Abstract
Acute lung injury (ALI) is a severe health issue with significant morbidity and mortality. Artemisinin is used for the treatment of fever and malaria in clinical practice. Dihydroartemisinin (DHA), the major active metabolite of artemisinin, plays a role in anti-organizational fibrosis and anti-neuronal cell death. However, whether DHA can attenuate ALI remains unclear. The current study thus examined the effects of DHA on ALI and primary macrophages. The results revealed that DHA attenuated lipopolysaccharide (LPS)-induced pulmonary pathological damage. DHA suppressed the LPS-induced infiltration of inflammatory cells, the elevation of myeloperoxidase activity, oxidative stress and the production of pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor-α, and IL-6. Furthermore, DHA reduced the LPS-induced inflammatory response by suppressing the degradation of I-κB and the nuclear translocation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)/p65 in vivo and in vitro. DHA activated the nuclear factor-erythroid 2 related factor 2 (Nrf2) pathway, which was suppressed by LPS treatment. The Nrf2 inhibitor, ML385, diminished the protective effects of DHA against LPS-induced inflammation in macrophages. On the whole, the findings of this study demonstrate that DHA exerts therapeutic effects against LPS-induced ALI by inhibiting the Nrf2-mediated NF-κB activation in macrophages. The present study also confirmed the therapeutic effects of DHA in mice with LPS-induced ALI. Thus, these findings demonstrate that DHA exhibits anti-inflammatory activities and may be a therapeutic candidate for the treatment of ALI.
Keywords: acute lung injury, inflammation, dihydroartemisinin, macrophage, NF-κB, nuclear erythroid-2 related factor 2
Introduction
Acute lung injury (ALI) is a leading cause of acute respiratory failure (1). ALI is characterized by extreme inflammation, the release of pro-inflammatory cytokines, excessive neutrophil infiltration and lung endothelial/epithelial cell injury, resulting in edema and gas exchange deterioration (2). However, the clinical mortality rate of the severe form of ALI, acute respiratory distress syndrome (ARDS), remains >40.0% (1). Therefore, more effective therapeutic strategies for ARDS are urgently required.
Macrophages, the principal immune cells in the lungs, produce inflammatory molecules and carry out vital functions in the molecular mechanisms of ALI, such as boosting neutrophil infiltration and triggering inflammatory reactions (3). Neutrophils trigger the release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 (4). These pro-inflammatory cytokines induce the production of oxidants, which are associated with the activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), eventually contributing to ALI (5,6). Accordingly, oxidative stress is increased in lipopolysaccha-ride (LPS)-induced ALI (7). The transcription factor, nuclear factor-erythroid 2 related factor 2 (Nrf2), plays a critical role in protection against ALI by inducing the expression of antioxidant and detoxifying enzymes and proteins (8). For example, it has been reported that Nrf2 attenuates ALI and inflammation by supressing Toll-like receptor (TLR)4 and Akt signaling (9).
Artemisinin is isolated from Artemisia annua, a Chinese traditional medicinal herb. Artesunate is a water-soluble hemisuccinate derivative of artemisinin. Studies have reported that artesunate inhibits ischemia-reperfusion-induced lung inflammation and LPS-induced ALI (10,11). Dihydroartemisinin (DHA), the major active metabolite of artemisinin or arte-sunate, is an effective and widely distributed anti-malarial drug with good absorption (12,13). DHA is more stable and ten times more effective than artesunate (14). Recent studies have demonstrated that DHA not only exerts an anti-malarial effect, but also exerts anticancer, anti-organizational fibrosis and anti-neuronal cell death effects (15-17). However, whether DHA can attenuate ALI and affect NF-κB signaling activation in macrophages remains unclear.
The present study thus hypothesized that DHA may attenuate LPS-induced ALI and evaluated the effects of DHA on LPS-treated macrophages to elucidate the mechanisms through which DHA attenuates LPS-induced ALI.
Materials and methods
Ethics statement
The Ethics Committee of the Center for Scientific Research with Animal Models at Central South University (Changsha, China) approved the experiments, which were performed in accordance with the guidelines of the National Institutes of Health. Mice were anesthetized with pentobarbital sodium (80 mg/kg, intraperitoneal injection), and all necessary efforts were taken to minimize suffering prior to the experiments.
Animal experiments
Male adult C57bl/6 mice were kept in climate-controlled quarters with a 12-h light/dark cycle and a relative humidity of 40-60%, and were provided with food and water ad libitum at a temperature of 25°C. The mice were housed for 1 week for environmental adaptation prior to experimentation. The mice were randomly divided into 4 groups (weight, 20-25 g; age, 8 weeks; n=24 in each group) as follows: i) The control group; ii) ALI group; iii) DHA group; and iv) ALI + DHA group. The ALI model was induced by the intratracheal injection of LPS (E. coli O111:B4; 5 mg/kg; Sigma-Aldrich; Merck KGaA) in 50 µl saline as described in our previous studies (18,19). Mice in the control group received saline. Mice in the DHA group received DHA (75 mg/kg; Sigma-Aldrich; Merck KGaA) only. Mice in the LPS + DHA group were treated with DHA (75 mg/kg) via intragastric administration 1 h prior to LPS administration. A total of 12 h following the LPS administration, the mice were anesthetized by an intraperitoneal (i.p.) injection of sodium pentobarbital (80 mg/kg) and blood was collected for further analysis. Following the collection of lung tissue, the mice were then sacrificed by an i.p. injection of 200 mg/kg sodium pentobarbital.
Survival experiment
For survival analysis, another 80 mice were randomly divided into 4 groups as follows: i) The control group; ii) ALI group; iii) DHA group; and iv) ALI + DHA group (n=20 in each group). The mice were treated with LPS at a lethal dose (25 mg/kg, intratracheal) (20-23). Mice in the DHA group received DHA (75 mg/kg; Sigma-Aldrich; Merck KGaA) only. Mice in the LPS + DHA group were treated with DHA (75 mg/kg) via intragastric administration 2 h following the adminstration of LPS. The survival rate was monitored every 6 h, as previously described (19).
Histological analysis
Lung tissue was excised and immersed in 4% paraformaldehyde for 24 h at 4°C. Paraffin-embedded lung tissue was sectioned at a thickness of 4 µm and was then stained with haematoxylin and eosin (cat. no. G1120; Solarbio) for 5 min at room temperature for pathological analysis. According to a previous study, the severity of inflammation was graded between 0 and 4 as follows: 1, <25% lung involvement; 2, 25-49% lung involvement; 3, 50-75% lung involvement; and 4, >75% lung involvement (24). Lung injury was scored by 3 pathologists blinded to the treatments.
Bronchoalveolar lavage fluid (BALF) acquisition and analysis
BALF was collected by lavaging the lungs with 1 ml PBS 3 times and was centrifuged at 800 x g for 5 min at 4°C. Total cells, macrophages and neutrophils were counted with a hemocytometer following Wright-Giemsa staining for 10 min at room temperature. The cell-free supernatant of BALF was harvested, and total protein content was determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Inc.).
Lung wet-to-dry weight ratio
The lungs were excised from the mice and blood was removed by blotting the tissue with filter papers until dry. After weighing (wet weight), the lungs were placed in an incubator at 60°C for 48 h and then weighed again (dry weight). The wet-to-dry ratio of the lungs was calculated to reflect edema.
Lactate dehydrogenase (LDH) activity assay
LDH activity in BALF was determined with an LDH Cytotoxicity assay kit (cat. no. A020; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. The absorbance at 490 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc.).
Measurement of myeloperoxidase (MPO), malondialde- hyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) levels
The MPO, MDA, SOD and GSH levels were detected using the related kits (MPO: cat. no. A044; MDA: cat. no. A003; SOD: cat. no. A001; GSH: cat. no. A005; Nanjing Jiancheng Bioengineering Institute). All procedures were performed according to the manufacturer's protocols.
Primary peritoneal macrophages
Male C57bl/6 mice (8-week-old, n=20) were used to extract primary peritoneal macrophages. At 3 days following the intraperitoneal injection of 3 ml 3% thioglycolate (Sigma-Aldrich; Merck KGaA), the animals were euthanized by CO2 inhalation (flow rate of CO2, 20%) and peritoneal macrophages were harvested by peritoneal lavage with cooled RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.). Cells were collected by centrifugation (room temperature, 1,200 g, 10 min) and resuspended with culture medium. Cells were plated into 6- or 12-well plates (1x106 cells/well) and the culture medium was discarded 2 h later. After being allowed to rest overnight, the cells were treated with DHA (20 µM) for 1 h followed by treatment with LPS (100 ng/ml) for a further 6 h at 37°C. To investigate the effects of ML385 (an Nrf2 inhibitor; MedChemExpres) on macrophages, primary macrophages were pre-treated with ML385 (20 µM) for 30 min, and the cells were then treated with DHA (20 µM) for 1 h followed by treatment with LPS (100 ng/ml) for a further 6 h.
Immunofluorescence
Primary peritoneal macrophages were plated on polylysine-coated coverslips. Following fixing with 4% paraformaldehyde at 4°C and permeabilization with Triton X-100, macrophages were incubated overnight at 4°C with anti-CD68 antibody (cat. no. ab125212; 1:50; Abcam) and anti-F4/80 antibody (cat. no. ab6640; 1:50; Abcam). FITC (cat. no. ab6717; 1:200; Abcam) and Cy3 (cat. no. ab6953; 1:200; Abcam)-conjugated secondary antibody were then applied for 1 h at room temperature. Following nuclear staining with DAPI (cat. no. C0065; Solarbio), the macrophages were viewed under a fluorescence microscope (Thermo Fisher Scientific, Inc.).
Cytokine measurements
The levels of TNF-α, IL-1β and IL-6 in mouse sera and the culture supernatants of primary peritoneal macrophages were detected using enzyme-linked immunosor-bent assay (ELISA) kits (TNF-α, cat. no. BMS607-3; IL-1β, cat. no. BMS6002; IL-6, cat. no. BMS603-2; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
Total RNA extraction and reverse transcription-quantitative polymerase chain reaction
Total RNA was extracted from the lung tissue or macrophages using RNAiso reagent (cat. no. 5301100; Takara Bio, Inc.). Total RNA (1 µg) was used for the synthesis of cDNA using a PrimeScript RT Reagent kit with gDNA Eraser (cat. no. RR047A; Takara Bio, Inc.). qPCRs were run using SYBR-Green Real-time PCR Master mix (Thermo Fisher Scientific, Inc.) on a Bio-Rad real-time PCR system (CFX96 Touch™; Bio-Rad Laboratories, Inc.). The amplification conditions were as follows: Pre-degeneration at 95°C for 10 min, then 40 cycles of denaturing at 95°C for 15 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec, and a final extension at 72°C for 5 min. Relative fold expression levels were normalized to GAPDH and calculated using the 2-∆∆Cq method (25). The primer sequences were as follows: TNF-α forward, ACA GCA AGG GAC TAG CCA GGA G and reverse, GGA GTG CCT CTT CTG CCA GT; IL-1β forward, GGG CCT CAA AGG AAA GAA TC and reverse, TAC CAG TTG GGG AAC TCT GC; IL-6 forward, CTG GGG ATG TCT GTA GCT CA and reverse, CTG TGA AGT CTC CTC TCC GG; and β-actin forward, TCT TTG CAG CTC CTT CGT TG and reverse, TCC TTC TGA CCC ATT CCC AC.
Western blot analysis
The lung tissues or cell samples were lysed in RIPA buffer (cat. no. P0013K; Beyotime) at 4°C for 30 min, and the total proteins were quantified using a BCA kit (cat. no. P0010; Beyotime). Briefly, 30 µg proteins were separated by 12% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (EMD Millipore). The membranes were blocked with 5% fat-free milk for 2 h and then probed at 4°C overnight with the following primary antibodies: Anti-phosphorylated(p)-I-κB (1:1,000; cat. no. 2859; Cell Signaling Technology, Inc.), anti-I-κB (1:1,000; cat. no. 4812; Cell Signaling Technology, Inc.), anti-Nrf2 (1:1,000; cat. no. YT3189; ImmunoWay), anti-heme oxygenase 1 (HO-1; 1:1,000; cat. no. 43966; Cell Signaling Technology, Inc.), anti-p65 (1:1,000; cat. no. 10745; Proteintech), anti-β-actin (1:1,000; cat. no. 4970; Cell Signaling Technology, Inc.) and anti-p-p65 (1:1,000; cat. no. 3033; Cell Signaling Technology, Inc). After washing with TBST 3 times, the membranes were incubated with secondary antibodies (1:7,500; cat. no. ab6721; Abcam) at room temperature for 1 h.
Enhanced chemiluminescence (EMD Millipore) was used to detect the protein content. Images were obtained using a ChemiDoc XRS system (Bio-Rad Laboratories, Inc.).
Measurement of ROS production
The lung tissues were homogenized and stained with 50 µM of DCFH-DA (cat. no. S0033; Beyotime) at 37°C in the dark for 30 min. DCF fluorescence intensities were detected by a multi-detection reader (Thermo Fisher Scientific, Inc.) at an excitation and emission wavelength of 485 and 535 nm.
Statistical analysis
Data were analyzed using SPSS 19.0 software (SPSS, Inc.). All data are expressed as the means ± standard error of the mean. Means were compared by two-way ANOVA followed by Tukey's post-hoc test to assess significance. Survival analysis was carried out using the Kaplan-Meier log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
DHA attenuates lung tissue injury in mice with LPS-induced ALI
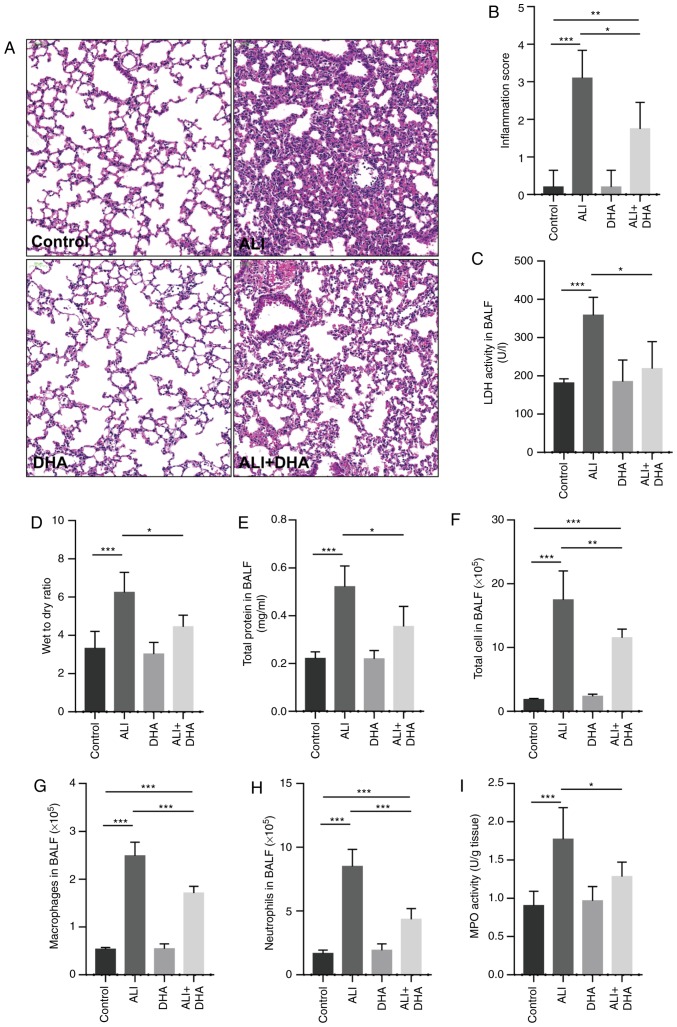
The lung tissues of the control mice were with intact alveoli, while the lung tissues from the mice with ALI exhibited obvious pulmonary edema, alveolar disarray and inflammatory cell infiltration in the alveolar cavity. Following treatment with DHA, the pathological changes induced by LPS in the lungs were attenuated (Fig. 1A). DHA treatment also significantly reduced the lung injury score and LDH activity in the BALF of mice with ALI (Fig. 1B and C). LPS significantly increased the lung wet-to-dry weight ratio and the total protein content in BALF, and these were reduced by DHA treatment (Fig. 1D and E), indicating that DHA attenuated LPS-induced edema. In addition, it was identified that DHA treatment significantly decreased the numbers of total cells, macrophages and neutrophils in the BALF of mice with LPS-induced ALI (Fig. 1F-H). It was also revealed that DHA treatment significantly reduced MPO activity in the lungs of mice with LPS-induced ALI (Fig. 1I). These results indicate that DHA attenuates the lung injury induced by LPS in mice.
Figure 1.
DHA attenuates lung tissue injury in mice with LPS-induced ALI. Mice were treated with saline or DHA (75 mg/kg). After 1 h, mice were administered with LPS (5 mg/kg, intratracheal) or saline for 12 h. (A) Lung histopathological changes were detected by hematoxylin and eosin staining (scale bar, 100 µm) and (B) lung inflammation was scored (n=4-7). (C) LDH activity in BALF was determined to assess lung tissue damage (n=4-7). (D) Lung wet-to-dry ratio and (E) total protein in BALF were measured to determine lung edema (n=6-9). The numbers of (F) total cells, (G) macrophages and (H) neutrophils in BALF were determined (n=5-7). (I) MPO activity in lung tissue was determined (n=6-8). Data are expressed as the means ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. DHA, dihydroartemisinin; LPS, lipopolysaccharide; ALI, acute lung injury; BALF, bronchoalveolar lavage fluid; MPO, myeloperoxidase; LDH, lactate dehydrogenase.
DHA reduces the inflammatory response and oxidative stress in the lungs of LPS-exposed mice
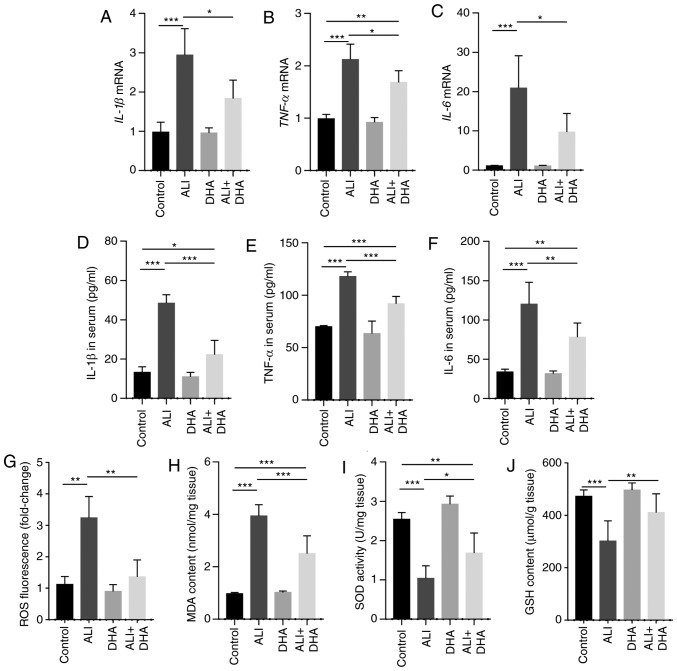
IL-1β, TNF-α and IL-6 are pro-inflammatory cytokines that are critical to the development of ALI (26). The results of the present study demonstrated that mice with ALI exhibited a significant increase in the mRNA levels of IL-1β, TNF-α and IL-6 in the lungs following LPS administration; however, these effects were significantly suppressed by DHA treatment (Fig. 2A-C). Accordingly, DHA reduced the protein levels of IL-1β, TNF-α and IL-6 in the serum of mice with LPS-induced ALI (Fig. 2D-F). LPS induced a significant increase in ROS generation and MDA content in the lungs, which was signifi-cantly suppressed by DHA treatment (Fig. 2G and H). By contrast, SOD activity and GSH content were decreased in the ALI group and were partially restored by treatment with DHA (Fig. 2I and J). These results indicate that DHA protects against LPS-induced lung injury by reducing inflammation and oxidative stress.
Figure 2.
DHA reduces the inflammatory response and oxidative stress in the lungs of LPS-administered mice. Mice were treated with saline or DHA (75 mg/kg). After 1 h, mice were treated with LPS (5 mg/kg, intratracheal) or saline for 12 h. (A) IL-1β, (B) TNF-α and (C) IL-6 mRNA levels in the lungs were determined by RT-qPCR (n=6, 7). (D) IL-1β, (E) TNF-α, and (F) IL-6 protein levels in the serum were determined by ELISA (n=4-6). (G) ROS production, (H) MDA content, (I) SOD activity and (J) GSH content in lung tissue were determined (n=6-8). Data are expressed as the means ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. DHA, dihydroartemisinin; LPS, lipopolysaccharide; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
DHA inhibits inflammatory cytokine release and oxidative stress induced by LPS in primary macrophages
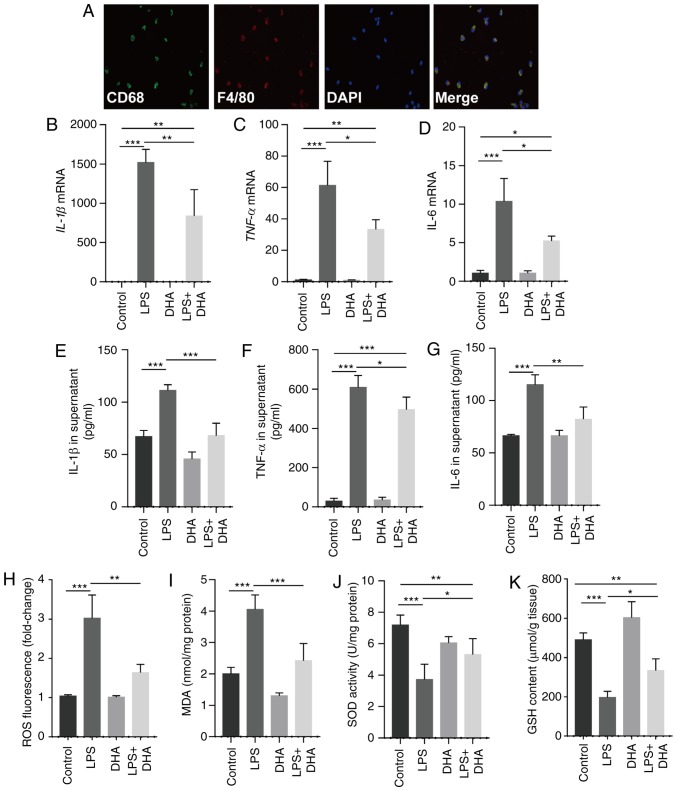
Treatment with DHA (20 µM) suppressed the increase in the IL-1β, TNF-α and IL-6 mRNA expression levels in primary macrophages and protein levels in the culture supernatant, which were induced by LPS (100 ng/ml) (Fig. 3A-G). Furthermore, ROS generation and MDA levels were increased in the macrophages treated with LPS, whereas these responses were significantly suppressed by DHA (Fig. 3H and I). In addition, DHA treatment partially restored the decreased SOD activity and GSH content in macrophages exposed to LPS (Fig. 3J and K). Collectively, these findings suggest that DHA reduces the LPS-induced inflammatory response and oxidative stress in primary macrophages.
Figure 3.
DHA inhibits pro-inflammatory cytokine release and oxidative stress induced by LPS in primary macrophages. LPS (100 ng/ml)-treated primary macrophages were treated with DHA (20 µM) for 6 h. (A) Representative images of immunofluorescence analyses performed on primary macrophages using anti-CD68 (green) and anti-F4/80 (red) antibodies. The mRNA expression levels of (B) IL-1β, (C) TNF-α and (D) IL-6 in primary macrophages were determined (n=4-6). (E) IL-1β, (F) TNF-α and (G) IL-6 protein levels in the culture supernatant of primary macrophages were determined (n=4-6). (H) ROS production, (I) MDA content, (J) SOD activity and (K) GSH content in primary macrophages were determined (n=4-6). Data are expressed as the means ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. DHA, dihydroartemisinin; LPS, lipopolysaccharide; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Inhibition of the NF-κB pathway by DHA is dependent on Nrf2 in primary macrophages
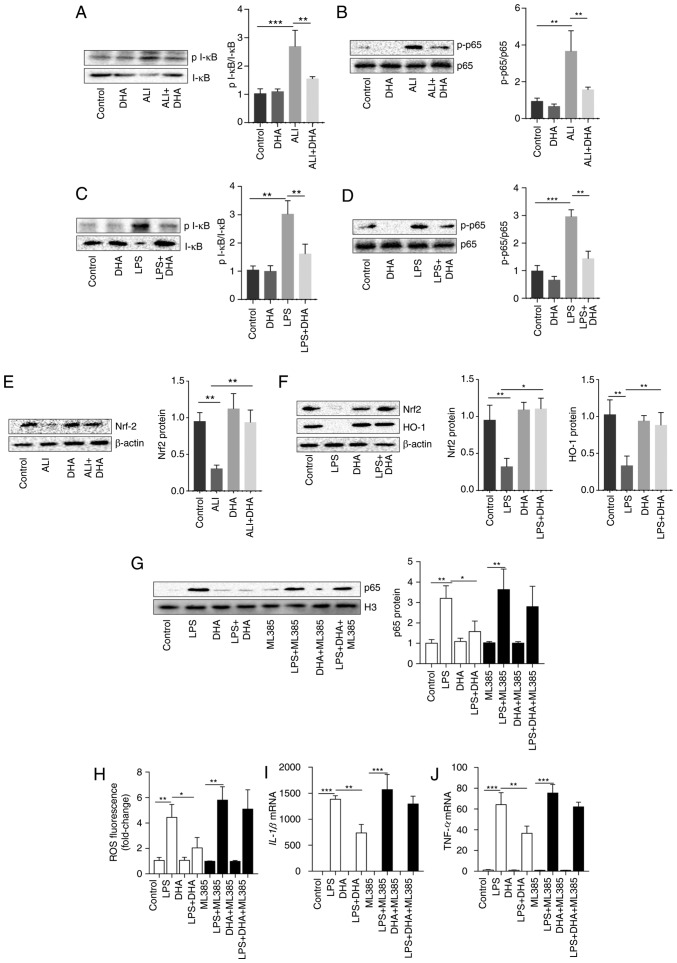
DHA inhibited I-κB degradation and reduced the increase in p-p65 expression induced by LPS in the lung tissue of mice and primary macrophages (Fig. 4A-D). As shown in Fig. 4E and F, DHA activated Nrf2 and HO-1, which were suppressed by LPS in lung tissue and macrophages. To determine the role of Nrf2 in the protective effects of DHA, Nrf2 was blocked in primary macrophages using the Nrf2 inhibitor, ML385. It was identified that the inhibition of NF-kB/p65 translocation by DHA was markedly diminished by ML385 treatment (Fig. 4G). Furthermore, the LPS-induced increase in ROS production, and in the IL-1β and TNF-α mRNA levels was not suppressed by DHA in primary macrophages pre-treated with ML385 (Fig. 4H-J). These results indicate that Nrf2 is vital for the protective effects of DHA against LPS-induced ALI.
Figure 4.
Inhibition of the NF-κB pathway by DHA is dependent on Nrf2 in primary macrophages. Mice were treated with saline or DHA (75 mg/kg). After 1 h, mice were treated with LPS (5 mg/kg, intratracheal) or saline for 12 h. (A and B) p-I-κB and p-p65 protein levels in lung tissue were determined (n=4). LPS (100 ng/ml)-treated primary macrophages were treated with DHA (20 µM) for 6 h. (C and D) p-I-κB and p-p65 protein levels in primary macrophages were determined (n=3). (E) Nrf2 protein level in lung tissue was determined (n=4). (F) Nrf2 and HO-1 protein levels in primary macrophages were determined (n=4-6). (G) Nuclear p65 protein level in primary macrophages following ML385 treatment was determined (n=5 or 6). (H) ROS production in primary macrophages after ML385 treatment was determined (n=5 or 6). The mRNA expression levels of (I) IL-1β and (J) TNF-α in primary macrophages after ML385 treatment were determined (n=4-6). Data are expressed as the means ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001. DHA, dihydroartemisinin; LPS, lipopolysaccharide; ROS, reactive oxygen species; Nrf2, nuclear factor-erythroid 2 related factor 2; p-phosphorylated; HO-1, heme oxygenase 1.
Therapeutic effect of DHA on LPS-induced ALI mice
Clinically, pharmacotherapy does not commence until there is an approved diagnosis of ALI. In this study, the therapeutic effects of DHA on mice with ALI were subsequently examined. It was identified that treatment with DHA 2 h post-exposure to LPS significantly improved the survival rate of mice with LPS-induced ALI (Fig. 5). This result suggests a therapeutic effect of DHA against ALI in mice.
Figure 5.
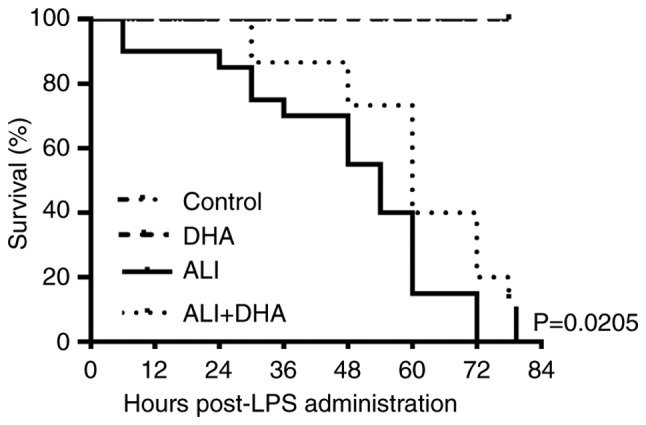
Therapeutic effects of DHA on ALI mice induced by LPS. DHA (75 mg/kg) was administered to mice 2 h after the injection of LPS (25 mg/kg, intratracheal) or saline. The mortality was monitored every 6 h (n=20 each group). At 84 h following the injection of LPS, the control group and DHA group were left with 20 mice. The LPS group had no mice left, while the ALI + DHA group had 7 mice left. Control group vs. LPS group, P<0.0001. ALI group vs. ALI + DHA group, P=0.0205<0.05. DHA, dihydroartemisinin; ALI, acute lung injury; LPS, lipopolysaccharide.
Discussion
Arteminsinin and its derivative, artesunate, have been reported to exert protective effects against lung inflammation (10,11), which indicates the potential use of arteminsinin and its derivative in therapy. As a more effective agent than artesunate, DHA has been reported to exert anti-inflammatory and anti-fibrotic effects against bleomycin-induced pulmonary fibrosis in rats (27). The present study first reported that DHA attenuated lung tissue injury in a murine model of LPS-induced ALI and suppressed macrophage activation induced by LPS. Notably, it was identified that treatment with DHA 2 h post-exposure to LPS significantly improved the survival rate of LPS-exposed mice, indicating a therapeutic effect of DHA against ALI. Mechanistically, it was determined that the DHA-mediated suppression of inflammatory injury was dependent on Nrf2. The present findings suggest the use of DHA as a potential therapeutic agent for patients with ALI in the future.
Nrf2 plays a critical role in the regulation of oxida-tive stress, which is the key pathogenic mechanism of ALI (28-30). Generally, Nrf2 exists as a complex with Keap1 in the cytoplasm. When cells are sensitized to ROS, Nrf2 is released from the complex and translocates to the nucleus, promoting the expression of antioxidants, such as HO-1 and SOD (31). A deficiency in Nrf2 results in severe lung injury induced by ischemia-reperfusion or LPS (9,32). The present study also demonstrated that Nrf2 expression was downregulated in the lungs of mice with ALI. Furthermore, DHA increased the expression of downstream Nrf2, SOD and HO-1, indicating the activation of Nrf2. Additionally, inhibition of Nrf2 abolished the protective effects of DHA, indicating a role of Nrf2 in the therapeutic effects of DHA. The Nrf2/ARE signaling pathway also regulates the expression of anti-inflammatory genes and inhibits the progression of inflammation (33). Nrf2 negatively regulates LPS-induced NF-κB signaling activation (29). The present study demonstrated that Nrf2 blockade markedly diminished the inhibition of NF-κB/p65 translocation induced by DHA in macrophages, which indicated that DHA inhibits the NF-κB pathway in a Nrf2-dependent manner in macrophages.
The NF-κB pathway is a key target for the development of anti-inflammatory agents (34). Numerous natural products have been screened for anti-inflammatory activities by inhibiting NF-κB (35-37). Artemisinin significantly inhibits NF-κB activation by suppressing the phosphorylation and degradation of I-κBα and p65 nuclear translocation (38). Artesunate has been reported to suppress LPS-induced TLR4 expression and NF-κB activation in lung tissue and to upregulate Nrf2 and HO-1 expression in lung tissue in vivo (11). However, whether DHA can affect the activation of NF-κB and the underlying mechanisms in macrophages remain unclear. The present study first reported that DHA significantly mitigated NF-κB pathway activation in the lungs of ALI mice and in primary macrophages exposed to LPS. It has also been reported that DHA inhibits the NF-κB pathway in rat chondrocytes (39) and tumor cell invasion (40). While the exact mechanisms remain unclear, the present study provides a novel mechanism through which DHA inhibits the NF-κB pathway by activating Nrf2. This indicates that DHA is a potential anti-inflammatory and anti-oxidative agent.
Macrophages are the principal immune cells of inflammatory molecules in pulmonary tissue and exert a vital function in the molecular mechanisms of ALI, triggering inflammation reactions and boosting the infiltration of neutrophils (3). There is increasing evidence to suggest that macrophages, which act as the first line of defense in the lungs, are key factors in the pathogenesis of ALI (41). The depletion of macrophages has been found to mitigate lung injury significantly at 4 h following the administration LPS in mice by attenuating neutrophilic alveolitis and reducing pro-inflammatory cytokines (42). Under the LPS challenge, the pro-inflammatory M1 alveolar macrophages are mainly derived from the bone marrow. Those alveolar macrophages are the triggers of the uncontrolled inflammatory response during ALI. However, it is hard to harvest a sufficient amount of alveolar macrophages from healthy mice to conduct an experiment. In this study, the primary peritoneal macrophages were recruited to the peritoneal cavity by 3% thioglycolate. Thus, these macrophages are also derived from bone marrow. Notably, the adoptive transfer of peritoneal macrophages into the lungs results in the expression of certain alveolar macrophage-specific genes (43). In some studies, primary peritoneal macrophages are used to investigate the role of macrophages in lungs (19,44-46). The present study focused on the role of DHA in the LPS-challenged inflammatory response in macrophages in vitro. It was found that DHA inhibited inflammatory cytokine release and oxidative stress induced by LPS in primary peritoneal macrophages. Collectively, we hypothesized that primary murine peritoneal macrophages share, at least partly, the response to LPS-challenge with alveolar macrophages.
The limitations of the present were the following: First, only the protective effects of DHA against LPS-induced ALI in mice were examined. To further clarify the effects of DHA on ALI, the protective effects of DHA in other models of ALI should also be investigated. Second, the mechanisms underlying the suppression of ALI by DHA are not completely clear. In addition to the NF-κB pathway, the effects of DHA on the activation of the NLRP3 inflammasome should be examined, which is a vital mechanism underlying the uncontrolled inflammation during ALI (47). Artesunate has been identified to alleviate renal ischemia-reperfusion-induced lung inflammation by attenuating the activation of NLRP3 inflammasome (10).
In conclusion, this study demonstrates that DHA exerts protective and therapeutic effects against LPS-induced ALI by inhibiting the NF-κB signaling pathway in a Nrf2-dependent manner. The present findings provide further evidence that DHA may be a valuable therapeutic candidate for use in the treatment of ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81370974, 81500056 and 81500065) and the Hunan Provincial Natural Science Foundation of China (grant no. 2019JJ50785).
Availability of data and materials
The data used to support the findings of this study are presented in the present study or are available from the corresponding author upon request.
Authors' contributions
XTH designed and performed most of the experiments, analyzed and interpreted the data, and wrote the manuscript. WL, CXH, YaZ, CYZ, and CCS assisted during the acquisition, analysis, and interpretation of data and revised the manuscript. ZQL and YoZ assisted with data acquisition and revision of the manuscript. SYT performed experiments and prepared the manuscript. All authors have reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
The Ethics Committee of the Center for Scientific Research with Animal Models at Central South University (Changsha, China) approved the experiments, which were performed in accordance with the guidelines of the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pan C, Liu L, Xie JF, Qiu HB. Acute respiratory distress syndrome: Challenge for diagnosis and therapy. Chin Med J (Engl) 2018;131:1220–1224. doi: 10.4103/0366-6999.228765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, Wu YC, Meng M, Sun QS, Gao SM, Sun H. Linarin prevents LPS-induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and-NF-κB pathways. Int J Mol Med. 2018;42:1460–1472. doi: 10.3892/ijmm.2018.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol. 2013;190:3590–3599. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Liang X, Wang D, Zhang H, Liu L, Chen H, Li Y, Duan Q, Xie K. Combination therapy with nitric oxide and molecular hydrogen in a murine model of acute lung injury. Shock. 2015;43:504–511. doi: 10.1097/SHK.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 5.Takashima K, Matsushima M, Hashimoto K, Nose H, Sato M, Hashimoto N, Hasegawa Y, Kawabe T. Protective effects of intratracheally administered quercetin on lipopolysaccha-ride-induced acute lung injury. Respir Res. 2014;15:150. doi: 10.1186/s12931-014-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng G, He H, Chen Z, OuYang L, Xiao X, Ge J, Xiang B, Jiang S, Cheng S. Lianqinjiedu decoction attenuates LPS-induced inflammation and acute lung injury in rats via TLR4/NF-κB pathway. Biomed Pharmacother. 2017;96:148–152. doi: 10.1016/j.biopha.2017.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang K, Guo S, Yang C, Yang J, Chen Y, Shaukat A, Zhao G, Wu H, Deng G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int Immunopharmacol. 2018;64:140–150. doi: 10.1016/j.intimp.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: A potential role for Nrf2. Antioxid Redox Signal. 2008;10:321–332. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, Xu H, Jiang H, Hu R. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 2018;121:78–85. doi: 10.1016/j.freeradbiomed.2018.04.557. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Qu M, Yu L, Song P, Chang Y. Artesunate inhibits renal ischemia-reperfusion-mediated remote lung inflammation through attenuating ROS-induced activation of NLRP3 inflammasome. Inflammation. 2018;41:1546–1556. doi: 10.1007/s10753-018-0801-z. [DOI] [PubMed] [Google Scholar]
- 11.Zhao D, Zhang J, Xu G, Wang Q. Artesunate protects LPS-induced acute lung injury by inhibiting TLR4 expression and inducing Nrf2 activation. Inflammation. 2017;40:798–805. doi: 10.1007/s10753-017-0524-6. [DOI] [PubMed] [Google Scholar]
- 12.Keating GM. Dihydroartemisinin/Piperaquine: A review of its use in the treatment of uncomplicated Plasmodium falciparum malaria. Drugs. 2012;72:937–961. doi: 10.2165/11203910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Morris CA, Onyamboko MA, Capparelli E, Koch MA, Atibu J, Lokomba V, Douoguih M, Hemingway-Foday J, Wesche D, Ryder RW, et al. Population pharmacokinetics of artesunate and dihydroartemisinin in pregnant and non-pregnant women with malaria. Malar J. 2011;10:114. doi: 10.1186/1475-2875-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 15.Yang DX, Qiu J, Zhou HH, Yu Y, Zhou DL, Xu Y, Zhu MZ, Ge XP, Li JM, Lv CJ, et al. Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci. 2018;205:176–183. doi: 10.1016/j.lfs.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Lin SP, Li W, Winters A, Liu R, Yang SH. Artemisinin prevents glutamate-induced neuronal cell death via Akt pathway activation. Front Cell Neurosci. 2018;12:108. doi: 10.3389/fncel.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Li S, Li Y, Bai Y. Anticancer effects of dihydroartemisinin on human esophageal cancer cells in vivo. Anal Cell Pathol (Amst) 2018;2018:8759745. doi: 10.1155/2018/8759745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Liu T, Duan JX, Li P, Sun GY, Liu YP, Zhang J, Dong L, Lee KSS, Hammock BD, et al. Soluble epoxide hydrolase inhibitor attenuates lipopolysaccharide-induced acute lung injury and improves survival in mice. Shock. 2017;47:638–645. doi: 10.1097/SHK.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong WJ, Yang HH, Guan XX, Xiong JB, Sun CC, Zhang CY, Luo XQ, Zhang YF, Zhang J, Duan JX, et al. Inhibition of glycol-ysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J Cell Physiol. 2019;234:4641–4654. doi: 10.1002/jcp.27261. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Kwun MJ, Choi JY, Ahn KS, Oh SR, Lee YG, Christman JW, Sadikot RT, Han CW, Joo M. Therapeutic effect of the tuber of alisma orientale on lipopolysaccha-ride-induced acute lung injury. Evid-Based Complement Alternat Med. 2013;2013:863892. doi: 10.1155/2013/863892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K, Okada H, Takemura G, Takada C, Kuroda A, Yano H, Zaikokuji R, Morishita K, Tomita H, Oda K, et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in lipopolysaccharide-induced experimental endotoxemia. Am J Pathol. 2019;189:1526–1535. doi: 10.1016/j.ajpath.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Mahapatra S, Ying L, Ho PP, Kurnellas M, Rothbard J, Steinman L, Cornfield DN. An amyloidogenic hexapeptide derived from amylin attenuates inflammation and acute lung injury in murine sepsis. PLoS One. 2018;13:e0199206. doi: 10.1371/journal.pone.0199206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda J, Starr ME, Takahashi H, Du J, Chang LY, Crapo JD, Evers BM, Saito H. Decreased pulmonary extracellular superoxide dismutase during systemic inflammation. Free Radic Biol Med. 2008;45:897–904. doi: 10.1016/j.freeradbiomed.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Janz DR, Ware LB. Biomarkers of ALI/ARDS: Pathogenesis, discovery, and relevance to clinical trials. Semin Respir Crit Care Med. 2013;34:537–548. doi: 10.1055/s-0033-1351124. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Yuan W, Lv C, Li N, Liu T, Wang L, Sun Y, Qiu X, Fu Q. Dihydroartemisinin supresses inflammation and fibrosis in bleomycine-induced pulmonary fibrosis in rats. Int J Clin Exp Pathol. 2015;8:1270–1281. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X, Zhu XY, Liu YJ, Wang CN. H2S attenuates LPS-induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cell Physiol Biochem. 2016;40:1603–1612. doi: 10.1159/000453210. [DOI] [PubMed] [Google Scholar]
- 29.Fan L, Fan Y, Liu L, Tao W, Shan X, Dong Y, Li L, Zhang S, Wang H. Chelerythrine attenuates the inflammation of lipopolysaccharide-induced acute lung inflammation through NF-κB signaling pathway mediated by Nrf2. Front Pharmacol. 2018;9:1047. doi: 10.3389/fphar.2018.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Lv H, Wen Z, Ci X, Peng L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-κB pathway in macrophages and in acute lung injury. Front Immunol. 2017;8:1518. doi: 10.3389/fimmu.2017.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Willis TL, Button RW, Strang CJ, Fu Y, Wen X, Grayson PRC, Evans T, Sipthorpe RJ, Roberts SL, et al. Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response. Nat Commun. 2019;10:3759. doi: 10.1038/s41467-019-11671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophysica Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Shang L, Wang T, Tong D, Kang W, Liang Q, Ge S. Prolyl hydroxylases positively regulated LPS-induced inflammation in human gingival fibroblasts via TLR4/MyD88-mediated AKT/NF-κB and MAPK pathways. Cell Prolif. 2018;51:e12516. doi: 10.1111/cpr.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang XT, Li C, Peng XP, Guo J, Yue SJ, Liu W, Zhao FY, Han JZ, Huang YH, Yang Li, et al. An excessive increase in glutamate contributes to glucose-toxicity in β-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep. 2017;7:44120. doi: 10.1038/srep44120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D, Li X, Hu Y, Jiang H, Wu Y, Ding Y, Yu K, He H, Xu J, Sun L, Qian F. Tabersonine attenuates lipopolysac-charide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochem Pharmacol. 2018;154:183–192. doi: 10.1016/j.bcp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Seo EJ, Fischer N, Efferth T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer's disease. Pharmacol Res. 2018;129:262–273. doi: 10.1016/j.phrs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol Immunotoxicol. 2017;39:28–36. doi: 10.1080/08923973.2016.1267744. [DOI] [PubMed] [Google Scholar]
- 39.Jiang LB, Meng DH, Lee SM, Liu SH, Xu QT, Wang Y, Zhang J. Dihydroartemisinin inhibits catabolism in rat chon-drocytes by activating autophagy via inhibition of the NF-κB pathway. Sci Rep. 2016;6:38979. doi: 10.1038/srep38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW, Jeong HG. Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol. 2010;79:1714–1726. doi: 10.1016/j.bcp.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Xiu H, Zhang S, Zhang G. The Role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, Christman JW. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26:572–578. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- 43.Morales-Nebreda L, Misharin AV, Perlman H, Budinger GR. The heterogeneity of lung macrophages in the susceptibility to disease. Eur Respir Rev. 2015;24:505–509. doi: 10.1183/16000617.0031-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Jin Y, Chen H, Sun C, Fu W, Zheng L, Lu M, Chen P, Chen G, Zhang Y, et al. Discovery of caffeic acid phenethyl ester derivatives as novel myeloid differentiation protein 2 inhibitors for treatment of acute lung injury. Eur J Med Chem. 2018;143:361–375. doi: 10.1016/j.ejmech.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 45.Duan JX, Zhou Y, Zhou AY, Guan XX, Liu T, Yang HH, Xie H, Chen P. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol Immunol. 2017;91:105–113. doi: 10.1016/j.molimm.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Qi Z, Qi S, Ling L, Lv J, Feng Z. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–271. doi: 10.1016/j.intimp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Hosseinian N, Cho Y, Lockey RF, Kolliputi N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther Adv Respir Dis. 2015;9:188–197. doi: 10.1177/1753465815586335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are presented in the present study or are available from the corresponding author upon request.