Abstract
Synaptic dopamine (DA) is mainly regulated by the presynaptic DA transporter (DAT). Single-photon emission computerized tomography (SPECT) with the DAT radiotracer [123I]FP-CIT assesses changes in synaptic DA availability when endogenous DA displaces [123I]FP-CIT or competes for DAT. Here, we investigated the effects of haloperidol (HAL) and clozapine (CLZ) on [123I]FP-CIT binding in the rat striatum and midbrain to assess the utility of [123I]FP-CIT SPECT to quantify changes in synaptic DA availability. Rats underwent [123I]FP-CIT SPECT after intraperitoneal administration of normal saline (vehicle), HAL (1 and 7 mg/kg), CLZ (10 and 54 mg/kg) and bupropion (BUP, a DAT blocker, 20 and 100 mg/kg). In the striatum and midbrain, percent differences in the nondisplaceable binding potential (BPND) of [123I]FP-CIT compared to the vehicle were calculated for the various drugs and doses. In another experiment, changes in endogenous striatal DA concentration were measured by in vivo microdialysis under the conditions used in the SPECT study. BUP dose-dependently occupied DAT at considerable levels. Compared to the vehicle, HAL decreased [123I]FP-CIT BPND in the striatum (−25.29% and −2.27% for 1 and 7 mg/kg, respectively) and to a greater degree in the midbrain (−58.74% and −49.64% for 1 and 7 mg/kg, respectively), whereas the CLZ-treated group showed a decrease in the midbrain (−38.60% and −40.38% for 10 and 54 mg/kg, respectively) but an increase in the striatum (18.85% and 38.64% for 10 and 54 mg/kg, respectively). Antipsychotic-induced changes in endogenous striatal DA concentrations varied across drugs and doses. The data demonstrate that [123I]FP-CIT SPECT may be a useful preclinical technique for detecting increases in synaptic DA availability in the midbrain and striatum in response to HAL, with results comparable to those of in vivo microdialysis.
Keywords: [123I]FP-CIT SPECT, Dopamine availability, Haloperidol, Clozapine, In vivo microdialysis
INTRODUCTION
Synaptic dopamine (DA) availability is implicated in the pathology of various neurological and psychiatric diseases, such as Parkinson’s disease [1, 2] and schizophrenia [3, 4]. DA availability in the brain is mainly regulated by the DA transporter (DAT) [5], and DA receptors for its D2 and D3 subtypes expressed on the cell membrane of DA neurons (i.e., autoreceptors) also play a key role in regulating the activity of DAergic neurons and controlling DA synthesis, release, and reuptake [6]. Under the DA hypothesis, antipsychotic drugs act mainly by regulating the DAergic systems, as evidenced by the significant association between DA receptor antagonism and improved positive and/or negative symptoms. Some of them have been proven to regulate DA release [6–8]. An antipsychotic drug haloperidol (HAL) is known to increase DA synthesis and release in the striatum and related mesolimbic structures [9–12]. The proposed mechanism of action consists of presynaptic terminal blockade by DA autoreceptors [11], which abolishes feedback inhibition, leading to enhanced DA synthesis or release. Clozapine (CLZ), despite acting as a DA antagonist as part of its therapeutic effect against schizophrenia symptoms, also stimulates DA release in the ventral striatum (i.e., nucleus accumbens) of rats [13] and in the hippocampus of schizophrenia patients [14]. Presynaptic modulation induced by antipsychotic drugs affects the extracellular DA concentration by altering autoregulation [15]. An increased extracellular DA concentration can lead to stimulation of DA autoreceptors, which inhibits DA release [6, 16]. DA release and metabolism in the rat striatum in vivo were reported to be differentially modulated by typical and atypical antipsychotic drugs, e.g., HAL and CLZ, respectively [17, 18]. HAL and CLZ may have different effects on the release of DA according to their pharmacological characteristics and whether they are typical or atypical antipsychotics.
The competition between endogenous transmitters and radio-labeled ligands for in vivo binding to receptors and transporters may provide a method to measure endogenous neurotransmitter release in the living central nervous system with noninvasive molecular imaging techniques from nuclear medicine. This method is valuable for studying altered neuronal activity in anatomical and chemical systems in several neurologic and psychiatric conditions and for assessing the regulatory effect of drugs on their target systems. [123I]N-omega-fluoropropyl-2-beta-carbomethoxy-3-beta-(4-iodophenyl)nortropane ([123I]FP-CIT) single-photon emission computerized tomography (SPECT) is used in scientific studies in humans and is also used in preclinical studies to assess the integrity of the nigrostriatal system in the diagnosis of Parkinson’s disease [19–21] and schizophrenia [22, 23]. [123I]FP-CIT SPECT is increasingly used for diagnostics and drug development for neurological and psychiatric diseases as well as for precision medicine in cases of complicated medication status—for instance, a Parkinson’s disease patient receiving antipsychotic drugs. Consequently, there is a need for further experimental evidence supporting the utility of [123I]FP-CIT SPECT for quantitation of changes in DA availability. For example, it remains controversial whether changes in synaptic DA availability induced by acute treatment with antipsychotic drugs such as HAL can be quantitatively assessed by [123I]FP-CIT SPECT. In one study, DAT imaging with [123I]FP-CIT SPECT revealed acute changes in DAT activity with a 25% baseline reduction in the specific-to-nonspecific binding ratio at equilibrium (V3″) of [123I]FP-CIT after acute HAL administration (1 mg/kg i.p.), possibly because the increased levels of endogenous DA displaced [123I]FP-CIT or competed for presynaptic binding sites [24]. However, based on results reproduced from this experiment, another investigator argued that synaptic changes in DA levels resulting from acute HAL administration were not detectable using storage phosphor imaging with [123I]FP-CIT in rats [25] because the dose was too low to alter [123I]FP-CIT binding.
We investigated differences in the nondisplaceable binding potential of [123I]FP-CIT in the rat striatum and midbrain, which compose the nigrostriatal DA system, after HAL and CLZ compared to vehicle treatment in an attempt to assess the validity of [123I]FP-CIT SPECT as a measure of synaptic DA availability induced by HAL and CLZ. The reliability of this noninvasive imaging technique was further examined for the striatum by conducting an in vivo microdialysis study that directly measured the changes in DA concentration; this method, having been proposed as a measure of the magnitude of DA release, was selected for the important task of investigating the relationship between antipsychotic drug-induced DA release and [123I]FP-CIT displacement. We will discuss the combined results of the [123I]FP-CIT SPECT study (differences in [123I]FP-CIT binding) and the in vivo microdialysis study (changes in DA concentration).
MATERIALS AND METHODS
Animals and drugs
This study was approved by the Institutional Animal Care and Use Committee of the Seoul National University Bundang Hospital. Animals were purchased from Orient Bio Inc., Seoul, Korea. A total of 35 and 20 Sprague Dawley (SD) rats (male, 6-week-old, 260~300 g body weight) were used for [123I]FP-CIT SPECT and in vivo microdialysis studies, respectively. The animals were physiologically acclimated for one week in a clean room after arriving (12 h/12 h light/dark cycle, temperature 20~25°C, relative humidity 40~60%). The rats were fasted for at least 6 h before the [123I]FP-CIT SPECT study but were provided water ad libitum.
HAL, CLZ and bupropion (BUP) hydrochloride were purchased from Sigma-Aldrich Korea, Yongin, Korea. HAL and CLZ were dissolved in 1% tartaric acid and 1 N HCl. A DAT blocker, BUP, the positive control drug for HAL and CLZ, was used to test whether endogenous DA displaces [123I]FP-CIT or BUP competes with [123I] FP-CIT for DAT. BUP was dissolved in NS.
In the [123I]FP-CIT SPECT study, the animals were divided into four groups defined by different drugs (vehicle (n=5), HAL (n=10), CLZ (n=10), BUP (n=10) treatment groups). While the vehicle-treated group had no dose conditions, the others had low and high dose conditions for the drug treatment. The low and high doses of HAL, CLZ and BUP were 1 and 7 mg/kg body weight, 10 and 54 mg/kg body weight, and 20 and 100 mg/kg body weight, respectively (n=5 per drug and dose condition). The doses were selected based on previous studies showing that low and high doses induce ~25% increases in synaptic DA availability and greater than 80% DA receptor occupancy by drugs in the striatum, respectively [24–27].
In another experiment, changes in endogenous DA concentration in the striatum were monitored by in vivo microdialysis under the same conditions as in the [123I]FP-CIT SPECT study (n=5 per drug and dose condition).
In both experiments, drugs were injected intraperitoneally (i.p.) while the animals were awake.
[123I]FP-CIT SPECT/CT study
The [123I]FP-CIT SPECT/CT study was performed on a dedicated small-animal SPECT/CT system (NanoSPECT/CT, Mediso Inc., Budapest, Hungary). Helical small-animal SPECT scans were performed using a 4-head γ-camera outfitted with multipinhole collimators (1.4-mm-diameter pinholes) designed for rats. [123I] FP-CIT was injected at a dose (mean±SD) of 39.5±7.2 MBq 1 h after drug treatment; 2 h later (once [123I]FP-CIT had reached equilibrium in the striatum), SPECT/CT data were acquired from the animals for 30 min under 2% isoflurane anesthesia. After the scan, the SPECT data were reconstructed using iterative three-dimensional ordered subset expectation maximization with the single-slice rebinning method. CT-based attenuation correction was performed, as were scatter and random correction. The reconstructed images were 176×176×136 pixels with a voxel size of 0.6×0.6×0.6 mm (x, y, z). PMOD software (PMOD Technologies LLC., Geneva, Switzerland) was used for processing and analysis of SPECT and CT images. Images were spatially normalized to standard stereotaxic space with the predefined magnetic resonance imaging (MRI) rat brain template. The striatum, midbrain and cerebellum were defined using automated anatomical labeling embedded in PMOD software [28]. Synaptic DA availability in the striatum and midbrain was quantitatively assessed in terms of the nondisplaceable binding potential (BPND) of [123I]FP-CIT, which is proportional to the density of available binding sites (i.e., DAT). The cerebellum (which is known as a DAT-poor region or nondisplaceable binding site of [123I]FP-CIT) was set as the reference region for estimating BPND according to the following equation: BPND=(CT-CND)/CND=CT/CND-1, where CT and CND are the concentrations of [123I]FP-CIT in the striatum or midbrain and in the cerebellum, respectively.
In vivo microdialysis study
Extracellular DA concentrations in the striatum of freely moving rats were directly measured by in vivo microdialysis following HAL and CLZ treatment. Rats were anesthetized with a mixture of Alfaxan (0.3 ml/g, i.p.) and Rompun (0.05 ml/g, i.p.). A guide cannula (CMA/12; CMA Microdialysis, Solna, Sweden) was stereotaxically implanted such that it terminated in the center of the striatum (anteroposterior: 1.0 mm; lateral: 3.2 from the bregma; height: 3.0 from the dura) [29] and attached to the skull using skull screws and dental cement. The cannula was then closed with a tight-fitting stainless-steel obturator. Following 3~4 days of recovery, a 4-mm microdialysis probe (CMA/12; CMA Microdialysis) connected via a dual liquid swivel to a syringe pump was inserted into the guide cannula and perfused with artificial cerebrospinal fluid (Harvard Apparatus, Cambridge, UK) at a constant rate of 1.5 ml/min. In order to monitor changes in extracellular DA concentration on the same time scale as the [123I]FP-CIT SPECT study, dialysate samples were collected at 20-min intervals for 1 h at baseline and 3 h after drug treatment via an outlet tube connected to a microfraction collector (CMA Microdialysis). The dialysate (injection volume of 15 μL) was assayed for DA by high-performance liquid chromatography (HPLC) with an API 4000QTRAP detection system (ABSCIEX, Foster City, CA, USA). The mobile phase consisted of 0.1% formic acid in distilled water and HPLC-grade acetonitrile. A Luna hydrophilic interaction liquid chromatography (HILIC) column (3-μm particle size, 2.0×50 mm) was used, and the flow rate of the system was 0.25 ml/min. The DA level in dialysates is expressed as a percentage of the three baseline samples collected immediately before drug treatment. For the analysis, only results derived from healthy rats with correctly positioned dialysis probes were included in the data analysis. The mean DA level across the 3 baseline timepoints was designated as 0% at 0 min.
Statistical analysis
Data were analyzed using GraphPad Prism (version 7.0, Graph-Pad Software Inc., La Jolla, CA, USA). The statistical significance of differences in mean BPND between drug and dose conditions was tested by two-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test between dose conditions for each drug. In vivo microdialysis data were statistically tested with repeated-measures ANOVA, followed by Dunnett’s multiple comparison test for the factor of time. p<0.05 was considered statistically significant. Values are represented as the mean±SEM.
RESULTS
In the vehicle-treated group, the mean [123I]FP-CIT BPND was 1.64±0.13 and 1.69±0.32 in the striatum and midbrain, respectively; these values are comparable to the results of previous studies [24, 30]. BUP dose-dependently occupied DAT to a considerable degree, as evidenced by decreases in [123I]FP-CIT BPND of −16.50% (20 mg/kg) and −56.29% (100 mg/kg) in the striatum and −31.57% (20 mg/kg) and −53.08% (100 mg/kg) in the midbrain, implying that [123I]FP-CIT SPECT is a reliable and sensitive technique for measuring drug-induced changes in DAT activity and can conceptually allow the assessment of changes in synaptic DA availability in vivo when endogenous DA displaces [123I]FP-CIT or competes for DAT.
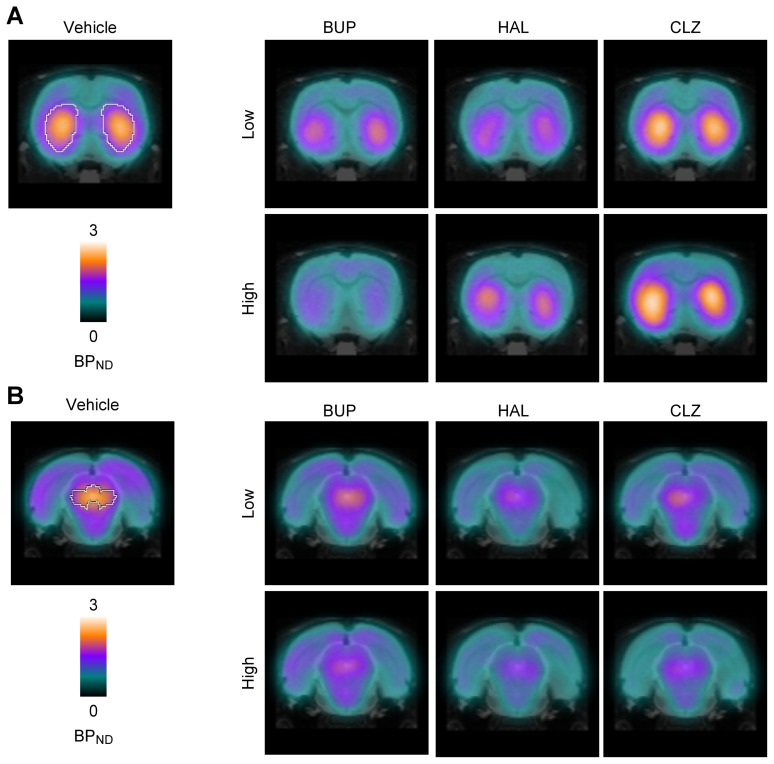
Treatment with HAL and CLZ markedly altered synaptic DA availability compared to the vehicle, as evidenced by changes in [123I]FP-CIT BPND in both the striatum and the midbrain. Intriguingly, the level of changes in [123I]FP-CIT BPND treatment varied across drugs, doses, and regions (Fig. 1 and Table 1). Compared to the vehicle, HAL decreased [123I]FP-CIT BPND in the striatum (−25.29% and −2.27% for 1 and 7 mg/kg, respectively) and to a greater degree in the midbrain (−58.74% and −49.64% for 1 and 7 mg/kg, respectively), whereas the CLZ-treated group showed an increase in the striatum (18.85% and 38.64% for 10 and 54 mg/kg, respectively) but a decrease in the midbrain (−38.60% and −40.38% for 10 and 54 mg/kg, respectively).
Fig. 1.
Group mean (n=5 per drug and dose condition) BPND parametric images comparing changes in [123I]FP-CIT BPND in the striatum (A) and midbrain (B) after treatment with BUP, HAL and CLZ. These changes are also summarized in graphs (C). VEH, BUP, HAL and CLZ represent the vehicle-, bupropion-, haloperidol- and clozapine-treated groups. The low and high doses of HAL, CLZ and BUP were 1 and 7 mg/kg body weight, 10 and 54 mg/kg body weight, and 20 and 100 mg/kg body weight, respectively. Values are the mean±SEM. White lines overlaid on the SPECT images of the striatum (A) and midbrain (B) for the vehicle-treated group show the margin of each region.
Table 1.
Differences in [123I]FP-CIT BPND
| Drug | Dose (mg/kg) | Striatum | Midbrain | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| BPND | % Difference | p | BPND | % Difference | p | ||
| VEH | - | 1.64±0.13 | - | - | 1.69±0.32 | - | - |
| BUP | 20 | 1.37±0.22 | −16.50±13.41 | 0.3954 | 1.16±0.16 | −31.57±9.26 | 0.2165 |
| 100 | 0.72±0.08 | −56.29±4.59 | 0.0006 | 0.79±0.12 | −53.08±7.21 | 0.0376 | |
| HAL | 1 | 1.22±0.09 | −25.29±5.60 | 0.0749 | 0.70±0.12 | −58.74±7.38 | 0.0041 |
| 7 | 1.60±0.06 | −2.27±3.65 | 0.9797 | 0.85±0.05 | −49.64±3.13 | 0.0215 | |
| CLZ | 10 | 1.95±0.20 | 18.85±12.00 | 0.2257 | 1.04±0.11 | −38.60±6.53 | 0.0668 |
| 54 | 2.27±0.13 | 38.64±7.87 | 0.0061 | 1.01±0.05 | −40.38±2.76 | 0.0676 | |
Values are the mean±SEM. VEH, vehicle; BUP, bupropion; HAL, haloperidol; CLZ, clozapine.
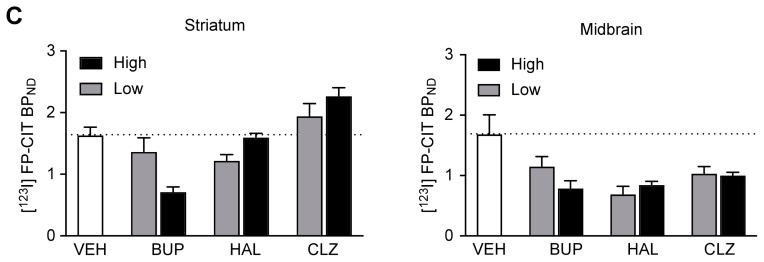
The changes in extracellular striatal DA concentrations by HAL (1 and 7 mg/kg) and CLZ (10 and 54 mg/kg) treatment were evaluated by in vivo microdialysis (Fig. 2). Despite the lack of statistical significance in the entire dataset by repeated-measures ANOVA, immediately after the treatment, a low dose of HAL (1 mg/kg) induced a 17.96%±24.90% increase in DA concentration compared to the baseline, after which DA returned to the baseline level during the study; in contrast, a high dose of HAL (7 mg/kg) induced a decrease in DA concentration that was preceded by a maximal 55.36%±67.22% baseline increase immediately after the treatment (Fig. 2A). We smoothed the temporal changes by using the time-averaged % changes. The time-averaged % change, which also has unit of % baseline, was calculated by averaging the % baseline values of individual dialysate samples along the axis of time, from the time of drug treatment to the end of the study. Overall % baseline values were summed and divided by the number of samples regardless of the time of measurement. The time-averaged percentages of change from baseline for the low and high doses of HAL were 7.48±3.48 and −17.24±9.29% baseline, respectively, corresponding to changes in [123I]FP-CIT BPND (Fig. 2B). Both doses of CLZ resulted in increased DA concentrations, but the time lag was longer than that of HAL, as the maximal changes appeared late in the measurement period, with 43.53±29.21% and 49.10±19.25% increases from baseline, respectively, whereas immediately after the treatment, the changes from baseline were 22.82±10.70% and 16.26±27.52% for 10 and 54 mg/kg CLZ, respectively (Fig. 2C). The time-averaged percentage changes from baseline for 10 and 54 mg/kg CLZ were 8.98±7.00% and 17.13±4.94%, respectively (Fig. 2D).
Fig. 2.
Time course of the effects of HAL (A) and CLZ (C) treatment on DA release in the rat striatum (n=5 per drug and dose condition). Time-averaged (from the time of drug treatment to the end of the study) percentage changes from baseline for each dose of HAL (B) and CLZ (D). HAL and CLZ represent the haloperidol- and clozapine-treated groups. The low and high doses of HAL and CLZ were 1 and 7 mg/kg body weight and 10 and 54 mg/kg body weight, respectively. The value is designated as 0% at time 0, and drugs were treated at time=0 min. Values are the mean±SEM.
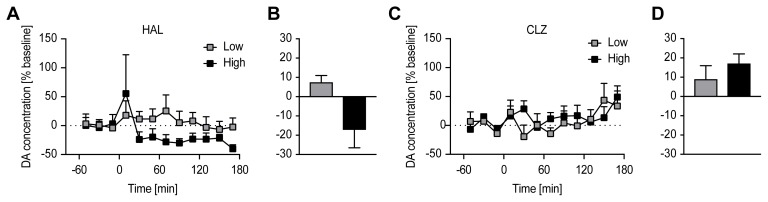
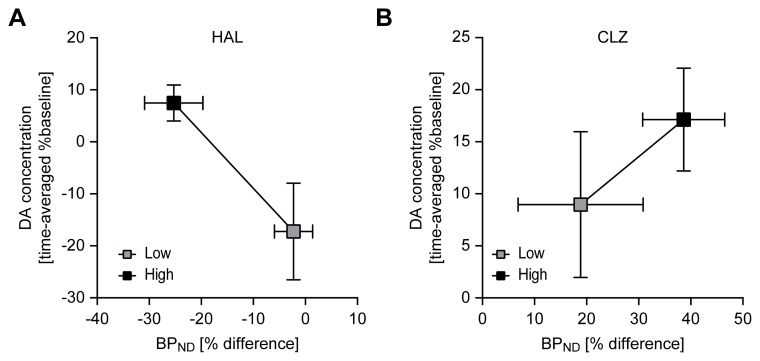
The relationships between percentage differences in [123I]FP-CIT BPND and time-averaged percentage changes from baseline extracellular DA concentration after treatment with varying doses of HAL and CLZ are depicted in Fig. 3 (A and B for HAL and CLZ, respectively). An inverse relationship between percentage differences in [123I]FP-CIT BPND and time-averaged % percentage change from baseline extracellular DA concentration (lower [123I] FP-CIT BPND, greater synaptic DA availability) appeared only in the HAL-treated group.
Fig. 3.
Relationship between percentage differences in [123I] FP-CIT BPND (x-axis) and time-averaged percentage changes from baseline extracellular DA concentration (y-axis) after treatment with varying doses of HAL and CLZ. HAL and CLZ represent the haloperidol- and clozapine-treated groups. The low and high doses of HAL and CLZ were 1 and 7 mg/kg body weight and 10 and 54 mg/kg body weight, respectively. Values are the mean±SEM.
DISCUSSION
Under the investigational assumption that if endogenous DA displaces radioligands or competes with them for presynaptic binding sites, radioligand binding to the DAT could be affected, we investigated differences in the [123I]FP-CIT BPND in the rat striatum and midbrain, which compose the nigrostriatal DA system, after HAL and CLZ treatments that increased synaptic DA availability. The reliability of this noninvasive imaging technique was further examined for the striatum by using an in vivo microdialysis study that directly measured the changes in DA concentration induced by HAL and CLZ.
Our results showed that [123I]FP-CIT SPECT allows the detection of apparent increases in synaptic DA concentration induced by low (1 mg/kg i.p.) and high doses (7 mg/kg i.p.) of HAL in both the striatum and midbrain in terms of reduced [123I]FP-CIT BPND. The [123I]FP-CIT SPECT results for the striatum were compatible with those obtained in the in vivo microdialysis study. For instance, elevated extracellular DA concentration corresponded to decreased or increased [123I]FP-CIT BPND in the striatum induced by 1 or 7 mg/kg HAL, respectively (Fig. 3A). Although the high dose (7 mg/kg) of HAL unexpectedly induced an 18% decrease from baseline in striatal DA concentration determined by in vivo microdialysis, this change corresponded to an elevation in [123I]FP-CIT BPND. The decreased DA concentration after the high-dose (7 mg/kg) HAL treatment may be attributable to desensitization of autoreceptors for the DA D2 and DA D3 subtypes and the consequent loss of feedback excitation by DA or to activation of monoamine oxidase B (MAO-B) to maintain the homeostasis of DAergic neurotransmission. MAO plays a major role in the inactivation of multiple catecholamines, including DA, and synaptic DA is mainly metabolized by MAO-B in the brain [31]. The 7 mg/kg HAL was high enough to abnormally increase synaptic DA concentration in rats and may cause MAO activation. One study demonstrated that HAL inhibited DA release in the striatum and in the nucleus accumbens using in vivo electrochemical techniques and provided evidence for the induction of a depolarization block of DA cell firing as a possible mechanism underlying this effect [32, 33].
The [123I]FP-CIT SPECT and in vivo microdialysis techniques detected changes in striatal synaptic DA availability and extracellular DA concentrations induced by CLZ treatment. However, CLZ reduced [123I]FP-CIT BPND in the midbrain while increasing the value in the striatum. Moreover, direct measurements of elevated striatal DA concentrations (8.98±7.00% and 17.13±4.94% increases from baseline induced by low and high doses of CLZ, respectively) did not affect [123I]FP-CIT BPND. The discrepancy shown here as increases in both [123I]FP-CIT BPND and DA concentration after CLZ treatment was unexpected (Fig. 3B), although we expected that the degree of change in [123I]FP-CIT BPND in the brain would differ between HAL and CLZ since their binding targets and temporal behavior with respect to the target were distinct. This may be due to differences in the pharmacodynamics profiles of CLZ, time course of drug action at the target site, and experimental protocol (time window) in the [123I]FP-CIT SPECT scan. The results from the in vivo microdialysis study showed that the increases in extracellular DA concentration induced by CLZ follow distinct time courses from the increases induced by HAL. The maximum striatal DA concentration was reached between 60 and 80 min after treatment with HAL, whereas CLZ showed a more prolonged effect on releasing DA. In the present study, HAL and CLZ were administered 30 min before [123I]FP-CIT application. Thus, the radioligand was administered before attaining the maximum striatal DA concentration induced by HAL but after reaching that induced by CLZ. Since [123I]FP-CIT—which requires 2 h to reach a state of equilibrium [34]—was injected 30 min after antipsychotic drug treatment, the time course of the drug action may have been missed. Thus, it is possible that the time window for [123I]FP-CIT detection did not cover the maximal DA concentration induced by CLZ treatment, leading to an error in the measurement of [123I]FP-CIT BPND. The elevation in [123I]FP-CIT BPND following CLZ treatment could instead represent a decrease in DA availability in the recovery phase of drug action. Although our data did not provide evidence supporting previous explanations (i.e., the DA concentration during the late phase was increased rather than decreased), this outcome could be due to variations between datasets acquired from different animals.
Although it is possible that there were experimental errors and unknown contamination factors that could have led to these results, the magnitude of error in the measured values (BPND and DA concentration) was quite acceptable. The reliability of increased striatal [123I]FP-CIT BPND could be validated from decreases in the midbrain of the same subject on CLZ treatment. In the midbrain, non-dose-dependent decreases in [123I]FP-CIT binding after treatment were consistently shown among drugs (although the DAT blocker BUP occupied DAT dose-dependently). Regardless of dose, the %difference was greater for HAL (−49.64~58.74% difference) than CLZ (−38.60 to −40.38% difference) in accordingly with pharmacologic characteristics in DA regulation of HAL and CLZ, implying their own typicality. That CLZ affects increasing binding affinity to DAT is unlikely, but it is not impossible, as some ligands occasionally act in this way. For example, an antiepileptic drug, tiagabine, which binds to the central benzodiazepine receptor, affects the increased binding affinity of radiolabeled ligand ([18F] flumazenil) to the central benzodiazepine receptor, known as the “GABA shift” [35]. On the other hand, the interaction between CLZ and [123I]FP-CIT could also be considered. The potential effect of CLZ on the group of antipsychotic drugs was examined; for instance, only CLZ induced a decrease in the protein kinase C level [36], but the effect of decreased protein kinase C level and interaction between [123I]FP-CIT and DAT is unexpected.
In addition, this study examined changes in synaptic DA availability after acute administration of HAL and CLZ in an attempt to resolve the previously reported controversy [12, 13] through an analogous approach to the experimental paradigm and to determine whether [123I]FP-CIT SPECT can be used to assess changes in endogenous DA concentration based on alterations in [123I]FP-CIT BPND to DAT as it is displaced by endogenous DA. Importantly, we partly overcame the experimental limitations of the earlier studies by performing in vivo microdialysis to eliminate technical (imaging vs. direct measurement) and conceptual (changes in DAT radiotracer binding potential vs. DA concentration) differences in our measurements and by including additional experimental variables such as another drug that has a common mode of action in increasing synaptic DA concentrations, varying doses of the drug, and multiple brain regions implicated in DAergic neurotransmission. These observations raised concerns about the validity of these measurements as an indicator of DA release. Both studies not only used HAL (1 mg/kg, i.p.) alone but also lacked a means of directly measuring DA concentration in the striatum. We proposed that changes in [123I]FP-CIT BPND in the midbrain region should also be assessed to clarify changes in the activity of nigrostriatal DA projections in response to antipsychotic drug treatment.
DAT imaging is increasingly used for diagnostics and drug development for neurological and psychiatric diseases as well as for precision medicine in cases of complicated medication status—for instance, a Parkinson’s disease patient receiving antipsychotic drugs. Consequently, there is a need for further experimental evidence supporting the utility of [123I]FP-CIT SPECT for quantitation of acute changes in available DA. Recently, there has been growing interest in assessing DAT binding in schizophrenia patients. However, the results obtained to date on striatal DAT binding in schizophrenia subjects have been inconsistent, with reports of elevated [37, 38], reduced [22, 38, 39], or unaltered [23, 40–44] DAT binding. Interestingly, unaltered [43], decreased [22, 39], or increased [37] DAT binding has also been observed in medicated patients. These results are difficult to interpret because factors such as illness duration and phase–which could vary between patients and investigations—are likely to affect the regulation of pre- and postsynaptic binding sites. Moreover, our findings suggest that antipsychotic drugs themselves may confound presynaptic binding data. Schizophrenia patients who are not responding to antipsychotic drug treatment can have a high percentage of occupied D2 receptors without any relief of symptoms [45]. In light of the present findings, it is conceivable that presynaptic autoreceptor or transporter function may be dysregulated in this subgroup of schizophrenia patients. In routine clinical studies as well as scientific studies, patients are frequently on medication and sometimes even take drugs of abuse [46]. Moreover, in preclinical studies, animals are anesthetized for their scans. Prescribed drugs, drugs of abuse, and anesthetics may influence the visual interpretation and/or quantification of [123I]FP-CIT SPECT scans.
The present study has several limitations. We used different methods to measure alterations in the DAergic neurotransmission system induced by treatment with the antipsychotic drugs HAL and CLZ. The two techniques employed have pros and cons with respect to their ability to measure alterations in the DAergic neurotransmission system, and they were used under fundamentally different experimental conditions (anesthetized animals vs. awake animals). Nonetheless, we felt that the utility of [123I]FP-CIT SPECT compared to the in vivo microdialysis technique should be demonstrated due to the lack of alternative in vivo imaging techniques other than [123I]FP-CIT SPECT. Experimental conditions were maintained as similar as possible between our [123I]FP-CIT SPECT and in vivo microdialysis studies. The methodological validity of [123I]FP-CIT SPECT for measuring altered DAT activity was partially demonstrated in the present study. We attempted to describe the reliability and sensitivity of [123I]FP-CIT SPECT and the DAT blocker BUP for the measurement of DAT activity. The dose-dependent responses in [123I]FP-CIT BPND after exposure to low and high doses of BUP demonstrated that BUP displaces [123I]FP-CIT or competes for the DAT in both the striatum and midbrain. This result directly indicates that radioligand binding to the DAT could be affected if endogenous DA displaces radioligands or competes with them for presynaptic binding sites. We did not perform an in vivo microdialysis study using BUP to validate the significance of the changes in the DA concentration and/or exclude the variation. Instead, we chose to measure changes in the DA concentration using a within-subject design. We felt that this experimental design could reduce experimental error, including variations in DA concentration measurements.
In conclusion, this study demonstrates that [123I]FP-CIT SPECT may be a useful preclinical technique for detecting increases in synaptic DA availability induced by HAL treatment in both the midbrain and the striatum, with results comparable to those obtained by in vivo microdialysis. Our most compelling hypothesis is that alterations in synaptic DA concentration are reflected as variations in DAT radioligand binding, at least in response to HAL and as detected by [123I]FP-CIT SPECT. For [123I]FP-CIT SPECT to be established as a standard technique to aid in the diagnosis of neurological and psychiatric diseases or monitor therapeutic responses, the effects of prescribed drugs on patients’ imaging results must be carefully investigated first.
ACKNOWLEDGEMENTS
This study was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI16C-0947), and by the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning, Republic of Korea (NRF-2018R1D1A1B07047994, 2016R1D1A1A02937028).
REFERENCES
- 1.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 2.Joe EH, Choi DJ, An J, Eun JH, Jou I, Park S. Astrocytes, microglia, and Parkinson’s disease. Exp Neurobiol. 2018;27:77–87. doi: 10.5607/en.2018.27.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens JR. Schizophrenia and dopamine regulation in the mesolimbic system. Trends Neurosci. 1979;2:102–105. doi: 10.1016/0166-2236(79)90041-9. [DOI] [Google Scholar]
- 4.Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000;97:7673–7675. doi: 10.1073/pnas.97.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa J, Meltzer HY. Differential effects of repeated treatment with haloperidol and clozapine on dopamine release and metabolism in the striatum and the nucleus accumbens. J Pharmacol Exp Ther. 1991;256:348–357. [PubMed] [Google Scholar]
- 8.Starke K, Späth L, Wichmann T. Effects of verapamil, diltiazem and ryosidine on the release of dopamine and acetylcholine in rabbit caudate nucleus slices. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:124–130. doi: 10.1007/BF00506191. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pehek EA, Yamamoto BK. Differential effects of locally administered clozapine and haloperidol on dopamine efflux in the rat prefrontal cortex and caudate-putamen. J Neurochem. 1994;63:2118–2124. doi: 10.1046/j.1471-4159.1994.63062118.x. [DOI] [PubMed] [Google Scholar]
- 11.Pehek EA. Comparison of effects of haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J Pharmacol Exp Ther. 1999;289:14–23. [PubMed] [Google Scholar]
- 12.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 13.Shilliam CS, Dawson LA. The effect of clozapine on extracellular dopamine levels in the shell subregion of the rat nucleus accumbens is reversed following chronic administration: comparison with a selective 5-HT(2C) receptor antagonist. Neuropsychopharmacology. 2005;30:372–380. doi: 10.1038/sj.npp.1300591. [DOI] [PubMed] [Google Scholar]
- 14.Chung YC, Park IS, Li Z, Dai J, Meltzer HY, Ichikawa J. Clozapine, but not haloperidol, increases hippocampal dopamine and acetylcholine release. Clin Psychopharmacol Neurosci. 2003;1:79–85. [Google Scholar]
- 15.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gainetdinov RR, Grekhova TV, Sotnikova TD, Rayevsky KS. Dopamine D2 and D3 receptor preferring antagonists differentially affect striatal dopamine release and metabolism in conscious rats. Eur J Pharmacol. 1994;261:327–331. doi: 10.1016/0014-2999(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 18.Westerink BH, Kawahara Y, De Boer P, Geels C, De Vries JB, Wikström HV, Van Kalkeren A, Van Vliet B, Kruse CG, Long SK. Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. Eur J Pharmacol. 2001;412:127–138. doi: 10.1016/S0014-2999(00)00935-3. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Fischer D, Blessmann G, Trosowski C, Béhé M, Schurrat T, Hartmann A, Behr TM, Oertel WH, Höglinger GU, Höffken H. Quantitative [(123)I]FP-CIT pinhole SPECT imaging predicts striatal dopamine levels, but not number of nigral neurons in different mouse models of Parkinson’s disease. Neuroimage. 2007;38:5–12. doi: 10.1016/j.neuroimage.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 20.Niñerola-Baizán A, Rojas S, Bonastre M, Tudela R, Lomeña F, Pavía J, Marin C, Ros D. In vivo evaluation of the dopaminergic neurotransmission system using [123I]FP-CIT SPECT in 6-OHDA lesioned rats. Contrast Media Mol Imaging. 2015;10:67–73. doi: 10.1002/cmmi.1608. [DOI] [PubMed] [Google Scholar]
- 21.Marshall V, Grosset DG. Role of dopamine transporter imaging in the diagnosis of atypical tremor disorders. Mov Disord 18 Suppl. 2003;7:S22–S27. doi: 10.1002/mds.10574. [DOI] [PubMed] [Google Scholar]
- 22.Mateos JJ, Lomeña F, Parellada E, Font M, Fernandez E, Pavia J, Prats A, Pons F, Bernardo M. Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first-episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psychopharmacology (Berl) 2005;181:401–406. doi: 10.1007/s00213-005-2250-2. [DOI] [PubMed] [Google Scholar]
- 23.Lavalaye J, Linszen DH, Booij J, Dingemans PM, Reneman L, Habraken JB, Gersons BP, van Royen EA. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001;47:59–67. doi: 10.1016/S0920-9964(00)00023-2. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaus S, Antke C, Kley K, Beu M, Wirrwar A, Müller HW. Pretreatment with haloperidol reduces (123)I-FP-CIT binding to the dopamine transporter in the rat striatum: an in vivo imaging study with a dedicated small-animal SPECT camera. J Nucl Med. 2009;50:1147–1152. doi: 10.2967/jnumed.109.061952. [DOI] [PubMed] [Google Scholar]
- 25.Booij J, van Loon G, de Bruin K, Voorn P. Acute administration of haloperidol does not influence 123I-FP-CIT binding to the dopamine transporter. J Nucl Med. 2014;55:647–649. doi: 10.2967/jnumed.113.132340. [DOI] [PubMed] [Google Scholar]
- 26.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. doi: 10.1177/070674370204700106. [DOI] [PubMed] [Google Scholar]
- 27.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 28.Schiffer WK, Mirrione MM, Biegon A, Alexoff DL, Patel V, Dewey SL. Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods. 2006;155:272–284. doi: 10.1016/j.jneumeth.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat in stereotaxic coordinates. Academic Press; New York: 1986. [Google Scholar]
- 30.Niñerola-Baizán A, Rojas S, Roé-Vellvé N, Lomeña F, Ros D, Pavía J. Dopamine transporter imaging in the aged rat: a [123I]FP-CIT SPECT study. Nucl Med Biol. 2015;42:395–398. doi: 10.1016/j.nucmedbio.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Fowler CJ, Benedetti MS. The metabolism of dopamine by both forms of monoamine oxidase in the rat brain and its inhibition by cimoxatone. J Neurochem. 1983;40:1534–1541. doi: 10.1111/j.1471-4159.1983.tb08123.x. [DOI] [PubMed] [Google Scholar]
- 32.Lane RF, Blaha CD. Chronic haloperidol decreases dopamine release in striatum and nucleus accumbens in vivo: depolarization block as a possible mechanism of action. Brain Res Bull. 1987;18:135–138. doi: 10.1016/0361-9230(87)90042-6. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez L, Hoebel BG. Haloperidol given chronically decreases basal dopamine in the prefrontal cortex more than the striatum or nucleus accumbens as simultaneously measured by microdialysis. Brain Res Bull. 1989;22:763–769. doi: 10.1016/0361-9230(89)90097-X. [DOI] [PubMed] [Google Scholar]
- 34.Booij J, Andringa G, Rijks LJ, Vermeulen RJ, De Bruin K, Boer GJ, Janssen AG, Van Royen EA. [123I]FP-CIT binds to the dopamine transporter as assessed by biodistribution studies in rats and SPECT studies in MPTP-lesioned monkeys. Synapse. 1997;27:183–190. doi: 10.1002/(SICI)1098-2396(199711)27:3<183::AID-SYN4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Kim W, Park HS, Moon BS, Lee BC, Kim SE. PET measurement of “GABA shift” in the rat brain: a preclinical application of bolus plus constant infusion paradigm of [18F] flumazenil. Nucl Med Biol. 2017;45:30–34. doi: 10.1016/j.nucmedbio.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Leśkiewicz M, Otczyk M, Lasoń W. Antipsychotic drugs inhibit the human corticotropin-releasing-hormone gene promoter activity in neuro-2A cells-an involvement of protein kinases. Neuropsychopharmacology. 2006;31:853–865. doi: 10.1038/sj.npp.1300911. [DOI] [PubMed] [Google Scholar]
- 37.Sjøholm H, Bratlid T, Sundsfjord J. 123I-beta-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology (Berl) 2004;173:27–31. doi: 10.1007/s00213-003-1700-y. [DOI] [PubMed] [Google Scholar]
- 38.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res. 2003;65:39–46. doi: 10.1016/S0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 39.Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Syvälahti E, Hietala J. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res. 2001;52:115–120. doi: 10.1016/S0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt GJ, Meisenzahl EM, Frodl T, La Fougère C, Hahn K, Möller HJ, Dresel S. The striatal dopamine transporter in first-episode, drug-naive schizophrenic patients: evaluation by the new SPECT-ligand[99mTc]TRODAT-1. J Psychopharmacol. 2005;19:488–493. doi: 10.1177/0269881105056530. [DOI] [PubMed] [Google Scholar]
- 41.Yoder KK, Hutchins GD, Morris ED, Brashear A, Wang C, Shekhar A. Dopamine transporter density in schizophrenic subjects with and without tardive dyskinesia. Schizophr Res. 2004;71:371–375. doi: 10.1016/j.schres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH SPECT study. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: a dual-isotope SPECT study. Am J Psychiatry. 2004;161:1496–1498. doi: 10.1176/appi.ajp.161.8.1496. [DOI] [PubMed] [Google Scholar]
- 43.Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Räkköläinen V, Syvälahti E, Hietala J. Striatal dopamine transporter binding in neurolepticnaive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 44.Laruelle M, Abi-Dargham A, van Dyck C, Gil R, D‘Souza DC, Krystal J, Seibyl J, Baldwin R, Innis R. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol Psychiatr. 2000;47:371–379. doi: 10.1016/S0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 45.Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Küfferle B, Barnas C, Stastny J, Praschak-Rieder N, Pezawas L, de Zwaan M, Quiner S, Pirker W, Asenbaum S, Podreka I, Brücke T. Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder--implications for psychopharmacology. World J Biol Psychiatry. 2002;3:133–146. doi: 10.3109/15622970209150614. [DOI] [PubMed] [Google Scholar]
- 46.Ham S, Kim TK, Chung S, Im HI. Drug abuse and psychosis: new insights into drug-induced psychosis. Exp Neurobiol. 2017;26:11–24. doi: 10.5607/en.2017.26.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]