Abstract
Context
Previous meta-analyses evaluating the association between nut consumption and the risk of cardiovascular disease (CVD) had substantial methodological limitations and lacked recently published large prospective studies; hence, making an updated meta-analysis highly desirable.
Objective
To update the clinical guidelines for nutrition therapy in relation to the European Association for the Study of Diabetes (EASD), a systematic review and meta-analysis of prospective studies was conducted using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to summarize the evidence of the association between total nuts, specific types of nuts, and the incidence of, and mortality from, CVD outcomes.
Data sources
Relevant articles were identified by searching the PubMed and Cochrane databases.
Data extraction
Two independent researchers screened the articles to identify those that met the inclusion criteria.
Data analysis
The inverse variance method with fixed-effect or random-effects models was used to pool data across studies (expressed as risk ratio [RR] and 95% confidence interval [CI]). Heterogeneity was tested and quantified using the Cochrane Q test and I2-statistic, respectively. The GRADE system was used to assess the quality of the evidence.
Results
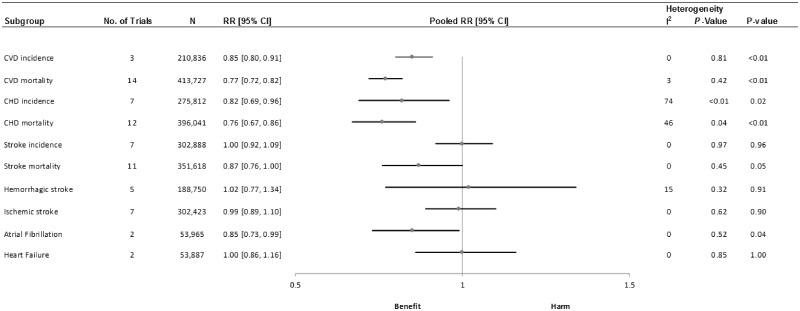
Nineteen studies were included in the analyses. The results revealed an inverse association between total nut consumption (comparing highest vs lowest categories) and CVD incidence (RR, 0.85; 95%CI, 0.800.91; I2, 0%), CVD mortality (RR, 0.77; 95%CI, 0.72–0.82; I2, 3%), coronary heart disease (CHD) incidence (RR, 0.82; 95%CI, 0.69–0.96; I2, 74%), CHD mortality (RR, 0.76; 95%CI, 0.67–0.86; I2, 46%), stroke mortality (RR, 0.83; 95%CI, 0.75–0.93; I2, 0%), and atrial fibrillation (RR, 0.85; 95%CI, 0.73–0.99; I2, 0%). No association was observed with stroke incidence and heart failure. The certainty of the evidence ranged from moderate to very low.
Conclusions
This systematic review and meta-analysis revealed a beneficial role of nut consumption in reducing the incidence of, and mortality from, different CVD outcomes.
Keywords: cardiovascular disease, meta-analysis, nuts, peanut butter, peanuts, tree nuts, walnuts
INTRODUCTION
Cardiovascular disease (CVD) is an important public health issue. According to the World Health Organization (WHO), it is the leading cause of death worldwide, affecting approximately 17.7 million people in 2015.1 Importantly, CVDs are susceptible to behavior modifications. In this sense, a healthy diet is one of the lifestyle components that could be promoted to help address this global health concern.2 Different healthy dietary patterns, such as the Mediterranean diet (MedDiet),3 the DASH diet4 or a vegetarian diet5 have nuts as a key food component. Nuts, despite their high fat content (mainly unsaturated fatty acids),6 are also rich in minerals, vitamins, fiber, and bioactive compounds.7 Given this exceptional nutritional profile, frequent nut consumption has been inversely associated with a lower risk of CVD in large prospective cohort studies, which have been summarized in several meta-analyses.8–20 Nonetheless, some of the previous meta-analyses had methodological limitations, such as the inclusion of studies with nuts plus seeds or legumes as exposure; the inclusion of studies combining different outcomes across analyses (eg, inclusion of studies with only fatal CHD [coronary heart disease] outcome in the CVD mortality analysis); and the inclusion of studies without the first category of exposure as reference. Moreover, since publication of the last meta-analyses, the results of two new large prospective cohort studies evaluating the association between nut consumption and CVD outcomes have been published.21,22 One study reported updated results from the Nurses’ Health Study I (NHSI), Nurses’ Health Study II (NSHII), and Health Professionals Follow-up Study (HPFS) comprising up to 32 years of follow-up and a large number of cases.21 Another reported the association between nut consumption and the incidence of 7 CVD outcomes in a population of 32 911 males.22 Importantly, most of the previous meta-analyses have focused on total nut intake, and only a few have taken into account the potential associations between specific types of nut consumption and the risk of CVD outcomes, which may vary considerably.
Therefore, taking into consideration the aforementioned issues and in order to develop evidence-based recommendations, the Diabetes and Nutrition Study group (DNSG) of the EASD (European Association for the Study of Diabetes) commissioned a systematic review and meta-analysis (SRMA) of prospective cohort studies using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to summarize the evidence of the association between the consumption of total nuts or specific types of nuts, and the incidence of, and mortality from, certain CVD outcomes. The shape of the associations with linear and non-linear dose-response analysis was also evaluated.
METHODS
The current systematic review and meta-analysis followed the methodological guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.23 Results are reported according to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.24 The protocol is available at http://www.crd.york.ac.uk/PROSPERO/ (identifier, PROSPERO 2018 CRD42018103360).
Search strategy
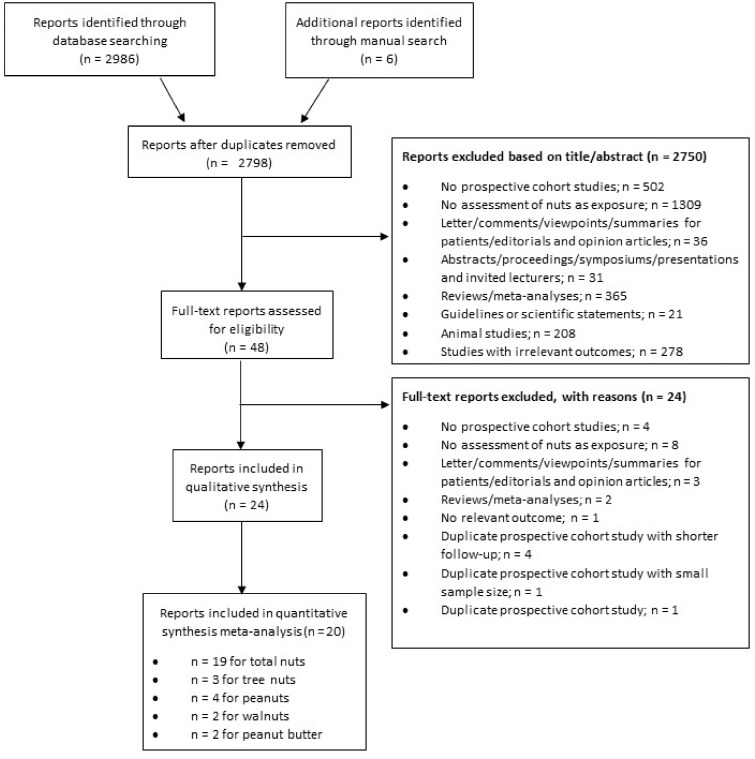
A systematic search, limited to human studies with no language restrictions, was conducted of the MEDLINE (PubMed) and Cochrane Library databases through 5 June 2018. An updated search was then performed on March 19, 2019. Table 1 shows the PICOS (participants, interventions/exposures, comparators, outcomes and study design) criteria used to identify studies eligible for inclusion. The electronic search was supplemented with a manual review of the reference lists of the retrieved articles. Figure 1 and Table S1 in the Supporting Information online summarize the search and selection process.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Participants | General population of adults | Aged <18 years |
| Intervention/exposure | Nut consumption (including total nuts, or subtypes of nuts, eg, walnuts, almonds, peanuts, peanut butter, hazelnuts) | Dietary intakes do not include total nut consumption or different subtypes of nut consumption |
| Comparison | Extreme quantiles | Risk estimate on continuous scale |
| Outcome | Incidence of, or mortality from, cardiovascular disease, coronary heart disease, stroke, heart failure, atrial fibrillation | Other cardiovascular disease outcomes |
| Study design | Prospective cohort studies | Cross-sectional, case-control, ecological, retrospective observational studies, clinical trials, and non-human studies |
Figure 1.
Flow diagram of the literature search and selection process
Study selection
An initial screening of all titles and abstracts of the retrieved articles was performed to evaluate compliance with the eligibility criteria. Inclusion criteria were prospective cohort studies with at least 1 year of follow-up, conducted in an adult population; with total nuts or specific types of nuts as exposure; with the incidence of, or mortality from, CVD, CHD, stroke, heart failure (HF), or atrial fibrillation (AF) as the outcome; and reporting effect estimators as odds ratios (ORs), risk ratios (RRs), or hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs). When more than 1 article from the same study was revealed, both papers were included if the end points were different (ie, AF in one article and stroke in the other). When multiple publications from the same study reported the same outcome, the study with the longest follow-up was selected for inclusion. Proceedings or published abstracts were not included in the present systematic review and meta-analysis.
Two publications were identified for the Nurses’ Health Study I and Health Professionals Follow-up Study, which evaluated the association between nut consumption and CVD outcomes in a whole population cohort study21 and in individuals with diabetes only.25 Therefore, for the present meta-analysis the study conducted in the whole cohort, where participants with diabetes where also included, was selected. Similarly, for The Netherlands Cohort Study, two different articles were also identified: one published in 201526 and another one in 2019.27 However, in both cases the total sample size and the period of follow-up were the same. Therefore, the 2015 publication26 was included because its main aim fitted better with the objective of the present systematic review and meta-analysis.
Data extraction
Two independent reviewers (N.B.-T. and I.P.-G.) reviewed the full text of the articles that were selected in the first phase of the screening process. Using a standardized proforma, the following relevant information was extracted from those studies that met all of the inclusion criteria: authors, journal and year of publication, study design, cohort name, country or study location, total sample size, characteristics of subjects, follow-up duration, sources of findings, type of exposure and method used for its assessment, outcome and method assessment, effect estimators (OR, RR, or HR and 95% confidence intervals), and statistical analyses. When necessary, authors were contacted by email to obtain additional information relevant to the analyses. Any disagreement was resolved by discussion or, if necessary, by a third author (J.S.-S.).
Quality of the included studies
The Newcastle-Ottawa scale (NOS) was utilized to assess the quality of the included studies.28 It is a rating scale from 0 to 9, where points are allocated according to 3 different domains: population selection, outcome assessment, and comparability of the groups. A maximum of 4, 3, and 2 points were allotted to each study after evaluation of the aforementioned domains. High-quality studies were considered those studies with a total score of at least 7 points. Disagreements in grading the quality of the studies were resolved through consensus between the reviewers.
Outcomes
The primary outcomes were CVD incidence (including only nonfatal or a combination of nonfatal and fatal outcomes of a composite of different CVD outcomes) and CVD mortality, which only included a composite of different fatal CVD end points. Secondary outcomes included incidence of nonfatal or a combination of nonfatal and fatal outcomes, and mortality from fatal outcomes, ie, CHD, stroke, AF, and HF.
Studies that reported fatal CHD and nonfatal myocardial infarction separately were combined using a fixed-effects model to generate an overall estimate for CHD incidence.29,30 In the same way, following the same procedure, ischemic stroke and intracerebral hemorrhage outcomes,22 and fatal ischemic stroke and fatal hemorrhagic stroke31 end points, were combined to obtain an overall estimate for stroke incidence and stroke mortality, respectively.
Statistical analyses
The generic inverse variance method with a random-effects model (≥5 comparisons) or fixed-effects model (<5 comparisons) was used to pool the natural log-transformed RRs for CVD incidence and mortality outcomes, to compare highest vs lowest categories of nut consumption. For one study32 that reported results using the second category of nut consumption, rather than the lowest one, as the reference, the RR and its corresponding 95%CI were recalculated following the Hamling et al33 method, using the first category as the reference.
Heterogeneity among studies was estimated using Cochran’s Q test and quantified by the I2 statistic. Statistical significance was set at P < 0.10, and an I2 value ≥ 50% was considered to reflect substantial heterogeneity.
Meta-regression analysis was performed in order to assess whether a priori specified study characteristics (ie, sex, follow-up, geographical area, NOS scale and its individual domains) may have affected the overall effect estimates. This subgroup analysis was only conducted if at least 10 study comparisons were available.23
Sensitivity analysis, excluding 1 study at a time and recalculating the summary estimates, was performed to ascertain the influence of individual studies on the summary estimates. If the removal of a study yielded a change in the level of significance, magnitude (by >10%), or direction of the pooled risk estimates, or changed the evidence of heterogeneity, then it was considered as influential.
Linear dose-response analysis for total nut consumption and different CVD outcomes was conducted following the 2-stage generalized least-squares trend (GLST) estimation method developed by Greenland and Longnecker34 and Orsini et al.35 In the first stage, the method fits the dose-response model within each study, and in the second stage it combines study-specific trends. Data on RRs and the corresponding 95%CIs, total number of participants, cases, and doses for at least 3 categories of nut consumption were needed to carry out this method. The mean or median of nut consumption from each exposure category was used if it was directly reported. For those studies that did not report this information, the midpoint between the upper and lower boundaries was assigned when ranges of nut consumption were available. For studies that reported open-ended extreme categories, a width equal to the adjacent category was assumed in order to estimate the upper or lower cutoff value. Some studies reported the information on nut consumption in grams, and others in servings. Therefore, servings were converted to grams, where 1 serving equated to 28 g, unless authors specified other serving sizes.
Potential nonlinear association between nut consumption and CVD outcomes was assessed using restricted cubic splines (MKSPLINE procedure), which were combined using multivariate meta-analysis. The departure from linearity was assessed by the Wald test constraining the regression coefficient for the second spline equal to zero.36
Publication bias was tested by the visual inspection of the funnel plots for asymmetry and statistically Begg’s test and Egger’s test. When few studies are included in the analysis, the power of the tests is too low; therefore, publication bias was only examined if more than 10 study comparisons were included in the analysis.23 Statistical significance was set at P < 0.05.
Data analysis was performed using Review Manager (RevMan) software version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014 and STATA version 15 software (StataCorp LP, College Station, Texas).
Grading the evidence
The GRADE system was used to rate the overall quality and the strength of the evidence. The quality of evidence for each outcome was categorized as high, moderate, low, or very low. This system regards observational studies as low-quality evidence.37 The level of evidence can be upgraded or downgraded according to different specified criteria. Determinants to downgrade included study design and execution limitations,38 inconsistency,39 indirectness,40 imprecision,41 and publication bias.42 Determinants to upgrade included large magnitude effect, dose-response gradient, and attenuation by plausible confounding effects.43 Discrepancies in ratings of the evidence quality were resolved by consensus between N.B.-T. and I.P.-G.
RESULTS
Study selection process
The present systematic review and meta-analysis included 19 prospective studies from the 2992 identified articles (Figure 1). Three study comparisons (1 report) were included in the meta-analysis for total CVD,21 14 study comparisons (9 reports) for CVD mortality,21,26,31,44–49 7 study comparisons (5 reports) for CHD,21,22,29,30,50 12 study comparisons (8 reports) for CHD mortality,21,22,26,30,31,44,48,51 7 study comparisons (5 reports) for stroke,21,22,32,52,53 11 study comparisons (7 reports) for stroke mortality,21,26,31,32,47,48,51 5 study comparisons (4 reports) for hemorrhagic stroke,22,52–54 7 study comparisons (5 reports) for ischemic stroke,21,22,32,52,53 2 study comparisons for AF,22,55 and 2 study comparisons for HF.22,56
Four studies57–60 that reported the risk estimate on continuous scale instead of categories of nut consumption were identified and therefore were not included in the high vs low categories of consumption analyses and the dose-response analyses.
Study characteristics
The characteristics of the included studies are presented in Table 221,22,26,29–32,44,46–60. Publication date ranged from 1992 to 2018. Six of the studies originated from Europe, 13 from America, 1 from Asia, 1 from Australia, 1 from both China and the USA, and 1 from both China and Germany. The duration of follow-up ranged from 4.3 to 28.7 years. All the studies assessed nut intake via a food frequency questionnaire. The vast majority of studies (70.9%) were of high quality according to the NOS scale. Tables S1–S5 in the Supporting Information online describe the characteristics of the included studies by type of nut consumption.
Table 2.
Characteristics of the included studies evaluating the association between nuts and risk of cardiovascular disease outcomes
| Study | Country | Study name | Population | Nut consumption assessment method | Type of nuts | Nut intake | Age, y | Follow-up (mean, median, or range), y | Outcome | Incident cases | Funding source | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraser et al (1992)29 | USA | Seventh-day Adventists | 26 473 M/F | Self-administered semiquantitative FFQ | Not specified |
|
≥25 | 6 | Nonfatal MI | 134 | Agency | 7 |
| CHD mortality | 463 | |||||||||||
| Albert et al (2002)30 | USA | Physicians’ Health Study | 21 454 M | Self-administered semiquantitative FFQ | Not specified |
|
40–84 | 17 | CHD mortality | 566 | Agency | 7 |
| Nonfatal MI | 1037 | |||||||||||
| Blomhoff et al (2006)44 | USA |
|
31 778 F | Self-administered FFQ | Nuts plus peanut butter |
|
55–69 | 15 | CVD mortality | 1675 | Agency | 6 |
| CHD mortality | 948 | |||||||||||
| Djoussé et al (2008)56 | USA | Physicians’ Health Study | 20 976 M | Unvalidated, self-administered FFQ | Not specified |
|
40–84 | 19.6 | Heart failure | 1093 | NR | 6 |
| Nettleton et al (2008)57 | USA | Atherosclerosis Risk in Communities Study | 14 153 M/F | Interview-administered FFQ | Nuts plus peanut butter | Continuous: 1 serving/d | 45–64 | 13 | Heart failure | 1140 | Agency | 8 |
| Djoussé et al (2010)52 | USA | Physicians’ Health Study | 21 078 M | Unvalidated, self-administered FFQ | Not specified |
|
40–84 | 21.1 | Stroke | 1424 | Agency | 6 |
| Ischemic stroke | 1189 | |||||||||||
| Hemorrhagic stroke | 219 | |||||||||||
| Bernstein et al (2012)54 | USA | Nurses’ Health Study I | 80 010 F | Validated, self-administered FFQ | Not specified |
|
30–55 | 26 | Hemorrhagic stroke | 1383 | Agency | 6 |
| Health Professionals Follow-Up Study | 43 150 M |
|
40–75 | 22 | Hemorrhagic stroke | 829 | ||||||
| Khawaja et al (2012)55 | USA | Physicians’ Health Study | 21 054 M | Unvalidated, self-administered FFQ | Not specified |
|
40–84 | 24 | Atrial fibrillation | 3317 | Agency | 7 |
| Yaemsiri et al (2012)58 | USA | Women’s Health Initiative Observational Study | 87 025 F | Self-administered FFQ | Not specified | Continuous: 1 serving/d | 50–79 | 7.6 | Ischemic stroke | 1049 | Agency | 8 |
| von Ruesten et al (2013)59 | Germany | EPIC-Potsdam study | 23 531 M/F | Self-administered semiquantitative FFQ | Peanuts, walnuts, brazil nuts | Continuous: 1 serving/d | 35–65 | 8 | CVD | 363 | Agency | 8 |
| Guasch-Ferré et al (2013)46 | Spain | PREDIMED study | 7216 M/F | Interview-administered, validated, semiquantitative FFQ | Almonds, peanuts, hazelnuts, pistachios, pine nuts |
|
55–80 | 4.8 | CVD mortality | 81 | Agency-Industry | 9 |
| Bonaccio et al (2015)48 | Italy | Moli-sani study |
|
Validated, self-administered FFQ | Walnuts, hazelnuts, almonds, peanuts |
|
>35 | 4.3 | CVD mortality | 104 | Agency | 6 |
| CHD mortality | 39 | |||||||||||
| Stroke mortality | 19 | |||||||||||
| di Giuseppe et al (2015)32 | Germany | EPIC-Potsdam study | 25 997 M/F | Validated, self-administered FFQ | Peanuts, walnuts, brazil nuts |
|
F: 49.2M: 52.5 | 8.3 | Stroke | 288 | Agency | 8 |
| Ischemic stroke | 235 | |||||||||||
| Stroke mortality | 36 | |||||||||||
| Gopinath et al (2015)51 | Australia | Blue Mountains Eye Study | 1312 F | Validated, self-administered FFQ | Not specified |
|
≥49 | 15 | CVD mortality | 258 | Agency | 7 |
| IHD mortality | 188 | |||||||||||
| Stroke mortality | 101 | |||||||||||
| 1581 M | CVD mortality | 288 | ||||||||||
| IHD mortality | 242 | |||||||||||
| Stroke mortality | 75 | |||||||||||
| Haring et al (2014)50 | USA | Atherosclerosis Risk in Communities Study | 12 066 M/F | Interview-administered FFQ | Not specified |
|
45–64 | 22 | CHD | 1147 | Agency | 9 |
| Haring et al (2015)53 | USA | Atherosclerosis Risk in Communities Study | 11 601 M/F | Interview-administered FFQ | Nuts plus peanut butter |
|
45–64 | 22.7 | Stroke | 699 | Agency | 8 |
| Hemorrhagic stroke | 114 | |||||||||||
| Ischemic stroke | 598 | |||||||||||
| Hshieh et al (2015)47 | USA | Physicians’ Health Study | 20 742 M | Unvalidated, self-administered FFQ | Not specified |
|
40–84 | 9.6 | CVD mortality | 760 | Agency | 6 |
| Stroke mortality | 143 | |||||||||||
| Luu et al (2015)31 | USA | Southern Community Cohort Study | 71 764 M/F | Semi-quantitative FFQ | Total nuts and peanut butter |
|
40–79 | 5.4 | CVD mortality | 1857 | Agency | 8 |
| IHD mortality | 793 | |||||||||||
| Ischemic stroke mortality | 121 | |||||||||||
| Hemorrhagic stroke mortality | 96 | |||||||||||
| China | Shanghai Women’s Health Study and Shanghai Men's Health Study | 134 265 M/F | Peanut |
|
40–70 and 40–74 | 12.2 and 6.5 | CVD mortality | 2587 | ||||
| IHD mortality | 631 | |||||||||||
| Ischemic stroke mortality | 588 | |||||||||||
| Hemorrhagic stroke mortality | 597 | |||||||||||
| van den Brandt and Schouten (2015)26 | Netherlands | Netherlands Cohort Study | 3202 M/F (subcohort) | Self-administered validated FFQ | Tree nuts, peanuts |
|
55–69 | 9 | CVD mortality | 2985 | NR | 7 |
| IHD mortality | 1488 | |||||||||||
| Stroke mortality | 565 | |||||||||||
| Wang et al (2016)60 | China | Linxian NIT cohort | 2445 M/F | FFQ | Peanuts, chestnuts, walnuts | Continuous: 3 servings/mo | 40–69 | 26 | Heart disease mortality | 355 | Agency | 6 |
| Germany | Stroke mortality | 452 | ||||||||||
| Eslamparast et al (2017)49 | Iran | Golestan Cohort Study | 28 257 F | Validated, self-administered, semiquantitative FFQ | Peanuts, tree nuts |
|
40–87 | 7 | CVD mortality | 911 | Agency | 7 |
| 20 855 M | 1105 | |||||||||||
| Guasch-Ferré et al (2017)21 | USA | Nurses’ Health Study I | 76 364 F | Validated, self-administered FFQ | Peanuts, other nuts, and walnuts (if available) |
|
30–55 | 28.7 | CVD | 6727 | Agency | 7 |
| CVD mortality | 1770 | |||||||||||
| CHD | 3552 | |||||||||||
| CHD mortality | 996 | |||||||||||
| Stroke | 3322 | |||||||||||
| Stroke mortality | 773 | |||||||||||
| Ischemic stroke | 1635 | |||||||||||
| Nurses’ Health Study II | 92 946 F | 25–42 | 21.5 | CVD | 1915 | |||||||
| CVD mortality | 82 | |||||||||||
| CHD | 670 | |||||||||||
| CHD mortality | 46 | |||||||||||
| Stroke | 1262 | |||||||||||
| Stroke mortality | 36 | |||||||||||
| Ischemic stroke | 220 | |||||||||||
| Health Professionals Follow-Up Study | 41 526 M | 40–75 | 22.5 | CVD | 5494 | |||||||
| CVD mortality | 2599 | |||||||||||
| CHD | 4168 | |||||||||||
| CHD mortality | 1921 | |||||||||||
| Stroke | 1326 | |||||||||||
| Stroke mortality | 367 | |||||||||||
| Ischemic stroke | 742 | |||||||||||
| Larsson et al (2018)22 | Sweden | Cohort of Swedish Men | 32 911 M | FFQ | Nuts (not including coconut or chestnuts) |
|
45–83 | 17 | MI | 4983 | Agency | 8 |
| MI mortality | 917 | |||||||||||
| Heart failure | 3160 | |||||||||||
| Atrial fibrillation | 7550 | |||||||||||
| Ischemic stroke | 3782 | |||||||||||
| Intracerebral hemorrhage | 543 |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; F, females; FFQ, food frequency questionnaire; IHD, ischemic heart disease; M, males; MI, myocardial infarction; NIT: Nutrition Intervention Trials; NOS, Newcastle-Ottawa scale; NR, Not reported; PREDIMED, Prevención con Dieta MEDiterránea.
High vs low categories of consumption analyses
Nuts and cardiovascular disease incidence
Three cohort comparisons, involving 210 836 participants and 14 136 cases, analyzed the association between nut consumption and the risk of CVD incidence. The summary RR (95%CI) for high vs low categories of nut consumption was 0.85 (0.80–0.91) with no evidence of interstudy heterogeneity (I2 = 0%; Pheterogeneity, 0.81) (Figure 2 and Figure S1 in the Supporting Information online). Regarding specific types of nuts, consumption of tree nuts ([RR, 0.85; 95%CI, 0.79–0.91]; I2, 0%; Pheterogeneity, 0.70), peanuts ([RR, 0.87; 95%CI, 0.81–0.93]; I2, 0%; Pheterogeneity, 0.67), and walnuts ([RR, 0.81; 95%CI, 0.71–0.92]; I2, 73%; Pheterogeneity, 0.03) was associated with a lower risk of CVD incidence after comparing highest vs lowest categories of consumption (Table 3 and Figures S2–S4 in the Supporting Information online). No association was reported between peanut butter consumption and the risk of CVD incidence ([RR, 0.98; 95%CI, 0.93–1.03]; I2, 89%; Pheterogeneity, <0.01) (Table 3 and Figure S5 in the Supporting Information online).
Figure 2.
Summary plots of effect estimates from prospective cohort studies evaluating the association between nut consumption and the risk of different cardiovascular outcomes. Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; RR, risk ratio
Table 3.
Summary RR of cardiovascular disease outcomes by different types of nut consumption (comparing highest vs lowest categories)
| No. of cohorts | No. of participants | No. of cases | RR (95%CI) | P for heterogeneity | I 2 (%) | |
|---|---|---|---|---|---|---|
| Tree nuts | ||||||
| CVD | 3 | 210 836 | 14 136 | 0.85 (0.79, 0.91) | 0.70 | 0 |
| CVD mortality | – | – | – | – | – | – |
| Stroke | 3 | 210 836 | 5910 | 1.00 (0.89, 1.11) | 0.93 | 0 |
| Stroke mortality | 3 | 118 962 | 1851 | 0.93 (0.77, 1.13) | 0.44 | 0 |
| CHD | 3 | 210 836 | 8390 | 0.77 (0.70, 0.84) | 0.08 | 61 |
| CHD mortality | – | – | – | – | – | – |
| Peanuts | ||||||
| CVD | 3 | 210 836 | 14 136 | 0.87 (0.81, 0.93) | 0.67 | 0 |
| CVD mortality | 2 | 134 265 | 5572 | 0.77 (0.70, 0.85) | 0.81 | 0 |
| Stroke | 3 | 210 836 | 5910 | 0.90 (0.81, 0.99) | 0.32 | 13 |
| Stroke mortality | 4 | 253 227 | 3036 | 0.83 (0.73, 0.95) | 0.07 | 57 |
| CHD | 3 | 210 836 | 8390 | 0.85 (0.79, 0.92) | 0.55 | 0 |
| CHD mortality | 2 | 134 265 | 2119 | 0.75 (0.64, 0.88) | 0.46 | 0 |
| Walnuts | ||||||
| CVD | 3 | 144 021 | 5255 | 0.81 (0.71, 0.92) | 0.03 | 73 |
| CVD mortality | – | – | – | – | – | – |
| Stroke | 3 | 144 021 | 5910 | 0.85 (0.71, 1.02) | 0.19 | 39 |
| Stroke mortality | – | – | – | – | – | – |
| CHD | 3 | 144 021 | 2685 | 0.79 (0.66, 0.94) | 0.04 | 69 |
| CHD mortality | – | – | – | – | – | – |
| Peanut butter | ||||||
| CVD | 3 | 210 836 | 14 136 | 0.98 (0.93, 1.03) | <0.01 | 89 |
| CVD mortality | – | – | – | – | – | – |
| Stroke | 3 | 210 836 | 5910 | 0.94 (0.87, 1.02) | <0.01 | 86 |
| Stroke mortality | – | – | – | – | – | – |
| CHD | 3 | 210 836 | 8390 | 1.00 (0.94, 1.07) | 0.17 | 43 |
| CHD mortality | – | – | – | – | – | – |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; RR, risk ratio.
Nuts and cardiovascular disease mortality
Fifteen cohort comparisons analyzed the association between nut consumption and CVD mortality, including 413 727 participants and 14 475 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.77 (0.72–0.82) with no evidence of interstudy heterogeneity (I2, 3%; Pheterogeneity, 0.42) (Figure 2 and Figure S6 in the Supporting Information online). Regarding specific types of nuts, only peanuts have been studied in relation to CVD mortality, showing a summary RR of 0.77 (95%CI, 0.70–0.85) for high vs low categories of consumption, with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.81) (Table 3 and Figure S7 in the Supporting Information online).
Nuts and coronary heart disease incidence
Seven cohort comparisons analyzed the association between nut consumption and CHD incidence, including 275 812 participants and 12 654 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.82 (0.69–0.96) with evidence of substantial interstudy heterogeneity (I2, 74%; Pheterogeneity <0.01) (Figure 2 and Figure S8 in the Supporting Information online). Regarding specific types of nuts, consumption of tree nuts ([RR, 0.77; 95%CI, 0.70–0.84]; I2, 61%; Pheterogeneity, 0.08), peanuts ([RR, 0.85; 95%CI, 0.79–0.92]; I2, 0%; Pheterogeneity, 0.55), and walnuts ([RR, 0.791; 95%CI, 0.66–0.94]; I2, 69%; Pheterogeneity, 0.04) was associated with a lower risk of CHD incidence after comparing highest vs lowest categories of consumption (Table 3 and Figures S9–S11 in the Supporting Information online). No association was reported between peanut butter consumption and the risk of CHD incidence ([RR, 1.00; 95%CI, 0.94–1.07]; I2, 43%; Pheterogeneity, 0.17) (Table 3 and Figure S12 in the Supporting Information online).
Nuts and coronary heart disease mortality
Thirteen cohort comparisons analyzed the association between nut consumption and CHD mortality, including 396 041 participants and 7877 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.76 (0.67–0.86) with evidence of moderate interstudy heterogeneity (I2, 46%; Pheterogeneity, 0.04) (Figure 2 and Figure S13 in the Supporting Information online). Regarding specific types of nuts, peanut consumption was inversely associated with the risk of CHD mortality after comparing high vs low categories of consumption ([RR, 0.75; 95%CI, 0.64–0.88]; I2, 0%; Pheterogeneity, 0.46) (Table 3 and Figure S14 in the Supporting Information online).
Nuts and stroke incidence
Seven cohort comparisons analyzed the association between nut consumption and stroke incidence, including 302 888 participants and 12 646 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 1.00 (0.92–1.09) with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.97) (Figure 2 and Figure S15 in the Supporting Information online). Regarding specific types of nuts, peanut consumption was associated with a lower risk of stroke incidence after comparing highest vs lowest categories of consumption ([RR, 0.90; 95%CI, 0.81–0.99]; I2, 13%; Pheterogeneity, 0.32) (Table 3 and Figure S16 in the Supporting Information online). No association was observed between tree nut, walnut, and peanut butter consumption and the risk of stroke incidence (Table 3 and Figures S17–S19 in the Supporting Information online).
Nuts and stroke mortality
Twelve cohort comparisons analyzed the association between nut consumption and stroke mortality, including 351 618 participants and 2332 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.83 (0.75–0.93) with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.45) (Figure 2 and Figure S20 in the Supporting Information online). Regarding specific types of nuts, peanut consumption was associated with a lower risk of stroke mortality after comparing highest vs lowest categories of consumption ([RR, 0.85; 95%CI, 0.79–0.92]; I2, 0%; Pheterogeneity, 0.55) (Table 3 and Figure S21 in the Supporting Information online). No association was reported between tree nut consumption and the risk of stroke death (Table 3 and Figure S22 in the Supporting Information online).
Nuts and hemorrhagic stroke
Five cohort comparisons analyzed the association between nut consumption and hemorrhagic stroke incidence, involving 188 750 participants and 3088 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 1.02 (0.77–1.34) with no evidence of interstudy heterogeneity (I2, 15%; Pheterogeneity, 0.32) (Figure 2 and Figure S23 in the Supporting Information online). No study analyzed the association between different types of nuts and the risk of hemorrhagic stroke.
Nuts and ischemic stroke
Seven cohort comparisons analyzed the association between nut consumption and ischemic stroke incidence, involving 302 423 participants and 8401 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.99 (0.89–1.10) with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.62) (Figure 2 and Figure S24 in the Supporting Information online). No study analyzed the association between different types of nuts and the risk of hemorrhagic stroke.
Nuts and atrial fibrillation
Two cohort comparisons analyzed the association between nut consumption and AF, involving 53 965 participants and 10 867 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 0.85 (0.73–0.99) with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.52) (Figure 2 and Figure S25 in the Supporting Information online). No study analyzed the association between different types of nuts and the risk of AF.
Nuts and heart failure
Two cohort comparisons analyzed the association between nut consumption and HF, involving 53 887 participants and 4253 cases. The summary RR (95%CI) for high vs low categories of nut consumption was 1.00 (0.86–1.16) with no evidence of interstudy heterogeneity (I2, 0%; Pheterogeneity, 0.85) (Figure 2 and Figure S26 in the Supporting Information online). No study analyzed the association between different types of nuts and the risk of HF.
Dose-response analyses
Figures S27–S34 in the Supporting Information online show the linear and non-linear dose-response analyses between total nut consumption and CVD outcomes. The summary RR (95%CI) for a 28-g/d increment was 0.87 (0.81–0.93) for CVD incidence, 0.71 (0.61–0.84) for CVD mortality, 0.75 (0.64–0.88) for CHD incidence, 0.67 (0.52–0.87) for CHD mortality, 1.06 (0.97–1.15) for stroke incidence, 1.01 (0.88–1.18) for stroke mortality, 1.05 (0.77–1.43) for hemorrhagic stroke, and 1.06 (0.86–1.31) for ischemic stroke.
Total nut consumption and the risk of CVD incidence (Figure S27 in the Supporting Information online), CVD mortality (Figure S28 in the Supporting Information online), stroke mortality (Figure S32 in the Supporting Information online), and hemorrhagic stroke (Figure S33 in the Supporting Information online) showed a non-linear association (Pnon-linearity <0.01). The reduction in the risk of CVD incidence was observed up to a consumption of 10 g/d, with no further reduction with higher consumptions (Table S6 in the Supporting Information online). For CVD mortality and CHD mortality, there was a steeper reduction in the risk at approximately 15–20 g/d, with no further reduction with a higher consumption (Table S6 in the Supporting Information online). The reduction in the risk of stroke mortality was observed up to a consumption of 5 g/d, with no significant reductions above this amount (Table S6 in the Supporting Information online). The association between total nuts and hemorrhagic stroke appeared to be J-shaped with a risk reduction up to 5 g/d, but there was a slight non-significant positive association at intakes of 25 g/d (Table S6).
There was no evidence of non-linear association for the other outcomes.
Sensitivity analyses
Table S7 in the Supporting Information online shows the sensitivity analysis by the removal of one study at a time. Regarding total nut consumption, no trial modified the magnitude, direction, or significance of the pooled estimates or the evidence for heterogeneity for total CVD incidence and mortality, stroke incidence and mortality, HF, or AF. Removal of the Guasch-Ferré et al study (NHSI)21 changed the pooled estimates of total CHD incidence from significant to nonsignificant. Removal of the Larsson et al22 study explained the heterogeneity for CHD death (I2, 16%; Pheterogeneity, 0.28). With regard to different types of nuts, removal of the Guasch-Ferré et al study (HPFS) and Guasch-Ferré study (NHSII)21 modified the significance of the pooled RR for peanut consumption and total stroke from significant to nonsignificant, and removal of the Bao et al45 study explained the heterogeneity (I2, 0%; Pheterogeneity, 0.77). In the case of peanut butter, removal of the Guasch-Ferré et al study (NHSII)21 explained the heterogeneity for total CVD (I2, 0%; Pheterogeneity, 0.60) and for total stroke (I2, 0%; Pheterogeneity, 0.53). Furthermore, removal of the Guasch-Ferré et al study (NHSI)21 changed the significance of the pooled estimates for total stroke from nonsignificant to significant. Regarding walnuts, removal of the Guasch-Ferré et al study (HPFS)21 changed the magnitude of the RR for total CVD and CHD and the pooled estimates became significant for total stroke. Removal of the Guasch-Ferré et al study (NHSI)21 changed the significance of the pooled estimates for total CVD and CHD from significant to nonsignificant. Finally, removal of the Guasch-Ferré et al study (NHSII)21 explained the heterogeneity (I2, 0%; Pheterogeneity, 0.53) for CHD and changed the significance of the pooled estimates from significant to nonsignificant.
Subgroup analyses
Subgroup analyses could only be conducted for CVD, CHD, and stroke death. Figures S35–S40 in the Supporting Information online show the a priori subgroup analyses for the aforementioned outcomes. The meta-regression analysis revealed no evidence of effect modification by sex, duration of follow-up, or NOS quality score and its individual domains. However, the risk of CVD death was modified by geographical area (P = 0.03). In studies conducted in America, nut consumption was inversely associated with the risk of CVD mortality (RR, 0.68; 95%CI, 0.61–0.77), whereas no association was observed in those studies conducted in Europe (RR, 0.91; 95%CI, 0.72–1.14) or Oceania (RR, 0.95; 95%CI, 0.71–1.28). Geographical area explained 38.9% of the total heterogeneity (I2, 47%; Pheterogeneity, 0.04). No effect modification by geographical area was observed for CHD mortality and stroke mortality.
Publication bias
Figures S41–S43 in the Supporting Information online show the funnel plots used to assess publication bias for death from CVD, CHD, and stroke (the only outcomes with more than 10 study comparisons in the analyses). There was no statistical evidence of small study effects based on visual inspection of the funnel plots with either Egger’s test or Begg’s test (all P > 0.05).
Grading of the evidence
Table 4 shows the GRADE assessment for the certainty of the evidence for the association between total nut consumption and the risk of CVD outcomes. The evidence was rated as moderate for CVD mortality and CHD mortality; low for CVD incidence and stroke mortality; and very low for CHD incidence, stroke incidence, hemorrhagic stroke, ischemic stroke, AF, and HF. Tables S8–S11 in the Supporting Information online show the GRADE assessment for the association between subtypes of nut consumption and the risk of CVD outcomes. The overall certainty of the evidence was graded as very low for all subtypes of nut consumption and CVD outcomes.
Table 4.
GRADE assessment of the systematic review and meta-analysis of prospective cohort studies assessing the association between total nut consumption and cardiovascular disease outcomes
| Outcome | No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerationsa | Relative (95%CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| CVD incidence | 3 | Observational studies | Not serious | Not serious | Seriousb | Not serious | Dose-response gradientc | RR 0.85 (0.80 to 0.91) | ⊕⊕⊕◯◯ LOW |
| CVD mortality | 14 | Observational studies | Not serious | Not serious | Not serious | Not serious | Dose-response gradientd | RR 0.77 (0.72 to 0.82) | ⊕⊕⊕◯ MODERATE |
| CHD incidence | 7 | Observational studies | Not serious | Seriouse | Seriousf | Seriousg | Dose-response gradienth | RR 0.82 (0.69 to 0.96) | ⊕◯◯◯ VERY LOW |
| CHD mortality | 12 | Observational studies | Not serious | Not serious | Not serious | Not serious | Dose-response gradienti | RR 0.76 (0.67 to 0.86) | ⊕⊕⊕◯ MODERATE |
| Stroke incidence | 7 | Observational studies | Not serious | Not serious | Seriousj | Seriousk | None | RR 1.00 (0.92 to 1.09) | ⊕◯◯◯ VERY LOW |
| Stroke mortality | 11 | Observational studies | Not serious | Not serious | Not serious | Seriousl | Dose-response gradientm | RR 0.87 (0.76 to 1.00) | ⊕⊕◯◯ LOW |
| Hemorrhagic stroke | 5 | Observational studies | Seriousn | Not serious | Seriouso | Seriousp | Dose-response gradientq | RR 1.02 (0.77 to 1.34) | ⊕◯◯◯ VERY LOW |
| Ischemic stroke | 7 | Observational studies | Not serious | Not serious | Seriousr | Seriouss | None | RR 0.99 (0.89 to 1.10) | ⊕◯◯◯ VERY LOW |
| Atrial fibrillation | 2 | Observational studies | Not serious | Not serious | Serioust | Seriousu | None | RR 0.85 (0.73 to 0.99) | ⊕◯◯◯ VERY LOW |
| Heart failure | 2 | Observational studies | Seriousv | Not serious | Seriousw | Seriousx | None | RR 1.00 (0.86 to 1.16) | ⊕◯◯◯ VERY LOW |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; GLST, generalized least squares trend; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; NOS, Newcastle-Ottawa scale, RR, risk ratio.
Publication bias could not be assessed in meta-analyses that included <10 trial comparisons. Therefore, for these outcomes, no downgrades were made for publication bias.
Serious indirectness for CVD incidence, as the included studies were conducted among health professionals and >50% of the weight (69.30%) was contributed by studies conducted among males.
Upgrade for a dose-response gradient, as the MKSPLINE dose-response analyses showed a significant nonlinear inverse relationship between total nut consumption and CVD incidence (P < 0.01); see Figure S27 in the Supporting Information online.
Upgrade for a dose-response gradient, as the MKSPLINE dose-response analyses showed a significant nonlinear inverse relationship between total nut consumption and CVD mortality (P < 0.01); see Figure S28 in the Supporting Information online.
Serious inconsistency for CHD incidence due to high degree of unexplained heterogeneity (I2 = 74%, P = 0.001).
Serious indirectness for CHD incidence, as >50% of the weight (55.4%) was contributed by studies conducted among health professionals.
Serious imprecision for CHD incidence, as the 95%CI (0.69–0.96) overlapped with the minimally important difference for clinical benefit (RR 0.95).
Upgrade for a dose-response gradient, as the GLST dose-response analyses revealed a significant linear inverse relationship between total nut consumption and CHD incidence (P < 0.01); see Figure S29 in the Supporting Information online.
Upgrade for a dose-response gradient, as the MKSPLINE dose-response analyses showed a significant nonlinear inverse relationship between total nut consumption and CHD mortality (P < 0.01); see Figure S30 in the Supporting Information online.
Serious indirectness for stroke incidence, as >50% of the weight (72.7%) was contributed by studies conducted among health professionals.
Serious imprecision for stroke incidence as the 95%CI (0.92–1.09) overlapped with the minimally important difference for clinical benefit (RR 0.95) and harm (RR 1.05).
Serious imprecision for stroke mortality, as the 95%CI (0.76–1.00) overlapped with the minimally important difference for clinical benefit (RR 0.95).
Upgrade for a dose-response gradient, as the MKSPLINE dose-response analyses showed a significant nonlinear inverse relationship between total nut consumption and stroke mortality (P = 0.029); see Figure S32 in the Supporting Information online.
Serious risk of bias for hemorrhagic stroke, as >50% of the weight (68.7%) was contributed by studies considered to be at high risk of bias (NOS < 7).
Serious indirectness for hemorrhagic stroke, as >50% of the weight (68.7%) was contributed by studies conducted among health professionals and >50% of the weight (55.7%) was contributed by studies conducted among males.
Serious imprecision for hemorrhagic stroke as the 95%CI (0.77–1.34) overlapped with the minimally important difference for clinical benefit (RR 0.95) and harm (RR 1.05).
Upgrade for a dose-response gradient, as the MKSPLINE dose-response analyses showed a significant nonlinear inverse relationship between total nut consumption and hemorrhagic stroke (P = 0.01); see Figure S33 in the Supporting Information online.
Serious indirectness for ischemic stroke, as >50% of the weight (66.1%) was contributed by studies conducted among health professionals.
Serious imprecision for ischemic stroke, as the 95%CI (0.89–1.10) overlapped with the minimally important difference for clinical benefit (RR 0.95) and harm (RR 1.05).
Serious indirectness for atrial fibrillation, as only 2 available studies were conducted among males.
Serious imprecision for atrial fibrillation, as the 95%CI (0.73–0.99) overlapped with the minimally important difference for clinical benefit (RR 0.95).
Serious risk of bias for heart failure, as >50% of the weight (65.40%) was contributed by a study considered to be at high risk of bias (NOS < 7).
Serious indirectness for heart failure, as only 2 available studies were conducted among males.
Serious imprecision for heart failure, as the 95%CI (0.86–1.16) overlapped with the minimally important difference for clinical benefit (RR 0.95) and harm (RR 1.05).
DISCUSSION
The results of the present systematic review and meta-analysis of prospective cohort studies showed a significant inverse association between total nut consumption and the risk of CVD incidence and mortality, CHD incidence and mortality, and AF. There was no association between total nut consumption and stroke incidence or mortality, hemorrhagic stroke, ischemic stroke, and HF. Regarding specific types of nuts, tree nut consumption was associated with a lower risk of CVD and CHD incidence, while peanut consumption was associated with a lower incidence of, and mortality from, CVD, stroke, and CHD, and walnut consumption with a lower incidence of CVD, stroke, and CHD. No association was observed between peanut butter consumption and CVD outcomes.
Several previous meta-analyses have focused on summarizing data regarding nut consumption and different CVD outcomes.8–20 It is important to highlight that some limitations in terms of methodology were present, such as the inclusion of studies with nuts plus seeds or fruits as exposure9,10,16,17 or the arbitrary combination of different end points across the analyses (eg, the inclusion of studies with a cause-specific CVD outcome in CVD).9,14,18–20 The present meta-analysis attempted to deal with these methodological issues by including exclusively those studies that reported only nut consumption as exposure. Additionally, in the analyses of CVD incidence, only those studies evaluating a composite of non-fatal, or a combination of nonfatal and fatal CVD events, were included. Similarly, for CVD mortality, only studies that evaluated a composite of fatal CVD events were considered. For secondary outcomes, the same definition criteria as in the primary outcomes were applied.
The results of the present study, regarding total nut consumption, are highly consistent with one of the most recent meta-analyses in this field,15 which showed an inverse association between nut consumption and different CVD outcomes, and which also took into account the aforementioned methodological issues at the time of performing their analyses. Recently, one study conducted only among individuals with diabetes observed similar results. Those individuals consuming ≥5 servings of total nuts per week presented a lower risk of CVD incidence (HR, 0.83; 95%CI, 0.71–0.98), CHD incidence (HR, 0.80; 95%CI, 0.67–0.96), and CVD mortality (HR, 0.66; 95%CI, 0.52–0.84) than those consuming less than 1 serving per month.25
Results regarding specific types of nuts are in line with those of previous meta-analyses, which also showed an inverse association between tree nut consumption and peanut consumption and the risk of CVD incidence, CVD mortality, CHD incidence, and CHD mortality,9,11,15 and no association with stroke incidence.9,15 In a recent analysis conducted only among individuals with diabetes, the results were similar for tree nut consumption, which was also associated with a lower risk of CVD incidence and mortality and CHD incidence. However, the findings revealed no association between peanut consumption and CVD outcomes.25 Although the present meta-analysis also evaluated the association between walnut consumption and peanut butter consumption and the risk of CVD, CHD, and stroke incidence, the data was sourced from only one report, which included information from three different cohorts: the NHSI, NHSII, and HPFS.21 The lack of association between peanut butter consumption and the risk of CVD outcomes may be due to the addition of salt and hydrogenated fats, which could counteract the beneficial effect of other nutrients present in raw peanuts. At present, owing to the limited number of studies included in previous meta-analyses and in the present analyses, and considering the high degree of interstudy heterogeneity, it is unclear whether different types of nuts are associated with CVD outcomes.
Different potential mechanisms have been proposed to explain the beneficial association observed between nut consumption and different CVD outcomes. Nuts are rich in unsaturated fatty acids, proteins, different minerals (including potassium and magnesium), vitamins (including vitamin C and E), and phenolic compounds. This unique nutritional profile means that nuts possess different properties that beneficially modify CVD risk factors and therefore reduce the risk of CVD. In fact, the ability to lower total cholesterol and low-density lipoprotein (LDL)-cholesterol levels is probably one of the best-known properties of nuts, as was demonstrated by a pooled analysis of 25 intervention trials,61 and more recently in one meta-analysis of 61 randomized controlled trials.62 Other possible mechanisms include a reduction in circulating levels of inflammatory cytokines (especially C-reactive protein), the modulation of nitric oxide production, an improvement in endothelial function, and a reduction in oxidative stress.63,64
The present analysis has some strengths that should be elucidated. First, a comprehensive systematic search strategy was used to identify all available prospective cohort studies. Second, studies reporting only nut consumption as exposure were included. Third, the certainty of the evidence was assessed using the GRADE approach.
However, the present systematic review and meta-analysis also has some limitations. Subgroup analyses for most of the outcomes could not be performed because less than 10 study comparisons were available. Measurement error in the evaluation of nut consumption could not be ruled out because all included studies used food frequency questionnaires for this purpose. Because of this limitation, along with the possibility of residual confounding because of the observational nature of the included studies, GRADE-assessed prospective cohort studies tend to be of lower quality than other types of prospective studies. Another important limitation is that the certainty of the evidence in the effect estimates, regarding total nut consumption, was moderate only for two outcomes (CVD mortality and CHD mortality), and it was considered as low and very low for the others, mainly owing to downgrading for indirectness and imprecision. Therefore, future research is very likely to change the confidence in the effect estimates.
CONCLUSION
The present systematic review and meta-analysis provides the most updated and comprehensive summary estimates of the association between total nut consumption, different subtypes, and CVD outcomes. The results suggest a beneficial role of total nut consumption in reducing the incidence of, and mortality from, different CVD outcomes. Future research should focus on specific types of nuts in order to better clarify their effect on CVD outcomes.
Supplementary Material
Acknowledgments
Author contributions. N.B.-T. and J.S.-S. designed the study. N.B.-T., J.S.-S., and I.P.-G. contributed to data acquisition. N.B.-T., J.S.-S., and I.P.-G. analyzed and interpreted data. N.B.-T., J.S.-S., I.P.-G., C.W.C.K., H.K., D.R., and J.L.S. drafted and critically revised the manuscript for important intellectual content. N.B.-T. and J.S.-S. are the guarantors of this work, have full access to the data of the study, and take responsibility for the accuracy of the data analysis and the integrity of the data.
Funding. The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) commissioned this systematic review and meta-analysis and provided funding and logistical support for meetings as part of their development of clinical practice guidelines for nutrition therapy. J.S.-S. was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Canadian Diabetes Association Clinician Scientist award, CIHR INMD/CNS New Investigator Partnership Prize, and Banting & Best Diabetes Centre Sun Life Financial New Investigator Award. With the exception of the Clinical Practice Guidelines Committee of the DNSG of the EASD, none of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript, or decision to publish. I.P.-G. has received grants from the Spanish Ministry of Education, Culture and Sports (FPU 17/01925).
Declaration of interest. J.S.-S. reports serving on the board of the International Nut and Dried Fruit Council, and the Eroski Foundation, and receiving grant support from these entities through his institution. He also reports serving on the Executive Committee of the Instituto Danone, Spain. He has received research funding from the Instituto de Salud Carlos III, Spain; Ministerio de Educación y Ciencia, Spain; Departament de Salut Pública de la Generalitat de Catalunya, Catalonia, Spain; and the European Commission. He has also received research funding from the California Walnut Commission, Sacramento CA, USA; Patrimonio Comunal Olivarero, Spain; La Morella Nuts, Spain; and Borges S.A., Spain. He reports receiving consulting fees or travel expenses from Danone, the California Walnut Commission, the Eroski Foundation, the Instituto Danone - Spain, Nuts for Life, the Australian Nut Industry Council, Nestlé, Abbott Laboratories, and Font Vella Lanjarón. He is on the Clinical Practice Guidelines Expert Committee of the European Association for the study of Diabetes (EASD), and has served on the Scientific Committee of the Spanish Food and Safety Agency, and the Spanish Federation of Food, Nutrition and Dietetic Societies. He is a member of the International Carbohydrate Quality Consortium (ICQC), and Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD. J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, and WhiteWave Foods. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Mott's LLP, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, and Wirtschaftliche Vereinigung Zucker e.V. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Sobeys Inc. C.W.C.K. has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, American Pistachio Growers, Barilla, Calorie Control Council, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, Pulse Canada, Saskatchewan Pulse Growers, and Unilever. He has received in-kind research support from the Almond Board of California, American Peanut Council, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Quaker (PepsiCo), Primo, Unico, Unilever, and WhiteWave Foods. He has received travel support and/or honoraria from the American Peanut Council, American Pistachio Growers, Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sabra Dipping Co., Saskatchewan Pulse Growers, Sun-Maid, Tate & Lyle, Unilever, and WhiteWave Foods. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, Oldways Preservation Trust, Paramount Farms, and Pulse Canada. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a director of the Toronto 3 D Knowledge Synthesis and Clinical Trials foundation. D.R. is the president of the Croatian Society for Diabetes and Metabolic Disorders of the Croatian Medical Association. He serves as an Executive Committee member of the Croatian Endocrine Society, Croatian Society for Obesity, and Croatian Society for Endocrine Oncology. He was a board member and secretary of IDF Europe and currently he is the chair of the IDF YLD Program. He has served as an Executive Committee member of the Diabetes and Nutrition Study Group of the EASD and currently he serves as a Executive Committee member of Diabetes and Cardiovascular Disease Study Group of the EASD. He has served as principal investigator or coinvestigator in clinical trials of AstraZeneca, Eli Lilly, MSD, Novo Nordisk, Sanofi Aventis, Solvay, and Trophos. He has received honoraria for speaking or advisory board engagements and consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Lifescan – Johnson & Johnson, Novartis, Novo Nordisk, MSD, Merck Sharp & Dohme, Pfizer, Pliva, Roche, Salvus, Sanofi Aventis, and Takeda. No competing interests were declared by N.B.-T., I.P.-G., and H.K.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Search strategy
Table S2 Characteristics of the included studies evaluating the association between tree nut consumption and the risk of cardiovascular disease outcomes, comparing highest vs lowest categories
Table S3 Characteristics of the included studies evaluating the association between peanut consumption and the risk of cardiovascular disease outcomes, comparing highest vs lowest categories
Table S4 Characteristics of the included studies evaluating the association between walnut consumption and the risk of cardiovascular disease outcomes, comparing highest vs lowest categories
Table S5 Characteristics of the included studies evaluating the association between peanut butter consumption and the risk of cardiovascular disease outcomes, comparing highest vs lowest categories
Table S6 Risk ratio and 95%CI from non-linear dose-response analysis of total nuts and cardiovascular disease, coronary heart disease, and stroke
Table S7 Sensitivity analysis by systematic exclusion of one study at a timea
Table S8 GRADE assessment of the systematic review and meta-analysis of prospective cohort studies assessing the association between tree nut consumption and cardiovascular disease outcomes
Table S9 GRADE assessment of the systematic review and meta-analysis of prospective cohort studies assessing the association between peanut consumption and cardiovascular disease outcomes
Table S10 GRADE assessment of the systematic review and meta-analysis of prospective cohort studies assessing the association between walnut consumption and cardiovascular disease outcomes
Table S11 GRADE assessment of the systematic review and meta-analysis of prospective cohort studies assessing the association between peanut butter consumption and cardiovascular disease outcomes
Figure S1 Association between nut consumption (highest vs lowest categories) and the risk of cardiovascular disease
Figure S2 Association between tree nut consumption (highest vs lowest categories) and the risk of cardiovascular disease
Figure S3 Association between peanut consumption (highest vs lowest categories) and the risk of cardiovascular disease
Figure S4 Association between walnut consumption (highest vs lowest categories) and the risk of cardiovascular disease
Figure S5 Association between peanut butter consumption (highest vs lowest categories) and the risk of cardiovascular disease
Figure S6 Association between nut consumption (highest vs lowest categories) and the risk of cardiovascular disease mortality
Figure S7 Association between peanut consumption (highest vs lowest categories) and the risk of cardiovascular disease mortality
Figure S8 Association between nut consumption (highest vs lowest categories) and the risk of coronary heart disease
Figure S9 Association between tree nut consumption (highest vs lowest categories) and the risk of coronary heart disease
Figure S10 Association between peanut consumption (highest vs lowest categories) and the risk of coronary heart disease
Figure S11 Association between walnut consumption (highest vs lowest categories) and the risk of coronary heart disease
Figure S12 Association between peanut butter consumption (highest vs lowest categories) and the risk of coronary heart disease
Figure S13 Association between nut consumption (highest vs lowest categories) and the risk of coronary heart disease mortality
Figure S14 Association between peanut consumption (highest vs lowest categories) and the risk of coronary heart disease mortality
Figure S15 Association between nut consumption (highest vs lowest categories) and the risk of stroke
Figure S16 Association between peanut consumption (highest vs lowest categories) and the risk of stroke
Figure S17 Association between tree nut consumption (highest vs lowest categories) and the risk of stroke
Figure S18 Association between walnut consumption (highest vs lowest categories) and the risk of stroke
Figure S19 Association between peanut butter consumption (highest vs lowest categories) and the risk of stroke
Figure S20 Association between nut consumption (highest vs lowest categories) and the risk of stroke mortality
Figure S21 Association between peanut consumption (highest vs lowest categories) and the risk of stroke death
Figure S22 Association between tree nut consumption (highest vs lowest categories) and the risk of stroke mortality
Figure S23 Association between nut consumption (highest vs lowest categories) and the risk of hemorrhagic stroke
Figure S24 Association between nut consumption (highest vs lowest categories) and the risk of ischemic stroke
Figure S25 Association between nut consumption (highest vs lowest categories) and the risk of atrial fibrillation
Figure S26 Association between nut consumption (highest vs lowest categories) and the risk of heart failure
Figure S27 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of cardiovascular disease incidence
Figure S28 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of cardiovascular disease mortality
Figure S29 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of coronary disease incidence
Figure S30 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of coronary disease mortality
Figure S31 Linear and non-linear dose-response relation between increasing total nut intak by 28-g increments and the risk of stroke incidence
Figure S32 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of stroke mortality
Figure S33 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of hemorrhagic stroke
Figure S34 Linear and non-linear dose-response relation between increasing total nut intake by 28-g increments and the risk of ischemic stroke
Figure S35 Subgroup analyses of total nut consumption and the risk of cardiovascular disease mortality
Figure S36 Risk of bias (Newcastle-Ottawa Scale [NOS]) subgroup analysis of total nut consumption and the risk of cardiovascular disease mortality
Figure S37 Subgroup analyses of total nut consumption and the risk of coronary heart disease mortality
Figure S38 Risk of bias (Newcastle-Ottawa Scale [NOS]) subgroup analysis of total nut consumption and the risk of coronary heart disease mortality
Figure S39 Subgroup analyses of total nut consumption and the risk of stroke mortality
Figure S40 Risk of bias (Newcastle-Ottawa Scale [NOS]) subgroup analysis of total nut consumption and the risk of stroke mortality
Figure S41 Funnel plot of the natural logarithm risk ratio for cardiovascular disease mortality
Figure S42 Funnel plot of the natural logarithm risk ratio for coronary heart disease mortality
Figure S43 Funnel plot of the natural logarithm risk ratio for stroke mortality
References
- 1. World Health Organization. WHO | Cardiovascular diseases (CVDs). 2017. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed March 27, 2018.
- 2. Yu E, Malik VS, Hu FB.. Cardiovascular disease prevention by diet modification. J Am Coll Cardiol. 2018;72:914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 4. Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–118. [DOI] [PubMed] [Google Scholar]
- 5. Craig WJ, Mangels AR; American Dietetic Association. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109:1266–1282. [DOI] [PubMed] [Google Scholar]
- 6. Ros E, Mataix J.. Fatty acid composition of nuts–implications for cardiovascular health. Br J Nutr. 2006;96:S29–S35. [DOI] [PubMed] [Google Scholar]
- 7. Segura R, Javierre C, Lizarraga MA, et al. Other relevant components of nuts: phytosterols, folate and minerals. Br J Nutr. 2006;96:S36–S44. [DOI] [PubMed] [Google Scholar]
- 8. Afshin A, Micha R, Khatibzadeh S, et al. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aune D, Keum N, Giovannucci E, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2017; 59:1071–1090. [DOI] [PubMed] [Google Scholar]
- 11. Chen G-C, Zhang R, Martínez-González MA, et al. Nut consumption in relation to all-cause and cause-specific mortality: a meta-analysis 18 prospective studies. Food Funct. 2017;8:3893–3905. [DOI] [PubMed] [Google Scholar]
- 12. Grosso G, Yang J, Marventano S, et al. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr. 2015;101:783–793. [DOI] [PubMed] [Google Scholar]
- 13. Luo C, Zhang Y, Ding Y, et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:256–269. [DOI] [PubMed] [Google Scholar]
- 14. Ma L, Wang F, Guo W, et al. Nut consumption and the risk of coronary artery disease: a dose-response meta-analysis of 13 prospective studies. Thromb Res. 2014;134:790–794. [DOI] [PubMed] [Google Scholar]
- 15. Mayhew AJ, de Souza RJ, Meyre D, et al. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr. 2016;115:212–225. [DOI] [PubMed] [Google Scholar]
- 16. Shao C, Tang H, Zhao W, et al. Nut intake and stroke risk: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2016;6:30394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi ZQ, Tang JJ, Wu H, et al. Consumption of nuts and legumes and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:1262–1271. [DOI] [PubMed] [Google Scholar]
- 18. Weng Y-Q, Yao J, Guo M-L, et al. Association between nut consumption and coronary heart disease. Coron Artery Dis. 2016;27:227–232. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Z, Xu G, Wei Y, et al. Nut consumption and risk of stroke. Eur J Epidemiol. 2015;30:189–196. [DOI] [PubMed] [Google Scholar]
- 20. Zhou D, Yu H, He F, et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100:270–277. [DOI] [PubMed] [Google Scholar]
- 21. Guasch-Ferré M, Liu X, Malik VS, et al. Nut consumption and risk of cardiovascular disease. J Am Coll Cardiol. 2017;70:2519–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsson SC, Drca N, Björck M, et al. Nut consumption and incidence of seven cardiovascular diseases. Heart. 2018;104:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. United Kingdom: The Cochrane Collaboration; 2011. [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 25. Liu G, Guasch-Ferré M, Hu Y, et al. Nut consumption in relation to cardiovascular disease incidence and mortality among patients with diabetes mellitus. Circ Res. 2019;124:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van den Brandt PA, Schouten LJ.. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: a cohort study and meta-analysis. Int J Epidemiol. 2015;44:1038–1049. [DOI] [PubMed] [Google Scholar]
- 27. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemiol. 2019;34:351–369. doi:10.1007/s10654-019-00483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2014. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 11, 2018.
- 29. Fraser GE, Sabaté J, Beeson WL, et al. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 30. Albert CM, Gaziano JM, Willett WC, et al. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med. 2002;162:1382–1387. [DOI] [PubMed] [Google Scholar]
- 31. Luu HN, Blot WJ, Xiang Y-B, et al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med. 2015;175:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. di Giuseppe R, Fjeld MK, Dierkes J, et al. The association between nut consumption and the risk of total and ischemic stroke in a German cohort study. Eur J Clin Nutr. 2015;69:431–435. [DOI] [PubMed] [Google Scholar]
- 33. Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–970. [DOI] [PubMed] [Google Scholar]
- 34. Greenland S, Longnecker MP.. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 35. Orsini N, Bellocco R, Greenland S.. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40–57. [Google Scholar]
- 36. Desquilbet L, Mariotti F.. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 37. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 38. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 39. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303–1310. [DOI] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 42. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 43. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. [DOI] [PubMed] [Google Scholar]
- 44. Blomhoff R, Carlsen MH, Andersen LF, et al. Health benefits of nuts: potential role of antioxidants. Br J Nutr. 2006;96:S52–S60. [DOI] [PubMed] [Google Scholar]
- 45. Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guasch-Ferré M, Bulló M, Martínez-González MÁ, et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. doi:10.1186/1741-7015-11-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hshieh TT, Petrone AB, Gaziano JM, et al. Nut consumption and risk of mortality in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonaccio M, Di Castelnuovo A, De Curtis A, et al. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli-sani study. Br J Nutr. 2015;114:804–811. [DOI] [PubMed] [Google Scholar]
- 49. Eslamparast T, Sharafkhah M, Poustchi H, et al. Nut consumption and total and cause-specific mortality: results from the Golestan Cohort Study. Int J Epidemiol. 2017;46:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haring B, Gronroos N, Nettleton JA, et al. Dietary protein intake and coronary heart disease in a large community based cohort: results from the Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2014;9:e109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gopinath B, Flood VM, Burlutksy G, et al. Consumption of nuts and risk of total and cause-specific mortality over 15 years. Nutr Metab Cardiovasc Dis. 2015;25:1125–1131. [DOI] [PubMed] [Google Scholar]
- 52. Djoussé L, Gaziano JM, Kase CS, et al. Nut consumption and risk of stroke in US male physicians. Clin Nutr. 2010;29:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haring B, Misialek JR, Rebholz CM, et al. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2015;46:3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bernstein AM, Pan A, Rexrode KM, et al. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khawaja O, Gaziano JM, Djousse L.. Nut consumption and risk of atrial fibrillation in the Physicians’ Health Study. Nutr J. 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Djoussé L, Rudich T, Gaziano JM.. Nut consumption and risk of heart failure in the Physicians’ Health Study I. Am J Clin Nutr. 2008;88:930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nettleton JA, Steffen LM, Loehr LR, et al. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J Am Diet Assoc. 2008;108:1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yaemsiri S, Sen S, Tinker L, et al. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol. 2012;72:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. von Ruesten A, Feller S, Bergmann MM, et al. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67:412–419. [DOI] [PubMed] [Google Scholar]
- 60. Wang J-B, Fan J-H, Dawsey SM, et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep. 2016;6:22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sabaté J, Oda K, Ros E.. Nut consumption and blood lipid levels. Arch Intern Med. 2010;170:821–827. [DOI] [PubMed] [Google Scholar]
- 62. Del Gobbo LC, Falk MC, Feldman R, et al. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coates A, Hill A, Tan S.. Nuts and cardiovascular disease prevention. Curr Atheroscler Rep. 2018;20:48. [DOI] [PubMed] [Google Scholar]
- 64. Bitok E, Sabaté J.. Nuts and cardiovascular disease. Prog Cardiovasc Dis. 2018;61:33–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.