Healthy persons with a tendency to suppress anger showed a reduced ability to inhibit pain during a neutral distractor. This could promote pain risk.
Keywords: Pain, Emotion inhibition, Emotion regulation, Pain risk, Attention
Abstract
Background
The tendency to inhibit anger (anger-in) is associated with increased pain. This relationship may be explained by the negative affectivity hypothesis (anger-in increases negative affect that increases pain). Alternatively, it may be explained by the cognitive resource hypothesis (inhibiting anger limits attentional resources for pain modulation).
Methods
A well-validated picture-viewing paradigm was used in 98 healthy, pain-free individuals who were low or high on anger-in to study the effects of anger-in on emotional modulation of pain and attentional modulation of pain. Painful electrocutaneous stimulations were delivered during and in between pictures to evoke pain and the nociceptive flexion reflex (NFR; a physiological correlate of spinal nociception). Subjective and physiological measures of valence (ratings, facial/corrugator electromyogram) and arousal (ratings, skin conductance) were used to assess reactivity to pictures and emotional inhibition in the high anger-in group.
Results
The high anger-in group reported less unpleasantness, showed less facial displays of negative affect in response to unpleasant pictures, and reported greater arousal to the pleasant pictures. Despite this, both groups experienced similar emotional modulation of pain/NFR. By contrast, the high anger-in group did not show attentional modulation of pain.
Conclusions
These findings support the cognitive resource hypothesis and suggest that overuse of emotional inhibition in high anger-in individuals could contribute to cognitive resource deficits that in turn contribute to pain risk. Moreover, anger-in likely influenced pain processing predominantly via supraspinal (e.g., cortico-cortical) mechanisms because only pain, but not NFR, was associated with anger-in.
Introduction
Anger is characterized as an intense feeling of displeasure, a cognitive appraisal of injustice, and behavioral action centered on repairing the injustice. In this way, anger is adaptive in its ability to direct attention toward, and resolving, perceived injustice [1]. However, at higher intensities, frequency, and duration, anger may prove maladaptive. Indeed, individuals who lash out during arguments may socially isolate themselves and/or incur criminal charges.
Emotional experiences can be altered through emotion regulation (ER) in order to feel less negative emotions (such as anger and sadness) and more positive emotions (such as pleasure and happiness) [2, 3]. Thus, anger management is a form of ER that helps: (a) alter the intensity and duration of anger-related outbursts, (b) prevent social exclusion and physical aggression, and (c) alter health-related outcomes.
Anger-in is a trait-like anger management style associated with the internalization and suppression of anger. Numerous studies have shown that it is correlated with chronic pain. For example, Hatch et al. [4] and Pilowsky and Spence [5] found that anger-in was more common among headache sufferers than healthy, pain-free individuals. Similarly, Duckro et al. [6] examined anger-in in a sample of chronic post-traumatic headache patients and found that anger suppression was associated with increased depression that in turn increased disability. Burns et al. [7] found the degree to which 127 chronic pain patients inhibited anger impaired adjustment to chronic pain, whereas Bruehl et al. [8] found that anger-in was associated with higher ratings of affective pain in patients with complex regional pain syndrome and patients with myofascial pain. Finally, in a study of 142 chronic pain patients, Kerns et al. [9] found that anger-in was a stronger predictor of pain behavior and pain intensity than depression, pain history, anger intensity, and other anger management styles.
Although these studies clearly show a linkage between anger-in and chronic pain, it is not clear whether anger-in predisposes a person to pain or whether it is a consequence. To partially address this issue, studies can examine the relationship between anger-in and measures of laboratory pain in healthy, pain-free individuals. For example, Gelkopf [10] assessed the relationship between anger-in and responses to cold pain (via the cold pressor task) and found that it was associated with reduced cold pressor pain tolerance, increased pain during the cold pressor, and greater heart rate reactions to pain. In 2003, Burns et al. [11] examined the relationship between anger-in and cold pressor pain during tasks intended to evoke anger, sadness, or joy. They found that anger-in was associated with increased cold pressor pain, regardless of the emotion-induction task. Then, in 2004, Burns et al. [12] found that anger-in was associated with lower cold pain tolerance but only when cold pain was assessed without anger provocation. By contrast, Quartana et al. [13] assessed pain using the cold pressor task after they asked participants to suppress their anger following anger induction. They found (in two studies) that anger suppression led to greater cold pain intensity. This study was later replicated by Quartana and Burns [14]. Together, these findings suggest that anger-in may promote pain, yet the mechanisms underlying this relationship are poorly understood.
Recent evidence suggests that the relationship between anger-in and pain may be mediated by negative affectivity (i.e., the negative affectivity hypothesis) [15]. Specifically, increased pain may result from the fact that persons with high anger-in tend to experience magnified subjective distress and negative emotions, leading to a need for tonic inhibition of those negative emotions [15]. Given that negative emotions are known to enhance pain, anger-in may enhance pain by magnifying the pain-enhancing effects of distress and negative emotions.
Alternatively, the relationship between anger-in and pain may be mediated by a deficit in cognitive resources (i.e., the cognitive resource hypothesis) [16]. Specifically, chronic engagement of emotion inhibition (like those with high anger-in) may place an increased demand on cognitive resources, thus reducing the capacity for attention, executive control, and further ER [16]. This may increase pain vulnerability by directing cognitive resources to constant emotion inhibition, thereby decreasing resources for pain management. Indeed, cognitive resources like attention are limited; therefore, high anger-in may result in resource deficits for future cognitive pain modulation, such as attentional modulation of pain [17–22].
The influence of emotion and attention on pain can be studied using a paradigm called emotional control of nociception (ECON). This well-validated paradigm uses picture contents that vary in pleasantness/valence (mutilation/injured bodies, neutral objects, erotica) to manipulate emotional state in order to assess how these states alter pain and the nociceptive flexion reflex (NFR; a physiological index of pain processing in the spinal cord) [23]. Studies using ECON find that pictures reliably evoke emotional modulation of pain and NFR. Specifically, unpleasant pictures eliciting negative emotions (e.g., mutilation) increase pain and NFR, whereas pleasant pictures eliciting positive emotions (e.g., erotica) inhibit pain and NFR. This implies that pain and pain signaling can be augmented in two ways: (a) by amplifying negative emotions and/or (b) by reducing positive emotions. Therefore, individuals unable to regulate their emotional state may be at greater risk for developing chronic pain due to an inability to decrease the effect of negative emotions and/or sustain positive emotions [24–26].
Although ECON does not involve anger provocation, it can still provide a context in which anger-in tendencies can exert their effects (i.e., suppression of negative emotion) because negative emotions are elicited during ECON. Thus, if the negative affectivity hypothesis of anger-in is correct, then individuals who chronically inhibit anger (i.e., those high on anger-in) will ironically experience greater distress in situations where they must regulate anger and other negative emotions. As a result, these persons should experience even greater pain amplification during ECON’s unpleasant pictures because they will have stronger negative emotional reactions.
The ECON paradigm can also be used to study how attention modulates pain by comparing pain/NFR evoked during neutral pictures (visual distractor) to pain/NFR evoked in the absence of a picture stimulus (no distractor). Ostensibly, viewing and processing the neutral pictures demands more cognitive resources than viewing no picture at all, so pain should be reduced during the neutral pictures (via distraction). This was demonstrated in a study by Roy and colleagues [21]. Given this, if the cognitive resource hypothesis is correct, then individuals who chronically inhibit anger will be unable to engage cognitive methods of pain regulation, like attentional modulation of pain. As a result, persons high on anger-in should not be able to reduce pain and NFR as a result of viewing neutral pictures (relative to no pictures).
To date, little is known about anger-in and its effect on the emotion, attention, and pain relationship. The proposed study will first attempt to replicate prior studies finding that high anger-in is associated with enhanced pain by assessing responses to a cold pressor task (tonic exposure to painfully cold water at 10°C). Next, ECON will be used to elucidate how anger-in affects the capacity to modulate pain via emotion and attention. If the negative affectivity hypothesis is supported, higher scores on anger-in will be associated with greater facilitation of pain/NFR during unpleasant/mutilation pictures when compared to persons low on anger-in. By contrast, if the cognitive resource hypothesis is supported, higher scores on anger-in will be associated with a reduced ability to attentionally modulate pain/NFR during neutral pictures relative to no pictures. And finally, by examining NFR as an outcome, this study will be able to determine whether anger-in exerts its effects by engaging cerebrospinal mechanisms to modulate pain processing at the spinal level because NFR is a correlate of spinal nociception [27].
Methods
Participants
Participants were recruited at the University of Tulsa as part of a larger study investigating risk factors associated with chronic pain in healthy, pain-free Native Americans and non-Hispanic Whites. Participants were recruited through flier distribution, word of mouth, newspaper advertisement, and email advertisement. Exclusion criteria included the following: younger than 18 years of age; history of cardiovascular, neuroendocrine, musculoskeletal, neurological disorders, or chronic pain; body mass index greater than 35; use of antidepressant, anxiolytic, analgesic, stimulant, or antihypertensive medications; current psychotic symptoms or substance abuse; and inability to speak/read English. Testing was completed over a two-day period with testing sessions approximately 4–6 hr in duration. Data collection occurred between March 2014 and December 2017.
Data for this study were drawn from the 253 participants enrolled in the larger parent study entitled the Oklahoma Study of Native American Pain Risk (OK-SNAP). To study the effect of anger-in on emotional and attentional modulation of pain and NFR, two groups were formed that were low and high on anger-in. Low anger-in was defined as individuals that scored at least 1 SD below the normative mean, whereas high anger-in was defined as individuals that scored at least 1 SD above the normative mean. These normative statistics were derived from means and SDs published in psychometric studies of the anger expression inventory (AEI; Manger-in = 16.21, SDanger-in = 3.87) [28, 29]. Using this method, 101 participants were categorized into high and low anger-in groups: 74 as low anger-in (n = 47 female) and 27 as high anger-in (n = 14 female).
This study was approved by the Institutional Review Boards of the University of Tulsa, Cherokee Nation, and the Oklahoma City Area Indian Health Service. Prior to testing, participants were given an overview of the procedures and told they could withdraw participation at any time. All participants provided verbal and written informed consent prior to enrollment and received $100 honorarium for completion of each testing day.
Testing Apparatus
The study was controlled by a computer with dual monitors, analog-to-digital board (USB-6212 BNC; National Instruments, Austin, TX, USA) and LabVIEW software (National Instruments). All participants completed electronic questionnaires and pain ratings using one monitor and computer mouse, whereas the experimenter (adjacent room) monitored physiology using a second monitor. Testing occurred in a sound-attenuated and electrically shielded room. All participants were monitored via video camera and wore sound-attenuated headphones to communicate with the experimenter and to hear prerecorded instructions.
A stimulator (Digitimer DS7A, Hertfordshire, UK) and a bipolar electrode (Nicolet, Model#019-40400, Madison, WI) delivered electrical stimulations over the retromalleolar pathway of the sural nerve of the left ankle. Electrical stimulations were administered in a train of five 1 ms rectangular wave pulses at 250 Hz, which were perceived as a single stimulation. Electrical stimulation timing was computer controlled and the maximum stimulus intensity was set to 50 mA.
Two active Ag-AgCl electrodes were applied over the biceps femoris of the left leg approximately 10 cm superior to the popliteal fossa to capture NFR-related electromyogram (EMG). All NFR EMG signals were collected, filtered (10–300 Hz), and amplified (×10,000) using a Grass Technologies (West Warwick, RI) Model 15LT amplifier (with AC Module 15A54). A common ground electrode was placed over the lateral epicondyle of the femur on the left leg. Skin was cleaned with alcohol wipes and exfoliated (NuPrep gel; Weaver and Company, Aurora, CO) to achieve impedance less than 5 kΩ for EMG and stimulating electrodes. Stimulating electrodes and EMG sensors were filled with conductive gel (EC60; Grass Technologies). Corrugator EMG to measure facial affect (see below) was collected using two miniature Ag-AgCl electrodes filled with conductive gel (EC60, Grass Technologies), placed above the left corrugator supercilii muscle, filtered (30–1,000 Hz), and amplified (×20,000). Skin conductance response (SCR; to measure emotional arousal, see below) was measured by placing two electrodes filled with isotonic paste (EC33, Grass Instruments) on the volar surface of the middle and ring fingers after participants washed and dried their hands with soap and water. All signals were sampled at 1,000 Hz.
Questionnaires
To assess background information and inclusion/exclusion criteria, participants completed a custom-built demographics and health status questionnaire.
The Pain Catastrophizing Scale (PCS) was administered to participants prior to pain testing to determine whether groups differed on this variable. The PCS is a 13-item measure that assesses the degree to which an individual experiences rumination, magnification, and helplessness in past painful experiences [30]. Total scores range between 0 and 52, with higher PCS scores indicating more catastrophic thinking.
The AEI was used to assess anger-in (28, 29). The AEI has been shown to have convergent validity with other measures of anger expression and inhibition [29]. The AEI is a 20-item scale with 8 items measuring anger-in (the tendency to inhibit anger), 8 items measuring anger-out (the tendency to express anger overtly), and 4 items measuring anger-control (the tendency to control anger); however, only the anger-in subscale was used for the current study. Anger-in scores range between 8 and 32, with higher scores indicating a greater tendency to inhibit anger.
Responses to Tonic Pain (Cold Pressor)
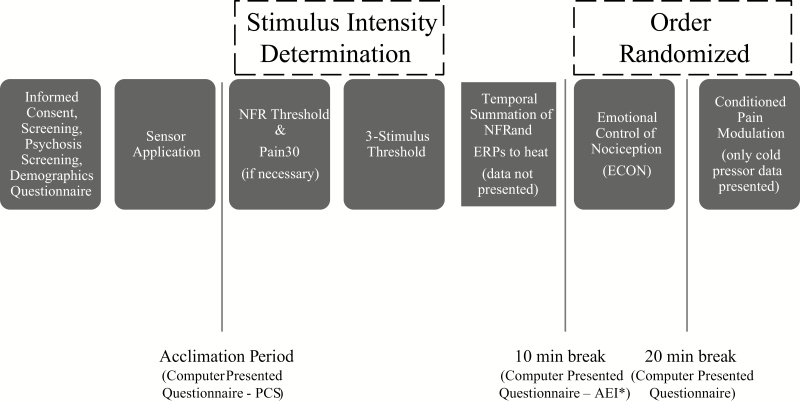
Given that the electric stimulations used during ECON were individually calibrated to each person (see description below), pain ratings in response to those stimuli may not provide an optimal measure of group differences in pain perception. To overcome this issue, the present study assessed pain ratings in response to a 2 min long hand/arm immersion in a circulating water bath set at 10°C (i.e., cold pressor task). The cold pressor is ideal for measuring pain responsivity because it is safe, mimics the experience of chronic pain conditions, and has excellent reliability and validity [31, 32]. For this study, the cold pressor was embedded in a larger task called conditioned pain modulation (CPM, Fig. 1).
Fig. 1.
Experimental procedures for emotional control of nociception (ECON) testing day. NFR = nociceptive flexion reflex. ERP = event-related potential.
The goal of the CPM task was to assess pain in response to painful electric stimuli before, during, and after participants submersed their hand in the cold pressor. Given that the current study only focuses on emotional and attentional modulation of pain, responses to CPM will not be presented and only ratings to the cold pressor will be reported. The cold pressor was a circulating water bath (Thermo Fisher Scientific, Pittsburgh, PA) that maintained a constant temperature of 10 ± 0.1°C. Participants were instructed to submerge their right hand up to the forearm in the cold water (which was always 6” deep) and to keep their hand palm down with fingers spread. They also were instructed to keep their hand still while it was in the water. The circulating water ensures that the temperature around the hand/arm does not warm, thus causing a gradually building, aching pain. After participants removed and dried their hand, they rated their pain intensity on a computer-presented visual analog scale (VAS) that ranged from “no pain sensation” to “the most intense pain sensation imaginable” with scores that were converted to values between 0 and 100.
Determination of Electric Stimulus Intensity Used During ECON
The stimulus intensity (mA) of electric stimulations delivered during ECON is set to the highest of three criteria: ×1.2 the intensity of NFR threshold, ×1 Pain30, and ×1.2 three-stimulus threshold. Setting the intensity above NFR threshold and three-stimulation threshold ensures that the stimulus intensity reliably evokes NFRs throughout the ECON task. Setting the intensity to a minimum of Pain30 ensures that the stimuli are at least mildly painful (because NFR threshold and three-stimulus threshold are below some individuals’ pain threshold). Given this, the procedures listed below were all assessed prior to ECON administration. Furthermore, because NFR threshold and three-stimulus threshold assess spinal nociception, they provide a method to assess group differences in the reactivity of the spinal cord to painful input.
Pain rating instructions were the same across all three tasks. Participants rated their pain intensity following electrical stimulations using the same computer-presented VAS that was used to rate the cold water pain (described previously).
NFR threshold
NFR is a spinally mediated withdrawal reflex evoked by Aδ fiber activation, wherein the limb (e.g., leg) withdraws from a noxious stimulus [18, 22, 27, 33]. Given that the reflex requires the activation of Aδ fibers but its reflex arc does not require supraspinal regions (it is observed in spinally transected individuals [34, 35]), the NFR is used as a correlate of spinal nociception. However, NFR can be modulated by corticospinal circuitry [36, 37].
NFR threshold was determined from a series of ascending–descending staircase stimulations. The first staircase began at 0 mA and increased in 2 mA increments until the first reflex was elicited. After the first reflex, stimulus intensity decreased in 1 mA intervals until the reflex disappeared. The following two ascending–descending staircases were administered with 1 mA increments. Intervals between electric stimuli varied randomly (8–12 s) to minimize predictability and reflex habituation. NFR was determined to be present if the mean rectified biceps femoris EMG in the 90–150 ms poststimulus window exceeded the mean rectified biceps femoris EMG in the 60 ms prestimulus baseline window by at least 1.4 SD of baseline EMG activity [33]. NFR threshold was defined as the average stimulus intensity (mA) of the two peaks and two troughs of the last two ascending–descending staircases. This is the stimulus intensity necessary to evoke a nociceptive (pain-related) response from spinal neurons.
Pain30
Pain30 was only assessed if the stimuli associated with NFR threshold did not evoke a VAS rating greater than or equal to 30 (i.e., mild pain). If assessed, the computer was programmed to start stimulus intensity at the NFR threshold intensity and then increase in 2 mA intervals until a rating of 30 was achieved.
Three-stimulus threshold
This procedure assessed NFR following a three-stimulus series (stimulus = train of five 1 ms pulses at 250 Hz) with a 0.5 s interval between stimulations in the series. It is similar to what others call temporal summation of NFR threshold [38, 39] and assesses the stimulation intensity required to evoke amplification of spinal cord neurons. The first series began at 0 mA and increased by 2 mA until an NFR was elicited by the third stimulus in the series. That stimulation intensity (in mA) was recorded as the three-stimulation threshold.
Emotional Control of Nociception
Twenty-four pictures were selected from the International Affective Picture System (IAPS): eight mutilation, eight neutral, and eight erotic. Each picture content was chosen because of its ability to reliably modulate pain and NFR [19]. Electrical stimulations were administered during and in between pictures to evoke pain and NFR. Eighteen stimulations were administered in total: 12 stimulations during 50% of the pictures (equally balanced across contents and 3–5 after picture onset) and 6 stimulations during interpicture intervals (to reduce predictability of stimulations and to assess attentional modulation). Each picture was presented for 6 s with a 12–22 s interpicture interval. Pictures were randomized with the limitation that pictures of the same content were not shown twice in a row. After each picture, participants were asked to rate their valence and arousal (and pain intensity if an electric stimulation was administered during the picture) using computer-presented Self-Assessment Manikin (SAM) scales (see descriptions of scales below). Although this paradigm is used to determine emotional effects on pain by presenting pictures of varying affective content, it may also be used to assess attentional effects on pain by comparing pain/NFR evoked during neutral pictures (a distractor) with pain/NFR evoked during interpicture intervals (no distractor).
Emotional reactions to pictures
Emotional experience was assessed using two continuous but orthogonal (independent) constructs known as valence and arousal [40]. Valence refers to the unpleasantness or pleasantness of an emotional experience, whereas arousal refers to the emotional activation/intensity that is evoked. A computerized version of the SAM was used to assess subjective appraisals of valence and arousal. This two-item questionnaire assesses valence/pleasure (1 = unpleasant, 5 = neutral, 9 = pleasant) and arousal (1 = calm to 9 = excited) ratings by moving an indicator on or between any of the five manikins for each 9-point scale.
To obtain a physiological measure of valence, the corrugator muscle was measured. This muscle controls the eyebrow and pulls it into a frown during unpleasant experiences and relaxes during pleasant experiences. Literature suggests that corrugator activity is inversely correlated with emotional valence ratings [41]. Given this, corrugator EMG was collected during pictures to determine a person’s facial display of valence. The mean rectified change during the 6 s of picture viewing relative to the rectified mean of the 1 s prior to picture onset was used to estimate corrugator EMG reactivity.
Sweat glands (controlled via sympathetic nervous system activation) are known to open and release sweat, thereby increasing the conductive properties of skin. Given this, SCR (a measure of electrodermal conductivity) can be used as an index of emotional/sympathetic arousal and positively correlates with emotional arousal ratings [42]. SCR was determined by finding the peak skin conductance level that occurred between 1 and 6 s after picture onset and subtracting the mean activity in the 1 s prior to picture onset.
Pain outcomes during ECON
Following painful electric stimuli, pain intensity was assessed using the VAS for pain intensity described earlier. Participants made their ratings by moving an indicator along a line and submitting their answers with a button press.
NFR magnitude was used to assess within-subject changes in spinal nociception. NFR magnitude was calculated in d units (d = [mean rectified EMG of 90–150 ms poststimulation interval minus the mean rectified EMG of −60 to 0 ms prestimulation interval) divided by (the average SD of rectified EMG from −60 to 0 ms prestimulation and SD of 90–150 ms poststimulation intervals]). The d-score method has been shown to produce a stronger correlation with pain report than other scoring methods and produces a more normal distribution [43].
Testing Procedures
The larger study was composed of two testing days. Figure 1 presents the tasks during the ECON testing day. Tasks such as temporal summation of heat, pain threshold/tolerance for electric, ischemic, cold, heat, and pressure stimuli were administered on the other day of this study. Day order was counterbalanced across participants but stratified by race and sex. In addition to the small breaks between each task, each testing day also had two longer breaks that lasted 10 and 20 mins with questionnaires administered. Some questionnaires were always administered on Day 1, while others were administered on Day 2 (see Fig. 1). The AEI was always administered on Day 2. Given this, some participants were not included in the current analysis if they did not return for Day 2 activities because ECON did not always occur on the same day as AEI administration. Prior to pain tasks on the first testing day, participants completed the demographics questionnaire and PCS.
Statistical Analyses
To determine group differences in demographic variables, chi-square analysis (nominal independent variables [IVs]) and independent sample t-tests (continuous IVs) were conducted using anger-in group (high vs. low) as the IV. An independent sample t-test was also used to examine group differences in cold pressor pain.
Multilevel models (MLMs; MIXED procedure, SPSS 20.0, IBM, Armonk, NY) were used to analyze valence, arousal, corrugator EMG, skin conductance response, pain ratings, and NFR magnitude during emotional and attentional modulation.
In accordance with each of the 24 pictures during ECON, valence, arousal, corrugator EMG, and SCR had 24 rows of data per participant. In accordance with the 12 stimulations delivered during pictures (4 per content), pain and NFR analysis during emotional modulation included 12 rows of data per participant. In accordance with 10 stimulations during attentional modulation (6 during no picture condition, 4 during neutral picture condition), pain and NFR analysis during attentional modulation included 10 rows of data per participant.
In MLMs, Level 1 units were picture responses (valence, arousal, corrugator, SCR) or pain responses (pain, NFR), depending on analysis type. Level 2 units were participants and included a random intercept to model Level 2 variance. The SPSS MIXED procedure implements Satterthwaite estimation procedures to produce noninteger denominator degrees of freedom that vary from analysis to analysis.
For emotional modulation analyses, picture content (mutilation, neutral, and erotica) and anger-in group (high vs low) were used as IVs. Pain/NFR, corrugator EMG, SCR, and valence/arousal ratings were used as dependent variables (DVs). For attentional modulation analysis, attention (neutral picture vs. no picture) and anger-in group (high vs. low) were used as IVs, whereas pain/NFR were used as DVs.
As an aside, we believe that it is generally preferred to keep a continuous variable continuous rather than create groups and discard potentially important variance. Thus, analyses were also conducted with anger-in as a continuous variable (i.e., an MLM version of Analysis of Covariance). Conclusions were the same, but because the primary aim was to examine anger-in differences on a categorical variable (i.e., picture content), keeping the anger-in variable continuous would have been problematic for interpreting the picture content mean differences because finding a significant Anger-in × Picture Content interaction would imply that the picture content means are adjusted incorrectly (i.e., a violation of the homogeneity of regression lines assumption of Analysis of Covariance). Moreover, even if this were not a problem, to interpret any significant interactions, arbitrary cut points on anger-in would have to be used to test and interpret the simple effects of picture content. For these reasons, anger-in was kept as a dichotomous variable (low vs. high) in all analyses.
Stimulus order (the order of electrical stimulations during ECON) was used as a continuous predictor in MLMs with pain/NFR as DVs to model habituation and/or sensitization effects unrelated to emotional or attentional modulation. Picture order (the order of picture presentation during ECON) was used as a continuous predictor in MLMs with valence, arousal, corrugator EMG, and SCR as DVs. This tactic improves statistical power and validity of the models.
All data were tested for normality. Within-cell outliers were identified using Wilcox’s MAD-median procedure using a 2.24 cutoff [44] and winsorized by replacing the outlier with the nearest nonoutlier value. SCR was log-transformed (Log[SCR+1]) due to significant skew. For follow-up tests, Fisher’s Least Significant Differences was used. Significant was α = 0.05 (two-tailed).
Results
Two participants from the low anger-in group discontinued testing prior to ECON and one participant from the low anger-in group was excluded from final analysis due to technical problems during ECON. Thus, a total of 98 participants were included in the final analysis: 71 participants who were low anger-in (n = 47 female) and 27 who were high anger-in (n = 14 female).
Background Variables (Table 1)
Table 1.
Participant characteristics by group
| Characteristic | Low anger-In (n = 71) | High anger-in (n = 27) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | p | ||
| Sex (female) | 47 | 66% | 14 | 52% | 0.19 | .25 | |
| Race/ethnicity | 2.01 | .37 | |||||
| Non-Hispanic White | 32 | 45% | 13 | 48% | - | - | |
| Native American | 34 | 48% | 14 | 52% | - | - | |
| Other | 5 | 7% | 0 | 0% | - | - | |
| M | SD | M | SD | t-value | p | Cohen’s d (95% CI for d) | |
| Age (years)* | 32.87 | 14.59 | 26.63 | 9.39 | 2.06 | .001 | −0.46 [−0.91,−0.02] |
| Body mass index (kg/m2) | 26.45 | 4.62 | 25.11 | 4.66 | 1.27 | .35 | −0.29 [−0.73,0.16] |
| Pain catastrophizing (0–52)** | 1.66 | 1.74 | 6.69 | 5.72 | −6.63 | <.001 | 1.50 [1.01,1.99] |
| Cold pressor pain intensity (0–100) | 50.42 | 25.48 | 61.96 | 25.43 | 2.01 | .047 | 0.45 [0.01, 0.90] |
| NFR threshold (mA) | 16.67 | 9.22 | 16.77 | 10.39 | −0.05 | .96 | 0.01 [−0.43,0.45] |
| 3-stimulus threshold (mA) | 13.67 | 6.96 | 15.7 | 7.48 | −1.27 | .21 | 0.28 [−0.16,0.73] |
| ECON stimulation intensity (mA) | 25.07 | 12.12 | 22.92 | 9.47 | 0.83 | .41 | −0.19 [−0.63,0.26] |
CI confidence interval; ECON emotional control of nociception; NFR nociceptive flexion reflex.
*p < .05; **p < .001.
No group differences were found for sex, race/ethnicity, body mass index, NFR threshold, three-stimulus threshold, and stimulation intensity administered during the ECON task. By contrast, groups did differ on age and pain catastrophizing. When catastrophizing was entered as a covariate in primary analyses, it was not significant in any analysis: cold pressor pain (p = 0.08), valence ratings (p = 0.49), corrugator EMG (p = 0.46), arousal ratings (p = 0.09), skin conductance (p = 0.75), emotional modulation of pain ratings (p = 0.39), emotional modulation of NFR (p = 0.10), attentional modulation of pain ratings (p = 0.29), or attentional modulation of NFR (p = 0.16). As a result, pain catastrophizing was dropped from all final models. The inclusion of age as a covariate did not change any conclusions, so it was also dropped. Even though sex was not significantly different between groups, sex can be an important predictor of pain and emotion-related outcomes [45, 46], so analyses were initially conducted controlling for sex. All conclusions were identical with sex included, so it too was dropped from the final models.
Tonic Cold Pressor Pain
Results indicated a significant difference in cold water pain (Table 1). The high anger-in group reported more pain in response to cold water than the low anger-in group. Thus, persons who chronically inhibit anger experienced enhanced pain (hyperalgesia) in response to the cold pressor task.
Emotional Reactions to Pictures (Figure 2)
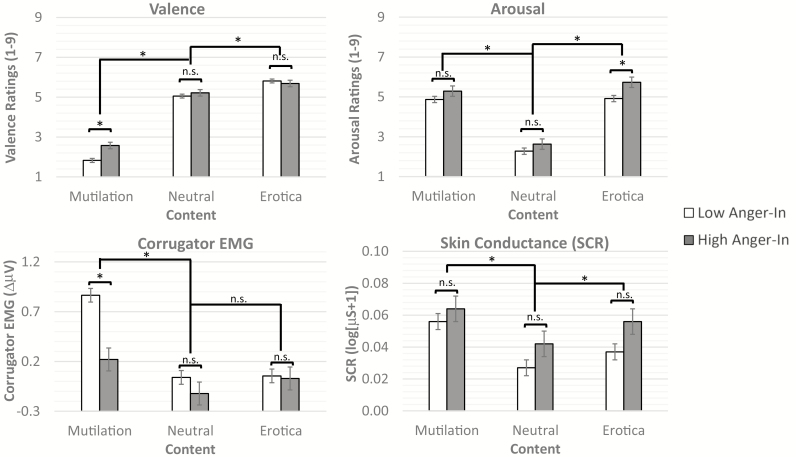
Fig. 2.
Valence (top left graph), arousal (top right graph), corrugator electromyogram (EMG, bottom left graph), and skin conductance (bottom right graph) responses to pictures by picture content (mutilation, neutral, erotica) and anger-in group (high anger-in, low anger-in). Valence ratings scale from 1 (unpleasant) to 9 (pleasant). Arousal ratings scale from 1 (calm) to 9 (excited). In general, mutilation pictures were rated as more unpleasant (lower valence), more arousing, and elicited more corrugator EMG and skin conductance response than neutral pictures. By contrast, erotica pictures were generally rated as more pleasant, more arousing, and elicited greater skin conductance response than neutral. Both anger-in groups showed similar valence ratings and corrugator response to neutral and positive pictures. However, participants who scored high on anger-in rated negative pictures as less unpleasant (higher valence ratings) and displayed less corrugator EMG activity than the low anger-in group. Moreover, participants who scored high on anger-in reported more arousal in response to erotic pictures. Both groups displayed similar SCRs to pictures. *Statistically significant at p < .05. n.s. = nonsignificant.
Analysis of valence ratings found a significant main effect of picture content (F[2, 2,136.80] = 1,051.08, p < 0.001). Mutilation pictures were more unpleasant (lower valence) than neutral (p < 0.001) and erotica pictures (p < 0.001), and erotic pictures were more pleasant (higher valence) than neutral pictures (p < 0.001). Although there was not a main effect of group (F[1, 99.99] = 2.51, p = 0.12), there was a significant Group × Content interaction (F[2, 2,136.79] = 14.52, p < 0.001). Those in the high anger-in group reported less unpleasantness (i.e., higher valence ratings) in response to the mutilation pictures than the low anger-in group (Mhigh = 2.58, SEMhigh= 0.16 vs. Mlow = 1.83, SEMlow= 0.10, p < 0.001; see Fig. 2).
Analysis of corrugator EMG found a significant main effect of picture content (F[2, 1,030.55] = 33.68, p < 0.001). Mutilation pictures elicited significantly more corrugator EMG than erotica and neutral (ps < 0.001), whereas erotica and neutral pictures did not significantly differ (p = 0.28). Moreover, a significant main effect of group (F[1, 97.97] = 7.67, p = 0.007) was qualified by a significant Group × Content interaction (F[2, 1,031.37] = 8.97, p < 0.001). Those in the high anger-in group responded with less corrugator EMG activity during mutilation pictures than the low anger-in group (F[1, 295.71] = 23.25, p < 0.001; Mhigh = 0.22, SEMhigh = 0.12 vs. Mlow = 0.87, SEMlow = 0.07, p < 0.001; see Fig. 2).
Analysis of arousal ratings found a significant main effect of picture content (F[2, 2,099.52] = 559.13, p < 0.001). Mutilation and erotic pictures were rated as more arousing than neutral pictures (ps < 0.001) and erotica was more arousing than mutilation (p = 0.008; Fig. 2). There was no main effect of group (F[1, 99.99) = 3.44, p = 0.07), but there was a significant Group × Content interaction (F[2, 2,099.53] = 3.44, p = 0.03). Those in the high anger-in group reported more arousal in response to the erotic pictures than the low anger-in group (Mhigh = 5.73, SEMhigh= 0.26 vs. Mlow = 4.92, SEMlow= 0.16, p = 0.009; see Fig. 2).
Analysis of skin conductance found a significant main effect of picture content (F[2, 1,002.31] = 23.83, p < 0.001) but no main effect of group (F[1, 96.81] = 2.69, p = 0.104) or Group × Content interaction (F[2, 1,004.95] = 1.20, p = 0.30). Mutilation pictures elicited significantly higher SCR than erotica and neutral (ps < 0.001), whereas erotica pictures elicited significantly higher SCR than neutral (p = 0.001; Fig. 2).
Emotional Modulation of Pain/NFR (Fig. 3)
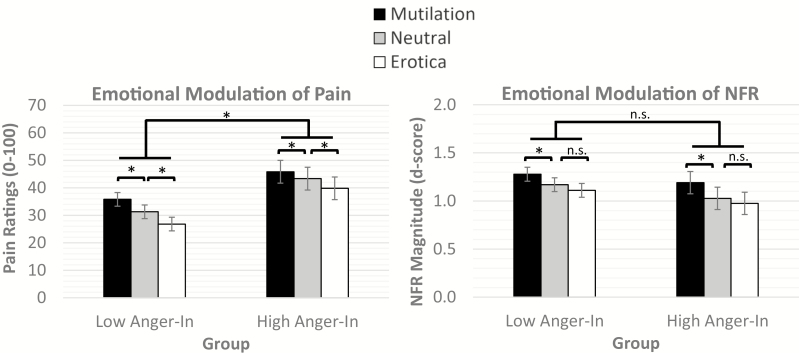
Fig. 3.
Emotional modulation of pain (left graph) and nociceptive flexion reflexes (NFR, right graph) by mutilation, neutral, and erotica pictures in anger-in groups (low anger-in, high anger-in). Suprathreshold electric stimulations elicited higher pain ratings during mutilation pictures than neutral pictures, whereas electric stimulations elicited lower pain ratings during erotic pictures than neutral pictures. Both groups displayed similar pain modulation in response to picture contents, but the high anger-in group reported more overall pain in response to the electric stimulations. Suprathreshold electric stimulations elicited larger NFRs during mutilation pictures than neutral and erotic pictures. However, NFRs during neutral and erotic pictures did not significantly differ. There were no significant group differences in emotional modulation of NFR. *Statistically significant at p < .05. n.s. = nonsignificant
Analysis of pain ratings found a significant main effect of content (F[2, 949.23] = 51.80, p < 0.001) and a significant main effect of group (F[1, 97.99] = 6.07, p = 0.02) but not a significant Content × Group interaction (F[2, 953.06] = 2.11, p = 0.12). Both groups reported that pain was higher during mutilation pictures than neutral and erotic pictures and lower during erotic pictures than neutral pictures (all ps < 0.001; Fig. 3). Additionally, the high anger-in group reported overall more pain in response to the electric stimulations than the low anger-in group (Mhigh = 43.00, SEMhigh= 4.07 vs. Mlow = 31.30, SEMlow= 2.45). This suggests that emotional modulation of pain was similar in the two groups, but persons who chronically inhibit anger experienced enhanced pain (hyperalgesia).
Analysis of NFR found a significant main effect of content (F[2, 992.14] = 14.81, p < 0.001) but not a significant main effect of group (F[1, 93.78] = 0.03, p = 0.35) or Content × Group interaction (F[2, 993.52] = 0.33, p = 0.72). For both groups, NFRs were larger during mutilation pictures than neutral and erotic pictures (ps < 0.001). However, no difference in NFR was exhibited between erotic and neutral pictures (p = 0.124; Fig 3).
Attentional Modulation of Pain/NFR (Fig. 4)
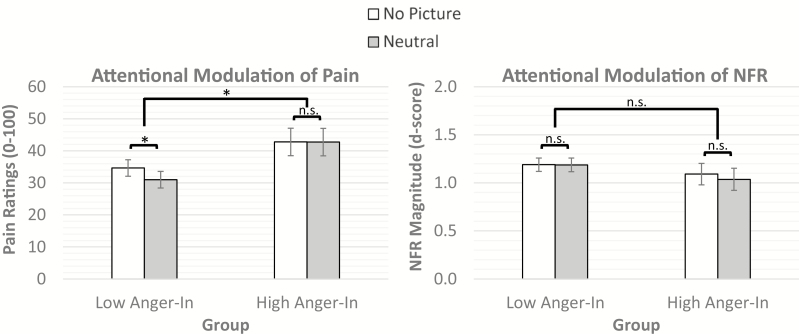
Fig. 4.
Attentional modulation of pain (left graph) and nociceptive flexion reflexes (NFR, right graph) by interpicture intervals (no picture) and neutral pictures (distractor) in anger-in groups (low anger-in, high anger-in). For the low anger-in group, pain ratings were lower during neutral pictures compared to no pictures, suggesting an effect of distraction/attention that inhibited pain. For the high anger-in group, pain ratings during no picture and neutral pictures were not significantly different; however, persons in the high anger-in group reported higher overall pain in response to the electric stimulations. NFRs were not significantly different between no pictures and neutral pictures in either group. *Statistically significant at p < .05. n.s. = nonsignificant
Analysis of attentional modulation of pain indicated a significant main effect of attention (F[1, 648.04] = 10.49, p = 0.001) that was qualified by a significant Group × Attention interaction (F[1, 649.75] = 9.99, p = 0.002). For individuals in the low anger-in group, pain ratings were lower during neutral pictures compared to those during the no picture intervals (p < 0.001; Fig 4). By contrast, for individuals who were in the high anger-in group, pain ratings during the no picture intervals were not significantly different from those during neutral pictures (p = 0.96). Further, there was a significant main effect of group (F[1, 98.13] = 3.98, p = 0.049) that indicated that the high anger-in group reported overall more pain in response to the electric stimulations than the low anger-in group (Mhigh = 42.75, SEMhigh= 4.26 vs. Mlow = 32.82, SEMlow= 2.56).
Analysis of attentional modulation of NFR found no significant main effect of group (F[1, 94.11] = 0.92, p = 0.34), attention (F[1, 816.03] = 0.73, p = 0.39), or Group × Attention interaction (F[1, 816.23] = 0.64, p = 0.43; Fig 4).
Discussion
Prior studies have shown that anger-in is associated with enhanced pain and pain-related outcomes [6, 10, 47]. The current study examined whether this relationship is explained by the negative affectivity hypothesis (persons high on anger-in experience greater pain during unpleasant events that in turn enhances pain) or the cognitive resource hypothesis (persons high on anger-in have limited attentional resources for pain modulation). To do so, participants were categorized as low or high on the anger-in subscale, and then the ECON paradigm was used to assess emotional and attentional modulation of pain and the NFR (a measure of spinal nociception).
ECON allowed us to test the negative affectivity hypothesis by determining whether anger-in groups differed in how pleasant (erotic), neutral, and unpleasant (mutilation) pictures influenced their emotional reactions and their pain/NFR (i.e., emotional modulation). By contrast, ECON allowed us to assess attentional modulation of pain/NFR (to test the cognitive resource hypothesis) by comparing pain/NFR in the absence of pictures (no distractor) to pain/NFR during neutral pictures (distractor).
Anger-In Enhances Pain
Our findings provide further support for a relationship between anger inhibition and enhanced pain. Specifically, the high anger-in group reported greater pain ratings in response to electric stimulations delivered during ECON as well as the cold pressor task. This effect varied between 10 and 12 VAS points and suggests that chronically inhibited anger (and perhaps other negative emotions, see below) amplifies pain perception (i.e., produces hyperalgesia) in response to both phasic (electric) and tonic (cold pressor) noxious stimuli.
Anger-In and Emotional Reactions to Pictures
Consistent with prior studies, mutilation pictures were rated as more unpleasant and arousing and elicited increased corrugator EMG activity (facial displays of negative emotion) and skin conductance (sympathetic arousal) when compared to neutral pictures. Further, erotic pictures were rated as more pleasant and arousing and elicited greater skin conductance relative to neutral pictures. However, there were important group differences in these reactions. Notably, those in the high anger-in group reported experiencing less displeasure (higher valence ratings), displayed less corrugator reactivity in response to mutilation pictures, and reported greater arousal in response to erotic pictures when compared to those in the low anger-in group.
These findings are consistent with other studies on ER in that strategies directed at inhibition of negative emotions generally reduce subjective emotional experience [48] and decrease facial displays of emotion (i.e., reduced corrugator activity) [49]. Interestingly, the group difference in arousal ratings in response to erotic pictures was not coupled with an increase in pleasure (or displeasure). Thus, increased arousal could be due to attempts at inhibiting their emotional reaction to erotica rather than a general augmentation of the pleasure response to those pictures. Importantly, these behavioral findings serve as a manipulation check for our IV and corroborate participants’ self-reports of their anger-in tendencies (as assessed by the AEI), thus strengthening the internal validity of the study.
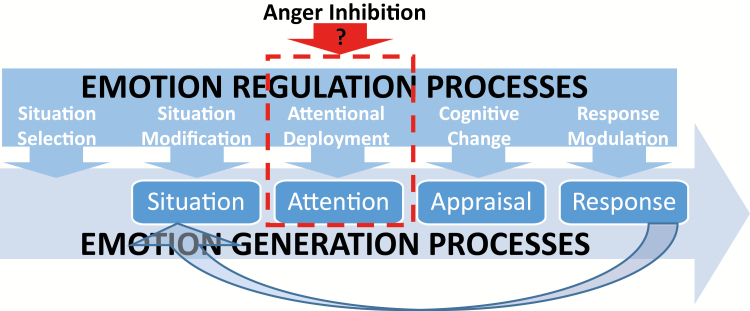
Interestingly, both groups exhibited similar skin conductance responses to pictures. To understand the potential implications of this, it is necessary to consider James Gross’ [50] process model of ER (see Fig 5). In this model, Gross distinguishes between antecedent-focused strategies of ER (e.g., distraction, reappraisal) that occur early in the emotion generative process versus response-focused strategies (e.g., behavioral suppression) that occur later in the emotion generative process, after the emotion is already underway. He argues that response-focused strategies are potentially unhealthy because they are less successful in quelling the emotion that was to be regulated (because the regulation attempt happens after emotion generation), but also because these strategies are effortful and increase physiological arousal and stress. As evidence of this, he and his colleagues have shown that persons who engage in response-focused strategies display heightened skin conductance responses and cardiac pulse amplitude when confronted with emotions to regulate [48, 51]. Given that our high anger-in participants did not show heightened sympathetic arousal relative to the low anger-in group (despite inhibiting their subjective unpleasantness and corrugator responses), this suggests that they may be using antecedent-focused strategies to regulate their negative emotions. However, given that we did not specifically measure their ER strategy or elicit anger in our study, we cannot determine exactly which strategy participants used and/or whether they would use a different strategy when confronted specifically with anger-provoking situations.
Fig. 5.
Anger inhibition in the context of James Gross’ (2002) modal model of emotion and process model of emotion regulation. This figure shows how anger inhibition may be a form of antecedent-focused emotion regulation that affects attentional deployment during emotion generation processes.
Anger-In and Emotional Modulation of Pain/NFR
Contrary to the negative affectivity hypothesis [15], emotional modulation of pain/NFR (the tendency for mutilation pictures to elicit greater pain/NFR and erotica pictures to elicit lower pain/NFR when compared to neutral pictures) was unaffected by anger-in such that emotional modulation was similar in both groups, even in response to the mutilation pictures.
Further, emotional modulation of NFR was observed in both groups, providing additional evidence that ECON procedures can engage cerebrospinal mechanisms to modulate spinal nociception [18, 22, 52]. Given the lack of group differences in emotional modulation of pain and NFR, these findings imply that high anger-in individuals do not exhibit enhanced negative-affectivity-induced pain or NFR facilitation.
Anger-In and Attentional Modulation of Pain/NFR
Although no differences in emotional modulation were observed, attentional modulation (the tendency for the presentation of a visual distractor, i.e., a neutral picture, to inhibit pain when compared with a no distractor control) was only observed in the low anger-in group. This is consistent with the model of cognitive resources proposed by Muraven and Baumeister [53]. They propose that attempts at self-regulation, particularly inhibition strategies, draw from a finite pool of cognitive resources, thus depleting further attempts to exert cognitive control. Moreover, they argue that these decrements in cognitive control are not due to changes in emotions that stem from the cognitive control attempt (e.g., from negative affect generated from trying to exert control) [53].
In light of this, participants who are high on anger-in appear to deplete their ability to engage in subsequent modulation of pain via attentional mechanisms but not via emotional mechanisms. Future research is needed to determine whether this deficit extends to other forms of cognitive modulation of pain (e.g., reappraisal, expectancies), but these initial findings suggest that risk for pain enhancement in persons who chronically inhibit anger may stem from a depletion of cognitive resources to attentionally modulate pain (Fig. 5). Interventions designed to help these individuals cope with anger and pain using less cognitively demanding strategies (e.g., methods to increase positive affect) may be an important next step in reducing their risk for pain and pain-related suffering.
It is currently unclear why we did not observe a group difference in attentional modulation of NFR. This may stem from the unreliability of attentional modulation of NFR. Specifically, some studies have noted that attention-demanding tasks inhibit NFR [54, 55], whereas others have found that they have no effect or even enhance NFR [56–59]. Thus, further research is needed to evaluate the reliability of attentional modulation of NFR.
Putative Neural Mechanisms for the Anger-In and Pain Relationship
This study also provides additional evidence that emotion and attention modulate pain through different mechanisms. In their innovative research on the topic, Villemure and Bushnell tested pain in the context of pleasant and unpleasant odors and asked participants to either focus on the odors or on the pain [60–62]. In doing so, they were able to independently manipulate emotion and attention as well as study the supraspinal correlates of these modulatory processes. They found that emotional odor modulation was associated with pain-evoked activity in the anterior cingulate cortex, medial thalamus, and primary and secondary somatosensory cortices. Further, the lateral inferior frontal cortex and periaqueductal gray were identified as regions central to emotional modulation [62]. By contrast, attentional modulation was associated with pain-evoked activity in the anterior insular cortex. The superior posterior parietal cortex and entorhinal cortex were identified as regions potentially central to attentional modulation of pain [62]. Given these findings, persons who tend to inhibit anger may have difficulty engaging this latter circuit to effectively modulate pain. This is consistent with research that implicates the insular cortex as an important region in inhibition of negative emotion [63, 64]. Thus, chronic inhibition of anger may hijack the anterior insular cortex and reduce its involvement in pain-modulation strategies.
It is noteworthy that we did not observe group differences in NFR threshold, three-stimulus threshold, emotional modulation of NFR, or attentional modulation of NFR. Given that all of these tasks assess aspects of spinal nociception, this indicates that anger-in does not exert its effects by engaging cerebrospinal mechanisms to amplify spinal nociceptive neurons. By contrast, we found that anger-in is associated with greater electric pain, cold pressor pain, and disrupted attentional modulation of pain. Together, this suggests that a purely supraspinal (e.g., cortico-cortical) mechanism accounts for how inhibition of anger affects pain (but see [65]). However, without measuring a supraspinal index of nociception (e.g., pain-related somatosensory evoked potential, functional magnetic resonance imaging of pain matrix [66, 67]), we cannot rule out the possibility that anger-in creates an upward bias of pain report without changing supraspinal pain signaling.
Limitations
The present study had multiple strengths such as its measurement of physiological and subjective outcomes, use of a well-validated emotional modulation paradigm, use of powerful MLM, and use of computer-presented questionnaires and prerecorded instructions to minimize experimental bias. Groups also included male and female participants who were ethnically/racially diverse, which improves generalizability of findings.
However, the study also had some limitations. First, participants were all healthy, pain-free individuals; therefore, it is unclear whether similar findings would be noted in clinical populations, like those with chronic pain. Second, sample size differences between high (n = 27) and low (n = 71) anger-in may have limited our statistical power. Third, use of high and low extremes for anger-in may inflate effect sizes, leading to an inability to generalize results outside of the specified levels (low anger-in and high anger-in). Fourth, while trait anger management styles were captured in the AEI, it is unclear whether participants truly employed anger inhibition during ECON procedures. Future studies should assess anger responses to pictures to determine whether anger was being inhibited.
Summary
This study found that individuals characterized as high on a measure of anger inhibition (anger-in) experienced greater pain in response to painful electric and cold stimuli, suggesting hyperalgesia. Moreover, these individuals displayed inhibited corrugator response and decreased unpleasantness during unpleasant (mutilation) pictures and greater reported arousal during pleasant (erotic) pictures. These individuals also experienced disrupted attentional modulation of pain, without showing a disruption of emotional modulation of pain, emotional modulation of NFR, or attentional modulation of NFR. Moreover, there were no group differences in NFR threshold or three-stimulus threshold (measures of spinal nociception). Together, these findings support a limited cognitive resource model such that chronic anger inhibition may lead to a deficit in subsequent self-regulatory resources, like attentional modulation. In turn, this may increase the risk of future chronic pain due to an inability to successfully cope with pain (i.e., the ability to distract themselves during pain). Moreover, the effects of anger-in on pain appear to be solely due to supraspinal (e.g., cortico-cortical) mechanisms because no measure of spinal nociception was associated with anger-in tendencies.
Acknowledgements
This research was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD007807. E. Lannon, S. Palit, and Y. Güereca were supported by a National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors report no conflicts of interest.
Authors’ Contributions T. A. Toledo, N. Hellman, E. W. Lannon, C. A. Sturycz, B. L. Kuhn, M. F. Payne, S. Palit and Y. M. Güereca, collected data and contributed to the writing of the manuscript. T. A. Toledo & J. L. Rhudy analyzed data. J. O. Shadlow & J. L. Rhudy supervised and designed the study.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Okifuji A. Anger and pain. In: Gebhart GF, Schmidt Robert F, eds. Encyclopedia of Pain. Springer, 2013: 147–151. [Google Scholar]
- 2. Tamir M. What do people want to feel and why? Pleasure and utility in emotion regulation. Curr Dir Psychol Sci. 2009;18:101–105. [Google Scholar]
- 3. Webb TL, Miles E, Sheeran P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol Bull. 2012;138:775–808. [DOI] [PubMed] [Google Scholar]
- 4. Hatch JP, Schoenfeld LS, Boutros NN, Seleshi E, Moore PJ, Cyr-Provost M. Anger and hostility in tension-type headache. Headache. 1991;31:302–304. [DOI] [PubMed] [Google Scholar]
- 5. Pilowsky I, Spence N. Pain, anger and illness behaviour. Pain. 1977;3:288–289. [DOI] [PubMed] [Google Scholar]
- 6. Duckro PN, Chibnall JT, Tomazic TJ. Anger, depression, and disability: A path analysis of relationships in a sample of chronic posttraumatic headache patients. Headache. 1995;35:7–9. [DOI] [PubMed] [Google Scholar]
- 7. Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Anger management style, hostility and spouse responses: Gender differences in predictors of adjustment among chronic pain patients. Pain. 1996;64:445–453. [DOI] [PubMed] [Google Scholar]
- 8. Bruehl S, Chung OY, Burns JW. Differential effects of expressive anger regulation on chronic pain intensity in CRPS and non-CRPS limb pain patients. Pain. 2003;104:647–654. [DOI] [PubMed] [Google Scholar]
- 9. Kerns RD, Rosenberg R, Jacob MC. Anger expression and chronic pain. J Behav Med. 1994;17:57–67. [DOI] [PubMed] [Google Scholar]
- 10. Gelkopf M. Laboratory pain and styles of coping with anger. J Psychol. 1997;131:121–123. [DOI] [PubMed] [Google Scholar]
- 11. Burns JW, Kubilus A, Bruehl S. Emotion induction moderates effects of anger management style on acute pain sensitivity. Pain. 2003;106:109–118. [DOI] [PubMed] [Google Scholar]
- 12. Burns JW, Bruehl S, Caceres C. Anger management style, blood pressure reactivity, and acute pain sensitivity: Evidence for “Trait x Situation” models. Ann Behav Med. 2004;27:195–204. [DOI] [PubMed] [Google Scholar]
- 13. Quartana PJ, Yoon KL, Burns JW. Anger suppression, ironic processes and pain. J Behav Med. 2007;30:455–469. [DOI] [PubMed] [Google Scholar]
- 14. Quartana PJ, Burns JW. Painful consequences of anger suppression. Emotion. 2007;7:400–414. [DOI] [PubMed] [Google Scholar]
- 15. Burns JW, Quartana PJ, Bruehl S. Anger inhibition and pain: Conceptualizations, evidence and new directions. J Behav Med. 2008;31:259–279. [DOI] [PubMed] [Google Scholar]
- 16. Schmeichel BJ. Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. J Exp Psychol Gen. 2007;136:241–255. [DOI] [PubMed] [Google Scholar]
- 17. Luck SJ, Hillyard SA, Mouloua M, Hawkins HL. Mechanisms of visual-spatial attention: Resource allocation or uncertainty reduction? J Exp Psychol Hum Percept Perform. 1996;22:725–737. [DOI] [PubMed] [Google Scholar]
- 18. Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): Do affective valence and arousal play a role? Pain. 2008;136:250–261. [DOI] [PubMed] [Google Scholar]
- 19. Rhudy JL, Bartley EJ, Williams AE. Habituation, sensitization, and emotional valence modulation of pain responses. Pain. 2010;148:320–327. [DOI] [PubMed] [Google Scholar]
- 20. Rhudy JL, DelVentura JL, Terry EL, et al. . Emotional modulation of pain and spinal nociception in fibromyalgia. Pain. 2013;154:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy M, Lebuis A, Peretz I, Rainville P. The modulation of pain by attention and emotion: A dissociation of perceptual and spinal nociceptive processes. Eur J Pain. 2011;15:e1–e10. [DOI] [PubMed] [Google Scholar]
- 22. Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. [DOI] [PubMed] [Google Scholar]
- 23. Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. [DOI] [PubMed] [Google Scholar]
- 24. Zautra A, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–795. [DOI] [PubMed] [Google Scholar]
- 25. Hamilton NA, Zautra AJ, Reich JW. Affect and pain in rheumatoid arthritis: Do individual differences in affective regulation and affective intensity predict emotional recovery from pain? Ann Behav Med. 2005;29:216–224. [DOI] [PubMed] [Google Scholar]
- 26. Lumley MA, Cohen JL, Borszcz GS, et al. . Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. [DOI] [PubMed] [Google Scholar]
- 28. Knight RG, Chisholm BJ, Paulin JM, Waal-Manning HJ. The Spielberger anger expression scale: Some psychometric data. Br J Clin Psychol. 1988;27(Pt 3):279–281. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD, Sydeman SJ. State-trait anxiety inventory and state-trait anger expression inventory. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 1994:292–321. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- 30. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 31. Edens JL, Gil KM. Experimental induction of pain: Utility in the study of clinical pain. Behav Ther. 1995;26:197–216. [Google Scholar]
- 32. Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain. 2004;5:233–237. [DOI] [PubMed] [Google Scholar]
- 33. Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 2007;128:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willer JC, Bussel B. Possible explanation for analgesia mediated by direct spinal effect of morphine. Lancet. 1980;1:158–159. [DOI] [PubMed] [Google Scholar]
- 35. Roby-Brami A, Bussel B, Willer JC, Le Bars D. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli. Probable involvement of a supraspinal loop. Brain. 1987;110(Pt 6):1497–1508. [DOI] [PubMed] [Google Scholar]
- 36. Palit S, Bartley EJ, Kuhn BL, et al. . Endogenous inhibition of pain and spinal nociception in women with premenstrual dysphoric disorder. J Pain Res. 2016;9:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartley EJ, Rhudy JL. Endogenous inhibition of the nociceptive flexion reflex (NFR) and pain ratings during the menstrual cycle in healthy women. Ann Behav Med. 2012;43:343–351. [DOI] [PubMed] [Google Scholar]
- 38. France CR, Froese SA, Stewart JC. Altered central nervous system processing of noxious stimuli contributes to decreased nociceptive responding in individuals at risk for hypertension. Pain. 2002;98:101–108. [DOI] [PubMed] [Google Scholar]
- 39. Arendt-Nielsen L, Nielsen J, Petersen-Felix S, Schnider TW, Zbinden AM. Effect of racemic mixture and the (S+)-isomer of ketamine on temporal and spatial summation of pain. Br J Anaesth. 1996;77:625–631. [DOI] [PubMed] [Google Scholar]
- 40. Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- 41. Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD and Nadel L, eds. Cognitive Neuroscience of Emotion. Oxford University Press; 2000:242–276. [Google Scholar]
- 42. Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. [DOI] [PubMed] [Google Scholar]
- 43. Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE. Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology. 2009;46:939–948. [DOI] [PubMed] [Google Scholar]
- 44. Wilcox RR, Keselman HJ. Modern robust data analysis methods: Measures of central tendency. Psychol Methods. 2003;8:254–274. [DOI] [PubMed] [Google Scholar]
- 45. Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- 47. Burns JW, Quartana P, Bruehl S. Anger suppression and subsequent pain behaviors among chronic low back pain patients: Moderating effects of anger regulation style. Ann Behav Med. 2011;42:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106:95–103. [DOI] [PubMed] [Google Scholar]
- 49. Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- 50. Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. [DOI] [PubMed] [Google Scholar]
- 51. Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. [DOI] [PubMed] [Google Scholar]
- 52. Roy M, Piché M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci U S A. 2009;106:20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychol Bull. 2000;126:247–259. [DOI] [PubMed] [Google Scholar]
- 54. Bathien N, Morin C. [Comparing variations of spinal reflexes during intensive and selective attention (author’s transl)]. Physiol Behav. 1972;9:533–538. [DOI] [PubMed] [Google Scholar]
- 55. Willer JC, Boureau F, Albe-Fessard D. Supraspinal influences on nociceptive flexion reflex and pain sensation in man. Brain Res. 1979;179:61–68. [DOI] [PubMed] [Google Scholar]
- 56. McIntyre D, Edwards L, Ring C, Parvin B, Carroll D. Systolic inhibition of nociceptive responding is moderated by arousal. Psychophysiology. 2006;43:314–319. [DOI] [PubMed] [Google Scholar]
- 57. Edwards L, Ring C, France CR, et al. . Nociceptive flexion reflex thresholds and pain during rest and computer game play in patients with hypertension and individuals at risk for hypertension. Biol Psychol. 2007;76:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Edwards L, Ring C, McIntyre D, et al. . Increases in arousal are associated with reductions in the human nociceptive flexion reflex threshold and pain ratings: Evidence for dissociation between nociception and pain. J Psychophysiol. 2006;20:259–266. [Google Scholar]
- 59. Petersen KL, al’Absi M, France C, Wittmers LE. Acute mental challenge reduces nociceptive flexion in men and women. Psychophysiol. 2001;38. [Google Scholar]
- 60. Villemure C, Bushnell MC. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain. 2002;95:195–199. [DOI] [PubMed] [Google Scholar]
- 61. Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: Deciphering the roles of emotion and attention. Pain. 2003;106:101–108. [DOI] [PubMed] [Google Scholar]
- 62. Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shiba Y, Oikonomidis L, Sawiak S, et al. . Converging prefronto-Insula-Amygdala pathways in negative emotion regulation in marmoset monkeys. Biol Psychiatry. 2017;82:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. [DOI] [PubMed] [Google Scholar]
- 65. Lannon E, Terry EL, Thompson K, Rhudy JL. Is anger management style associated with descending modulation of spinal nociception? J Appl Biobehav Res. 2017. [Google Scholar]
- 66. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. [DOI] [PubMed] [Google Scholar]
- 67. Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia–when the spine echoes what the brain expects. Pain. 2007;130:137–143. [DOI] [PubMed] [Google Scholar]