc9 are shown as a ribbon and coloured red and green. RHES-bound GTP is orange. (C) Htt-RHES-Ubc9 ternary complex obtained by best fitting of the RHES-Htt and RHES-Ubc9 binary complexes. RHES, Ubc9 and Htt are shown as in panels A and B. Htt, huntingtin; RHES, Ras Homolog Enriched in Striatum; Ubc9, ubiquitin carrier protein 9.
Table III.
RHES residues located at the interface with Htt and Ubc9 in the respective complexes obtained by docking simulations.
| RHES-Htt | RHES-Ubc9 |
|---|---|
| SER28 | ARG63 |
| ARG29 | THR153 |
| GLN47 | GLU157 |
| TYR48 | LEU158 |
| THR49 | VAL160 |
| PRO50 | SER161 |
| THR51 | GLY162 |
| ILE52 | ASP163 |
| GLU53 | GLU164 |
| ASP54 C | YS166 |
| PHE55 | ALA167 |
| ARG57 | TYR168 |
| LYS58 | PHE 169 |
| VAL59 | GLU181 |
| GLN68 | VAL185 |
| SER75 | SER188 |
| GLY76 | MET189 |
| ASN77 | |
| HIS78 | |
| PRO79 | |
| PRO81 | |
| MET83 | |
| LEU86 | |
| GLU104 |
Htt, huntingtin; RHES, Ras Homolog Enriched in Striatum; Ubc9, ubiquitin carrier protein 9.
Figure 3.
Htt-RHES-Ubc9 ternary complex in the context of the whole Htt structure. RHES, Ubc9 and Htt are shown as a ribbon and coloured red, green and sky blue, respectively. Predicted Htt sumoylation sites are shown as spheres and coloured yellow. Htt, huntingtin; RHES, Ras Homolog Enriched in Striatum; Ubc9, ubiquitin carrier protein 9.
Design of peptides interfering with RHES-Htt or RHES-Ubc9 interaction
Based on the results of the aforementioned analyses, peptides aimed at interfering with RHES-mHtt or RHES-Ubc9 interactions were designed.
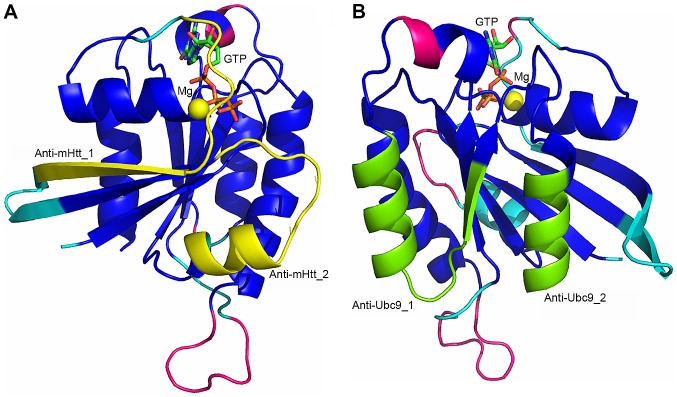
Two RHES regions, encompassing residues 47-59 and 75-86 (Fig. 4A), and partially overlapping with the G2-switch I and G3-switch II region, respectively, comprise 50 and 33% of the residues involved in the interaction with Htt predicted by docking simulations, respectively. Peptides comprising these residues are, therefore, expected to effectively interfere with RHES-mHtt interaction. Both peptide 47-59 (Anti-mHtt_1) and 75_86 (Anti-mHtt_2) have suitable properties to be used as such as RHES-mHtt interaction inhibitors: Short length (13 and 12 residues, respectively); high polar/hydrophobic residues ratio (6 charged, 5 polar and 5 hydrophobic residues in Anti-mHtt_1; 2 charged, 4 polar and 5 hydrophobic residues in Anti-mHtt_2); absence of branched hydrophobic-aliphatic residues next to one another; presence of proline residues, which confer rigidity to the peptide and, therefore, determine a reduction in entropy loss upon mHtt binding (see Fig. 1A). If required, Anti-mHtt peptides can be modified to improve their solubility and/or binding properties, by replacing residues at positions not predicted to be involved in mHtt binding (i.e., H56, F80, A82, R84 and R85) and/or substituting peptide bonds with non-peptidic moieties. In case Anti-mHtt peptides prove to be able to inhibit RHES-mHtt interaction, they can be alanine-scanned to identify residues essential for the interaction to be used as a guide for the design of non-peptidic molecules. In parallel to the use of peptidic compounds, the Anti-mHtt_1 and Anti-mHtt_2 regions can be used as a target for virtual screening of small molecule libraries.
Figure 4.
Designed Anti-mHtt and Anti-Ubc9 peptides mapped on the 3D model of RHES-GTP. The RHES-GTP model is represented by its solvent accessible surface area. (A) Peptides Anti-mHtt_1 and Anti-mHtt_2 are coloured yellow. (B) Peptides Anti-Ubc9_1 and Anti-Ubc9_2 are coloured green. In both panels, the rest of the RHES model is coloured as in Fig. 1B. Htt, huntingtin; RHES, Ras Homolog Enriched in Striatum; Ubc9, ubiquitin carrier protein 9; GTP, guanosine triphosphate.
As in the case of the RHES-Htt complex, two RHES regions comprise most of the residues involved in RHES-Ubc9 interaction (Fig. 4B); however, in the latter case one region is larger than the other. The region encompassing residues 153-169 (Anti-Ubc9_1), situated between the G4 and G5 motifs, comprises 12 (i.e., 71%) of the 17 residues predicted to be in contact with Ubc9. The region encompassing residues 181-189 (Anti-Ubc9_2), situated after the G5 motif, in the C-terminal region of the Ras domain, comprises just four (i.e., 24%) residues predicted to be in contact with Ubc9. Peptides comprising these regions and possibly just the Anti-Ubc9_1 region, are likely to effectively interfere with the RHES-Ubc9 interaction. The properties of peptides 153-169 (Anti-Ubc9_1; 17 residues) and 181-189 (Anti-Ubc9_2; 9 residues) are also favourable enough for them to be used as such as RHES-Ubc9 interaction inhibitors. However, in the case of these peptides, analysis of their properties allowed identification of modifications likely to be required to improve their solubility properties. Anti-Ubc9_1 contains three consecutive hydrophobic-aliphatic branched amino acids (L158, L159 and V160) and two consecutive aromatic residues (Y168 and F169), which may hamper solubility. However, L159 is not predicted to interact with Ubc9, therefore it can be replaced by a polar residue. To replace other hydrophobic residues and/or shorten the peptide, which is slightly longer than most peptides used for biomedical purposes, alanine-scanning mutagenesis must be performed to reveal which residues are actually essential for peptide activity. The Anti-Ubc9_2 peptide comprises six hydrophobic residues next to one another. Of these, only V185 is predicted to be involved in interactions with Ubc9, while the other five can be replaced with polar residues to increase peptide solubility. Anti-Ubc9_1 and Anti- Ubc9_2 regions can also be used as targets for virtual screening of small molecule libraries to identify non-peptide compounds aimed at inhibiting RHES-Ubc9 interaction.
Discussion
HD is known to be caused by the occurrence of a long poly-Q repeat in mHtt, but the molecular mechanisms underlying mHtt toxicity are not yet clear. While mHtt is expressed in all body tissues, HD neuropathology is specifically related to the brain and in particular to the corpus striatum. The only protein reported to be overexpressed in this brain region so far is RHES, a small GTP-binding protein containing an N-terminal domain homologous to the Ras family member and a C-terminal tail that is not present in other Ras proteins. RHES has a SUMO-E3 ligase activity and sumoylates mHtt, as well as other proteins, but not wild type Htt or the poly-Q containing protein ataxin. Sumoylated mHtt is then able to escape formation of insoluble aggregates and exert its cytotoxic and neurotoxic activity. Accordingly, several studies have demonstrated a protective role of RHES deletion towards HD symptoms in both cellular and animal models (5,14-16), supporting the notion that RHES is a very attractive therapeutic target against HD. In only one report knocking out RHES did not improve HD symptoms (17), likely because of the ability of the C-terminal tail of RHES to activate autophagy, which is protective towards HD (20).
Based on the above, a strategy to selectively inhibit RHES functions that enhance HD phenotypes while preserving the protective activity was envisaged. This goal can be achieved by interfering with the molecular interactions involved in mHtt sumoylation by RHES without hampering Beclin-1 binding.
Sumoylation is a multi-step process, analogous to ubiqui-tination, which leads to the formation of a covalent isopeptide bond between the carboxyl terminus of SUMO proteins and the ε-amino group of a lysine residue of target proteins, which is part of a ΨKxD/E motif (Ψ = large hydrophobic residue) (53). Briefly: i) SUMO proteins (SUMO-1, SUMO-2 and SUMO-3), which are synthesized as inactive precursors, are cleaved by SUMO-specific carboxy-terminal hydrolases that remove four C-terminal residues. Mature SUMO proteins, presenting a novel double-glycine C-terminus, are ii) linked via a thioester bond to the catalytic cysteine (C173) of the SAE1/SAE2 heterodimeric activating enzyme (E1), iii) transferred to the catalytic cysteine (C93) of the Ubc9 conjugating enzyme (E2) and iv) eventually conjugated to the target protein, in a reaction that can be aided by a ligase (E3). While Ubc9, the only known E2-ligase for sumoylation, can act as an E3-ligase itself, RHES facilitates or catalyses its sumoylation activity. Indeed, the role of E3-ligases is to interact with both the E2 enzyme and the target protein, thereby conferring substrate specificity to the E2-ligase. Additionally, RHES has been shown to physiologically regulate sumoylation by directly interacting with both E1 and Ubc9 and enhancing SUMO transfer to Lys residues in both directions (cross-sumoylation), i.e., from C173 of E1 to a lysine residue of Ubc9 and from C93 of Ubc9 to a lysine residue of E1 (13).
In principle, mHtt sumoylation by RHES may be hampered by interfering with each of the different steps of the sumoylation process, namely SUMO i) maturation, ii) activation, iii) conjugation, or iv) ligation. However, interference with some of these processes might have unwanted effects, which go beyond mHtt sumoylation. As an example, SAE1/SAE2 and Ubc9 are the only known SUMO-E1 and SUMO-E2 ligases, respectively; therefore, inhibition of this interaction would affect sumoylation of multiple targets, as would the inhibition of the cross-sumoylation between E1 and E2 catalysed by RHES.
The most specific strategy to interfere with mHtt sumoylation consists of the inhibition of the interaction between RHES and mHtt. To this end, RHES models in the GTP- and GDP-bound conformation were built by homology modelling, and a model of the complex between GTP-bound RHES and Htt was built by docking simulations. Analysis of these models allowed identification of RHES regions putatively involved in this interaction, as well as Anti-mHtt peptides expected to interfere with it.
Additionally, a model of the complex between RHES and Ubc9 was built by molecular docking, and a model of the ternary complex between RHES, Htt and Ubc9 by superposition of the RHES-Htt and RHES-Ubc9 complexes. Although there are no direct interactions between Htt and Ubc9 in the ternary complex, the two molecules are sufficiently close to each other for the interaction to occur if Htt underwent a conformational change with respect to that observed in the experimentally determined structure, which is only one of the many conformations that this protein is known to assume. Thus, the modelled ternary complex is compatible with the simultaneous formation of the two binary RHES complexes modelled by docking and provides indirect support for their validity. The RHES-Ubc9 model was also analysed to highlight RHES regions predicted to be involved in Ubc9 binding and design Anti-Ubc9 peptides aimed at interfering with it. In principle, inhibition of this E2-E3 interaction is less attractive than RHES-mHtt inhibition, because it could affect sumoylation of other potential RHES targets, as well as mHtt. However, Anti-Ubc9 peptides might act in synergy with the aforementioned Anti-mHtt peptides and be used jointly with them or as an alternative, in case Anti-mHtt peptides do not possess suitable activity or specificity.
Like the ubiquitination pathway (54), sumoylation can be antagonized by interfering with several processes. It has been shown that RHES sumoylation of mHtt can be hampered by interfering with RHES farnesylation, but not with its GTPase activity. In fact, mutation of the conserved cysteine 263 has been shown to abolish mHtt sumoylation, mHtt disaggregation and cytotoxicity (12). These effects are likely mediated by the lack of farnesylation, which is required for RHES to be localized at the membrane level, in parallel with other small G proteins whose activity is dependent on cell membrane attachment mediated by fatty acid addition to conserved cysteines in CXXX motifs. Conversely, mutation of serine 33, which is involved in the GTPase activity, does not prevent mHtt sumoylation, disaggregation and cytotoxicity, demonstrating that sumoylation and cytotoxicity are not associated with the GTPase activity (12).
Other RHES regions that may be targeted for HD therapy include the non-covalent SUMO binding motif identified within the Ras domain and the binding motif for the deubiquitinating enzyme identified within the N-terminal region. However, inhibition of the interaction with the mHtt target and/or the Ubc9 SUMO E2-ligase is the most direct way to interfere with mHtt sumoylation.
With the aim of assessing their therapeutic potential, the designed Anti-mHtt and Anti-Ubc9 peptides will be tested for their ability to: i) Interfere with RHES-mHtt and RHES-Ubc9 interaction, respectively, in vitro; ii) selectively inhibit mHtt sumoylation, while not preventing autophagy; and iii) revert the HD phenotype in cellular and/or animal models without causing undesired side-effects. In parallel, RHES regions encompassing the Anti-mHtt and Anti-Ubc9 peptides will be exploited to perform virtual screenings of available small molecule libraries to identify non-peptide compounds aimed at inhibiting mHtt sumoylation.
In principle, molecules able to selectively inhibit RHES SUMO-E3 ligase activity without interfering with the autophagy promoting and HD protective activity mediated by RHES C-terminal region, might effectively reduce the amount of soluble mHtt responsible for cytotoxicity and allow the development of therapeutic agents against the currently untreatable HD.
Supplementary Data
Acknowledgements
Not applicable.
Abbreviations
- DiRas1 and DiRas2
Distinct subgroup of the Ras family member 1 and 2
- HAP40
Htt-associated protein 40
- HD
Huntington's disease
- Htt
huntingtin
- mHtt
mutant Htt
- mTOR
mammalian target of rapamycin
- Rap1A
Ras-related protein 1A
- RHES
Ras homologue enriched in the striatum
- SAE1 and SAE2
SUMO-activating enzyme subunits 1 and 2
- SCRs
structurally conserved regions
- SUMO
small ubiquitin-related modifier
- Ubc9
SUMO conjugating enzyme, also referred to as 'ubiquitin carrier protein 9' or 'ubiquitin conjugating enzyme E2I'
Funding
The present research project was funded by the Italian Ministry of Health as a Finalised Research Project (Ricerca Finalizzata; entitled 'RARE in rarity: Advanced in vivo and in vitro technologies to Study Juvenile Huntington's Disease neuronal connectivity and its relationship with clinical and genetic factors: The RAREST-JHD project'; grant no. RF-2016-02364123), by the National Collection of Chemical Compounds and Screening Center, National Research Council of Italy (grant no. DSB.AD011.005) and by the Italian Ministry of Education, University and Research (MIUR) as a Scientific Research Program of Relevant National Interest (PRIN 2017), entitled 'Protein Bioinformatics for Human Health' (grant no. 2017483NH8_005). The funding bodies had no role in the design of the study, collection, analysis and interpretation of data, or manuscript writing.
Availability of data and materials
The coordinates of RHES-GTP and RHES-GDP models, as well as those of RHES-Htt and RHES-Ubc9 complex models, are available from the authors upon reasonable request.
Authors' contributions
MC built the RHES model and contributed to analyses. VB performed the docking simulations. GP, DSS and GC performed database and literature searches. FP performed data analysis, and contributed in writing the manuscript. AI contributed to the research design and writing the manuscript. VM contributed to the research design, data analysis and writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Testa CM, Jankovic J. Huntington disease: A quarter century of progress since the gene discovery. J Neurol Sci. 2019;396:52–68. doi: 10.1016/j.jns.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Gusella JF, MacDonald ME. Huntington's disease: CAG genetics expands neurobiology. Curr Opin Neurobiol. 1995;5:656–662. doi: 10.1016/0959-4388(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 3.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/S0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 4.Gong B, Lim MC, Wanderer J, Wyttenbach A, Morton AJ. Time-lapse analysis of aggregate formation in an inducible PC12 cell model of Huntington's disease reveals time-dependent aggregate formation that transiently delays cell death. Brain Res Bull. 2008;75:146–157. doi: 10.1016/j.brainresbull.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Lu B, Palacino J. A novel human embryonic stem cell-derived Huntington's disease neuronal model exhibits mutant huntingtin (mHTT) aggregates and soluble mHTT-dependent neurodegeneration. FASEB J. 2013;27:1820–1829. doi: 10.1096/fj.12-219220. [DOI] [PubMed] [Google Scholar]
- 6.Harrison LM, Lahoste GJ. The role of Rhes, Ras homolog enriched in striatum, in neurodegenerative processes. Exp Cell Res. 2013;319:2310–2315. doi: 10.1016/j.yexcr.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Sipione S, Cattaneo E. Modeling Huntington's disease in cells, flies, and mice. Mol Neurobiol. 2001;23:21–52. doi: 10.1385/MN:23:1:21. [DOI] [PubMed] [Google Scholar]
- 8.Shahani N, Swarnkar S, Giovinazzo V, Morgenweck J, Bohn LM, Scharager-Tapia C, Pascal B, Martinez-Acedo P, Khare K, Subramaniam S. RasGRP1 promotes amphetamine-induced motor behavior through a Rhes interaction network ('Rhesactome') in the striatum. Sci Signal. 2016;9:ra111. doi: 10.1126/scisignal.aaf6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valjent E. Striatal signaling: Two decades of progress. Front Neuroanat. 2012;6:43. doi: 10.3389/fnana.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross CA, Tabrizi SJ. Huntington's disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 11.Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific Ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. doi: 10.1002/(SICI)1097-4547(19990915)57:6<782::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramaniam S, Mealer RG, Sixt KM, Barrow RK, Usiello A, Snyder SH. Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation between the basic sumoylation enzymes E1 and Ubc9. J Biol Chem. 2010;285:20428–20432. doi: 10.1074/jbc.C110.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seredenina T, Gokce O, Luthi-Carter R. Decreased striatal RGS2 expression is neuroprotective in Huntington's disease (HD) and exemplifies a compensatory aspect of HD-induced gene regulation. PLoS One. 2011;6:e22231. doi: 10.1371/journal.pone.0022231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baiamonte BA, Lee FA, Brewer ST, Spano D, LaHoste GJ. Attenuation of Rhes activity significantly delays the appearance of behavioral symptoms in a mouse model of Huntington's disease. PLoS One. 2013;8:e53606. doi: 10.1371/journal.pone.0053606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mealer RG, Subramaniam S, Snyder SH. Rhes deletion is neuroprotective in the 3-nitropropionic acid model of Huntington's disease. J Neurosci. 2013;33:4206–4210. doi: 10.1523/JNEUROSCI.3730-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Sowada MJ, Boudreau RL, Aerts AM, Thedens DR, Nopoulos P, Davidson BL. Rhes suppression enhances disease phenotypes in Huntington's disease mice. J Huntingtons Dis. 2014;3:65–71. doi: 10.3233/JHD-140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A. Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci. 2012;15:191–193. doi: 10.1038/nn.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mealer RG, Murray AJ, Shahani N, Subramaniam S, Snyder SH. Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J Biol Chem. 2014;289:3547–3554. doi: 10.1074/jbc.M113.536912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naseri NN, Xu H, Bonica J, Vonsattel JP, Cortes EP, Park LC, Arjomand J, Gibson GE. Abnormalities in the tricarboxylic acid cycle in Huntington disease and in a Huntington disease mouse model. J Neuropathol Exp Neurol. 2015;74:527–537. doi: 10.1097/NEN.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golas MM, Sander B. Use of human stem cells in Huntington disease modeling and translational research. Exp Neurol. 2016;278:76–90. doi: 10.1016/j.expneurol.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Stricker-Shaver J, Novati A, Yu-Taeger L, Nguyen HP. Genetic rodent models of Huntington disease. Adv Exp Med Biol. 2018;1049:29–57. doi: 10.1007/978-3-319-71779-1_2. [DOI] [PubMed] [Google Scholar]
- 24.UniProt Consortium Activities at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2014;42(Database Issue):D191–D198. doi: 10.1093/nar/gkt1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul S, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 30.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr Sect D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 31.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandurangan AP, Stahlhacke J, Oates ME, Smithers B, Gough J. The SUPERFAMILY 2.0 database: A significant proteome update and a new webserver. Nucleic Acids Res. 2019;47:D490–D494. doi: 10.1093/nar/gky1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy A, Kucukural A, Zhang Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(Web Server Issue):W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED protein analysis Workbench. Nucleic Acids Res. 2013;41(Web Server Issue):W349–W357. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, et al. ELM 2016-data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res. 2016;44:D294–D300. doi: 10.1093/nar/gkv1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 40.Chou KC, Shen HB. Signal-CF: A subsite-coupled and window-fusing approach for predicting signal peptides. Biochem Biophys Res Commun. 2007;357:633–640. doi: 10.1016/j.bbrc.2007.03.162. [DOI] [PubMed] [Google Scholar]
- 41.Frank K, Sippl MJ. High-performance signal peptide prediction based on sequence alignment techniques. Bioinformatics. 2008;24:2172–2176. doi: 10.1093/bioinformatics/btn422. [DOI] [PubMed] [Google Scholar]
- 42.Shen HB, Chou KC. Signal-3L: A 3-layer approach for predicting signal peptides. Biochem Biophys Res Commun. 2007;363:297–303. doi: 10.1016/j.bbrc.2007.08.140. [DOI] [PubMed] [Google Scholar]
- 43.Pierce BG, Wiehe K, Hwang H, Kim BH, Vreven T, Weng Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30:1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce B, Weng Z. ZRANK: Reranking protein docking predictions with an optimized energy function. Proteins. 2007;67:1078–1086. doi: 10.1002/prot.21373. [DOI] [PubMed] [Google Scholar]
- 45.Beauclair G, Bridier-Nahmias A, Zagury JF, Saïb A, Zamborlini A. JASSA: A comprehensive tool for prediction of SUMOylation sites and SIMs. Bioinformatics. 2015;31:3483–3491. doi: 10.1093/bioinformatics/btv403. [DOI] [PubMed] [Google Scholar]
- 46.Sayers EW, Agarwala R, Bolton EE, Brister JR, Canese K, Clark K, Connor R, Fiorini N, Funk K, Hefferon T, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2019;47:D23–D28. doi: 10.1093/nar/gky1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moult J, Fidelis K, Kryshtafovych A, Schwede T, Tramontano A. Critical assessment of methods of protein structure prediction (CASP)-Round XII. Proteins. 2018;86(Suppl 1):S7–S15. doi: 10.1002/prot.25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vetter IR. Ras superfamily small G proteins: Biology and mechanisms 1. Springer; Vienna, Vienna: 2014. The structure of the G domain of the Ras superfamily; pp. 25–50. [Google Scholar]
- 49.Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thapliyal A, Verma R, Kumar N. Small G proteins Dexras1 and RHES and their role in pathophysiological processes. Int J Cell Biol. 2014;2014:308535. doi: 10.1155/2014/308535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandi V, Di Lella V, Marino M, Ascenzi P, Polticelli F. A comprehensive in silico analysis of huntingtin and its interactome. J Biomol Struct Dyn. 2018;36:3155–3171. doi: 10.1080/07391102.2017.1381646. [DOI] [PubMed] [Google Scholar]
- 52.Saudou F, Humbert S. The biology of Huntingtin. Neuron. 2016;89:910–926. doi: 10.1016/j.neuron.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Krumova P, Weishaupt JH. Sumoylation in neurodegenerative diseases. Cell Mol Life Sci. 2013;70:2123–2138. doi: 10.1007/s00018-012-1158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landré V, Rotblat B, Melino S, Bernassola F, Melino G. Screening for E3-ubiquitin ligase inhibitors: Challenges and opportunities. Oncotarget. 2014;5:7988–8013. doi: 10.18632/oncotarget.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The coordinates of RHES-GTP and RHES-GDP models, as well as those of RHES-Htt and RHES-Ubc9 complex models, are available from the authors upon reasonable request.