Abstract
Pistacia weinmannifolia (Anacardiaceae) has been used in herbal medicine for the treatment of influenza, dysentery and enteritis in China. It was recently observed that P. weinmannifolia root extract (PWRE) exerts anti-inflammatory effects both in in vitro and in vivo models. Based on the results from previous studies, the present study investigated the protective effect of PWRE on airway inflammation and mucus hypersecretion. Treatment with PWRE significantly decreased the number of eosinophils and the levels of Th2 cytokines, such as interleukin (IL)-4, IL-5 and IL-13, in the bronchoalveolar lavage fluid (BALF) of OVA-exposed mice. PWRE decreased the high serum levels of total and OVA-specific immunoglobulin E. PWRE also effectively inhibited the influx of inflammatory cells into the lung, as well as airway mucus hypersecretion. In addition, the increased level of monocyte chemoattractant protein-1 was significantly decreased with the PWRE treatment in the BALF of OVA-exposed mice and in lipopolysaccharide-stimulated RAW264.7 macrophages. These protective effects of PWRE on OVA-induced pulmonary inflammation were accompanied by the downregulation of mitogen associated protein kinases and nuclear factor-κB activation. Thus, the results from the present study indicate that PWRE could be valuable adjuvant for the treatment of asthma.
Keywords: Pistacia weinmannifolia root, allergic asthma, airway inflammation, mucus hypersecretion, NF-κB
Introduction
Allergic asthma is a chronic inflammatory disease and a major health issue, and its prevalence is increasing worldwide (1). The major features of asthma pathophysiology include airway inflammation and mucus hypersecretion (2,3). It is well known that the increased levels of eosinophil recruitment and T helper lymphocytes 2 (Th2) cytokines, such as interleukin-4 (IL-4), IL-5 and IL-13, are closely associated with sustained airway inflammation (4). Macrophages-derived chemokines such as monocyte chemoattractant protein-1 (MCP-1) increased the recruitment of inflammatory cells including eosinophils in asthma pathogenesis (5,6) The increased concentration of immunoglobulin E (IgE) has a pivotal role in allergic reactions and is much higher in asthmatic patients (7). Changes in the number of goblet cells and production of mucus are key to airway inflammation and obstruction (8). The mitogen-activated protein kinase (MAPK) signaling pathways have an important role in the inflammatory processes of allergic asthma (9). The activation of c-Jun N-terminal kinase (JNK) has been implicated in IgE class switching (10). Extracellular signal-regulated kinase (ERK) and p38 have been reported to play a role in the production of cytokines, including IL-5 (11). Nuclear factor (NF)-κB plays an important role in inflammatory cell influx, Th2 cytokine levels and inflammatory molecules in allergic asthma (12,13).
In recent years, the approaches to improve the side effects of medicine have focused on research into allergic asthma (14) and natural herbal extracts are attracting increased attention due to their prominent biological activities and minimal side effects (15). Pistacia weinmannifolia (PW) is used as a herbal medicine in China (16,17) and its major metabolites possess biological activities, such as inhibitory activities against histamine release (16,18,19). In a previous study, it was confirmed that the anti-inflammatory activities of P. weinmannifolia root extract (PWRE) in PMA/tumour necrosis factor-α-stimulated airway epithelial cells and in pulmonary inflammatory response induced by cigarette smoke and lipopolysaccharide (LPS) (20). Based on these results and those of other studies (16-20), which reflect the anti-inflammatory activities of PWRE on pulmonary inflammation, it was hypothesized that PWRE could exert a protective effect against ovalbumin (OVA)-induced lung inflammation. Therefore, the aim of the present study was to evaluate the regulatory effects of PWRE against eosinophil recruitment and Th2 cytokines, IgE and mucus overproduction, which are the major characteristics of allergic asthma.
Materials and methods
Preparation of PWRE
PWRE was prepared as previously described (20). P. weinmannifolia roots (PWRs) were collected from the Yunnan province of China. A voucher specimen recorded as D180305001 was deposited at the International Biological Material Research Center, Korea Research Institute of Bioscience and Biotechnology. The active substance of PWR was extracted by the processing method described in the International Conference on Harmonisation and Ministry of Food and Drug Safety guidelines (20). The collected roots were dried immediately following sampling and then ground to a powder. The raw materials were then packed in laminated bags and delivered to Korea. The PWREs were provided by the BTC Corporation. The powdered samples were extracted with 50% ethanol at 80°C and the product was dried in a freeze dryer (-70°C) to produce dried extracts (~19%) [Korea Good Manufacturing Practice (KGMP), lot no. BTC-PWE-180118].
Induction of ovalbumin (OVA) and alum-induced lung inflammation in murine models
Healthy female BALB/c mice (n=30, 6 weeks old; body weight, 16-18 g) were purchased from Koatech Co., Ltd., and used after 1 week of acclimatization with free access to food and water in specific pathogen-free conditions (22-23°C; 55-60% humidity; 12-h light/dark cycle). The experimental procedure was performed according to the methods described by Park et al (21). Briefly, the mice were sensitized twice intraperitoneally on day 0 and 14 with 30 µg OVA and 3 mg Alums (Thermo Fisher Scientific, Inc.) dissolved in a solution of 0.2 ml PBS. On days 21-23, the mice were aerosol challenged with 1% OVA (alum-free saline solution, 60 min/day) with a nebulizer (NE-U12; OMRON Corp.). The PWRE or montelukast (MON) was given by oral gavage for 6 consecutive days (from day 18 to 23). The mice were sacrificed on day 25. The mice were randomly divided into 4 groups (n=6 per subgroup) as follows: i) The normal control (NC) group; ii) the OVA group (intraperitoneally sensitized with OVA-Alum); iii) the MON group (intraperitoneally sensitized with OVA-Alum) + MON (30 mg/kg, per os); and iv) the PW group (intraperitoneally sensitized with OVA-Alum) + PWRE (7.5 or 15.0 mg/kg, per os). MON was used as a positive control. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology and performed in compliance with the National Institutes of Health Guidelines for the care and use of laboratory animals and Korean national laws for animal welfare. The humane endpoints are the condition of rapid loss of weight (>20% of normal body weight) and/or rapid or labored breathing.
Counting the inflammatory cells
BALF collection was performed in order to count the inflammatory cells and evaluate the levels of inflammatory cytokines as previously described (22). The mice were anesthetized with Zoletil 50® (30-50 mg/kg IP; Virbac) and Xylazine (5-10 mg/kg IP; Bayer Korea) on day 25 based on prior anesthesia condition (20). Briefly, on day 25, the trachea was cannulated and infused with 0.7 ml PBS for the collection of BALF (infusion was performed twice with a total volume of 1.4 ml) and blood was collected for the detection of IgE. Mice were sacrificed under Zoletil/Xylazine anaesthesia and exsanguinated. In order to distinguish the different cells, 0.1 ml of BALF was centrifuged at 246 × g for 5 min at room temperature to transfer the cells to the glass slide and then the glass slide was stained with Diff-Quik® solution (IMEB, Inc.) according to the manufacturer's protocol.
Measuring the Th2 cytokines and IgE production
The levels of IL-4, IL-5 and IL-13 in the BALF were determined using ELISA kits (R&D Systems, Inc.; IL-4, cat. no. M4000B; IL-5, cat. no. M5000; IL-13, cat. no. M1300CB) according to the manufacturer's protocol. Blood (0.4 ml) was collected in order to determine the serum IgE levels. The concentration of the total or OVA-specific IgE in the serum was determined using an ELISA (Biolegend, Inc.; Total IgE, cat. no. 432404; R&D Systems, Inc., OVA-specific IgE, cat. no. 439807). The absorbance was measured at 450 nm with a Spark™ 10 M multimode microplate reader (Tecan System Inc.).
Western blot analysis
Lung tissues were removed 48 h after the final OVA inhalation and incubated in CelLytic™ MT Cell Lysis reagent (cat. no. c3228; Sigma-Aldrich; Merck KGaA) containing protease and phosphatase inhibitors (cat. nos. 11836153001 and 04906837001; Roche Diagnostics) in order to obtain the proteins. The protein concentration was measured with the Pierce bicinchoninic acid Protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The proteins (50 µg/lane) were separated via SDS-PAGE (10-12% gels) and then transferred to PVDF membranes (EMD Millipore). The membranes were blocked in 5% skimmed milk dissolved in TBS and 0.05% Tween-20 (TBST) for 1 h at room temperature and probed overnight with primary antibodies at 4°C. The primary antibodies used were as follows: Anti-phosphorylated (p)-extracellular signal-regulated kinase (ERK; 1:1,000; cat. no. 9101; 1:1,000; Cell Signaling Technology, Inc.), anti-p-p38 (cat. no. 9211; 1:1,000; Cell Signaling Technology, Inc.), anti-p-NF-κB p65 (cat. no. 3033; 1:1,000; Cell Signaling Technology, Inc.), anti-p-inhibitor of NF-κB (p-IκBα; cat. no. 2859; 1:1,000; Cell Signaling Technology, Inc.), anti-β-actin (1:2,500; cat. no. 4967; 1:1,000; Cell Signaling Technology, Inc.), anti-ERK (cat. no. sc-154; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-p-c-Jun N-terminal kinase (JNK; cat. no. sc-6254; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-JNK (cat. no. sc-474; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-p38 (cat. no. sc-7149; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-MCP-1 (cat. no. sc-28879; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-NF-κB p65 (cat. no. sc-372; 1:1,000; Santa Cruz Biotechnology, Inc.) and anti-IκBα (cat. no. MA5-15132; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.). The membranes were washed five times with TBST for 10 min and developed with horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse & anti-rabbit; 1:2,000; cat. nos. 115-035-003 and 111-035-003; Jackson ImmunoResearch Laboratories, Inc.) at room temperature (RT) for 1 h. The membranes were developed with an ECL kit (Thermo Fisher Scientific, Inc.). All bands were visualized using a LAS-4000 luminescent image analyzer (Fujifilm) and quantified by densitometry using Fuji Multi Gauge software version 3.0 (Fujifilm).
Histological analysis of lung tissue
A total of 24 h after the final administration of PWRE and MON, the mice were sacrificed, and the lung tissues were collected. For histological evaluation, the lung tissues were fixed in 10% (v/v) neutral-buffered formalin solution at room temperature for 48 h and embedded in paraffin. The lung tissues were then sliced into 4-µm thick sections with a rotary microtome and stained with hematoxylin (BBC Biochemical Inc.) and eosin (Thermo Fisher Scientific Inc.; H&E) solutions at RT for 30 sec each to estimate the inflammatory response. The lung sections then were visualized using a light microscope (magnification, ×100; scale bar, 50 µm) to estimate the recruitment of inflammatory cells. Periodic acid-Schiff (PAS) staining (IMEB, Inc., cat. no. K7308) was performed to estimate the mucus secretion. The degree of inflammatory score and mucus production in each group was assessed by two independent observers in the laboratory using a semi-quantitative scope. The H&E staining was scored as follows: 0, no recruitment of inflammatory cells; 1, small amount of recruitment; 2, moderate recruitment; 3, large amount of recruitment. The PAS staining was scored as follows: 0, no mucus production; 1, mild mucus production; 2, moderate mucus production; 3, distinct mucus production; 4, severe mucus production.
Cell culture
The macrophage cell line RAW264.7 was obtained from the American Type Culture Collection. The cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100 µg/ml streptomycin and were incubated at 37°C in a humidified chamber with 5% CO2. The cells were activated with lipopolysaccharide (LPS; 0.5 µg/ml) 1 h after PWRE treatment (1.25, 2.5 and 5 µg/ml). The dose of LPS was based on a previous study (23). The level of MCP-1 in the culture supernatant was determined by ELISA.
Statistical analysis
All values are expressed as the mean ± standard deviation of at least three independent experiments. The statistical significance was determined by a two-tailed Student's t-test for comparisons between two groups. One-way analysis of variance followed by Dunnett's multiple groups. Data were analyzed using SPSS 20.0 (IBM Corp.). P<0.05 was considered to indicate a statistically significant result.
Results
Effect of PWRE on alleviating the eosinophil numbers in the BALF
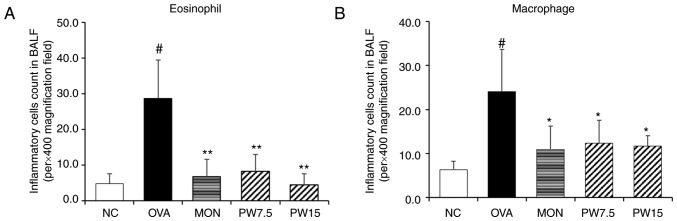
The significant increase in eosinophils and macrophages has been well established in OVA-induced pulmonary inflammatory response (24,25). Therefore, the present study focused on the inhibitory effect of PWRE on the cell numbers. To distinguish the inflammatory cells and count the cell numbers, Diff-Quik® staining was performed according to the manufacturer's protocol. As presented in Fig. 1, the numbers of eosinophil and macrophages were significantly increased in the OVA-exposed group compared with the NC group (P<0.05). Conversely, this increase in inflammatory cell numbers was significantly decreased in the PWRE-treated group (P<0.05; Fig. 1A and B).
Figure 1.
Effect of PWRE on the numbers of inflammatory cells in the BALF of OVA-challenged mice. The count of (A) eosinophils and (B) macrophages in the BALF was determined by Diff-Quik® staining. Data are expressed as the mean ± standard deviation (n=6). #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. OVA-induced group. NC, normal control mice; OVA group, mice administered ovalbumin; MON group, mice administered MON (30 mg/kg) + OVA; PW 7.5, mice administered P. weinmannifolia root extract (7.5 mg/kg) + OVA. OVA, ovalbumin; MON, montelukast; PWRE, P. weinmannifolia root extract; PW 7.5, 7.5 mg/kg PW + OVA, PW15, 15 mg/kg PW + OVA; BALF, bronchoalveolar lavage fluid; NC, negative control.
Effect of PWRE on attenuating Th2 cytokines in the BALF
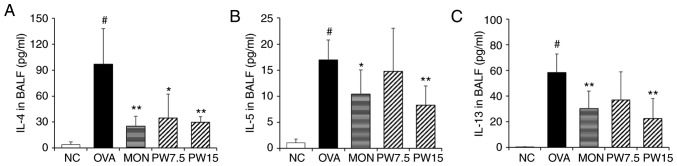
The present study next investigated the regulatory effect of PWRE on the production of Th2 cytokines that are deeply associated with the pathophysiology of asthma. ELISAs were performed in order to evaluate the levels of Th2 cytokines. It was revealed that IL-4, IL-5 and IL-13 were significantly increased in the OVA group when compared with the NC group (P<0.05; Fig. 2A-C). However, treatment with PWRE decreased the levels of these cytokines induced by OVA. In particular, the inhibitory effects of 15 mg/kg PWRE on the production of cytokines were similar to those of 30 mg/kg MON, which was used as a positive control.
Figure 2.
Effect of PWRE on the production of Th2 cytokines in the BALF. The levels of Th2 cytokines, such as (A) IL-4, (B) IL-5 and (C) IL-13, were determined by ELISA kits. The absorbance was measured at 450 nm with a microplate reader. #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. OVA-induced group. PWRE, P. weinmannifolia root extract; IL, interleukin; OVA, ovalbumin; MON, montelukast; BALF, bronchoalveolar lavage fluid; NC, negative control; Th2, T-helper 2.
Effect of PWRE on downregulating IgE production
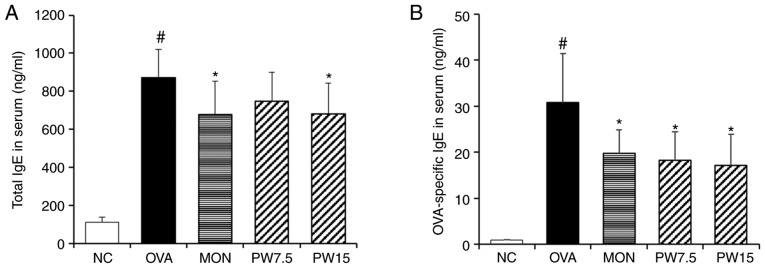
The serum total IgE level is highly elevated in allergic patients such as bronchial asthma and is known to increase with the onset and aggravation of the disease (26,27). A specific IgE test is needed together with total IgE for proper evaluation of allergic diseases (28). Based on the importance of the IgE-mediated immune response in asthma (29), the present study investigated the inhibitory activity of PWRE on OVA-induced IgE production. As presented in Fig. 3, the concentration of total IgE or OVA-specific IgE in the serum were significantly increased in the asthmatic group compared with those in the NC group (P<0.05), whereas treatment with PWRE effectively decreased the levels of total IgE and OVA-specific IgE (Fig. 3).
Figure 3.
Effect of PWRE on the production of IgE in the serum. The levels of (A) the total or (B) OVA-specific IgE in serum were evaluated by ELISA kits. The absorbance was measured at 450 nm with a microplate reader. #P<0.05 vs. NC group; *P<0.05 vs. OVA group. PWRE, P. weinmannifolia root extract; IgE, immunoglobulin E; OVA, ovalbumin; MON, montelukast; NC, negative control.
Effect of PWRE on inhibiting inflammatory cell influx and mucus hypersecretion
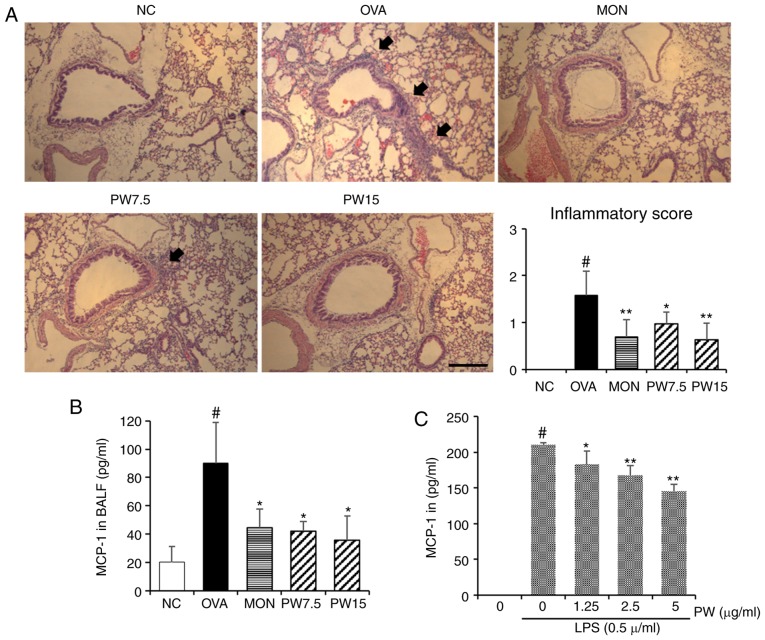
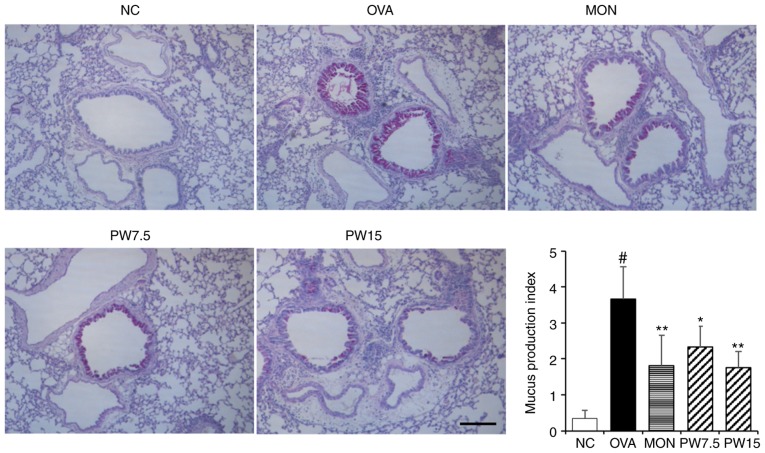
In order to investigate whether PWRE suppresses the OVA-induced inflammatory cell influx into the lungs, paraffin lung sections were stained with H&E in the present study. A significantly increased level of inflammatory cell influx was observed in the OVA group compared with the NC group (P<0.05; Fig. 4A). Notably, this level was down-regulated in the PWRE-treated group. The arrows point to the influx of inflammatory cells. The increased secretion of MCP-1 is closely associated with airway inflammation by inducing the influx of inflammatory cells (5,30). Therefore, the present study next assessed the inhibitory effect of PWRE on OVA-induced MCP-1 secretion. As presented in Fig. 4B, the marked increase in MCP-1 was observed in the BALF of the OVA group, whereas treatment with PWRE inhibited this secretion. In order to further investigate the regulatory effect of PWRE on MCP-1 secretion, the inhibitory effect of PWRE on MCP-1 was assessed in LPS-stimulated RAW264.7 macrophages. As presented in Fig. 4C, the administration of LPS significantly increased the MCP-1 secretion (P<0.05). However, pretreatment with PWRE significantly downregulated this secretion (P<0.05; Fig. 4C). Mucus hypersecretion is an prominent characteristic in the pathophysiology of allergic asthma (31). Therefore, the present study assessed whether PWRE led to an attenuation of the OVA-induced mucus overproduction. The paraffin lung sections were stained with the PAS staining reagent to measure the mucus production around the airways. As presented in Fig. 5, the levels of mucus production were significantly increased in the OVA group when compared with the NC group (P<0.05). However, a decrease in this level was observed in the PWRE group (Fig. 5). The mucus was stained a purple color by the PAS staining reagent.
Figure 4.
Effect of PWRE on the influx of inflammatory cells into the lungs and on the downregulation of MCP-1 secretion in LPS-stimulated RAW264.7 macrophages. (A) Hematoxylin and eosin staining was used to determine the level of inflammatory cell influx (peribronchial lesion; magnification, ×100; scale bar, 50 µm) and the degree of the inflammation score was assessed by two independent observers. An ELISA was used to determine the MCP-1 secretion level in the (B) BALF samples of allergic asthma and in the (C) LPS-stimulated RAW264.7 macrophages. #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. OVA group. PWRE, P. weinmannifolia root extract; MCP-1, monocyte chemoattractant protein-1; OVA, ovalbumin; MON, montelukast; BALF, bronchoalveolar lavage fluid; NC, negative control; LPS, lipopolysaccharide.
Figure 5.
Effect of PWRE on OVA-induced hyperproduction of mucus in the lungs. PAS staining was used to assess mucus production (peribronchial lesion, magnification, ×100; scale bar, 50 µm) and the degree of mucus production was assessed by two independent observers. #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. OVA group. PWRE, P. weinmannifolia root extract; NC, negative control; OVA, ovalbumin; MON, montelukast.
Effect of PWRE on decreasing MAPKs and NF-κB activation in the lungs
In order to investigate whether the airway inflammatory response was mediated by MAPK-responsive mechanisms, the present study evaluated the levels of ERK, JNK and p38 phosphorylation. As presented in Fig. 6, the levels of JNK, p38 and ERK were significantly upregulated in the OVA group compared with the NC group (P<0.05). However, 15 mg/kg PWRE significantly downregulated the enhanced activation of JNK, p38 and ERK in the lungs (P<0.05; Fig. 6). In order to further investigate the mechanism of PWRE, the NF-κB signaling pathway was assessed in the present study. As presented in Fig. 7, the activation of NF-κB p65 and IκBα was significantly upregulated in the OVA-exposed group compared with the NC group. However, this increase was effectively blocked by the PWRE treatment.
Figure 6.
Effect of PWRE on OVA-induced activation of MAPK molecules in the lungs. (A) The levels of JNK, p38 and ERK activation in the lung tissues were determined via western blot analysis. (B-D) Quantitative analysis of p-JNK, p-p38 and p-ERK was performed by densitometric analysis. #P<0.05 vs. NC group; *P<0.05 vs. OVA group. PWRE, P. weinmannifolia root extract; MAPKs, mitogen-activated protein kinases; JNK, c-Jun N-terminal kinase; p-ERK, phosphorylated-extracellular signal-regulated kinase; OVA, ovalbumin; MON, montelukast.
Figure 7.
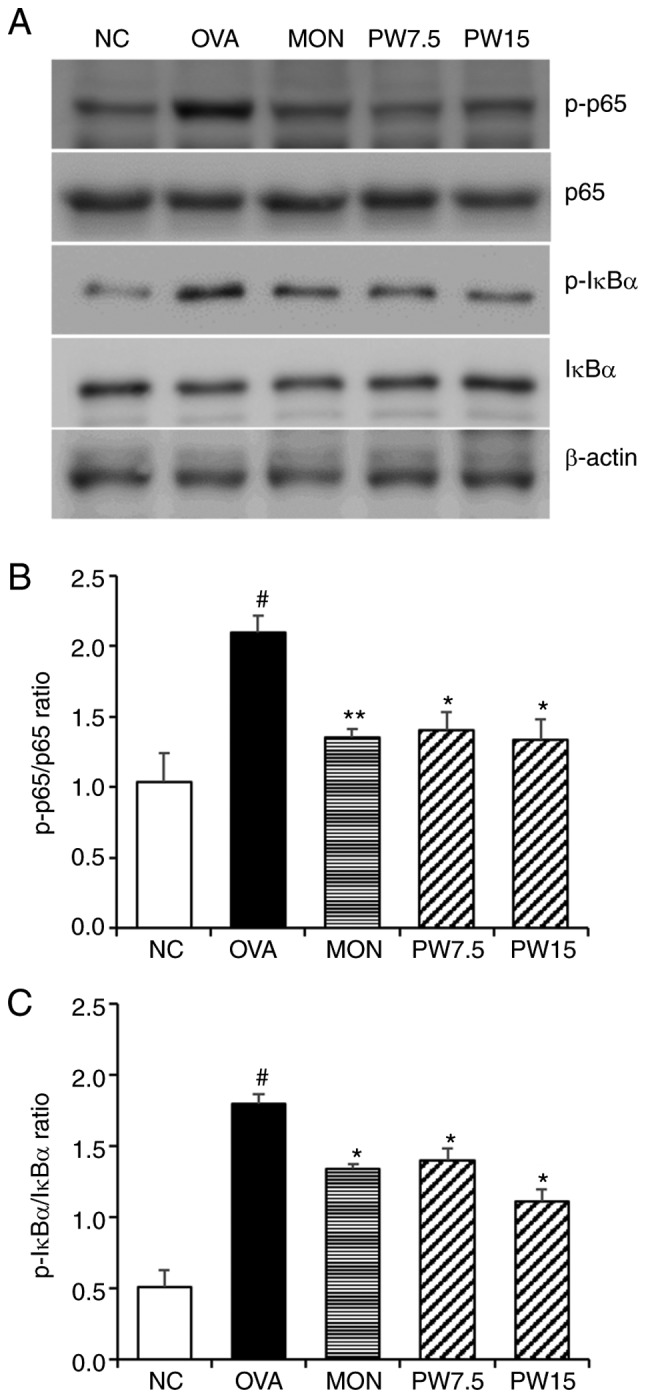
Effect of PWRE on OVA-induced activation of NF-κB p65 and IκBα molecules in the lungs. (A) The levels of NF-κB and IκB activation in the lung tissues were assessed via western blot analysis. (B) Quantitative analysis of p-NF-κB p65 and (C) p-IκB was performed by densitometric analysis. #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. OVA group. PWRE, P. weinmannifolia root extract; NF-κB, nuclear factor κB. IκB, inhibitor of NF-κB; OVA, ovalbumin; MON, montelukast.
Effect of PWRE on LPS-stimulated MAPKs and NF-κB activation in RAW264.7 macrophages
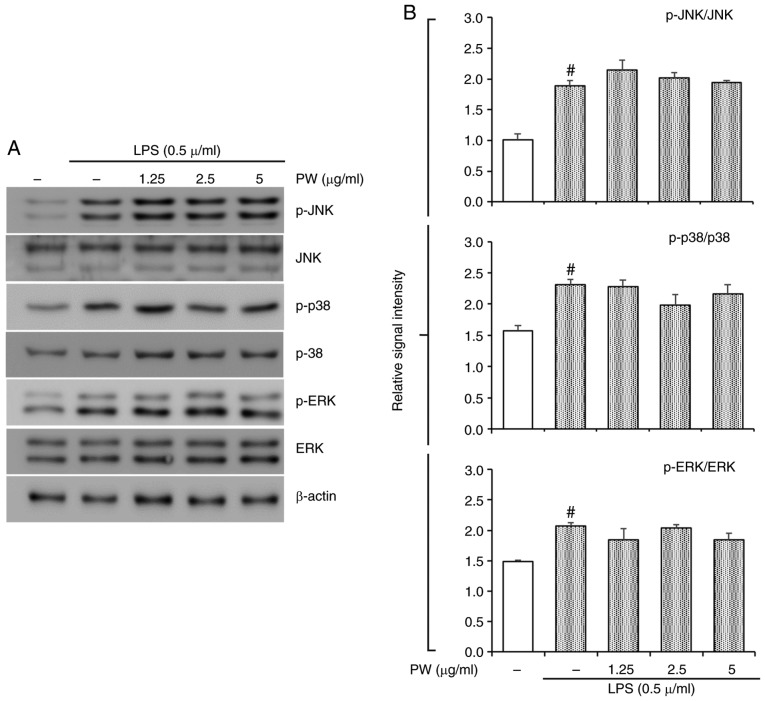
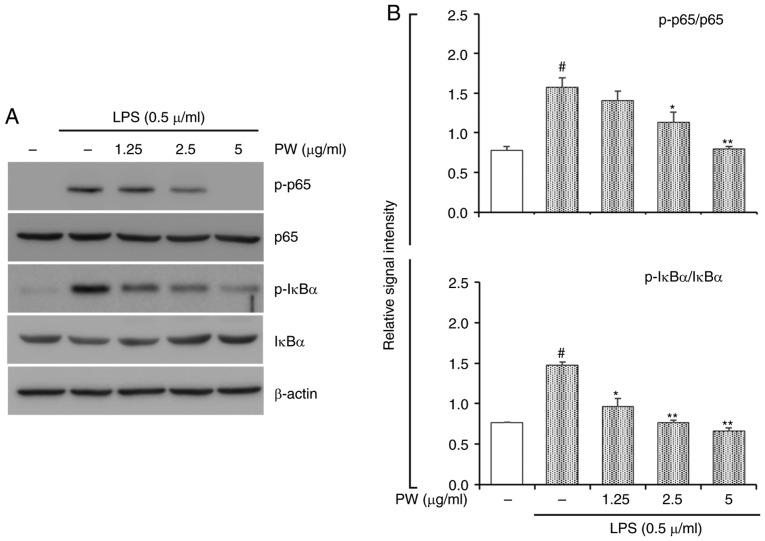
In the present study, PWRE exerted a protective effect in pulmonary inflammation in OVA-exposed mice. Its effects were accompanied by MAPK and NF-κB inactivation (Figs. 6 and 7). In particular, NF-κB activation was effectively downregulated upon PWRE administration. The results from the present study also demonstrated that PWRE regulates MCP-1 production in the BALF of OVA-exposure mice and in LPS-stimulated RAW264.7 macrophages (Fig. 4B and C). The regulatory effect of PWRE on LPS-stimulated MAPKs and NF-κB activation was therefore investigated in RAW264.7 macrophages. The administration of LPS significantly upregulated the activation of MAPKs and NF-κB (P<0.05; Figs. 8 and 9). However, the levels of JNK, p38 and ERK activation was not significantly downregulated by PWRE pretreatment (Fig. 8). Similar to those results presented in Fig. 7, the activation of NF-κB p65 and IκBα was significantly downregulated by ≥2.5 µg/ml PWRE pretreatment (P<0.05; Fig. 9).
Figure 8.
Effect of PW on LPS-stimulated activation of MAPK in RAW264.7 cells. (A) The levels of JNK, p38 and ERK activation were determined via western blot analysis. (B) Quantitative analysis of p-JNK, p-p38 and p-ERK was performed by densitometric analysis. #P<0.05 vs. NC group. PW, P. weinmannifolia root extract; LPS, lipopolysaccharide; p-ERK, phosphorylated-extracellular signal regulated kinase; JNK, c-jun n-terminal kinase; NC, negative control; MAPK, mitogen-associated protein kinase.
Figure 9.
Effect of PW on LPS-stimulated activation of NF-κB p65 and IκBα in RAW264.7 cells. (A) The levels of NF-κB and IκB activation were evaluated via western blot analysis. (B) Quantitative analysis of p-NF-κB p65 and p-IκB was performed by densitometric analysis. #P<0.05 vs. NC group; *P<0.05 and **P<0.01 vs. LPS only group. PW, P. weinmannifolia root extract; LPS, lipopolysaccharide; NF, nuclear factor.
Discussion
Previously, studies have demonstrated that PWRE exerts anti-inflammatory effects via downregulation of inflammatory molecules including IL-6 and IL-8, which are important parameters in chronic obstructive pulmonary disease (16,18,20). The present study extended the results of these previous publications, which demonstrate the protective effects of PWRE in OVA-induced pulmonary inflammation.
The airway inflammatory response is well known as a major cause of allergic asthma and is caused by a variety of inflammatory cells and molecules. IL-4 has been reported to differentiate native T cells into Th2 cells and induce class switching in B cells to IgE production (32,33). IL-5 has an important role in the maturation and recruitment of eosinophils, and IL-13 is recognized as a dominant factor for IgE class switching, eosinophil inflammation and mucus production (9). Eosinophil infiltration is well known as an indispensable indicator in airway inflammation and the increase of eosinophil cationic proteins leads to airway hyper-responsiveness (34). The high level of macrophages is also well known as a characteristic of the allergic asthma murine model and macrophage-derived MCP-1 is known as a potent eosinophil chemoattractant (5,30). Therefore, the regulation of eosinophil influx, Th2 cytokine secretion and IgE production are important therapeutic approaches in the treatment of asthma. OVA has been used as an allergen in asthma animal models and the utility of OVA-induced asthma model has been well established and this model has been widely used to evaluate anti-asthmatic effects and immunological mechanisms involved in the pathogenesis of asthma (35). In this study an allergic asthma mouse model, in which the levels of Th2 cytokines, IgE and mucus production were successfully upregulated by OVA compared with the NC control was established. In the present study, it was confirmed that PWRE administration attenuated OVA-induced eosinophils and macrophage recruitment. OVA-induced IL-4, IL-5, IL-13 and IgE were suppressed by the treatment of PWRE. In addition, the increased levels of MCP-1 were downregulated following PWRE treatment in both in vivo and in vitro studies. Therefore, the results from the present study suggest that PWRE has a protective role against OVA-induced pulmonary inflammation.
In normal circumstances, goblet cell-derived mucus exerts protective roles against harmful agents. However, the excessive production of mucus could easily obstruct breathing (36,37). Therefore, the regulation of mucus hypersecretion may be a valuable therapeutic strategy in alleviating airway obstruction. MUC5AC is a major oligomeric mucin in airway mucus and its level is upregulated in patients with asthma (38). The inhibitory activities of PWRE on MUC5AC secretion in PMA-stimulated airway epithelial cells have already been confirmed (20). Therefore, the regulatory effect of PWRE on mucus overproduction was expected in the present study and it was observed that PWRE ameliorated the OVA-induced mucus hypersecretion.
The MAPK and NF-κB signaling pathways are known as key mediators in allergic asthma, and are closely associated with the activation of various immune cells (39,40). Accumulating evidence emphasizes the importance of the inhibition of the MAPK pathway in airway inflammatory diseases such as asthma (9). Accordingly, the inhibitory effect of PWRE on MAPKs activation was assessed in the present study. It was subsequently confirmed that OVA-induced MAPKs activation was significantly decreased by PWRE treatment. In LPS-stimulated RAW264.7 macrophages, PWRE did not exert any inhibitory effects on MAPKs activation. It is well established that the activation of IκB leads to airway inflammation by inducing NF-κB activation and production of inflammatory molecules (41-43); therefore, the present study next investigated the ability of PWRE to inactivate NF-κB and IκB. Notably, PWRE exerted an inhibitory effect on OVA-induced NF-κB p65 and IκBα activation. Similar to the results presented, the inhibitory effect of PWRE was observed in IκBα and NF-κB activation in LPS-stimulated RAW264.7 macrophages. Therefore, the results from the present study suggest that the molecular mechanism underlying the protective effects of PWRE on pulmonary inflammation primarily regard the downregulation of NF-κB activation.
In the present study, PWRE inhibited the pulmonary inflammatory response by diminishing the recruitment of inflammatory cells and the concentration of IL-4, IL-5, IL-13 and IgE. PWRE also downregulated the levels of MCP-1 and mucus production. Notably, the effects of PWRE were accompanied by MAPKs and NF-κB inactivation. Abnormal weight changes and toxicological changes (such as intraperitoneal changes) were not observed after administration of PWRE. Therefore, the results from the present study suggest that PWRE may ameliorate airway inflammation and mucus hypersecretion in allergic asthma as a potential anti-inflammatory adjuvant. However, there was no evaluation of accurate count of inflammatory cells using flow cytometry. The levels of T-cell activation and eotaxin production in the pathogenesis of OVA-induced pulmonary inflammation have also not been investigated. It is necessary to confirm the inhibitory effect of PWER on MCP-1 in alveolar macrophages. These limitations should be addressed in the near future. In addition, the present study has limitations on the efficacy of PWRE in OVA-induced pulmonary inflammation. Therefore, clinical trials should be performed to elucidate this efficacy.
Acknowledgments
Not applicable.
Abbreviations
- OVA
ovalbumin
- BALF
bronchoalveolar lavage fluid
- IL-4
interleukin-4
- IL-5
interleukin-5, IL-13, interleukin-13
- IgE
immunoglobulin E
- MCP-1
monocyte chemoattractant protein-1
- MAPKs
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- p38
ERK, extracellular-signal-regulated kinase
- NF-κB
nuclear factor-κB
- IκB
inhibitor of NF-κB
Funding
The present study was supported by the Ministry of Trade, Industry and Energy and the Korea Institute for the Advancement of Technology (grant no. N0002410, 2017).
Availability of data and materials
All data generated and/or analyzed during the present study are included in this published article.
Authors' contributions
JWL performed the in vivo experiments and wrote the manuscript. JHM, MGK and SMK performed the in vivo experiments and contributed to the interpretation of the results. OKK performed the in vitro experiments. TKO, JKL and TYK contributed to the acquisition of data. SWL, SC, WYL, HWR and KSA made substantial contributions to the conception and design of the present study, acquisition of data, and the analysis and interpretation of data. SRO designed the present study and was involved in revising it critically for important intellectual content. All authors discussed the results and read and approved the final version of the manuscript.
Ethics approval and consent to participate
All experiments were approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (permit no. KRIBB-AEC-18054).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Xiong J, Liu S, Pan Y, Zhang B, Chen X, Fan L. Combination of fish oil and ethanol extracts from Spirulina platensis inhibits the airway inflammation induced by ovalbumin in mice. J Funct Foods. 2018;40:707–714. doi: 10.1016/j.jff.2017.12.014. [DOI] [Google Scholar]
- 2.Ye P, Yang XL, Chen X, Shi C. Hyperoside attenuates OVA-induced allergic airway inflammation by activating Nrf2. Int Immunopharmacol. 2017;44:168–173. doi: 10.1016/j.intimp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Pathophysiology of asthma. Br J Clin Pharmacol. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu X, Zhang Q, Du Q, Shen H, Zhu Z. Pinocembrin attenuates allergic airway inflammation via inhibition of NF-κB pathway in mice. Int Immunopharmacol. 2017;53:90–95. doi: 10.1016/j.intimp.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Schneider D, Hong JY, Bowman ER, Chung Y, Nagarkar DR, McHenry CL, Goldsmith AM, Bentley JK, Lewis TC, Hershenson MB. Macrophage/epithelial cell CCL2 contributes to rhinovirus-induced hyperresponsiveness and inflammation in a mouse model of allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L162–L169. doi: 10.1152/ajplung.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MG, Kim SM, Min JH, Kwon OK, Park MH, Park JW, Ahn HI, Hwang JY, Oh SR, Lee JW, Ahn KS. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int Immunopharmacol. 2019;74:105706. doi: 10.1016/j.intimp.2019.105706. [DOI] [PubMed] [Google Scholar]
- 7.Mizusaki A, Nishi K, Nishiwaki H, Ishida M, Tamamoto T, Sugahara T. Suppressive effect of ethanol extract from passion fruit seeds on IgE production. J Funct Foods. 2017;32:176–184. doi: 10.1016/j.jff.2017.02.030. [DOI] [Google Scholar]
- 8.Zhu X, Li Q, Hu G, Wang J, Hu Q, Liu Z, Wu G, Zhong Y. BMS-345541 inhibits airway inflammation and epithelial-mesen-chymal transition in airway remodeling of asthmatic mice. Int J Mol Med. 2018;42:1998–2008. doi: 10.3892/ijmm.2018.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Z, Nie H, Xu Y, Peng J, Zeng Y, Wei Y, Wen X, Qiu J, Zhong W, Deng X, He J. Therapeutic effects of rosmarinic acid on airway responses in a murine model of asthma. Int Immunopharmacol. 2016;41:90–97. doi: 10.1016/j.intimp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Jabara HH, Geha RS. Jun N-terminal kinase is essential for CD40-mediated IgE class switching in B cells. J Allergy Clin Immunol. 2005;115:856–863. doi: 10.1016/j.jaci.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Subhashini, Chauhan PS, Dash D, Paul BN, Singh R. Intranasal curcumin ameliorates airway inflammation and obstruction by regulating MAPKinase activation (p38, Erk and JNK) and prostaglandin D2 release in murine model of asthma. Int Immunopharmacol. 2016;31:200–206. doi: 10.1016/j.intimp.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004;4:1817–1828. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Zou XL, Pei DA, Yan JZ, Xu G, Wu P. A20 overexpression inhibits lipopolysaccharide-induced NF-κB activation, TRAF6 and CD40 expression in rat peritoneal mesothelial cells. Int J Mol Sci. 2014;15:6592–6608. doi: 10.3390/ijms15046592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Miao Z, Tian Y, Wang H, Wang S, He T, Yang Y, Wang P, Ma M, Yang T, et al. Limethason reduces airway inflammation in a murine model of ovalbumin-induced chronic asthma without causing side effects. Exp Ther Med. 2018;15:2269–2276. doi: 10.3892/etm.2018.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haslam E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J Nat Prod. 1996;59:205–215. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Sun H, Hou A, Zhao Q, Wei T, Xin W. Antioxidant properties of two gallotannins isolated from the leaves of Pistacia weinmannifolia. Biochim Biophys Acta. 2005;1725:103–110. doi: 10.1016/j.bbagen.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Wu X, Ji Y, Yang J. Isolation and characterization of microsatellite loci in Pistacia weinmannifolia (Anacardiaceae) Int J Mol Sci. 2011;12:7818–7823. doi: 10.3390/ijms12117818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minami K, Nakasugi T, Sun HD, Hou AJ, Ihara M, Morimoto M, Komai K. Isolation and identification of histamine-release inhibitors from Pistacia weinmannifolia J. Pisson ex Franch J Nat Med. 2006;60:138–140. doi: 10.1007/s11418-005-0017-z. [DOI] [Google Scholar]
- 19.Ci X, Chu X, Chen C, Li X, Yan S, Wang X, Yang Y, Deng X. Oxytetracycline attenuates allergic airway inflammation in mice via inhibition of the NF-κB pathway. J Clin Immunol. 2011;31:216–227. doi: 10.1007/s10875-010-9481-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Ryu HW, Lee SU, Kim MG, Kwon OK, Kim MO, Oh TK, Lee JK, Kim TY, Lee SW, et al. Pistacia weinmannifolia ameliorates cigarette smoke and lipopolysaccharide-induced pulmonary inflammation by inhibiting interleukin-8 production and NF-κB activation. Int J Mol Med. 2019;44:949–959. doi: 10.3892/ijmm.2019.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HA, Kwon OK, Ryu HW, Min JH, Park MW, Park MH, Paik JH, Choi S, Paryanto I, Yuniato P, et al. Physalis peru- viana L. inhibits ovalbumin-induced airway inflammation by attenuating the activation of NF-κB and inflammatory molecules. Int J Mol Med. 2019;43:1830–1838. doi: 10.3892/ijmm.2019.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Seo KH, Ryu HW, Yuk HJ, Park HA, Lim Y, Ahn KS, Oh SR. Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and LPS-induced murine model of acute lung injury. J Ethnopharmacol. 2018;210:23–30. doi: 10.1016/j.jep.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Yuk HJ, Lee JW, Park HA, Kwon Ok, Seo KH, Ahn KS, Oh SR, Ryu HW. Protective effects of coumestrol on lipopolysaccharide-induced acute lung injury via the inhibition of proinflammatory mediators and NF-κB activation. J Funct Foods. 2017;34:181–188. doi: 10.1016/j.jff.2017.04.027. [DOI] [Google Scholar]
- 24.He J, Lv L, Wang Z, Huo C, Zheng Z, Yin B, Jiang P, Yang Y, Li J, Gao Y, Xue J. Pulvis Fellis Suis extract attenuates ovalbumin-induced airway inflammation in murine model of asthma. J Ethnopharmacol. 2017;207:34–41. doi: 10.1016/j.jep.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Kao ST, Wang SD, Lin CC, Lin LJ. Jin Gui Shen Qi Wan, a traditional Chinese medicine, alleviated allergic airway hypersensitivity and inflammatory cell infiltration in a chronic asthma mouse model. J Ethnopharmacol. 2018;227:181–190. doi: 10.1016/j.jep.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Hizawa N, Yamaguchi E, Jinushi E, Konno S, Kawakami Y, Nishimura M. Increased total serum IgE levels in patients with asthma and promoter polymorphisms at CTLA4 and FCER1B. J Allergy Clin Immunol. 2001;108:74–79. doi: 10.1067/mai.2001.116119. [DOI] [PubMed] [Google Scholar]
- 27.Xia Z, Zhang Y, Li C, Xu Y, Dong J, Wang L, He Q, Zou X, Wu H, Han J, et al. Traditional Tibetan medicine Anzhijinhua San attenuates ovalbumin-induced diarrhea by regulating the serotonin signaling system in mice. J Ethnopharmacol. 2019;236:484–494. doi: 10.1016/j.jep.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Yang N, Shang YX. Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model. Int Immunopharmacol. 2019;72:422–428. doi: 10.1016/j.intimp.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Paiva Ferreira LKD, Paiva Ferreira LAM, Alves AF, Leite FC, de Araújo Silva LA, Vieira GC, Rodrigues LC, Piuvezam MR. MHTP, 2-Methoxy-4-(7-methoxy- 1,2,3,4-tetrahydroiso-quinolin-1-yl) phenol, a synthetic alkaloid, induces IFN-γ production in murine model of ovalbumin-induced pulmonary allergic inflammation. Inflammation. 2018;41:2116–2128. doi: 10.1007/s10753-018-0855-y. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TH, Maltby S, Simpson JL, Eyers F, Baines KJ, Gibson PG, Foster PS, Yang M. TNF-α and macrophages are critical for respiratory syncytial virus-induced exacerbations in a mouse model of allergic airways disease. J Immunol. 2016;196:3547–3558. doi: 10.4049/jimmunol.1502339. [DOI] [PubMed] [Google Scholar]
- 31.Bakar NA, Anyanji VU, Mustapha NM, Lim SL, Mohamed S. Seaweed (Eucheuma cottonii) reduced inflammation, mucin synthesis, eosinophil infiltration and MMP-9 expressions in asthma-induced rats compared to Loratadine. J Funct Foods. 2015;19:710–722. doi: 10.1016/j.jff.2015.10.011. [DOI] [Google Scholar]
- 32.Alvaro M, Sancha J, Larramona H, Lucas JM, Mesa M, Tabar AI, Martinez-Cañavate A, Immunotherapy Working Group. Sociedad Española de Inmunología Clínica y Alergia Pediátrica (SEICAP) Allergen-specific immunotherapy: Update on immunological mechanisms. Allergol Immunopathol (Madr) 2013;41:265–272. doi: 10.1016/j.aller.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Hu M, Zhang Q, Yu J, Su W. Effects of anthraqui-nones from Cassia occidentalis L. on ovalbumin-induced airways inflammation in a mouse model of allergic asthma. J Ethnopharmacol. 2018;221:1–9. doi: 10.1016/j.jep.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 34.SAun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: Utility and limitations. J Asthma Allergy. 2017;10:293–301. doi: 10.2147/JAA.S121092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Wang M, Li S, Wu H, Shen Q, Zhang S, Fang L, Liu R. Nebulized lidocaine ameliorates allergic airway inflammation via downregulation of TLR2. Mol Immunol. 2018;97:94–100. doi: 10.1016/j.molimm.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Huang S, Kang J, Lin J, Lai K, Sun Y, Xiao W, Yang L, Yao W, Cai S, et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition) Int J Chron Obstruct Pulmon Dis. 2018;13:399–407. doi: 10.2147/COPD.S144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Huang L, Wang N, Yi H, Wang H. Sulfur dioxide exposure enhances Th2 inflammatory responses via activating STAT6 pathway in asthmatic mice. Toxicol Lett. 2018;285:43–50. doi: 10.1016/j.toxlet.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 39.Huang CQ, Li W, Wu B, Chen WM, Chen LH, Mo GW, Zhang QF, Gong L, Li J, Zhang HC, et al. Pheretima aspergillum decoction suppresses inflammation and relieves asthma in a mouse model of bronchial asthma by NF-κB inhibition. J Ethnopharmacol. 2016;189:22–30. doi: 10.1016/j.jep.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Wang L, Chen B, Zhuo Q, Bao C, Lin L. Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. Int Immunopharmacol. 2017;51:158–164. doi: 10.1016/j.intimp.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW, Chun W, Kwon OK, Park HA, Lim Y, Lee JH, Kim DY, Kim JH, Lee HK, Ryu HW, et al. 3,4,5-Trihydroxycinnamic acid attenuates lipopolysaccharide (LPS)-induced acute lung injury via downregulating inflammatory molecules and upregulating HO-1/AMPK activation. Int Immunopharmacol. 2018;64:123–130. doi: 10.1016/j.intimp.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Lee K, Choi J, Choi BK, Gu YM, Ryu HW, Oh SR, Lee HJ. Picroside II Isolated from Pseudolysimachion rotundum var. subintegrum inhibits glucocorticoid refractory serum amyloid A (SAA) expression and SAA-induced IL-33 secretion. Molecules. 2019;24 doi: 10.3390/molecules24102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during the present study are included in this published article.